Abstract
A number of topoisomerase II-targeted anticancer drugs, including amsacrine, utilize an acridine or related aromatic core as a scaffold. Therefore, to further explore the potential of acridine-related compounds to act as topoisomerase II poisons, we synthesized a series of novel trifluoromethylated 9-amino-3,4-dihydroacridin-1(2H)-one derivatives and examined their ability to enhance DNA cleavage mediated by human topoisomerase IIα. Derivatives containing a H, Cl, F, and Br at C7 enhanced enzyme-mediated double-stranded DNA cleavage ~5.5- to 8.5-fold over baseline, but were less potent than amsacrine. The inclusion of an amino group at C9 was critical for activity. The compounds lost their activity against topoisomerase IIα in the presence of a reducing agent, displayed no activity against the catalytic core of topoisomerase IIα, and inhibited DNA cleavage when incubated with the enzyme prior to the addition of DNA. These findings strongly suggest that the compounds act as covalent, rather than interfacial, topoisomerase II poisons.
Keywords: acridin-2-ones, DNA topoisomerase IIα, anticancer drugs, covalent poison, DNA cleavage
Graphical abstract
Eukaryotic type II topoisomerases are ubiquitous enzymes that play critical roles in a number of genetic processes, including DNA replication, transcription, recombination, and chromosome segregation.1–6 These enzymes resolve the problems associated with the topological constraints of DNA (i.e., under- or overwinding, knotting, and tangling) by transiently cleaving both strands of the double helix.1–6 Humans encode two type II topoisomerase isoforms, topoisomerase IIα and IIβ.
Topoisomerase IIα is essential for the survival of proliferating cells, increases in concentration over the cell cycle (peaking in G2/M), and is the isoform that is involved in replicative processes. In contrast, topoisomerase IIβ is non-essential at the cellular level, is expressed in proliferating and quiescent cells, and maintains a constant expression level throughout the cell cycle. Although the precise cellular function of topoisomerase IIβ is not well defined, it appears to play an important role in the expression of hormonally regulated genes.1–5
Type II topoisomerases are the target for a number of widely used anticancer drugs.1,2,7–10 These drugs function by an insidious mechanism. Rather than robbing the cell of essential topoisomerase II functions, they act by increasing levels of covalent topoisomerase II-cleaved DNA complexes (i.e., cleavage complexes), which are requisite intermediates in the catalytic cycle of the enzyme. Because these agents convert the type II enzymes into cellular toxins that fragment the genome, they are called topoisomerase II poisons to distinguish them from catalytic inhibitors. Topoisomerase IIα is an important cytotoxic target for anticancer drugs.
