Abstract
Although adult obesity is known to increase endometrial cancer risk, evidence for childhood obesity is limited. We prospectively examined the association between body fatness throughout life and endometrial cancer risk. 47,289 members of the Nurses’ Health Study (NHS) and 105,386 of the Nurses’ Health Study II (NHS II) recalled their body fatness at ages 5, 10, and 20 using a pictogram. Childhood and adolescent body fatness were derived as the average at ages 5 and 10, and ages 10 and 20, respectively. We obtained adult weight from concurrent questionnaires. We calculated hazard ratios (HR) of endometrial cancer using Cox proportional hazards models. During follow-up, 757 incident cases of endometrial cancer were diagnosed. Body fatness in childhood, at age 10, in adolescence, and at age 20 were positively associated with endometrial cancer risk (HR for ≥ Level 5 versus ≤ Level 2 in adolescence: 1.83 (95% CI 1.41-2.37). After adjusting for most recent BMI, none of the associations persisted. Weight change since age 18 was positively associated with endometrial cancer risk [HR for ≥ 25 kg gain versus stable: 2.54 (95% CI 1.80-3.59). Adult BMI was strongly associated with endometrial cancer risk [HR BMI ≥ 35 kg/m2 versus BMI ≤ 25 kg/m2: 4.13 (95% CI 3.29-5.16)]. In postmenopausal women, the association with BMI was significantly stronger among non-users of hormone therapy. In conclusion, obesity throughout life is positively associated with endometrial cancer risk, with adult obesity one of the strongest risk factors. Maintaining a healthy weight throughout life remains important.
INTRODUCTION
Endometrial cancer is the most common gynecological cancer in the United States, with an estimated 52,630 new cases in 2014.1 About 40% of endometrial cancer is attributed to overweight and obesity2, and thought to occur predominantly via estrogen,3, 4 but also via insulin-dependent,3 and inflammatory5 pathways. While the evidence for an association between adult obesity and endometrial cancer risk is convincing,6 the role of early life obesity is unclear.
Childhood obesity could influence development of endometrial cancer in several ways. It may result in earlier menarche,7 which increases the risk of endometrial cancer.8 Childhood obesity may reduce levels of sex-hormone binding globulin,9 which increases levels of non-SHBG-bound estrogen.10 Conversely, body fatness in childhood is associated with lower levels of insulin growth factor – 1 (IGF-1) in adults,11 which may decrease incidence of adult cancers, including endometrial cancer.
We therefore examined the association between body fatness from early childhood to adulthood, adult weight gain, and endometrial cancer risk, and whether the association between childhood body fatness and endometrial cancer was independent of adult body size. Finally, we stratified the associations by menopausal status, and among postmenopausal women, by postmenopausal hormone therapy (HT) use.
METHODS
Study Population
The study population consists of participants from the Nurses’ Health Study (NHS) and the Nurses’ Health Study II (NHS II), two prospective cohort studies that began in 1976 and 1989, respectively. Details of the NHS have been described.12 Briefly, the NHS included 121,700 female registered nurses, aged between 30 and 55 in 1976, and residing in the United States. The NHS II included 116,430 women, aged between 25 and 42 in 1989, also residing in the United States. Participants provided information on demographics, lifestyle, and medical history at baseline and every two years thereafter via questionnaires. For this study, NHS participants were followed from 1988 - 2010 and NHS II participants from 1989 – 2009, with baseline years defined according to when childhood body fatness was queried. At baseline, we excluded women who were missing information on body fatness at age 5, 10, or 20 (NHS n = 35,503; NHS II n = 3,012), BMI at age 18 (NHS n = 7,410; NHS II n =1,114), reported a prior hysterectomy (NHS n = 21,934; NHS II n =5904), a previous diagnosis of any cancer except non-melanoma skin cancer (NHS n = 5,707; NHS II n = 1,013), and women whose age was unknown (NHS n = 124; NHS II n = 0). Women missing BMI at baseline (NHS n = 849; NHS II n = 402) could enter the study in later follow-up cycles if BMI was subsequently available. The final population comprised 47,289 participants from NHS and 105,356 participants from NHS II.
Ascertainment of endometrial cancer cases
Participants reported any new diagnosis of endometrial cancer via questionnaires. These were confirmed via medical record review, including diagnosis date, histology, and stage. Case definition was restricted to invasive adenocarcinomas, as these comprise the majority of cases of endometrial cancer. All cases were confirmed in both cohorts.
Assessment of body fatness
NHS and NHS II participants recalled their body fatness at ages 5, 10, and 20 years using a figure developed by Stunkard (Figure 1)13, which can be helpful in estimating weight status in the absence of BMI. To reduce the effects of random error, we also evaluated the average of the levels at ages 5 and 10 (i.e. average childhood level) and the average of the levels at ages 10 and 20 (i.e. average adolescent level).
Figure 1.
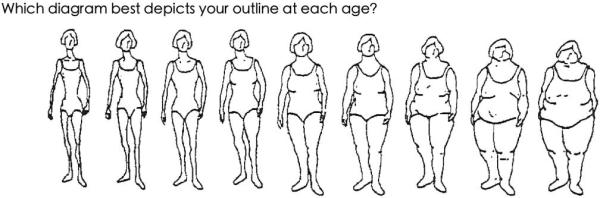
Nine-level figure for assessing body fatness at ages 5, 10, and 20 years
The long-term recall of childhood body fatness was validated during a follow-up of the Third Harvard Growth Study.14 Using the same figure, interviewed subjects who were aged 71 – 76 years at follow-up, were asked to select the outline that best reflected their body fatness at ages 5, 10, 15, and 20. In women, crude Pearson correlations between recalled body shape and measured BMI at approximately the same ages were 0.60 for age 5, 0.75 for age 10, and 0.66 for age 20. The results did not change appreciably, although attenuated, after adjusting for recent BMI.
Weight and height at age 18 and weight throughout follow-up were obtained via self-report. In a study of 184 NHS participants, the correlation between self-reported and measured weights was high, at 0.96.15 In another validation study of 118 NHS II participants, recalled weight and height were compared with college or nursing school entrance records.16 The correlation between reported weight and measured weight was 0.84, and that between self-reported height and measured height was 0.94. Therefore, the validity of self-reports is high in both cohorts.
Recent BMI was calculated as the weight from the questionnaire period prior to case diagnosis in kilograms divided by the square of the height in meters. Weight change since age 18 was calculated as the difference between reported weight from the questionnaire prior to case diagnosis and weight at age 18.
Spearman correlations between recent BMI and body fatness at ages 5, 10, and 20 were 0.15, 0.21, and 0.28 respectively in NHS, and 0.22, 0.32, and 0.40 respectively in NHS II.
Assessment of covariates
The potential risk factors included in our analyses were age, age of menarche, oral contraceptive use, age at last birth/number of pregnancies lasting 6+ months, menopausal status, HT use, smoking, physical activity, hypertension, diabetes, height, and first-degree family history of endometrial cancer and colon or rectal cancer. With the exception of family history of endometrial cancer, which was collected in 1996 for NHS and 2001 for NHS II, physical activity in adolescence and early adulthood, collected in 2004 for NHS and 1997 for NHS II, and age of menarche and height, collected in 1976 for NHS and 1989 for NHS II, all variables were updated during follow-up.
Statistical Analysis
Participants contributed person-time from baseline until 1 June 2010 for NHS and 1 June 2009 for NHS II or until they were censored due to endometrial or any other cancer (except nonmelanoma skin cancer), a hysterectomy, death, or loss to follow-up.
Body fatness at ages 5 and 10 were categorized as Level 1, 2, 3, 4, and 5-9. Because of the relatively small number of cases in NHS II, we combined the lower two levels of body fatness at age 20. Childhood and adolescent body fatness, which were the average of body fatness at ages 5 and 10, and ages 10 and 20, respectively, were categorized into four groups [Levels 1- 2, 2.5-3, 3.5-4.5, and 5-9].
Most recent body size was assessed using BMI in four groups: ≤ 24.9, 25.0-29.9, 30.0-34.9, and ≥35.0 kg/m2. If missing, BMI was imputed by BMI reported in the previous cycle. Weight change was categorized into 8 groups: ≤ −2, −2 – < 2 (stable weight), 2 – < 5, 5 – < 10, 10 – < 15, 15 – < 20, 20 – < 25, and ≥ 25 kg.
Cox proportional hazards regression was used to estimate multivariable hazard ratios (HR) of endometrial cancer, for body fatness at age 5, 10, and 20, in childhood, adolescence, recent BMI, and weight change, using indicator variables. We performed tests for linear trend by using a single ordinal variable with values l through 4 or 5, to represent the levels of body fatness/size at each time point. For weight change, the test for linear trend was performed using an ordinal variable with values 1 through 8.
The basic model was stratified on age (in months), 2-year follow-up cycle, and cohort. We additionally adjusted for smoking, oral contraceptive use, menopausal status and HT use, age at menopause, parity and age at last birth, physical activity during adolescence (average between middle school and high school physical activity), physical activity during early adulthood, recent physical activity, hypertension, diabetes, family history (mother or sister) of endometrial cancer, family history (parent or sibling) of colon or rectal cancer, and height (see Tables 2 - 5 footnotes for categories).
