Abstract
Background
The relationship of LPA single nucleotide polymorphisms (SNPs), apolipoprotein(a) isoforms and lipoprotein(a) [Lp(a)] levels with major adverse cardiovascular (MACE) events in different ethnic groups is not well known.
Methods
LPA SNPs, apolipoprotein(a) isoforms, Lp(a) and oxidized phospholipids on apolipoprotein B-100 (OxPL-apoB) levels were measured in 1792 Black, 1030 White and 597 Hispanic subjects enrolled in the Dallas Heart Study. Their interdependent relationships and prospective association with MACE after median 9.5-year follow-up were determined.
Results
LPA SNP rs3798220 was most prevalent in Hispanics (42.38%), rs10455872 in Whites (14.27%) and rs9457951 in Blacks (32.927%). The correlation of each of these SNPs with the major apolipoprotein(a) isoform size was highly variable and in different directions among ethnic groups. In the entire cohort, Cox regression analysis with multivariable adjustment revealed that quartiles 4 of Lp(a) and OxPL-apoB were associated with hazard ratios (HR) (95% CI) for time to MACE of 2.35 (1.50-3.69), p<0.001) and 1.89 (1.26-2.84), p=0.003), respectively, versus quartile 1. Addition of the major apolipoprotein(a) isoform and the 3 LPA SNPs to these models attenuated the risk, but significance was maintained for both Lp(a) and OxPL-apoB. Evaluating specific ethnic groups, in Blacks Lp(a) was a positive predictor and the size of the major apolipoprotein(a) isoform and inverse predictor, in Whites the size of the major apolipoprotein(a) isoform was an inverse predictor and in Hispanics OxPL-apoB was a predictor of time to MACE.
Conclusion
The prevalence and association of LPA SNPs with size of apolipoprotein(a) isoforms, Lp(a) and OxPL-apoB levels are highly variable and ethnicity-specific. The relationship to MACE is best explained by elevated plasma Lp(a) or OxPL-apoB levels, despite significant ethnic differences in LPA genetic markers.
Keywords: lipoprotein(a), ethnicity, isoforms, single nucleotide polymorphisms, and cardiovascular events, oxidized phospholipids
Subject terms: Lipids and cholesterol, biomarkers, race and ethnicity, atherosclerosis
Introduction
Lipoprotein(a) [Lp(a)] is an independent, genetic and likely causal risk factor for myocardial infarction, stroke, peripheral arterial disease1,2 and calcific aortic valve stenosis.3,4 Circulating Lp(a) levels are largely genetically determined by a variety of differences in the LPA gene. There are several levels of LPA gene regulation that determine plasma Lp(a) levels, including biochemical influences on transcription factors, variations in LPA single nucleotide polymorphisms (SNPs) and the inter and intra individual heterogeneity in kringle IV type 2 (KIV2) isoform repeats. Physiologic, dietary and environmental factors play a relatively minor role and can either lower or raise Lp(a) plasma levels.5 Furthermore, it is well appreciated that significant ethnic differences exist in Lp(a) levels, with highest levels present in subjects of African descent and generally followed in decreasing order of lower levels in southern Asians, Whites, Hispanics and east Asians.2,6,7
LPA SNPs rs3798220 and rs10455872 in Whites and rs9457951 in Blacks have been shown to be associated with increased plasma Lp(a) levels In separate studies.8,9 However, the independent association of LPA SNPs and apolipoprotein(a) isoforms with major adverse cardiovascular events (MACE) in populations as a whole and in different ethnic groups is not well established. The goals of this study were to evaluate these three major, well-defined LPA SNPs in their relationship to Lp(a) levels and apolipoprotein(a) isoforms in different ethnic groups and to assess their relationship of LPA SNPs to incident CVD events. This gap in the clinical database of Lp(a) is relevant in view of novel agents that can reduce Lp(a) levels, such as antibodies directed to proprotein convertase subtilisin kexin type 9 (PCSK9) to bind circulating PCSK9 generating immune complexes and preventing physiological action10,11 and antisense oligonucleotides directed to apolipoprotein(a) messenger ribonucleic acid to reduce apolipoprotein(a) protein synthesis and thereby prevent assembly of Lp(a).12 The Dallas Heart Study (DHS) provides a unique, prospective database with median 9.5 year follow-up to assess these relationships among White, Black and Hispanic subjects to provide insights into the atherogenicity of Lp(a). We hypothesized that elevated circulating Lp(a) levels rather than ethnic LPA genetic differences would be the key determinant in predicting incident cardiovascular events.