Amsacrine was the first anticancer agent shown to act by poisoning topoisomerase II.11 The drug is composed of an acridine ring coupled to a 4′-amino-methane-sulfon-m-anisidide head group. Structure-activity studies suggest that the poisoning activity of amsacrine is embodied in the head group, while the acridine moiety enhances drug activity primarily by promoting strong interactions with DNA.12
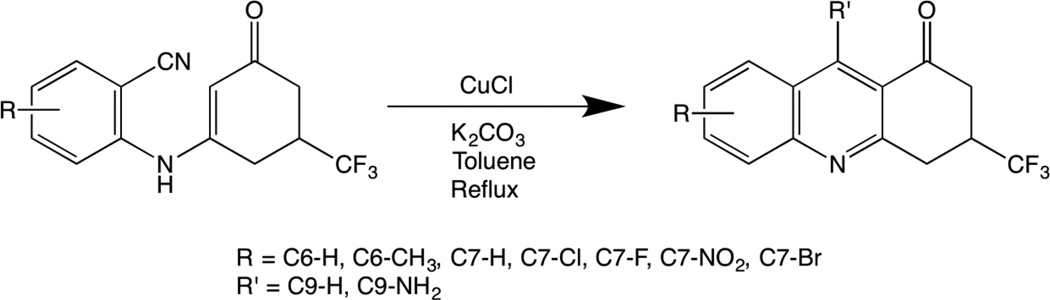
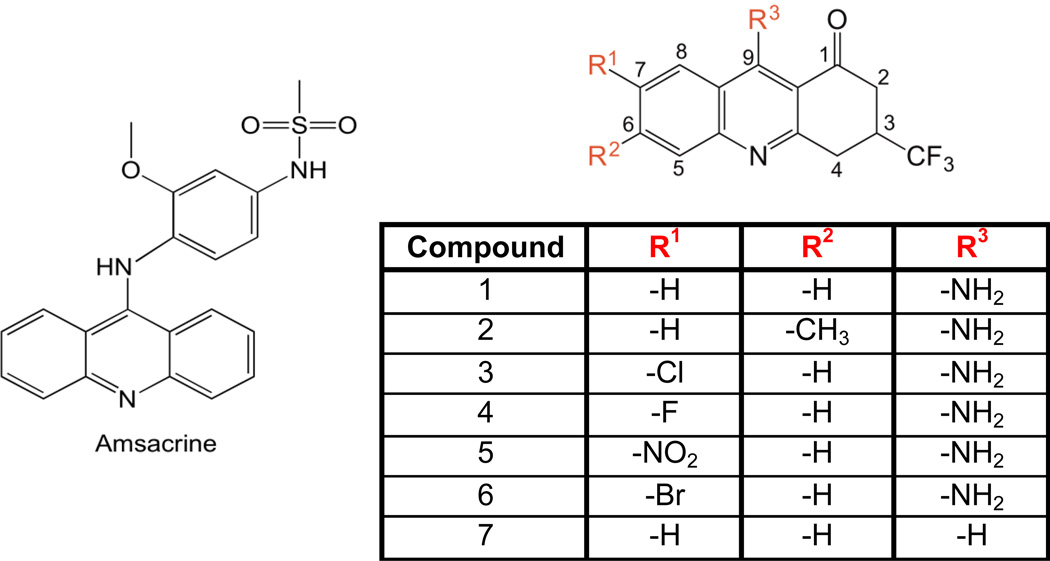
A number of other topoisomerase II-targeted agents also utilize an acridine or related aromatic core.1,2,7–10,13 Although most of these drug cores are attached to an aromatic head group, some agents (such as mitoxantrone) lack a head group entirely. Therefore, to further explore the potential of acridine-related compounds to act as topoisomerase II poisons, we synthesized a series of novel trifluoromethylated 9-amino-3,4-dihydroacridin-1(2H)-ones (referred to as trifluoromethylated 9-amino acridin-2-ones) and analyzed the activity of these compounds against human topoisomerase IIα. The trifluoromethyl group at C3 was included to provide additional H-bonding groups and because the presence of fluorines often provides improved pharmacological properties, such as increased membrane permeability, enhanced hydrophobic binding interactions, and improved metabolic stability (discussed in Fadeyi et al.14). The syntheses of these compounds started with the corresponding enaminobenzonitrile14 utilizing the generalized scheme shown in Fig. 1. Structures of the trifluoromethylated acridin-2-ones, along with that of amsacrine for comparison, are shown in Fig. 2. The detailed syntheses and physical and chemical characterizations of the compounds are described in the accompanying Supplementary Data.
Figure 1.
Generalized scheme used to synthesize the trifluoromethylated acridin-2-one derivatives used in the current study.
Figure 2.
Structures of amsacrine (left) and the trifluoromethylated acridin-2-ones used in the current study. The trifluoromethylated acridin-2-one skeleton is shown (top right) and the substituents at positions R1–3 (red) for compounds 1–7 are listed in the table.
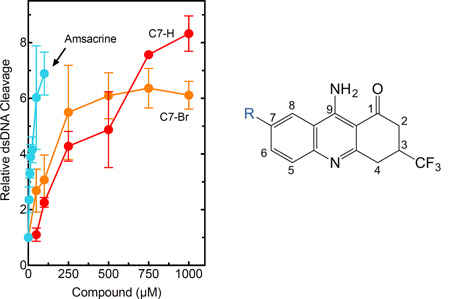
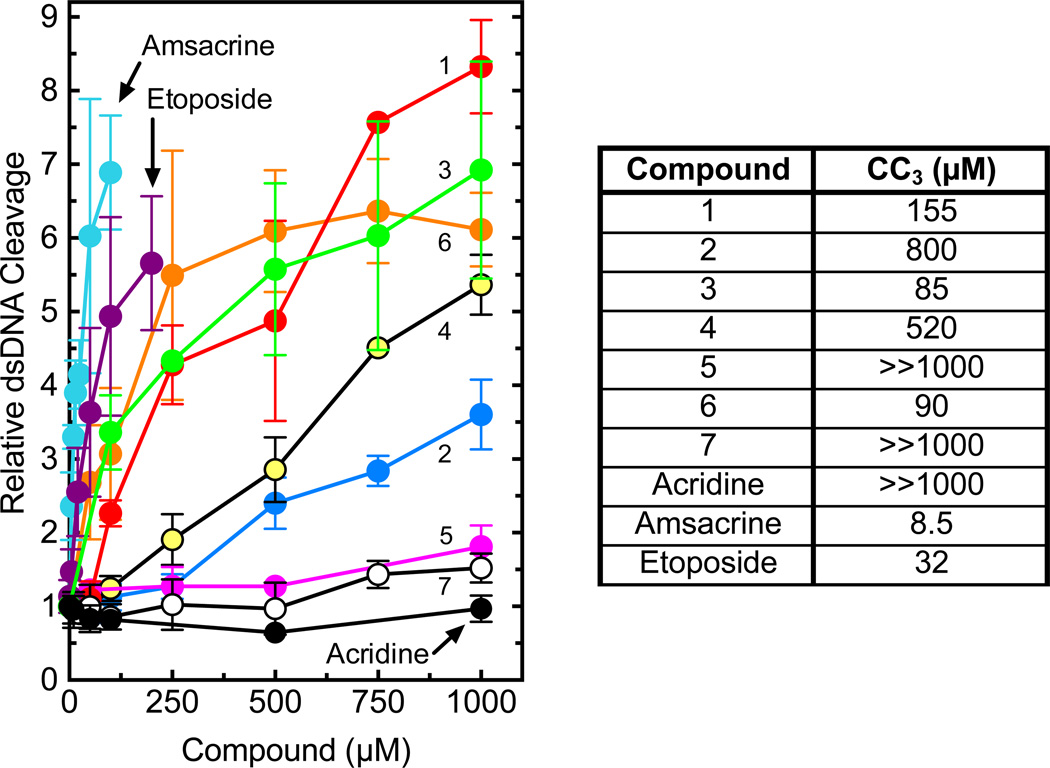