Table 2.
Age-and multivariable adjusted hazard ratios and 95% confidence intervals for endometrial cancer by life-course body fatness among NHS (1988-2010) and NHS II (1989-2009) participants
| No. cases/person-years | Age-adjusted HR | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + Menstrual cycle characteristics4 (95% CI) | Multivariate adjusted HR3 + recent BMI5,6 (95% CI) | |
|---|---|---|---|---|---|
| Body fatness1 at age 5, Level | |||||
| 1 | 264 / 731,584 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 193 / 731,679 | 1.10 | 1.10 (0.91-1.33) | 1.09 (0.90-1.32) | 1.09 (0.90-1.32) |
| 3 | 136 / 553,864 | 1.02 | 1.02 (0.83-1.26) | 1.00 (0.81-1.23) | 0.91 (0.73-1.11) |
| 4 | 95 / 304,037 | 1.21 | 1.19 (0.94-1.51) | 1.16 (0.91-1.47) | 0.95 (0.74-1.21) |
| 5+ | 69 / 176,878 | 1.30 | 1.27 (0.97-1.66) | 1.23 (0.94-1.61) | 0.92 (0.70-1.21) |
| P trend | 0.05 | 0.08 | 0.15 | 0.30 | |
| Body fatness1 at age 10, Level | |||||
| 1 | 190 / 554,482 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 196 / 736,442 | 1.07 | 1.09 (0.89-1.33) | 1.07 (0.87-1.31) | 1.04 (0.85-1.27) |
| 3 | 147 / 532,689 | 1.17 | 1.18 (0.95-1.47) | 1.14 (0.92-1.42) | 1.00 (0.80-1.25) |
| 4 | 100 / 374,512 | 1.12 | 1.10 (0.86-1.41) | 1.06 (0.83-1.36) | 0.83 (0.64-1.06) |
| 5+ | 124 / 299,917 | 1.58 | 1.54 (1.22-1.94) | 1.47 (1.17-1.86) | 1.05 (0.82-1.33) |
| P trend | <0.001 | 0.001 | 0.01 | 0.65 | |
| Body fatness1 at age 20, Level | |||||
| 1,2 | 270 / 839,815 | 1.00 | 1.00 | 1.00 | 1.00 |
| 3 | 222 / 915,989 | 0.95 | 0.95 (0.79-1.13) | 0.93 (0.77-1.11) | 0.82 (0.68-0.98) |
| 4 | 158 / 492,248 | 1.31 | 1.29 (1.05-1.57) | 1.24 (1.02-1.52) | 0.94 (0.77-1.16) |
| 5+ | 107 / 249,990 | 1.75 | 1.65 (1.31-2.07) | 1.61 (1.28-2.02) | 0.93 (0.73-1.20) |
| P trend | <0.001 | <0.001 | <0.001 | 0.62 | |
| Average childhood body fatness (ages 5 – 10)2, Level | |||||
| 1-2 | 381 / 1,263,067 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2.5-3 | 168 / 589,824 | 1.17 | 1.17 (0.97-1.41) | 1.14 (0.95-1.37) | 1.03 (0.85-1.24) |
| 3.5-4.5 | 138 / 483,640 | 1.17 | 1.14 (0.94-1.39) | 1.11 (0.91-1.35) | 0.89 (0.72-1.08) |
| 5+ | 70 / 161,512 | 1.47 | 1.43 (1.11-1.85) | 1.39 (1.07-1.80) | 0.97 (0.75-1.27) |
| P trend | 0.003 | 0.01 | 0.02 | 0.44 | |
| Average adolescent body fatness (ages 10 – 20)2, Level | |||||
| 1-2 | 261 / 816,097 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2.5-3 | 208 / 831,429 | 1.03 | 1.03 (0.86-1.24) | 1.01 (0.84-1.21) | 0.92 (0.76-1.11) |
| 3.5-4.5 | 211 / 697,746 | 1.28 | 1.27 (1.05-1.52) | 1.22 (1.01-1.47) | 0.93 (0.77-1.13) |
| 5+ | 77 / 152,771 | 1.92 | 1.83 (1.41-2.37) | 1.76 (1.35-2.28) | 1.03 (0.78-1.36) |
| P trend | <0.001 | <0.001 | <0.001 | 0.81 | |
| BMI at age 18, kg/m2 | |||||
| ≤ 19.9 | 216 / 931,989 | 1.00 | 1.00 | 1.00 | 1.00 |
| 20.0-21.4 | 181 / 646,539 | 1.07 | 1.10 (0.90-1.34) | 1.08 (0.88-1.31) | 0.94 (0.77-1.15) |
| 21.5-22.9 | 135 / 405,503 | 1.17 | 1.19 (0.95-1.48) | 1.15 (0.93-1.44) | 0.89 (0.71-1.11) |
| ≥23.0 | 225 / 514,012 | 1.70 | 1.64 (1.35-1.99) | 1.58 (1.30-1.92) | 0.91 (0.74-1.13) |
| <0.001 | <0.001 | <0.001 | 0.34 | ||
| Recent BMI, kg/m2 | |||||
| ≤ 24.9 | 242 / 1,323,670 | 1.00 | 1.00 | 1.00 | N/A7 |
| 25.0-29.9 | 202 / 679,660 | 1.22 | 1.23 (1.01-1.49) | 1.22 (1.00-1.47) | |
| 30.0-34.9 | 150 / 295,189 | 2.14 | 2.12 (1.71-2.63) | 2.08 (1.68-2.58) | |
| ≥35.0 | 163 / 199,524 | 4.34 | 4.13 (3.29-5.16) | 4.05 (3.24-5.07) | |
| <0.001 | <0.001 | <0.001 | |||
| Weight change since age 18, kg | |||||
| Loss (≤ − 2) | 32 / 196,640 | 0.76 | 0.76 (0.48-1.21) | 0.76 (0.48-1.21) | 0.60 (0.37-0.95) |
| Stable (−2 to + 2) | 41 / 225,427 | 1.00 | 1.00 | 1.00 | 1.00 |
| Gain (+ 2 – < 5) | 55 / 305761 | 1.11 | 1.10 (0.73-1.65) | 1.10 (0.73-1.65) | 1.15 (0.76-1.73) |
| (+ 5 – < 10) | 104 / 482,117 | 1.16 | 1.16 (0.81-1.66) | 1.16 (0.81-1.67) | 1.26 (0.87-1.81) |
| (+ 10 – < 15) | 98 / 404,216 | 1.13 | 1.11 (0.77-1.60) | 1.11 (0.77-1.60) | 1.22 (0.85-1.77) |
| (+ 15 – < 20) | 100 / 290,139 | 1.45 | 1.40 (0.97-2.02) | 1.40 (0.97-2.02) | 1.54 (1.07-2.23) |
| (+20 – < 25) | 75 / 207,044 | 1.48 | 1.43 (0.97-2.10) | 1.42 (0.96-2.09) | 1.57 (1.06-2.31) |
| (≥ + 25) | 252 / 386,699 | 2.83 | 2.54 (1.80-3.59) | 2.52 (1.78-3.55) | 2.71 (1.92-3.82) |
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | |
Participants were asked to recall their body fatness at ages 5, 10, and 20, using a nine-level drawing (Figure 1), where Level 1 corresponds to the leanest and Level 9 the most overweight.
Average childhood and adolescent body fatness were the average of the levels at ages 5 and 10, and ages 10 and 20 respectively.
Adjusted for smoking history [never, past, current], oral contraceptive use [never, <3 years, 3-5 years, > 5years], postmenopausal hormone use [premenopausal (reference), post-menopausal – never; postmenopausal – past; post-menopausal current – estrogen only; postmenopausal current – estrogen & progesterone / other], age at menopause [premenopausal/unknown, < 45 years, 45-< 47 years, 47 - <49 years, 49- <51 years, 51- <53 years, 53+ years], family history of colon/rectal cancer, family history of endometrial cancer, height (inches; continuous), parity and age at last birth[1-2 & <30 yrs, 1-2 & ≥ 30 yrs, 3-4 & < 30 yrs, 3-4 & ≥ 30 yrs, ≥ 5 children], physical activity [adolescent & age 20 models: <21, 21-<36, 36-<53, 54-<81, 81+ METS/week; weight change /recent BMI model: <3, 3-<9, 9-<18, 18-<27, 27+ METS/week]
Menstrual cycle characteristics: age of menarche [≤11, 12, 13, ≥14 years], menstrual cycle regularity at age 18 [regular, irregular/no periods]
BMI: body mass index
Weight change model adjusted for weight at age 18, not recent BMI
Recent BMI models not adjusted for recent BMI
Table 5.
