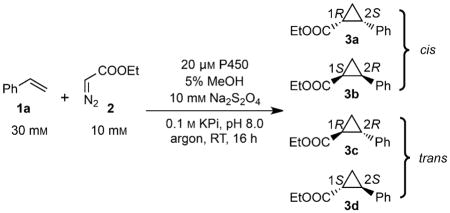
Table 1.
Activities and stereoselectivities of P450 variants for the reaction of styrene with ethyl diazoacetate.
| |||||
|---|---|---|---|---|---|
| Catalyst | Yield | TTN[a] | dr [cis:trans] | ee cis [%][b] | ee trans [%][c] |
| hemin | 16 | 79 | 13:87 | −2 | −4 |
| P450BM3 | 1 | 7 | 12:88 | 0 | −2 |
| P450BM3-T268A | 67 | 338 | 1:99 | −18 | −97 |
| P450BM3-CIS-T438S | 62 | 311 | 93:7 | −97 | −79 |
| P450BioI | 27 | 135 | 12:88 | 8 | 13 |
| P450BioI-T238A | 48 | 241 | 71:29 | 95 | −24 |
| P450cam | 41 | 207 | 88:12 | −43 | 9 |
| P450cam-T252A | 30 | 151 | 71:29 | −86 | −5 |
| P450eryF (A245) | 70 | 349 | 89:11 | −99 | −19 |
| CYP142 | 49 | 246 | 44:56 | −84 | −6 |
| CYP142-T234A | 54 | 272 | 90:10 | −97 | −14 |
| CYP164A2 | 7 | 34 | 14:85 | 5 | 2 |
| CYP164A2-T260A | 70 | 350 | 18:82 | −82 | −9 |
| CYP107N1 | 7 | 36 | 9:91 | 0 | −4 |
| CYP107N1-T251A | 48 | 238 | 9:91 | −3 | −3 |
| P450nor | 5 | 27 | 12:88 | −6 | −3 |
| P450nor-T243A | 13 | 66 | 10:90 | −3 | −2 |
| P450EpoK | 49 | 247 | 11:89 | −15 | −22 |
| P450EpoK-T258A | 44 | 219 | 18:82 | −25 | −14 |
| P450PikC | 50 | 249 | 8:92 | −2 | 3 |
| P450PikC-T247A | 46 | 231 | 7:93 | −15 | 32 |
| P450RhF | 52 | 258 | 9:91 | −3 | −2 |
| P450RhF-T275A | 34 | 171 | 12:88 | −11 | −2 |
| P450TxtE | 37 | 187 | 10:90 | 6 | −2 |
| P450TxtE-T250A | 45 | 225 | 10:90 | −2 | −2 |
| P450TylH1 | 48 | 242 | 13:87 | 16 | 8 |
| P450TylH1-T279A | 50 | 251 | 10:90 | 1 | −28 |
TTN =total turnover number.
(1R,2S)–(1S,2R).
(1R,2R)–(1S,2S).
TTNs and stereoselectivities were determined by chiral GC analysis.