As a first step toward analyzing the effects of the trifluoromethylated acridin-2-one derivatives on human topoisomerase IIα, we determined the ability of these compounds to enhance enzyme-mediated double-stranded cleavage of negatively supercoiled DNA (Fig. 3). Several of the trifluoromethylated acridin-2-one derivatives induced high levels of enzyme-mediated DNA cleavage, although none were as potent as amsacrine or etoposide (another well-characterized topoisomerase II poison). Acridine, which represents the aromatic core of amsacrine, did not enhance DNA cleavage. On the basis of the cleavage data, a number of structure-activity relationships among the compounds became evident.
Figure 3.
Effects of trifluoromethylated acridin-2-one derivatives on the DNA cleavage activity of human topoisomerase IIα (left). Results with amsacrine, etoposide, and acridine as shown as controls. Activity is reported as the relative increase in double-stranded DNA (dsDNA) cleavage as compared to reactions carried out in the absence of compounds. Error bars represent the standard deviation of at least three independent experiments. The table (right) provides the concentrations (CC3) of compounds 1–7, acridine, amsacrine, and etoposide that are required to triple levels of cleavage complexes as compared to baseline levels generated in the absence of the compounds. These values are used as a measure of potency.
First, the presence of the 9-amino moiety was critical for the activity of the trifluoromethylated acridin-2-one derivatives. Removal of this group (converting compound 1 to compound 7) decreased cleavage enhancement from >8-fold to ~1.5-fold and increased the CC3 (level of compound required to triple baseline levels of cleavage complex; used as a measure of potency because this value is within the linear range of activity for the compounds examined) from 155 µM to >>1000 µM. Second, the inclusion of either a methyl group at C6 (compound 2) or a nitro moiety at C7 (compound 5) was deleterious and decreased both the level of DNA cleavage and the potency of the compounds. Third, the inclusion of halogens at C7 affected the activity of the compounds against topoisomerase IIα. The presence of Cl, F, or Br (compounds 3, 4, or 6, respectively) resulted in a modest reduction (<35%) in cleavage levels (at 1000 µM compound) compared to compound 1. However, the chloro- and bromo-substituted compounds displayed an ~1.8-fold increase in potency. In contrast, the potency of the fluoro-substituted compound decreased ~3.4-fold.