Supplemental Table 1. Multivariable adjusted-hazard ratios and 95% confidence intervals for endometrial cancer by early life body fatness among NHS (1988-2010) and NHS II (1989 – 2009) participants
| NHS | NHS II | |||||
|---|---|---|---|---|---|---|
| No. cases/person-years | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + current BMI4 (95% CI) | No. cases/person-years | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + current BMI4 (95% CI) | |
| Body fatness1 at age 5, Level | ||||||
| 1 | 236 / 321,965 | 1.00 | 1.00 | 28 / 409,534 | 1.00 | 1.00 |
| 2 | 145 / 187,983 | 1.08 (0.87-1.33) | 1.06 (0.85-1.30) | 48 / 543,662 | 1.44 (0.90-2.31) | 1.39 (0.87-2.22) |
| 3 | 89 / 144,263 | 0.89 (0.70-1.14) | 0.80 (0.62-1.02) | 47 / 409,546 | 1.73 (1.08-2.77) | 1.45 (0.90-2.33) |
| 4 | 70 / 87,130 | 1.11 (0.86-1.47) | 0.92 (0.70-1.20) | 25 / 216,892 | 1.65 (0.95-2.84) | 1.20 (0.70-2.08) |
| 5+ | 50 / 61,578 | 1.13 (0.83-1.54) | 0.85 (0.62-1.17) | 19 / 115,273 | 2.02 (1.12-3.64) | 1.27 (0.69-2.34) |
| P trend | 0.54 | 0.13 | 0.01 | 0.55 | ||
| P interaction6 | 0.03 | |||||
| Body fatness1 at age 10, Level | ||||||
| 1 | 169 / 243,447 | 1.00 | 1.00 | 21 / 310,967 | 1.00 | 1.00 |
| 2 | 160 / 212,542 | 1.12 (0.90-1.39) | 1.06 (0.85-1.31) | 36 / 523,851 | 1.14 (0.66-1.96) | 1.04 (0.60-1.78) |
| 3 | 101 / 144,897 | 1.07 (0.83-1.37) | 0.91 (0.71-1.17) | 46 / 387,750 | 1.88 (1.11-3.15) | 1.43 (0.85-2.42) |
| 4 | 74 / 105,684 | 1.07 (0.81-1.41) | 0.82 (0.62-1.09) | 26 / 268,800 | 1.42 (0.79-2.53) | 0.94 (0.52-1.70) |
| 5+ | 86 / 96,349 | 1.36 (1.04-1.77) | 0.97 (0.74-1.28) | 38 / 203,538 | 2.34 (1.36-4.03) | 1.34 (0.76-2.36) |
| P trend | 0.07 | 0.32 | 0.001 | 0.43 | ||
| P interaction6 | 0.01 | |||||
| Body fatness1 at age 20, Level | ||||||
| 1-2 | 226 / 325,929 | 1.00 | 1.00 | 44 / 513,813 | 1.00 | 1.00 |
| 3 | 183 / 271,833 | 1.02 (0.83-1.24) | 0.89 (0.73-1.08) | 39 / 644,085 | 0.76 (0.49-1.17) | 0.61 (0.39-0.95) |
| 4 | 112 / 138,461 | 1.20 (0.96-1.51) | 0.91 (0.72-1.15) | 46 / 353,744 | 1.57 (1.03-2.38) | 1.00 (0.64-1.55) |
| 5+ | 69 / 66,695 | 1.43 (1.08-1.88) | 0.89 (0.67-1.20) | 38 / 183,263 | 2.15 (1.36-3.40) | 0.93 (0.55-1.59) |
| P trend | 0.01 | 0.36 | <0.001 | 0.75 | ||
| P interaction6 | 0.01 | |||||
| Average childhood body fatness (ages 5 – 10)2, Level | ||||||
| 1-2 | 327 / 452,126 | 1.00 | 1.00 | 54 / 810,829 | 1.00 | 1.00 |
| 2.5-3 | 117 / 159,702 | 1.05 (0.85-1.30) | 0.93 (0.75-1.16) | 51 / 430,074 | 1.77 (1.21-2.61) | 1.48 (1.00-2.18) |
| 3.5-4.5 | 96 / 134,150 | 1.03 (0.82-1.30) | 0.84 (0.66-1.05) | 42 / 349,457 | 1.62 (1.08-2.44) | 1.13 (0.74-1.72) |
| 5+ | 50 / 56,941 | 1.25 (0.93-1.69) | 0.90 (0.66-1.22) | 20 / 104,545 | 2.19 (1.30-3.69) | 1.29 (0.75-2.23) |
| P trend | 0.23 | 0.16 | 0.001 | 0.43 | ||
| P interaction6 | 0.01 | |||||
| Average adolescent body fatness (ages 10 – 20)2, Level | ||||||
| 1-2 | 223 / 325,593 | 1.00 | 1.00 | 38 / 490,419 | 1.00 | 1.00 |
| 2.5-3 | 168 / 236,570 | 1.07 (0.88-1.32) | 0.96 (0.78-1.18) | 40 / 594,805 | 0.95 (0.61-1.48) | 0.79 (0.50-1.24) |
| 3.5-4.5 | 148 / 193,645 | 1.18 (0.95-1.45) | 0.90 (0.73-1.12) | 63 / 504,038 | 1.62 (1.08-2.45) | 1.01 (0.65-1.57) |
| 5+ | 51 / 47,111 | 1.56 (1.14-2.13) | 0.97 (0.70-1.33) | 26 / 105,643 | 2.44 (1.45-4.12) | 1.09 (0.61-1.94) |
| P trend | 0.01 | 0.49 | <0.001 | 0.62 | ||
| P interaction6 | 0.01 | |||||
| Recent BMI, kg/m2 | ||||||
| ≤ 24.9 | 190 / 381,392 | 1.00 | N/A5 | 52 / 942,295 | 1.00 | N/A5 |
| 25.0-29.9 | 168 / 260,339 | 1.26 (1.02-1.56) | 34 / 419,079 | 1.17 (0.75-1.81) | ||
| 30.0-34.9 | 121 / 107,586 | 2.18 (1.71-2.78) | 29 / 187,606 | 2.00 (1.25-3.20) | ||
| ≥35.0 | 111 / 53,602 | 4.14 (3.18-5.39) | 52 / 145,926 | 3.95 (2.55-6.11) | ||
| P trend | < 0.001 | <0.001 | ||||
| P interaction6 | 0.001 | |||||
Participants were asked to recall their body fatness at ages 5, 10, and 20, using a nine-level drawing (Figure 1), where Level 1 corresponds to the leanest and Level 9 the most overweight.
Average childhood and adolescent body fatness were the average of the levels at ages 5 and 10, and ages 10 and 20 respectively.
Adjusted for pack-years of smoking [0,>0-20,>20-40,>40] –NHS; [never, past, current – NHS II], oral contraceptive use [never, <3 years, 3-5 years, > 5years], HT use [premenopausal, post-menopausal – never; postmenopausal – past; post-menopausal current – estrogen only; postmenopausal current – estrogen + progesterone - NHS]; postmenopausal status [pre/post], postmenopausal hormone use [never/ever estrogen; never/ever estrogen + progesterone; never/ever other] – NHS II, age at menopause [premenopausal/unknown, < 45 years, 45-< 47 years, 47 - <49 years, 49- <51, 51- <53 years, 53+ years], family history of colon/rectal cancer, family history of endometrial cancer, height (inches; continuous), parity and age at last birth [Nulliparous, 1-2 & <30 yrs, 1-2 & ≥ 30 yrs, 3-4 & < 30 yrs, 3-4 & ≥ 30 yrs, ≥ 5 children], physical activity [adolescent & age 20 models: <21, 21-<36, 36-<53, 54-<81, 81+; current BMI model: <3, 3-<9, 9-<18, 18-<27, 27+ METS/week]
BMI: body mass index
Recent BMI models not adjusted for recent BMI.
Wald test for heterogeneity between cohorts by body size (examined in base multivariate model).
Since menstrual cycle characteristics (age at menarche and menstrual cycle regularity at age 18) and recent BMI are potentially intermediates between childhood body fatness and endometrial cancer, these were assessed in separate models.
To examine whether the association between body fatness/size and endometrial cancer risk was heterogeneous across the cohorts, we created an interaction term between an ordinal variable to represent body fatness/size at each age and cohort, and evaluated the Wald statistic. Although there was heterogeneity in the association between body fatness/size and endometrial cancer risk between NHS and NHS II, the differences were modest and all associations were in the same direction (Table 5), i.e., interactions were quantitative and not qualitative.17 Therefore, we pooled the data by combining the data from both cohorts and adding an indicator variable to denote cohort.
In secondary analyses in each cohort, we further adjusted for coffee,18 dairy,19 acrylamide,20 alcohol intake,21 and talc use (NHS only).22 Results from these analyses were not appreciably different from the final models, and were therefore not included in our final tables.
We examined whether the association between body fatness and endometrial cancer risk differed by menopausal status, and among postmenopausal women, by HT use. We created interaction terms between ordinal variables representing body fatness/size at each age and menopausal status (premenopausal/postmenopausal) or HT use (ever/never) and examined the Wald statistic.
RESULTS
In NHS, 590 cases of endometrial cancer were diagnosed during 803,134 person-years of follow-up; of these, 549 were postmenopausal. In NHS II, 167 cases were diagnosed during 1,694,905 person-years, of which 60 were postmenopausal.
