Abstract
Motility is a fundamental property of mammalian cells that normally is observed in tissue culture by time lapse microscopy where resolution is limited by the wavelength of light. This paper examines a powerful electrical technique by which cell motion is quantitatively measured at the nanometer level. In this method, the cells are cultured on small evaporated gold electrodes carrying weak ac currents. A large change in the measured electrical impedance of the electrodes is observed when cells attach and spread on these electrodes. When the impedance is tracked as a function of time, fluctuations are observed that are a direct measure of cell motion. Surprisingly, these fluctuations continue even when the cell layer becomes confluent. By comparing the measured impedance with a theoretical model, it is clear that under these circumstances the average motions of the cell layer of 1 nm can be inferred from the measurements. We refer to this aspect of cell motility as micromotion.
Full text
PDF
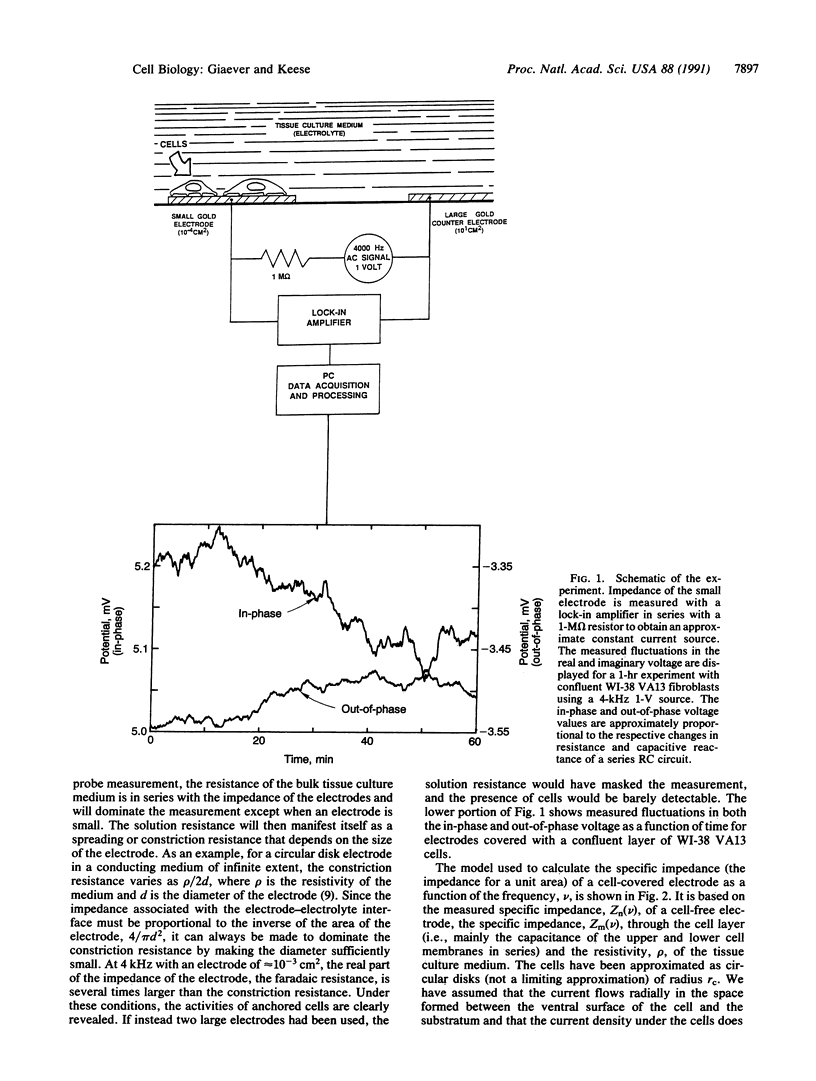
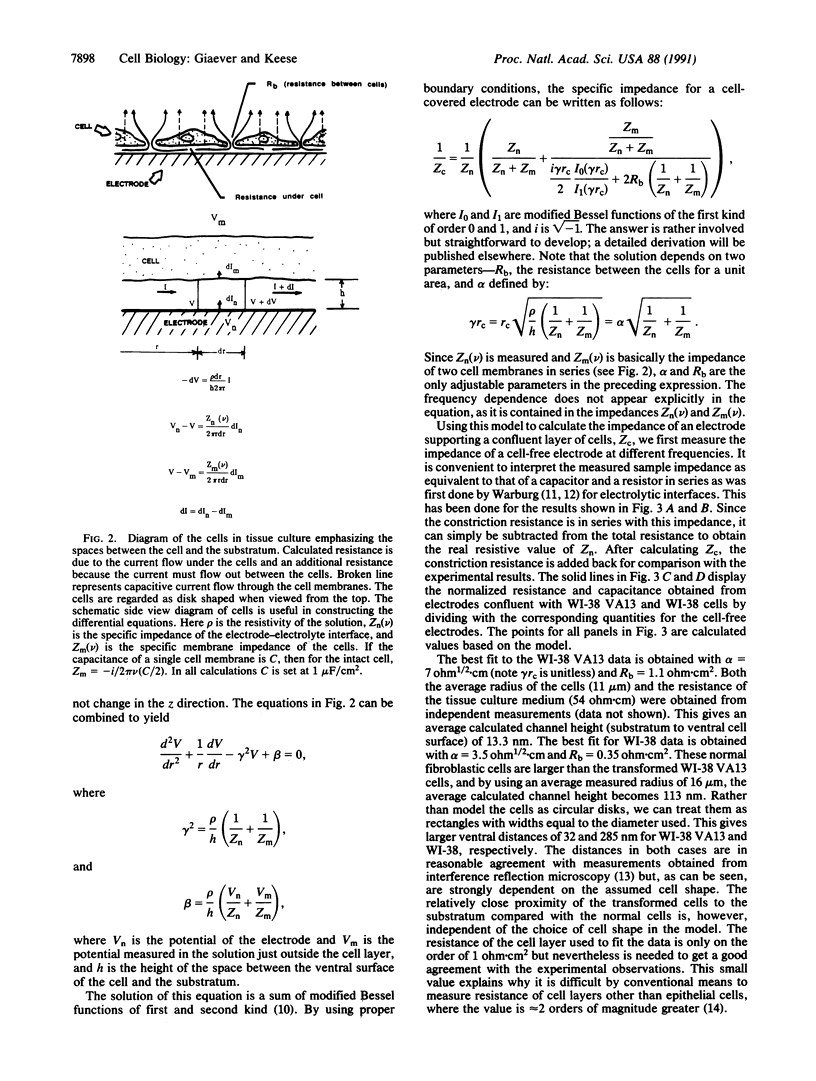
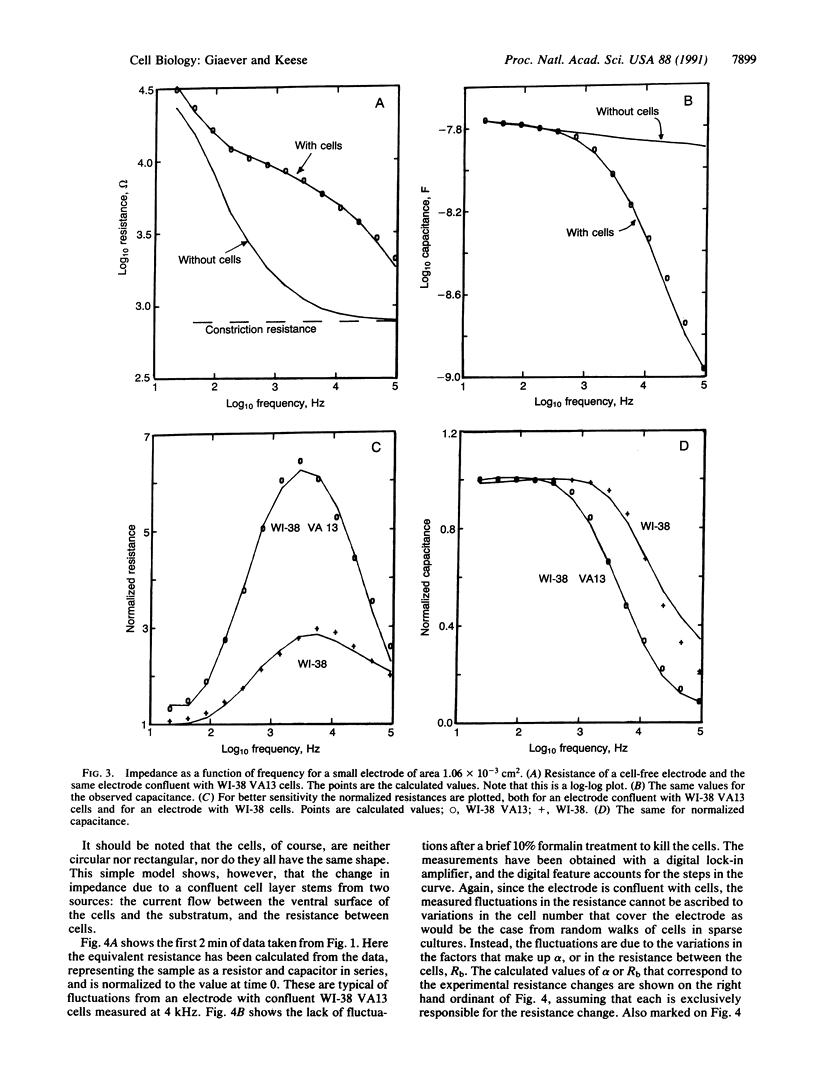
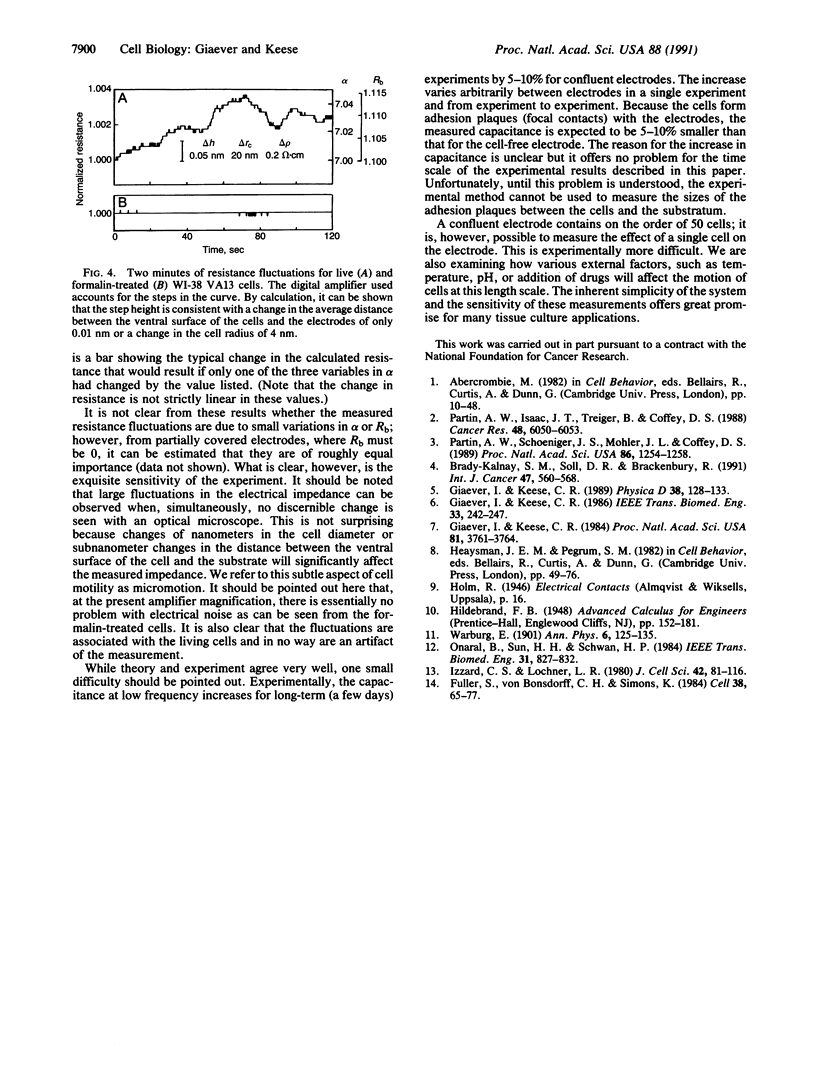
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady-Kalnay S. M., Soll D. R., Brackenbury R. Invasion of Rous sarcoma virus-transformed retinal cells: role of cell motility. Int J Cancer. 1991 Feb 20;47(4):560–568. doi: 10.1002/ijc.2910470414. [DOI] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3761–3764. doi: 10.1073/pnas.81.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture. IEEE Trans Biomed Eng. 1986 Feb;33(2):242–247. doi: 10.1109/TBME.1986.325896. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980 Apr;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Onaral B., Sun H. H., Schwan H. P. Electrical properties of bioelectrodes. IEEE Trans Biomed Eng. 1984 Dec;31(12):827–832. doi: 10.1109/TBME.1984.325245. [DOI] [PubMed] [Google Scholar]
- Partin A. W., Isaacs J. T., Treiger B., Coffey D. S. Early cell motility changes associated with an increase in metastatic ability in rat prostatic cancer cells transfected with the v-Harvey-ras oncogene. Cancer Res. 1988 Nov 1;48(21):6050–6053. [PubMed] [Google Scholar]
- Partin A. W., Schoeniger J. S., Mohler J. L., Coffey D. S. Fourier analysis of cell motility: correlation of motility with metastatic potential. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1254–1258. doi: 10.1073/pnas.86.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]