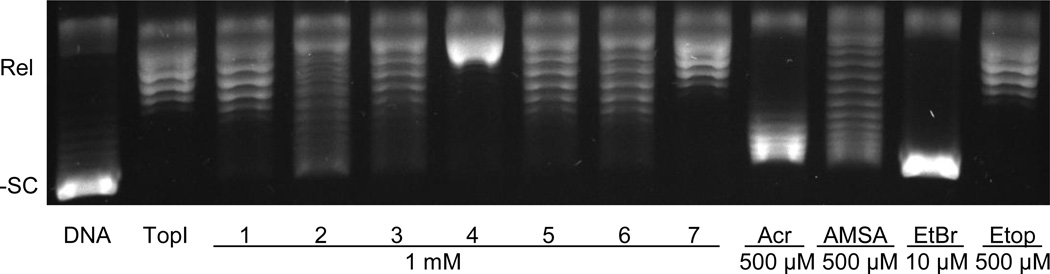
In some cases, enhanced interactions between topoisomerase II poisons and DNA leads to greater drug activity against the enzyme. Therefore, the ability of the trifluoromethylated acridin-2-one derivatives to intercalate into DNA was assessed (Fig. 4). In the concentration range examined, the compounds were weak intercalators at best (as determined by the shift in DNA topoisomer bands toward the position of negatively supercoiled DNA). Furthermore, there does not appear to be any correlation between the ability of the trifluoromethylated acridin-2-ones to intercalate and their activity against topoisomerase IIα. Compounds 2 and 4, for example, displayed similar DNA cleavage enhancement but very different intercalation patterns. Furthermore, compound 5 displayed similar intercalation to compound 1, but had a significantly lower potency against topoisomerase IIα. Because compounds 5 and 7 displayed virtually no activity against topoisomerase IIα, they were not subjected to further analysis.
Figure 4.
Intercalation of trifluoromethylated acridin-2-one derivatives. Results of a topoisomerase I-DNA relaxation assay are shown. Intercalation is indicated by the shift in the position of the plasmid from relaxed (Rel) DNA, which is generated upon incubation of negatively supercoiled DNA (-SC) with topoisomerase I. Each lane is labeled with the number of the trifluoromethylated acridin-2-one compound that was tested. Three intercalators, acridine (Acr, 500 µM), ethidium bromide (EtBr, 10 µM), and amsacrine (AMSA, 500 µM), and a non-intercalator, etoposide (Etop, 500 µM), are shown as positive and negative controls, respectively. Assays that contained only -SC DNA (DNA) or -SC DNA and topoisomerase I with no compound (TopI) are shown. Gel is representative of three independent experiments.
Clinically relevant topoisomerase II-targeted drugs, such as amsacrine and etoposide, act as interfacial topoisomerase II poisons.15 These drugs interact at the enzyme-DNA interface in a noncovalent fashion, intercalate into the cleaved DNA scissile bond, and stabilize the cleavage complex by inhibiting the ability of the type II enzyme to ligate the DNA backbone. Therefore, to determine whether the trifluoromethylated 9-amino acridin-2-one derivatives act by a similar mechanism, the effects of these compounds on topoisomerase IIα-mediated DNA ligation were characterized (Fig. 5). In marked contrast to amsacrine and etoposide, none of the trifluoromethylated 9-amino acridin-2-ones that enhanced DNA cleavage had any significant effect on rates of ligation.
Figure 5.
Effects of trifluoromethylated 9-amino acridin-2-one derivatives on DNA ligation mediated by topoisomerase IIα. Reaction mixtures were carried out in the absence or presence of 1 mM compound. Reactions that contained 50 µM amsacrine or etoposide are shown as positive controls. Error bars represent the standard deviation of at least three independent experiments.
The lack of ligation inhibition does not preclude the possibility that the trifluoromethylated 9-amino acridin-2-one derivatives act as interfacial poisons. However, the finding suggests that these compounds may increase levels of topoisomerase IIα-DNA cleavage complexes by a different mechanism. As an alternative to the interfacial mechanism, a number of topoisomerase II poisons act through covalent interactions with the type II enzyme. Covalent poisons adduct topoisomerase II at cysteine (and potentially other) residues outside of the DNA cleavage-ligation active site of the enzyme.2,16–19 They appear to increase levels of cleavage complexes by altering the ability of the N-terminal domain of topoisomerase II to act as a protein clamp.20,21 Covalent poisons display several hallmark characteristics that distinguish them from interfacial poisons.16,20,22–26 Because covalent poisons are reactive and require redox cycling as part of the adduction reaction, their activities are abrogated by the presence of reducing agents. Furthermore, because covalent poisons act by altering the N-terminal portion of the enzyme, they do not enhance DNA cleavage by topoisomerase II constructs that lack this domain. Finally, although covalent poisons enhance DNA scission when added to the topoisomerase II-DNA complex, they irreversibly inhibit enzyme-mediated cleavage when incubated with the protein prior to the addition of DNA. This likely occurs (at least in part) through the closing of the N-terminal protein gate, which precludes the binding of circular DNAs by the enzyme.