Table 1 describes the socio-demographic and reproductive characteristics of the study population. The mean age at baseline for NHS participants was 54 years compared to 34 years for NHS II participants. In NHS, women who reported a body fatness of Level 5 or higher at age 5 or age 10 were slightly younger than those who reported the lowest level of body fatness, although no such difference was observed in NHS II (Table 1). In both cohorts, age at menarche was slightly earlier for those reporting a body fatness of Level 5 or higher at any age.
Table 1.
Cumulative age-standardized characteristics of NHS (1998-2010) and NHS II (1989-2009) participants by body fatness at ages 5, 10, and 20.
| Nurses’ Health Study | ||||||
|---|---|---|---|---|---|---|
| Age 5 | Age 10 | Age 20 | ||||
| Level 1 (n=19187) | Level 5+ (n=3690) | Level 1 (n=14447) | Level 5+ (n=5701) | Level 1 (n=5313) | Level 5+ (n=4018) | |
| Age1 | 63.5 (9.2) | 62.4 (9.2) | 63.7 (9.2) | 61.6 (9.1) | 64.0 (9.1) | 62.8 (9.2) |
| Age at menarche, years | 12.7 (1.4) | 12.3 (1.4) | 12.8 (1.4) | 12.2 (1.4) | 12.9 (1.4) | 12.3 (1.4) |
| Regular menstrual cycles at age 18, % | 77.9 | 77.0 | 77.4 | 76.9 | 76.2 | 75.5 |
| BMI at age 18, mean, kg/m2 | 20.3 (2.4) | 24.1 (3.8) | 19.8 (2.1) | 24.1 (3.7) | 18.9 (1.8) | 26.0 (3.8) |
| Adult body mass index, kg/m2 | 25.5 (4.7) | 28.1 (6.4) | 25.0 (4.4) | 28.2 (6.3) | 24.5 (3.9) | 29.6 (7.1) |
| Parous, % | 93.2 | 92.6 | 93.0 | 92.6 | 92.0 | 91.4 |
| Number of children2 | 3.2 (1.5) | 3.0 (1.5) | 3.2 (1.5) | 3.0 (1.4) | 3.2 (1.5) | 3.0 (1.4) |
| Age at last birth2 | 31.6 (4.5) | 31.2 (4.3) | 31.6 (4.5) | 31.1 (4.3) | 31.8 (4.5) | 31.4 (4.4) |
| Postmenopausal, % | 86.3 | 86.2 | 86.3 | 86.0 | 86.5 | 86.0 |
| Age at menopause3 | 50.2 (3.6) | 50.3 (3.9) | 50.3 (3.5) | 50.3 (3.9) | 50.1 (3.6) | 50.4 (3.8) |
| Ever used HT therapy3, % | 58.8 | 57.2 | 59.8 | 58.7 | 59.7 | 55.6 |
| Ever used oral contraceptives, % | 47.9 | 48.9 | 48.2 | 50.4 | 46.9 | 48.2 |
| Ever a smoker, % | 54.7 | 62.4 | 53.7 | 63.2 | 54.3 | 62.5 |
| History of hypertension, % | 34.8 | 36.9 | 33.8 | 37.5 | 33.5 | 39.8 |
| History of diabetes, % | 6.1 | 7.5 | 5.6 | 7.8 | 5.7 | 9.8 |
| Family history of endometrial cancer, % | 3.4 | 2.6 | 3.3 | 2.8 | 3.2 | 2.7 |
| Family history of colorectal cancer, % | 7.9 | 7.6 | 8.1 | 7.6 | 8.5 | 7.7 |
| Adolescent physical activity, METS/week | 43.2 (34.1) | 38.7 (31.1) | 43.7 (34.7) | 37.9 (30.2) | 43.7 (35.0) | 37.8 (30.2) |
| Early adulthood physical activity, METS/week | 31.4 (30.7) | 29.5 (28.6) | 31.5 (31.0) | 28.7 (27.8) | 31.5 (31.8) | 28.1 (27.9) |
| Current physical activity, METS/week | 18.1 (23.8) | 17.8 (23.0) | 18.0 (23.1) | 18.2 (22.9) | 18.0 (23.3) | 16.8 (21.5) |
| Height, in | 64.5 (2.4) | 64.7 (2.5) | 64.5 (2.5) | 64.6 (2.4) | 64.5 (2.6) | 64.7 (2.4) |
| Nurses’ Health Study II | ||||||
|---|---|---|---|---|---|---|
| Age 5 | Age 10 | Age 20 | ||||
| Level 1 (n=25809) | Level 5+ (n=7268) | Level 1 (n=19614) | Level 5+ (n=12786) | Level 1 (n=4661) | Level 5+ (n=11493) | |
| Age1 | 42.2 (7.4) | 42.4 (7.3) | 42.3 (7.4) | 42.4 (7.3) | 43.5 (7.2) | 41.9 (7.3) |
| Birth weight >= 8.5 lb, % | 8.2 | 15.9 | 8.5 | 14.0 | 8.8 | 14.1 |
| Age at menarche, years | 12.7 (1.5) | 12.0 (1.4) | 12.8 (1.5) | 12.0 (1.4) | 12.8 (1.5) | 12.1 (1.5) |
| Regular menstrual cycles at age 18, % | 76.9 | 74.6 | 76.9 | 75.0 | 72.7 | 72.8 |
| BMI at age 18, mean, kg/m2 | 20.0 (2.6) | 24.3 (4.9) | 19.3 (2.1) | 24.3 (4.7) | 18.3 (1.7) | 26.6 (4.6) |
| Adult body mass index, kg/m2 | 24.5 (5.1) | 29.3 (7.6) | 23.5 (4. 2) | 29.4 (7.6) | 22.5 (3.5) | 31.8 (8.5) |
| Parous, % | 72.1 | 65.6 | 72.3 | 67.0 | 71.6 | 57.9 |
| Number of children2 | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.1 (0.9) |
| Age at last birth2 | 30.6 (4.7) | 30.6 (4.8) | 30.7 (4. 7) | 30.6 (4.7) | 30.6 (4.7) | 31.0 (5.0) |
| Ever used oral contraceptives, % | 86.1 | 82.9 | 86.3 | 83.1 | 85.0 | 79.8 |
| Postmenopausal, % | 14.0 | 14.2 | 13.9 | 14.1 | 14.5 | 14.1 |
| Age at menopause3 | 48.1 (4.6) | 47.8 (5.0) | 48.4 (4.6) | 48.1 (5.0) | 48.0 (4.4) | 48.1(5.2) |
| Ever used HT therapy3, % | 22.5 | 23.7 | 22.3 | 24.0 | 23.7 | 23.8 |
| Ever a smoker, % | 34.0 | 41.4 | 33.7 | 41.0 | 36.2 | 39.7 |
| History of hypertension, % | 10.1 | 15.1 | 8.8 | 15.4 | 8.1 | 19.0 |
| History of diabetes, % | 1.7 | 3.1 | 1.4 | 3.4 | 1.0 | 5.2 |
| Family history of endometrial cancer, % | 2.3 | 2.7 | 2.4 | 2.5 | 2.5 | 2.6 |
| Family history of colorectal cancer, % | 4.3 | 4.4 | 4.3 | 4.5 | 4.5 | 4.4 |
| Adolescent physical activity, METS/week | 53.8 (36.8) | 45.6 (33.5) | 54.8 (37.0) | 43.7 (32.4) | 53.9 (38.2) | 44.7 (32.9) |
| Early adulthood physical activity, METS/week | 43.7 (33.8) | 40.9 (33.1) | 44.1 (34.0) | 40.1 (32.5) | 43.9 (35.4) | 37.6 (30.7) |
| Current physical activity, METS/week | 22.2 (30.5) | 21.4 (30.2) | 22.4 (31.0) | 20. 8 (28.4) | 23.2 (33.1) | 19.6 (27.3) |
| Height, in | 64.8 (2.6) | 65.0 (2.6) | 64.9 (2.7) | 65.0 (2.6) | 64.8 (2.8) | 65.1 (2.67) |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age-adjusted
Among parous women only.
Among post-menopausal women only
Body fatness at age 5 was marginally associated with endometrial cancer risk after adjusting for covariates (Table 2). Childhood, age 10, adolescent, and age 20 body fatness, were significantly associated with risk after adjusting for covariates except recent BMI. For example, in adolescence, the HR for ≥ Level 5 versus ≤ Level 2 was 1.83 (95% CI 1.41-2.37; ptrend < 0.001). Higher BMI at age 18 also was strongly associated with an increased risk of endometrial cancer after adjusting for covariates except recent BMI; HR for BMI ≥ 23.0 kg/m2 versus BMI < 19.9 kg/m2 was 1.64 (95% CI 1.35-1.99; ptrend < 0.001). Recent BMI during adulthood was the strongest risk factor: HR for BMI ≥ 35.0 kg/m2 versus BMI < 25 kg/m2 was 4.13 (95% CI 3.29-5.16; ptrend < 0.001).
After adjusting for menstrual cycle characteristics, childhood, age 10, adolescent, and age 20 body fatness were still associated with risk, although attenuated slightly: HR in adolescence for ≥ Level 5 versus ≤ Level 2 was 1.76 (95% CI 1.35-2.28; ptrend < 0.001) (Table 2). After adjusting for recent BMI, none of these associations persisted: HR in adolescence for ≥ Level 5 versus ≤ Level 2 was 1.03 (95% CI 0.78-1.36; ptrend = 0.81).