Methods
Study subjects
The characteristics of the DHS subjects were previously described in detail.7 The DHS is a multiethnic, probability-based sample of the Dallas county population in which Blacks were systematically over-sampled so they represented ∼50% of the final sample size. In this study, 3,419 blood samples were available with complete data of LPA SNPs, apolipoprotein(a) isoforms, Lp(a) levels and accompanying ethnic, biomarker and major adverse cardiovascular event (MACE) data from 2001-2010 for subsequent analyses. The Institutional Review Board of the University of Texas Southwestern Medical Center approved the study, and all participants provided informed consent in accordance with institutional guidelines. The Human Subject protection program at University of California San Diego approved the measurement of oxidative biomarkers.
Determination of LPA SNPs
The LPA variants rs3798220, rs10455872, rs9457951, rs1801693, rs41272110 and G+1/inKIV-8A were genotyped using a TaqMan assay on a 7900HT Fast Real-Time PCR instrument (Applied Biosystems, Foster City, CA) at 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 seconds and 60°C for 1.5 minutes.
Determination of Lp(a) levels and apolipoprotein (a) isoforms
Measurement of Lp(a) levels was performed with a well-validated assay that is independent of apolipoprotein(a) isoform size and reported as in nmol/L.13 Apolipoprotein(a) isoforms were measured as the total number of KIV repeats as previously described.13 The analyses were based on size of the major apolipoprotein(a) KIV isoform visualized on agarose gel electrophoresis.7 In this study, the major apolipoprotein(a) isoform was associated with the smaller of the 2 alleles in 87% of subjects.
Determination of Oxidized Phospholipids on Apolipoprotein B-100 Levels
Oxidized phospholipids on apolipoprotein B-100 (OxPL-apoB) were measured as previously described in detail by chemiluminescent ELISA using the murine monoclonal antibody E06, which binds to the phosphocholine (PC) headgroup of oxidized but not native phospholipids.14-16 The OxPL-apoB values are expressed as relative lights units reflecting the amount of E06 bound to OxPL on apoB particles captured on microtiter well plates with antibody MB47. The OxPL-apoB measure primarily (∼85-90%) reflects the OxPL on Lp(a), in which OxPL are bound both covalently to apo(a) and in the lipid phase of apoB, with the remaining (0-15%) OxPL being present on non-Lp(a) apoB particles.17 It is to be emphasized that the OxPL-apoB measure only represents those OxPL recognized by E06 (i.e. E06 immunoreactivity) and does not represent non-E06 detectable OxPL present on apoB particles.17
Determination of Major Adverse Cardiovascular Events
The subjects were followed from January 2001 until December 31 2010 for a median of 9.5 years of follow-up. Event adjudication beyond 2010 is not currently available for the DHS. Major adverse cardiovascular events (MACE) was defined as cardiac death, non-fatal MI, stroke/TIA, unstable angina requiring hospitalization and arterial vascularization that included coronary artery bypass surgery, percutaneous coronary intervention, carotid endarterectomy, carotid stenting and peripheral artery revascularization. Of the 3419 individuals, all had complete LPA SNP, apolipoprotein(a) isoform and biomarker data. Additionally, all-cause death adjudicated until Dec 31 2010, however, this endpoint was not included in the MACE analyses as the causes of non-cardiac death were highly heterogeneous and would appropriately test the Lp(a) cardiovascular hypothesis. From this group of 3419 individuals, 2929 had the MACE endpoint adjudicated until the latest follow-up of Dec 31 2010, therefore the MACE analyses were performed on these subjects. There were no significant baseline differences in the individuals with or without adjudicated MACE events.
Statistical Analyses
Analyses were performed with SPSS 23.0 software package. Continuous variables were presented as means ± standard deviations (SD) or medians and interquartile range and dichotomous variables as percentages. Differences in baseline attributes between subjects were analyzed with ANOVA and χ2-test. Correlations between variables were determined with the Spearman test. The base-2 logarithms (log2) of Lp(a), size of the major apolipoprotein(a) isoform, OxPL-apoB and triglycerides were used to account for skewness in the distributions. Thus, hazard ratios for these variables reflect the change in hazard for an increase of 1 log2 (the equivalent of a doubling of the value) in the measure. Multivariable Cox regression analysis was used to estimate the associations between LPA SNPs, the size of the major apolipoprotein(a) isoform, Lp(a) and OxPL-apoB levels and time to MACE, with adjustment for sex, age in deciles, smoking status, the presence or absence of hypertension and diabetes, body mass index and levels of LDL-C per 25 mg/dL increase, HDL-C per 10 mg/dL increase, and log2 triglycerides. If a patient had more than one MACE event, only the first event was counted.
Results
Baseline Characteristics of the Study Group by Ethnicity
Table 1 displays the baseline characteristics of the entire group and by individual ethnic group. Blacks tended to be older and have a higher prevalence of hypertension, diabetes and current smoking. Lp(a) and OxPL-apoB levels were highest in Blacks, followed by Whites, then Hispanics. A similar but inverse association was noted in the major apolipoprotein(a) isoform among ethnic groups. Blacks also had higher HDL-C and lower triglyceride levels compared to the other groups.