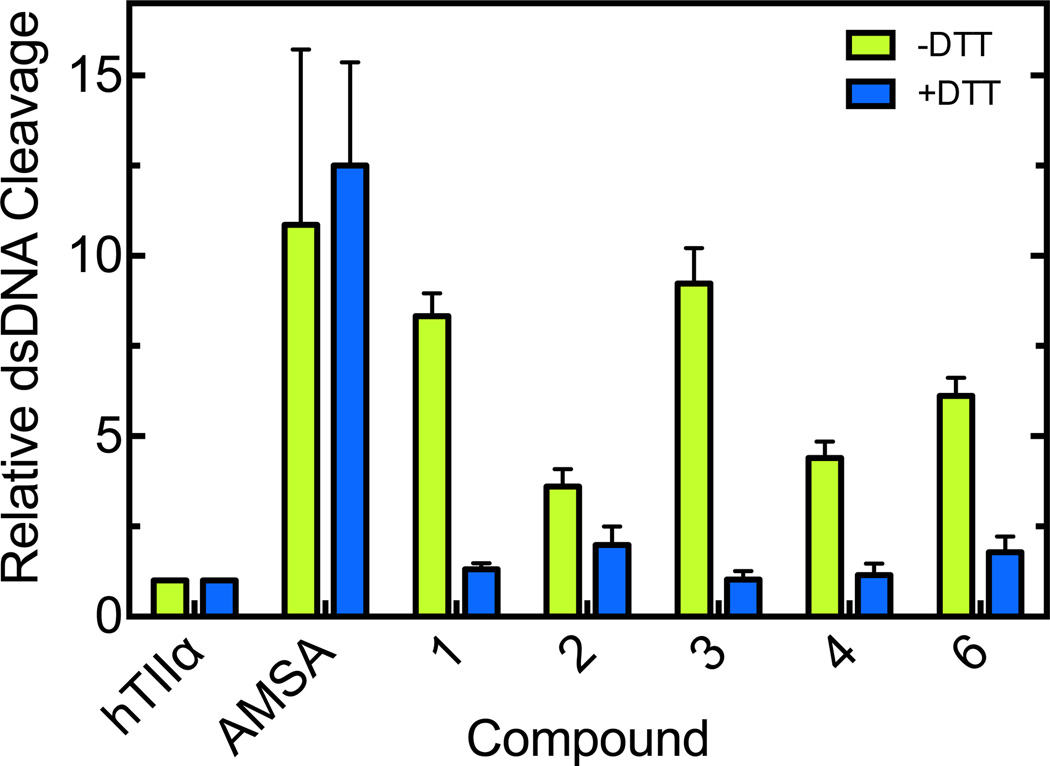
In order to identify the mechanism by which the trifluoromethylated 9-amino acridin-2-one derivatives alter topoisomerase II function, we first determined the effects of dithiothreitol (DTT) on the ability of these compounds to enhance topoisomerase IIα-mediated DNA cleavage (Fig. 6). The reducing agent dramatically decreased levels of acridin-2-one-induced DNA cleavage. In contrast, DTT had no effect on the activity of the interfacial poison amsacrine. These results strongly suggest that the trifluoromethylated 9-amino acridin-2-one derivatives alter topoisomerase IIα-mediated DNA cleavage by acting as covalent poisons. Therefore, two additional experiments were carried out to confirm this mechanism.
Figure 6.
Effects of a reducing agent (DTT) on acridin-2-one-induced DNA cleavage mediated by topoisomerase IIα. DNA cleavage reactions were carried out in the presence of 1 mM trifluoromethylated 9-amino acridin-2-one derivatives in the absence (green) or presence (blue) of 0.5 mM DTT. Reactions carried out in the absence of compounds (hTIIα) or in the presence of 50 µM amsacrine (AMSA) are shown as controls. Error bars represent the standard deviation of at least three independent experiments.