Weight change since age 18 was positively associated with endometrial cancer risk: the HR for 25+ kg weight gain versus stable weight was 2.54 (95% CI 1.80-3.59; ptrend < 0.001) (Table 2). After adjusting for weight at age 18, the association was slightly stronger: compared to women who maintained a stable weight, HR for women who gained 25+ kg was 2.71 (95% CI 1.92-3.82; ptrend < 0.001). Women who at age 18 had a BMI ≥ 21 kg/m2 were at greater risk (pinteraction < 0.001); HR = 2.86 (95% CI 1.80-4.54; ptrend < 0.001), versus 2.13 (95% CI 1.26-3.61; ptrend < 0.001) for BMI < 21 kg/m2. We also examined the association between weight change since age 18 and endometrial cancer risk, adjusting for recent weight instead of weight at age 18. After adjusting for recent weight, the association between weight change since age 18 and endometrial cancer was attenuated and no longer significant (HR for 25+ kg weight gain versus stable weight was 0.78 (95% CI 0.52-1.16; ptrend = 0.23).
We also stratified by menopausal status (Table 3). In postmenopausal women, body fatness was associated with endometrial cancer risk at age 10, during adolescence, and at age 20. In adolescence, the HR for ≥ Level 5 versus ≤ Level 2 was 1.56 (95% CI 1.15-2.12; ptrend = 0.002). Recent BMI was also associated with endometrial cancer risk. The associations were stronger in premenopausal women: body fatness was associated with endometrial cancer risk at every age, although the test for interaction was significant only for adolescence, age 20, and recent BMI. In adolescence, the HR for ≥ Level 5 versus ≤ Level 2 was 3.03 (95% CI 1.71-5.37; ptrend = 0.002). However, the associations attenuated and became non-significant after controlling for recent BMI.
Table 3.
Age- and multivariable-adjusted hazard ratios and 95% confidence intervals for endometrial cancer by life-course body fatness, stratified by menopausal status
| POSTMENOPAUSAL WOMEN | PREMENOPAUSAL WOMEN | |||||||
|---|---|---|---|---|---|---|---|---|
| No. cases/person-years | Age-adjusted HR | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + recent BMI4 (95% CI) | No. cases/person-years | Age-adjusted HR | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + recent BMI4 (95% CI) | |
| Body fatness1 at age 5, Level | ||||||||
| 1 | 233 / 331,397 | 1.00 | 1.00 | 1.00 | 28 /364,247 | 1.00 | 1.00 | 1.00 |
| 2 | 146 / 216,055 | 1.05 | 1.05 (0.85-1.29) | 1.03 (0.83-1.27) | 43 /476,598 | 1.41 | 1.43 (0.88-2.32) | 1.42 (0.87-2.30) |
| 3 | 103 / 167,183 | 0.98 | 0.98 (0.78-1.24) | 0.89 (0.70-1.13) | 31 /356,807 | 1.25 | 1.14 (0.67-1.94) | 0.95 (0.55-1.63) |
| 4 | 76 / 99,000 | 1.21 | 1.20 (0.92-1.55) | 0.99 (0.76-1.29) | 16 /188,861 | 1.16 | 1.03 (0.54-1.94) | 0.73 (0.38-1.38) |
| 5+ | 51 / 67,464 | 1.16 | 1.15 (0.84-1.56) | 0.89 (0.65-1.21) | 18 /99,916 | 2.50 | 2.21 (1.20-4.07) | 1.31 (0.70-2.48) |
| P trend | 0.21 | 0.25 | 0.40 | 0.04 | 0.15 | 0.69 | ||
| P interaction6 | 0.19 | |||||||
| Body fatness1 at age 10, Level | ||||||||
| 1 | 167 / 252,273 | 1.00 | 1.00 | 1.00 | 21 /275,430 | 1.00 | 1.00 | 1.00 |
| 2 | 160 / 238,483 | 1.08 | 1.09 (0.88-1.36) | 1.04 (0.83-1.30) | 32 /459,346 | 1.07 | 1.11 (0.63-1.95) | 1.04 (0.59-1.84) |
| 3 | 106 / 164,862 | 1.06 | 1.08 (0.84-1.38) | 0.93 (0.72-1.19) | 37 /339,548 | 1.61 | 1.53 (0.88-2.67) | 1.18 (0.67-2.07) |
| 4 | 78 / 119,026 | 1.10 | 1.09 (0.83-1.43) | 0.86 (0.65-1.13) | 21 /235,247 | 1.26 | 1.06 (0.56-1.99) | 0.69 (0.36-1.31) |
| 5+ | 98 / 106,454 | 1.56 | 1.53 (1.19-1.97) | 1.12 (0.86-1.46) | 25 /176,856 | 1.97 | 1.77 (0.97-3.22) | 0.90 (0.47-1.70) |
| P trend | 0.004 | 0.01 | 0.98 | 0.02 | 0.10 | 0.38 | ||
| P interaction6 | 0.42 | |||||||
| Body fatness1 at age 20, Level | ||||||||
| 1,2 | 227 / 347,818 | 1.00 | 1.00 | 1.00 | 36 /449,844 | 1.00 | 1.00 | 1.00 |
| 3 | 189 / 299,323 | 1.02 | 1.02 (0.84-1.24) | 0.90 (0.74-1.10) | 31 /568,858 | 0.73 | 0.71 (0.44-1.16) | 0.59 (0.36-0.97) |
| 4 | 119 / 155,345 | 1.26 | 1.23 (0.98-1.54) | 0.94 (0.74-1.18) | 38 /311,023 | 1.72 | 1.60 (0.99-2.57) | 0.99 (0.60-1.65) |
| 5+ | 74 / 78,613 | 1.54 | 1.46 (1.12-1.91) | 0.92 (0.69-1.22) | 31 /156,705 | 3.04 | 2.36 (1.40-3.97) | 1.02 (0.56-1.87) |
| P trend | <0.001 | 0.004 | 0.51 | <0.001 | <0.001 | 0.65 | ||
| P interaction6 | 0.01 | |||||||
| Average childhood body fatness (ages 5 – 10)2, Level | ||||||||
| 1,1.5,2 | 324 / 484,396 | 1.00 | 1.00 | 1.00 | 51 /714,738 | 1.00 | 1.00 | 1.00 |
| 2.5,3 | 127 / 181,803 | 1.11 | 1.12 (0.91-1.37) | 1.00 (0.81-1.23) | 39 /376,434 | 1.47 | 1.36 (0.88-2.09) | 1.13 (0.73-1.75) |
| 3.5,4,4.5 | 106 / 152,859 | 1.14 | 1.12 (0.90-1.40) | 0.91 (0.73-1.14) | 28 /304,397 | 1.28 | 1.09 (0.68-1.76) | 0.71 (0.43-1.17) |
| 5+ | 52 / 62,041 | 1.33 | 1.30 (0.97-1.75) | 0.96 (0.71-1.30) | 18 /90,859 | 2.68 | 2.31 (1.33-4.02) | 1.22 (0.67-2.21) |
| P trend | 0.04 | 0.06 | 0.52 | 0.004 | 0.03 | 0.78 | ||
| P interaction6 | 0.10 | |||||||
| Average adolescent body fatness (ages 10 – 20)2, Level | ||||||||
| 1,1.5,2 | 225 / 344,630 | 1.00 | 1.00 | 1.00 | 32 / 431,026 | 1.00 | 1.00 | 1.00 |
| 2.5,3 | 166 / 264,517 | 1.02 | 1.02 (0.84-1.25) | 0.92 (0.75-1.13) | 37 / 523,422 | 1.08 | 1.05 (0.65-1.69) | 0.89 (0.55-1.45) |
| 3.5,4,4.5 | 165 / 218,560 | 1.27 | 1.26 (1.02-1.54) | 0.98 (0.80-1.21) | 44 / 441,532 | 1.45 | 1.30 (0.81-2.08) | 0.80 (0.48-1.33) |
| 5+ | 53 / 53,391 | 1.63 | 1.56 (1.15-2.12) | 0.98 (0.71-1.35) | 23 / 90,448 | 3.81 | 3.03 (1.71-5.37) | 1.22 (0.63-2.36) |
| P trend | <0.001 | 0.002 | 0.86 | <0.001 | 0.002 | 0.98 | ||
| P interaction6 | 0.05 | |||||||
| Recent BMI, kg/m2 | ||||||||
| ≤ 24.9 | 196 / 406,469 | 1.00 | 1.00 | N/A5 | 41 / 853,801 | 1.00 | 1.00 | N/A5 |
| 25.0-29.9 | 171 / 283,070 | 1.22 | 1.23 (1.00-1.52) | 29 / 361,032 | 1.26 | 1.28 (0.78-2.08) | ||
| 30.0-34.9 | 123 / 122,265 | 2.12 | 2.10 (1.65-2.67) | 25 / 155,642 | 2.47 | 2.48 (1.47-4.17) | ||
| ≥35.0 | 119 / 69,295 | 4.12 | 3.97 (3.06-5.14) | 41 / 115,954 | 5.22 | 4.43 (2.68-7.34) | ||
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P interaction6 | 0.005 | |||||||
Participants were asked to recall their body fatness at ages 5, 10, and 20, using a nine-level drawing (Figure 1), where Level 1 corresponds to the leanest and Level 9 the most overweight.