Table 1.
Baseline characteristics of the study groups as a whole and by ethnicity.
| Entire Group (N=3419) | Black (N=1792) | White (N=1030) | Hispanic (N=597) | P-Value for ethnicity | |
|---|---|---|---|---|---|
| Age, years (SD) | 43.8 (10.1) | 44.5 (10.2) | 44.7 (10.0) | 40.0 (9.2) | <0.001 |
| Male, N (%) | 1501 (43.9) | 755 (42.1) | 496 (48.2) | 250 (42.0) | 0.004 |
| BMI | 30.8 (7.6) | 31.6 (8.2) | 29.0 (6.7) | 30.3 (6.6) | <0.001 |
| HTN, N (%) | 963 (28.2) | 660 (36.8) | 218 (21.2) | 85 (14.2) | <0.001 |
| Diabetes, N (%) | 393 (11.5) | 254 (14.2) | 66 (6.4) | 71 (11.9) | <0.001 |
| Current smoking, N (%) | 1004 (29.4) | 598 (33.4) | 284 (27.6) | 122 (20.4) | <0.001 |
| Laboratory variables | |||||
| Lp(a), nmol/L | 49.9 (19.6-110.5) | 79.0 (43-132) | 26.9 (10-69) | 21.3 (9-46) | <0.001 |
| OxPL-apoB, RLU | 4025 (2564-8734) | 6445 (3419-11389) | 3014 (2233-5306) | 2689 (2058-3766) | <0.001 |
| Size of major isoform, # kringles | 24 (19.5-28) | 23.0 (20-26) | 24.0 (19-29) | 27.0 (21-32) | <0.001 |
| Total Cholesterol, mg/dl | 180.3 (39.7) | 177.7 (40.3) | 183.6 (38.3) | 182.3 (40.1) | <0.001 |
| LDL-C, mg/dl | 106.2 (35.5) | 104.7 (36.8) | 108.2 (34.3) | 107.2 (33.3) | 0.029 |
| HDL-C, mg/dl | 49.9 (14.9) | 52.3 (15.4) | 48.4 (15.1) | 45.7 (11.2) | <0.001 |
| VLDL-C, mg/dl | 24.2 (18.9) | 20.8 (17.7) | 26.9 (18.1) | 29.4 (21.5) | <0.001 |
| Triglycerides, mg/dl | 96.0 (67-146) | 85.0 (62-123) | 109.0 (75-166) | 119.0 (81-176) | <0.001 |
| Minor allele frequency, N (%) | |||||
| rs3798220 | 324 (9.48) | 27 (1.51) | 44 (4.27) | 253 (42.38) | <0.001 |
| rs10455872 | 213 (6.22) | 33 (1.84) | 147 (14.27) | 33 (5.53) | <0.001 |
| rs9457951 | 615 (17.99) | 590 (32.92) | 4 (0.0039) | 21 (3.52) | <0.001 |
| rs1801693 | 1341 (40.2) | 417 (23.9) | 532 (52.9) | 392 (67.1) | <0.001 |
| rs41272110 | 455 (13.4) | 80 (4.48) | 272 (26.72) | 103 (17.37) | <0.001 |
| G+1/inKIV-8A | 186 (5.5) | 102 (5.74) | 55 (5.40) | 29 (4.94) | 0.75 |
Values are given as mean (SD) or median (IQR). For rs1801693, rs41272110 and G+1/inKIV-8A 3379, 3333 and 3395 subjects were genotyped.
The prevalence of LPA SNPs is shown in Table 1 and all were significantly different among ethnic groups except G+1/inKIV-8A. Rs3798220 was most prevalent in Hispanics, rs10455872 in Whites, rs9457951 in Blacks, rs1081693 in Hispanics, rs41272110 in Whites.
All patients had adjudicated total mortality data, but the 490 patients without an adjudicated MACE event did not have differences in Lp(a) or OxPL-apoB than patients with adjudicated MACE (Supplemental Table 1). They tended to be younger, male, smokers, with more hypertension, diabetes, higher triglycerides, but lower BMI, total cholesterol and LDL-C.
Relationship of LPA SNPs to Lp(a), OxPL-apoB, and Apolipoprotein(a) Isoforms by Ethnicity
Table 2 displays the relationship of LPA SNPs to Lp(a), apolipoprotein(a) isoforms and OxPL-apoB.
Table 2.