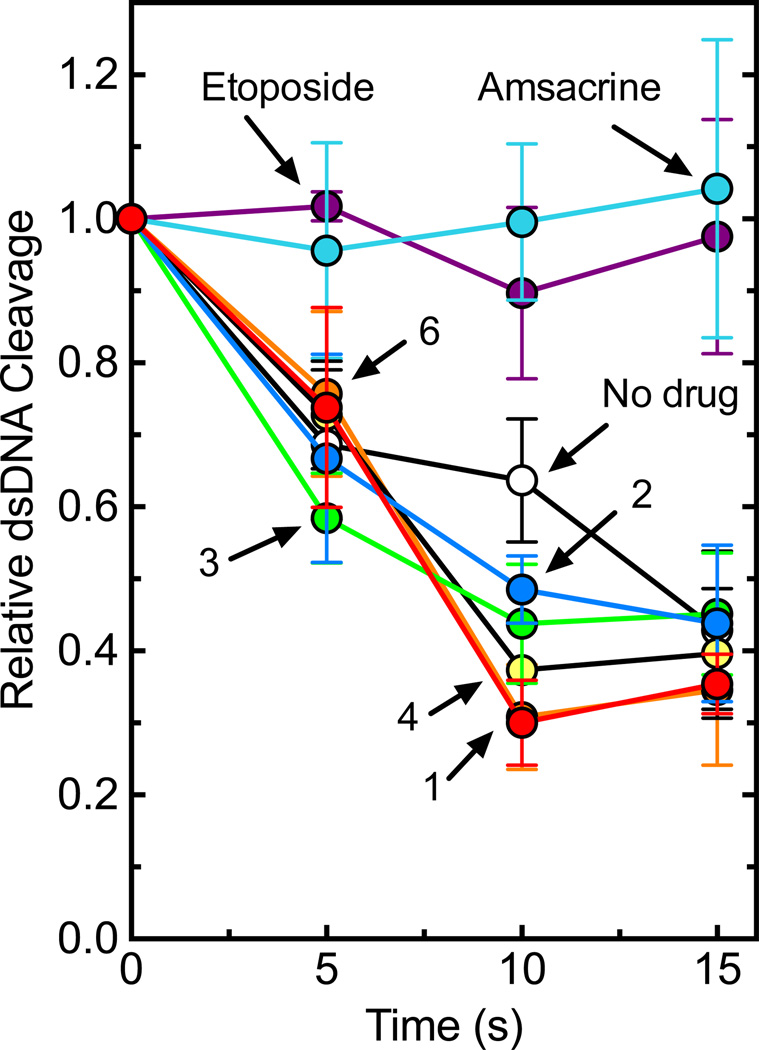
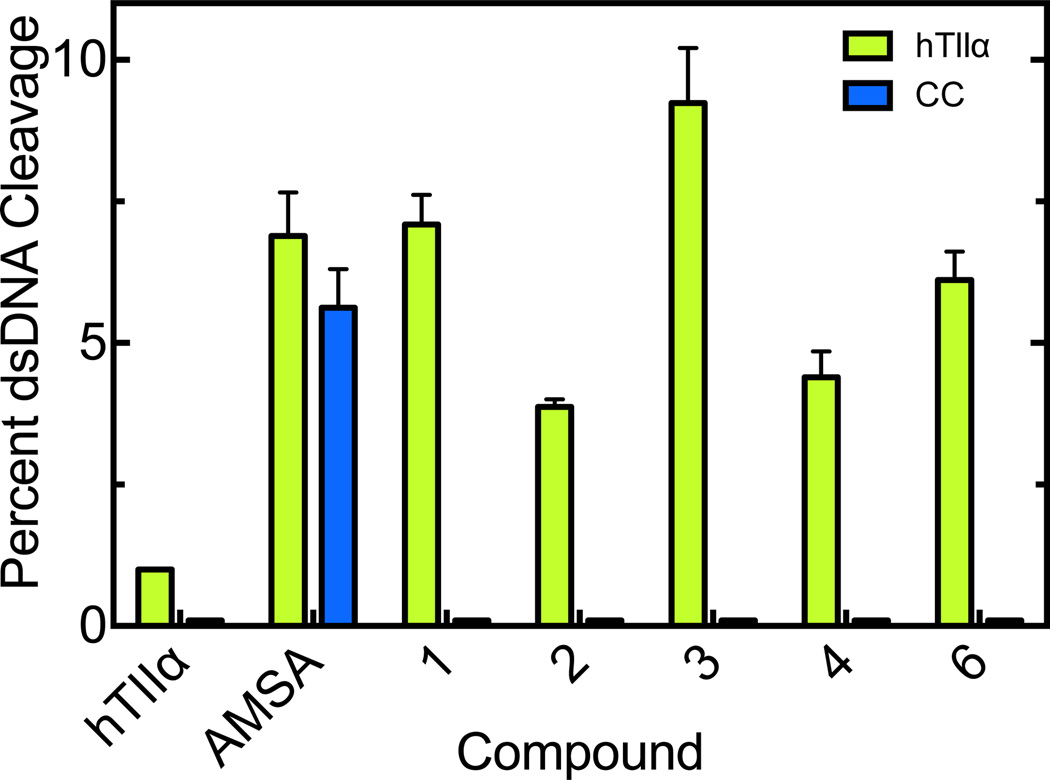
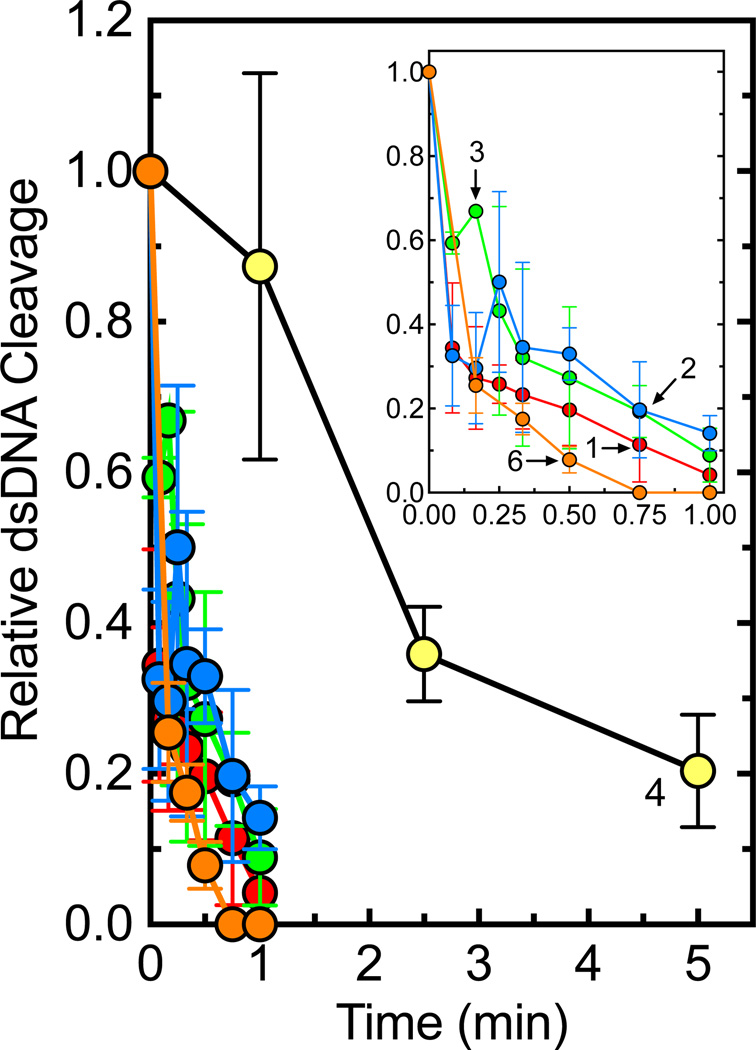
We assessed the effects of the trifluoromethylated 9-amino acridin-2-one derivatives on the catalytic core of topoisomerase IIα, a construct that lacks both the C- and N-terminal domains (Fig. 7). Although interfacial poisons such as amsacrine maintain their activity against the catalytic core, the trifluoromethylated 9-amino acridin-2-one derivatives did not induce enzyme-mediated DNA cleavage above background levels. As a final experiment, the trifluoromethylated 9-amino acridin-2-one derivatives were incubated with topoisomerase IIα prior to the addition of DNA (Fig. 8). All of the compounds rapidly inhibited the DNA cleavage activity of the enzyme.
Figure 7.
Effects of trifluoromethylated 9-amino acridin-2-one derivatives on DNA cleavage mediated by the catalytic core of topoisomerase IIα. DNA cleavage reactions using full-length topoisomerase IIα (green) or the catalytic core of the enzyme (blue) were carried out in the presence of 1 mM acridin-2-one derivatives. Reactions carried out in the absence of compounds (hTIIα) or in the presence of 50 µM amsacrine (AMSA) are shown as controls. Baseline levels of DNA cleavage mediated by the catalytic core are lower than those observed for the intact enzyme. Error bars represent the standard deviation of at least three independent experiments.
Figure 8.
Effects of trifluoromethylated 9-amino acridin-2-one derivatives on DNA cleavage mediated by topoisomerase IIα when incubated with the enzyme prior to the addition of DNA. Cleavage reactions were initiated after topoisomerase IIα was incubated with 1 mM derivatives for 1–5 min. The inset displays an expansion of time points between 0–1 min for compounds 1, 2, 3, and 6. Error bars represent the standard deviation of at least three independent experiments.