Average childhood and adolescent body fatness were the average of the levels at ages 5 and 10, and ages 10 and 20 respectively.
Adjusted for smoking history [never, past, current], oral contraceptive use [never(ref), <3 years, 3-5 years, > 5years], postmenopausal hormone use (postmenopausal women only) [never, past, current – estrogen only, current – estrogen only, current – estrogen & progesterone / other], age at menopause (postmenopausal women only) [unknown, < 45 years, 45-< 47 years, 47 - <49 years (ref), 49- <51 years, 51- <53 years, 53+ years], family history of colon/rectal cancer, family history of endometrial cancer, height (inches; continuous), parity and age at last birth[Nulliparous, 1-2 & <30 yrs, 1-2 & ≥ 30 yrs, 3-4 & < 30 yrs, 3-4 & ≥ 30 yrs, ≥ 5 children], physical activity [adolescent & age 20 models: <21, 21-<36, 36-<53, 54-<81, 81+ METS/week; recent BMI model: <3, 3-<9, 9-<18, 18-<27, 27+ METS/week]
BMI: body mass index
Recent BMI models not adjusted for recent BMI
Wald test for interaction between body size and menopausal status (examined in base multivariate model)
Finally, we stratified by HT use among postmenopausal women (Table 4). With the exception of body fatness at age 5 and in childhood, the association of endometrial cancer with body fatness varied by HT use (all pinteraction <0.05). Early life body fatness was unrelated to endometrial cancer among women who reported using HT after menopause. However, recent BMI was still strongly associated with endometrial cancer risk. Among HT non-users, body fatness at age 10, during adolescence, and at age 20 were positively associated with endometrial cancer risk, although non-significant after adjusting for recent BMI. Recent BMI was very strongly associated with endometrial cancer risk, with postmenopausal women with BMI ≥ 35 kg/m2 at highest risk: adjusted HR versus BMI < 25 kg/m2, was 9.31 (95% CI 5.64-15.35; ptrend < 0.001).
Table 4.
Age- and multivariable-adjusted hazard ratios and 95% confidence intervals for endometrial cancer by lifetime body fatness among postmenopausal participants, stratified by HT use
| EVER USERS | NEVER USERS | |||||||
|---|---|---|---|---|---|---|---|---|
| No. cases/person-years | Age-adjusted HR | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + recent BMI (95% CI) | No. cases/person-years | Age-adjusted HR | Multivariate-adjusted HR3 (95% CI) | Multivariate adjusted HR3 + recent BMI4 (95% CI) | |
| Body fatness1 at age 5, Level | ||||||||
| 1 | 144 / 180,840 | 1.00 | 1.00 | 1.00 | 66 / 126,607 | 1.00 | 1.00 | 1.00 |
| 2 | 88 / 127,499 | 0.99 | 0.98 (0.75-1.29) | 0.98 (0.74-1.28) | 51 / 77,264 | 1.18 | 1.23 (0.84-1.81) | 1.19 (0.80-1.76) |
| 3 | 55 / 96,848 | 0.85 | 0.85 (0.62-1.17) | 0.81 (0.59-1.12) | 41 / 61,834 | 1.21 | 1.19 (0.80-1.79) | 0.97 (0.64-1.48) |
| 4 | 36 / 56,055 | 0.89 | 0.88 (0.61-1.28) | 0.79 (0.54-1.15) | 34 / 37,613 | 1.78 | 1.78 (1.16-2.73) | 1.30 (0.83-2.02) |
| 5+ | 33 / 36,514 | 1.26 | 1.27 (0.86-1.87) | 1.12 (0.76-1.66) | 14 / 26,915 | 0.95 | 0.89 (0.49-1.61) | 0.61 (0.33-1.12) |
| P trend | 0.89 | 0.88 | 0.56 | 0.17 | 0.25 | 0.49 | ||
| P interaction5 | 0.14 | |||||||
| Body fatness1 at age 10, Level | ||||||||
| 1 | 112 / 140,185 | 1.00 | 1.00 | 1.00 | 40 / 94,813 | 1.00 | 1.00 | 1.00 |
| 2 | 93 / 137,784 | 0.93 | 0.94 (0.71-1.25) | 0.92 (0.69-1.21) | 56 / 86,585 | 1.39 | 1.42 (0.94-2.17) | 1.28 (0.84-1.96) |
| 3 | 58 / 93,847 | 0.89 | 0.90 (0.65-1.24) | 0.84 (0.60-1.16) | 40 / 62,074 | 1.36 | 1.34 (0.85-2.12) | 1.01 (0.63-1.61) |
| 4 | 37 / 66,716 | 0.80 | 0.80 (0.55-1.17) | 0.71 (0.48-1.03) | 34 / 45,490 | 1.78 | 1.69 (1.05-2.72) | 1.11 (0.67-1.82) |
| 5+ | 56 / 59,223 | 1.42 | 1.42 (1.02-1.97) | 1.21 (0.86-1.70) | 36 / 41,271 | 1.93 | 1.78 (1.11-2.85) | 1.06 (0.65-1.73) |
| P trend | 0.30 | 0.30 | 0.98 | 0.003 | 0.01 | 0.92 | ||
| P interaction5 | 0.03 | |||||||
| Body fatness1 at age 20, Level | ||||||||
| 1-2 | 151 / 198,137 | 1.00 | 1.00 | 1.00 | 57 / 127,424 | 1.00 | 1.00 | 1.00 |
| 3 | 108 / 171,336 | 0.90 | 0.91 (0.70-1.16) | 0.84 (0.65-1.09) | 68 / 110,831 | 1.35 | 1.32 (0.92-1.90) | 1.08 (0.74-1.56) |
| 4 | 63 / 86,785 | 1.05 | 1.04 (0.77-1.40) | 0.90 (0.66-1.22) | 51 / 59,369 | 1.83 | 1.67 (1.12-2.49) | 1.03 (0.67-1.58) |
| 5+ | 34 / 41,498 | 1.15 | 1.13 (0.77-1.65) | 0.89 (0.60-1.33) | 30 / 32,610 | 2.08 | 1.72 (1.08-2.75) | 0.81 (0.48-1.35) |
| P trend | 0.54 | 0.60 | 0.42 | <0.001 | 0.01 | 0.51 | ||
| P interaction5 | 0.01 | |||||||
| Average childhood body fatness (ages 5 – 10)2, Level | ||||||||
| 1-2 | 204 / 272,896 | 1.00 | 1.00 | 1.00 | 94 / 179,987 | 1.00 | 1.00 | 1.00 |
| 2.5-3 | 69 / 104,267 | 0.99 | 1.00 (0.76-1.32) | 0.95 (0.72-1.25) | 49 / 67,768 | 1.30 | 1.27 (0.89-1.82) | 1.02 (0.70-1.48) |
| 3.5-4.5 | 50 / 87,104 | 0.86 | 0.85 (0.62-1.17) | 0.77 (0.56-1.05) | 48 / 57,618 | 1.65 | 1.55 (1.08-2.23) | 1.08 (0.73-1.58) |
| 5+ | 33 / 33,488 | 1.45 | 1.45 (1.00-2.12) | 1.25 (0.85-1.84) | 15 / 24,859 | 1.12 | 1.03 (0.59-1.80) | 0.66 (0.36-1.15) |
| P trend | 0.49 | 0.50 | 0.80 | 0.05 | 0.12 | 0.41 | ||
| P interaction5 | 0.12 | |||||||
| Average adolescent body fatness (ages 10 – 20)2, Level | ||||||||
| 1-2 | 150 / 195,308 | 1.00 | 1.00 | 1.00 | 56 / 126,767 | 1.00 | 1.00 | 1.00 |
| 2.5-3 | 95 / 151,533 | 0.90 | 0.91 (0.70-1.18) | 0.85 (0.65-1.11) | 60 / 97,753 | 1.37 | 1.35 (0.93-1.97) | 1.16 (0.79-1.70) |
| 3.5-4.5 | 83 / 122,339 | 0.99 | 1.00 (0.76-1.31) | 0.87 (0.66-1.15) | 69 / 84,007 | 1.83 | 1.73 (1.19-2.50) | 1.12 (0.76-1.66) |
| 5+ | 28 / 28,575 | 1.47 | 1.42 (0.94-2.15) | 1.12 (0.73-1.72) | 21 / 21,709 | 2.14 | 1.80 (1.06-3.05) | 0.88 (0.50-1.56) |
| P trend | 0.34 | 0.37 | 0.72 | <0.001 | 0.002 | 0.95 | ||
| P interaction5 | 0.01 | |||||||
| Recent BMI, kg/m2 | ||||||||
| ≤ 24.9 | 150 / 243,398 | 1.00 | 1.00 | N/A5 | 26 / 138,819 | 1.00 | 1.00 | N/A5 |
| 25.0-29.9 | 111 / 156,920 | 1.16 | 1.16 (0.90-1.50) | 56 / 108,637 | 2.51 | 2.35 (1.46-3.78) | ||
| 30.0-34.9 | 59 / 63,369 | 1.65 | 1.64 (1.19-2.26) | 53 / 51,279 | 4.95 | 4.25 (2.65-7.13) | ||
| ≥35.0 | 36 / 34,068 | 2.35 | 2.23 (1.49-3.34) | 71 / 31,499 | 11.15 | 9.31 (5.64-15.35) | ||
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P interaction6 | <0.001 | |||||||
Participants were asked to recall their body fatness at ages 5, 10, and 20, using a nine-level drawing (Figure 1), where Level 1 corresponds to the leanest and Level 9 the most overweight.