Taken together, the above experiments strongly suggest that the trifluoromethylated 9-amino acridin-2-one derivatives enhance topoisomerase IIα-mediated DNA cleavage by acting as covalent topoisomerase II poisons. The chemistry that underlies the adduction of topoisomerase IIα by the acridin-2-ones is not immediately apparent. However, given the essential nature of the 9-amino moiety for the activity of these compounds, it may involve amino-imino tautomerism or the hydrolysis of the amino group to form a more reactive species. It is notable that the 4′-amino-methane-sulfon-m-anisidide head group of amsacrine (which also includes a primary aromatic amine) displays the ability to act as a covalent topoisomerase II poison.
On the basis of comparisons to amsacrine, it will be interesting to determine whether the addition of a head group to the acridin-2-one derivatives at the 9-amino position will convert these compounds to interfacial poisons that could act as scaffolds for the development of novel topoisomerase II-targeted drugs.
Supplementary Material
Acknowledgments
This research was supported by grant GM033944 (N.O.) from the National Institutes of Health and funds from the U.S. Department of Education Title III Grant, Tennessee State University (C.O.O.). L.I.L. was a trainee under NIH grant GM008554. We are grateful to Rachel E. Ashley and Elizabeth G. Gibson for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
Supplementary data
Experimental details for the synthesis and characterization of the trifluoromethylated acridin-2-one derivatives, as well as sources of materials and methods for biochemical assays, are available in the accompanying Supplementary Data.
References
- 1.Deweese JE, Osheroff MA, Osheroff N. Biochem. Mol. Biol. Educ. 2008;37:2. doi: 10.1002/bmb.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deweese JE, Osheroff N. Nucleic Acids Res. 2009;37:738. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitiss JL. Nat. Rev. Cancer. 2009;9:327. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos SM, Tretter EM, Schmidt BH, Berger JM. Nat. Rev. Mol. Cell. Biol. 2011;12:827. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SH, Chan NL, Hsieh TS. Ann. Rev. Biochem. 2013;82:139. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- 6.Pommier Y, Sun Y, Huang SN, Nitiss JL. Nat. Rev. Mol. Cell Biol. 2016 doi: 10.1038/nrm.2016.111. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitiss JL. Nat. Rev. Cancer. 2009;9:338. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pommier Y, Leo E, Zhang H, Marchand C. Chem. Biol. 2010;17:421. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pommier Y. ACS Chem. Biol. 2013;8:82. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendleton M, Lindsey RH, Jr, Felix CA, Grimwade D, Osheroff N. Ann. N.Y. Acad. Sci. 2014;1310:98. doi: 10.1111/nyas.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson EM, Tewey KM, Liu LF. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1361. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketron AC, Denny WA, Graves DE, Osheroff N. Biochemistry. 2012;51:1730. doi: 10.1021/bi201159b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamage SA, Spicer JA, Atwell GJ, Finlay GJ, Baguley BC, Denny WA. J. Med. Chem. 1999;42:2383. doi: 10.1021/jm980687m. [DOI] [PubMed] [Google Scholar]
- 14.Fadeyi OO, Adamson ST, Myles EL, Okoro CO. Bioorg. Med. Chem. Lett. 2008;18:4172. doi: 10.1016/j.bmcl.2008.05.078. [DOI] [PubMed] [Google Scholar]
- 15.Pommier Y, Marchand C. Nat. Rev. Drug. Discov. 2012;11:25. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Biochemistry. 2001;40:3316. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- 17.Bender RP, Osheroff N. Chem. Res. Toxicol. 2007;20:975. doi: 10.1021/tx700062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin RK, Zhou N, Lyu YL, Tsai YC, Lu CH, Kerrigan J, Chen YT, Guan Z, Hsieh TS, Liu LF. J. Biol. Chem. 2011;286:33591. doi: 10.1074/jbc.M111.258137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ketron AC, Osheroff N. Phytochem. Rev. 2014;13:19. doi: 10.1007/s11101-013-9291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Biochemistry. 2006;45:10140. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- 21.Mondrala S, Eastmond DA. Chem. Biol. Interact. 2010;184:259. doi: 10.1016/j.cbi.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey RH, Jr, Bromberg KD, Felix CA, Osheroff N. Biochemistry. 2004;43:7563. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- 23.Ketron AC, Gordon ON, Schneider C, Osheroff N. Biochemistry. 2013;52:221. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashley RE, Osheroff N. Chem. Res. Toxicol. 2014;27:787. doi: 10.1021/tx400453v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsey RH, Pendleton M, Ashley RE, Mercer SL, Deweese JE, Osheroff N. Biochemistry. 2014;53:6595. doi: 10.1021/bi5010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vann KR, Sedgeman CA, Gopas J, Golan-Goldhirsh A, Osheroff N. Biochemistry. 2015;54:4531. doi: 10.1021/acs.biochem.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.