Average childhood and adolescent body fatness were the average of the levels at ages 5 and 10, and ages 10 and 20 respectively.
Adjusted for smoking history [never, past, current], oral contraceptive use [never, <3 years, 3-5 years, > 5years], postmenopausal hormone use (ever-users only) [past (ref), current – estrogen only, current – estrogen only, current – estrogen & progesterone / other], age at menopause [unknown, < 45, 45-< 47, 47 - <49, 49-<51, 51- <53, 53+ years], birth weight [<5.5, 5.5-<7, 7-8.4, 8.5-9.9, 10 lb], family history of colon/rectal cancer, family history of endometrial cancer, height (inches; continuous), parity and age at last birth [Nulliparous, 1-2 & <30 yrs, 1-2 & ≥ 30 yrs, 3-4 & < 30 yrs, 3-4 & ≥ 30 yrs, ≥ 5 children], physical activity [adolescent & age 20 models: <21, 21-<36, 36-<53, 54-<81, 81+ METS/week; recent BMI model: <3, 3-<9, 9-<18, 18-<27, 27+ METS/week]
BMI: body mass index
Recent BMI models not adjusted for recent BMI
Wald test for interaction between body size and postmenopausal hormone use (examined in base multivariate model).
DISCUSSION
In our study, recent body size, as expected, was strongly associated with endometrial cancer risk, especially among obese women who have never used HT. Weight gain since age 18 years was strongly associated with risk, particularly for women who were heavier at age 18. Early life body fatness was positively associated with endometrial cancer risk, although the association was mediated by adult BMI. Finally, the association between early life body fatness and endometrial cancer risk was stronger in premenopausal than postmenopausal women, although it no longer persisted after adjusting for recent BMI.
Consistent with a number of studies,23, 24 we observed that recent obesity was strongly related to endometrial cancer risk. In premenopausal women, obesity is associated with an increased number of anovulatory cycles,25 which is associated with an increased risk of endometrial cancer.3, 23 In postmenopausal women, obesity may influence endometrial cancer risk by the increased conversion of androstendione into estrone,26 a biologically active estrogen that can be converted to estradiol. In both pre- and postmenopausal women, obesity may also increase risk via an increase in IGF-1 levels.27
Our study confirms the findings from a previous case-control study in Hawaii28 and a prospective cohort study in the United Kingdom,29 that the association between body size during adolescence and early adulthood may be mediated by adult BMI. In a case-control study in China,30 Xu et al reported an increased risk of endometrial cancer for women who were heavier than their peers during adolescence, although this was limited to women with a BMI of ≥ 25 kg/m2, and likely not independent of recent BMI. In a prospective cohort study of US teenagers,31 Blitzer et al reported a positive association of teen-age body size with endometrial cancer risk; however, current body size was not taken into account. To our knowledge, body fatness before age 10 had not been previously examined.
Consistent with a number of studies,24, 30, 32, 33 but not all,34 weight change since age 18 was positively associated with endometrial cancer risk. A larger body size is correlated with lower levels of SHBG9 and consequently an increase in non-SHBG bound estrogen,10 which can increase the risk of endometrial cancer3. Weight loss is associated with a return to normal SHBG levels,35 suggesting that the converse is true for weight gain. The stronger association between weight gain and endometrial cancer risk among women who were heavier at age 18 likely reflects the positive association between body fatness and endometrial cancer risk. The association was not significant after controlling for current weight, suggesting that this association is mediated by current weight.
We also observed (before adjusting for recent BMI) that the association between early life body fatness and endometrial cancer risk was stronger in premenopausal than postmenopausal women, consistent with the findings of Xu et al.30 Early life obesity is associated with hyperandrogenism,36, 37 which is believed to be a risk factor for endometrial cancer among relatively younger women,38 although this has not been observed in some studies.39, 40 Further, younger women may more accurately recall their body fatness, and thus a lesser degree of non-differential exposure misclassification. Lastly, the correlation between early life body fatness and adult BMI was stronger in the younger NHS II population, likely due partly to a birth cohort effect,41 their younger age, and the shorter time between the two measurements.
Consistent with our findings, other studies have also reported that the association between body size and endometrial cancer risk is modified by HT use.42-45 Serum estradiol and estrone levels among HT users compared with non-users are around 3 to 4 times higher,46 compared with about 1.4 to 1.6 times elevated levels for obese women relative to normal weight women.47 Thus, among women who use HT, the contribution of estrogen levels to the overall risk of endometrial cancer by obesity may not be as apparent.6 Our observed interaction between body fatness in early life and HT use may simply reflect the positive correlation between body size in early life and adulthood.
Limitations of our study include the use of remote recall of early life body fatness, which may result in misclassification. However, since body weight was recalled prior to diagnosis of endometrial cancer, any misclassification would be non-differential. Significant independent associations48-50 observed with this measure give credence to its validity.
Strengths include the prospective analysis of life-course body size (and the first to examine body fatness prior to age 10) and endometrial cancer risk. Further, we have measured and adjusted for several potential confounders, although the possibility of unmeasured or residual confounding cannot be eliminated.
In conclusion, obesity throughout life is positively associated with endometrial cancer risk. Although adult obesity appears to be most strongly associated with endometrial cancer risk, maintaining a healthy weight from childhood remains important, as early life obesity is strongly correlated with adult obesity, which has many adverse health outcomes.
Supplementary Material
Novelty and Impact Statement.
Adult body size is a well-established risk factor for endometrial cancer, the most common gynecological malignancy. However, data are limited on the association with childhood obesity. In one of the first prospective studies on the subject, we examined the association between body size throughout the life-course and endometrial cancer risk among participants of the Nurses’ Health Studies. Body size throughout life was positively associated with endometrial cancer risk, most strongly in adulthood.
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the Nurses’ Health Study and the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data.
The Nurses’ Health Study is supported by National Institutes of Health grant P01 CA87969. The Nurses’ Health Study II is supported by National Institutes of Health grants UM1 CA176726, UM1 50385, R01 50385, and CA082838
REFERENCES
- 1.National Cancer Institute [13 Apr 2014];Endometrial Cancer. Available at http://www.cancer.gov/cancertopics/types/endometrial.
- 2.Bergström A, Pisani P, Tenet V, Wolk A, Adami H-O. Overweight as an avoidable cause of cancer in Europe. International Journal of Cancer. 2001;91:421–30. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers. 2002;11:1531–43. Prev. [PubMed] [Google Scholar]
- 4.Zhang Z, Zhou D, Lai Y, Liu Y, Tao X, Wang Q, Zhao G, Gu H, Liao H, Zhu Y, Xi X, Feng Y. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Letters. 2012;319:89–97. doi: 10.1016/j.canlet.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Luhn P, Dallal CM, Weiss JM, Black A, Huang W-Y, Lacey JV, Hayes RB, Stanczyk FZ, Wentzensen N, Brinton LA. Circulating Adipokine Levels and Endometrial Cancer Risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiology Biomarkers & Prevention. 2013;22:1304–12. doi: 10.1158/1055-9965.EPI-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body Mass Index, Hormone Replacement Therapy, and Endometrial Cancer Risk: A Meta-Analysis. Cancer Epidemiology Biomarkers & Prevention. 2010;19:3119–30. doi: 10.1158/1055-9965.EPI-10-0832. [DOI] [PubMed] [Google Scholar]
- 7.Leitão RB, Rodrigues LP, Neves L, Carvalho GS. Development of adiposity, obesity and age at menarche: an 8-year follow-up study in Portuguese schoolgirls. Int J Adolesc Med Health. 25:55–63. doi: 10.1515/ijamh-2013-0007. [DOI] [PubMed] [Google Scholar]
- 8.Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, Olsen A, Overvad K, Clavel-Chapelon F, Fournier A, Chabbert-Buffet N, Boeing H, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. International Journal of Cancer. 127:442–51. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- 9.Gascon F, Valle M, Martos R, Zafra M, Morales R, Castano MA. Childhood obesity and hormonal abnormalities associated with cancer risk. European Journal of Cancer Prevention. 2004;13:193–97. doi: 10.1097/01.cej.0000130021.16182.c3. [DOI] [PubMed] [Google Scholar]
- 10.Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn WR. The serum transport of steroid hormones. Recent Prog. Horm. Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- 11.Poole EM, Tworoger SS, Hankinson SE, Schernhammer ES, Pollak MN, Baer HJ. Body Size in Early Life and Adult Levels of Insulin-like Growth Factor 1 and Insulin-like Growth Factor Binding Protein 3. American Journal of Epidemiology. 174:642–51. doi: 10.1093/aje/kwr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The Nurses' Health Study. Am J Nurs. 1978;78:1039–40. [PubMed] [Google Scholar]
- 13.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Key SRI, Sigman R, Matthysse S, editors. The Genetics of Neurological and Psychiatric Disorders. Raven Press; New York: 1983. [PubMed] [Google Scholar]
- 14.Must AWW, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993 Jul;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 15.Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, CH. H. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–8. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 16.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995 Aug;19:570–2. [PubMed] [Google Scholar]
- 17.Peto R. Statistical aspects of cancer trials. In: Halnam KE, editor. Treatment of Cancered. Chapman & Hall; London: 1982. [Google Scholar]
- 18.Je Y, Hankinson SE, Tworoger SS, DeVivo I, Giovannucci E. A Prospective Cohort Study of Coffee Consumption and Risk of Endometrial Cancer over a 26-Year Follow-Up. Cancer Epidemiology Biomarkers & Prevention. 20:2487–95. doi: 10.1158/1055-9965.EPI-11-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganmaa D, Cui X, Feskanich D, Hankinson SE, Willett WC. Milk, dairy intake and risk of endometrial cancer: A 26-year follow-up. International Journal of Cancer. 2011 doi: 10.1002/ijc.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson KM, Mucci LA, Rosner BA, Willett WC. A Prospective Study on Dietary Acrylamide Intake and the Risk for Breast, Endometrial, and Ovarian Cancers. Cancer Epidemiology Biomarkers & Prevention. 2010;19:2503–15. doi: 10.1158/1055-9965.EPI-10-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JJ, Hazra A, Giovannucci E, Hankinson SE, Rosner B, De Vivo I. One-carbon metabolism factors and endometrial cancer risk. Br J Cancer. 2013;108:183–87. doi: 10.1038/bjc.2012.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karageorgi S, Gates MA, Hankinson SE, De Vivo I. Perineal Use of Talcum Powder and Endometrial Cancer Risk. Cancer Epidemiology Biomarkers & Prevention. 2010;19:1269–75. doi: 10.1158/1055-9965.EPI-09-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer DW. The Epidemiology of Endometrial and Ovarian Cancer. Hematology/Oncology Clinics of North America. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiderpass E, Persson I, Adami H-O, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes and Control. 2000;11:185–92. doi: 10.1023/a:1008946825313. [DOI] [PubMed] [Google Scholar]
- 25.Key TJ, Allen NE, Verkasalo PK, Banks E. Energy balance and cancer: the role of sex hormones. Proceedings of the Nutrition Society. 2001;60:81–89. doi: 10.1079/pns200068. [DOI] [PubMed] [Google Scholar]
- 26.Ziel HK, Finkle WD. Association of estrone with the development of endometrial carcinoma. Am J Obstet Gynecol. 1976;124:735–40. doi: 10.1016/s0002-9378(16)33345-2. [DOI] [PubMed] [Google Scholar]
- 27.Kaaks R, Lukanova A, MS K. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers. 2002;11:1531–43. Prev. [PubMed] [Google Scholar]
- 28.Le Marchand L, Wilkins LR, Mi MP. Early age body size, adult weight gain and endometrial cancer risk. Int J Cancer. 1991;88:807–11. doi: 10.1002/ijc.2910480603. [DOI] [PubMed] [Google Scholar]
- 29.Yang TYO, Cairns BJ, Allen N, Sweetland S, Reeves GK, Beral V. Postmenopausal endometrial cancer risk and body size in early life and middle age: prospective cohort study. Br J Cancer. 2012;107:169–75. doi: 10.1038/bjc.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, Xiang Y, Zheng W, Ruan Z, Cheng J, Gao YT, Shu X. Weight history and risk of endometrial cancer among Chinese women. Int J Epidemiol. 2006;35:159–66. doi: 10.1093/ije/dyi223. [DOI] [PubMed] [Google Scholar]
- 31.Blitzer PH, Blitzer EC, Rimm AA. Association between teen-age obesity and cancer in 56,111 women: All cancers and endometrial carcinoma. Preventive Medicine. 1976;5:20–31. doi: 10.1016/0091-7435(76)90005-0. [DOI] [PubMed] [Google Scholar]
- 32.Park SL, Goodman MT, Zhang Z-F, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. International Journal of Cancer. 126:490–99. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens V, Jacobs E, Patel A, Sun J, Gapstur S, McCullough M. Body weight in early adulthood, adult weight gain, and risk of endometrial cancer in women not using postmenopausal hormones. Cancer Causes & Control. 2014;25:321–28. doi: 10.1007/s10552-013-0333-7. [DOI] [PubMed] [Google Scholar]
- 34.Shu XO, Brinton LA, Zheng W, Swanson CA, Hatch MC, Gao Y-T, Fraumeni JF. Relation of Obesity and Body Fat Distribution to Endometrial Cancer in Shanghai, China. Cancer Research. 1992;52:3865–70. [PubMed] [Google Scholar]
- 35.Enriori CL, Orsini W, del Carmen Cremona M, Etkin AE, Cardillo LR, Reforzo-Membrives J. Decrease of circulating level of SHBG in postmenopausal obese women as a risk factor in breast cancer: reversible effect of weight loss. Gynecol Oncol. 1986;23:77–86. doi: 10.1016/0090-8258(86)90118-6. [DOI] [PubMed] [Google Scholar]
- 36.Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–41. doi: 10.1159/000342545. [DOI] [PubMed] [Google Scholar]
- 37.Vilmann LS, Thisted E, Baker JL, Holm JC. Development of Obesity and Polycystic Ovary Syndrome in Adolescents. Hormone Research in Paediatrics. 2012;78:269–78. doi: 10.1159/000345310. [DOI] [PubMed] [Google Scholar]
- 38.Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case–control study. Cancer Causes & Control. 2010;21:2303–08. doi: 10.1007/s10552-010-9658-7. [DOI] [PubMed] [Google Scholar]
- 39.Holm NSL, Glintborg D, Andersen MS, Schledermann D, Ravn P. The prevalence of endometrial hyperplasia and endometrial cancer in women with polycystic ovary syndrome or hyperandrogenism. Acta Obstetricia et Gynecologica Scandinavica. 2012;91:1173–76. doi: 10.1111/j.1600-0412.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 40.Navaratnarajah R, Pillay OC, Hardiman P. Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med. 2008;26:62–71. doi: 10.1055/s-2007-992926. [DOI] [PubMed] [Google Scholar]
- 41.Bell A, Jones K. Don't birth cohorts matter? A commentary and simulation exercise on Reither, Hauser, and Yang's (2009) age–period–cohort study of obesity. Social Science & Medicine. doi: 10.1016/j.socscimed.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Canchola A, Chang E, Bernstein L, Largent J, Reynolds P, Deapen D, Ursin G, Horn-Ross P. Body size and the risk of endometrial cancer by hormone therapy use in postmenopausal women in the California Teachers Study cohort. Cancer Causes & Control. 2010;21:1407–16. doi: 10.1007/s10552-010-9568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses' Health Study cohort 1976–2004. International Journal of Cancer. 2010;126:208–16. doi: 10.1002/ijc.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCullough ML, Patel AV, Patel R, Rodriguez C, Feigelson HS, Bandera EV, Gansler T, Thun MJ, Calle EE. Body Mass and Endometrial Cancer Risk by Hormone Replacement Therapy and Cancer Subtype. Cancer Epidemiology Biomarkers & Prevention. 2008;17:73–79. doi: 10.1158/1055-9965.EPI-07-2567. [DOI] [PubMed] [Google Scholar]
- 45.Chang S-C, Lacey JV, Brinton LA, Hartge P, Adams K, Mouw T, Carroll L, Hollenbeck A, Schatzkin A, Leitzmann MF. Lifetime Weight History and Endometrial Cancer Risk by Type of Menopausal Hormone Use in the NIH-AARP Diet and Health Study. Cancer Epidemiology Biomarkers & Prevention. 2007;16:723–30. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 46.Edlefsen KLJR, Prentice RL, Janssen I, Rajkovic A, O'Sullivan MJ, Anderson G. The effects of postmenopausal hormone therapy on serum estrogen, progesterone, and sex hormone-binding globulin levels in healthy postmenopausal women. Menopause. 2010;17:622–9. doi: 10.1097/gme.0b013e3181cb49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endogenous Hormones Breast Cancer Collaborative Group Body Mass Index, Serum Sex Hormones, and Breast Cancer Risk in Postmenopausal Women. Journal of the National Cancer Institute. 2003;95:1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 48.Baer H, Colditz G, Rosner B, Michels K, Rich-Edwards J, Hunter D, Willett W. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Research. 2005;7:R314–R25. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magnusson CMK, Roddam AW, Pike MC, Chilvers C, Crossley B, Hermon C, McPherson K, Peto J, Vessey M, Beral V. Body fatness and physical activity at young ages and the risk of breast cancer in premenopausal women. Br J Cancer. 2005;93:817–24. doi: 10.1038/sj.bjc.6602758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body Fatness at Young Ages and Risk of Breast Cancer Throughout Life. American Journal of Epidemiology. 2010;171:1183–94. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