Relationship of LPA rs3798220, rs10485774 and rs9459571 to Lp(a), apolipoprotein(a) isoforms and OxPL-apoB.
| Black | ||||
|---|---|---|---|---|
|
|
||||
| rs3798220 | TT (N = 1765) | TC (N= 27) | CC (N = 0) | P-Value |
| Lp(a), nmol/L | 79.3 (43.8-132.2) | 49.1 (13.5-122.6) | - | 0.15 |
| OxPL-apoB, RLU | 6507 (3510-11416) | 4305 (2501-8733) | - | 0.11 |
| Major apolipoprotein(a) isoform, #KIV repeats | 23.0 (20-26) | 23.0 (21-33) | - | 0.042 |
| White | ||||
|
|
||||
| TT (N = 985) | TC (N= 43) | CC (N = 1) | ||
|
|
||||
| Lp(a), nmol/L | 25.9 (9.5-58.1) | 187.3 (113.4-229.1) | 197.1 (-) | <0.001 |
| OxPL-apoB, RLU | 2963 (2217-4701) | 12493 (8521-16202) | 13222 (-) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 24.5 (19-29) | 17.0 (17-19) | 17.0 (-) | <0.001 |
| Hispanic | ||||
|
|
||||
| TT (N = 343) | TC (N= 212) | CC (N = 41) | ||
|
|
||||
| Lp(a), nmol/L | 25.2 (10.2-61.0) | 18.8 (8.1-36.6) | 13.2 (6.4-22.9) | <0.001 |
| OxPL-apoB, RLU | 2873 (2145-4567) | 2586 (1972-3360) | 2445 (1777-2901) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 25.0 (20-30) | 30.25 (24-34) | 33.0 (31.5-34) | <0.001 |
| Black | ||||
|
|
||||
| rs10485774 | AA (N = 1759) | AG (N= 33) | GG (N = 0) | P-Value |
|
| ||||
| Lp(a), nmol/L | 77.9 (43.4-132.1) | 207.0 (117-261.9) | - | <0.001 |
| OxPL-apoB, RLU | 6314 (3473-11300) | 11854 (6397-17005) | - | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 23.0 (20-26) | 17 (16-17.5) | - | <0.001 |
| White | ||||
|
|
||||
| AA (N = 882) | AG (N= 139) | GG (N = 8) | ||
|
|
||||
| Lp(a), nmol/L | 21.2 (8.1-41.8) | 141.4 (108.4-172.3) | 219.6 (205.6-276.9) | <0.001 |
| OxPL-apoB, RLU | 2826 (2134-3895) | 7949 (5496-10745) | 12534 (8792-16924) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 25.5 (21-29) | 17.0 (16-18) | 17.25 (16.1-17.5) | <0.001 |
| Hispanic | ||||
|
|
||||
| AA (N = 563) | AG (N= 31) | GG (N = 2) | ||
|
|
||||
| Lp(a), nmol/L | 19.6 (8.1-40.7) | 151.7 (102.6-198.2) | 151.3 (-) | <0.001 |
| OxPL-apoB, RLU | 2602 (2046-3530) | 7966 (4773-12056) | 8583 (-) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 28.0 (22-32) | 17.0 (16-17) | 17.0 (-) | <0.001 |
| Black | ||||
| rs9459571 | CC (N = 1202) | CG (N= 547) | GG (N = 43) | P-Value |
|
| ||||
| Lp(a), nmol/L | 73.8 (40.6-121.9) | 94.4 (50.8-153.4) | 97.0 (62.2-186.6) | <0.001 |
| OxPL-apoB, RLU | 5502 (3247-9864) | 8602 (4241-14223) | 8032 (5052-17154) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 22.5 (20-26) | 24.0 (20-27) | 24.0 (19-24) | 0.008 |
| White | ||||
| CC (N = 1025) | CG (N= 3) | GG (N = 1) | ||
|
|
||||
| Lp(a), nmol/L | 26.7 (10.1-68.7) | 89.8 (-) | 35.8 (-) | 0.38 |
| OxPL-apoB, RLU | 3015 (2233-5286) | 8223 (-) | 1952 (-) | 0.43 |
| Major apolipoprotein(a) isoform, #KIV repeats | 24.0 (19-29) | 26.0 (-) | 27.0 (-) | 0.85 |
| Hispanic | ||||
|
|
||||
| CC (N = 575) | CG (N= 20) | GG (N = 1) | ||
|
|
||||
| Lp(a), nmol/L | 20.6 (8.2-43.7) | 76.4 (32.6-146.6) | 507 (-) | <0.001 |
| OxPL-apoB, RLU | 2656 (2053-3674) | 6508 (2993-11918) | 18675 (-) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 28.0 (21-32) | 21.5 (6508) | 14 (-) | 0.003 |
|
| ||||
| Black | ||||
| rs1801693 | AA (N = 1326) | AG (N= 391) | GG (N = 26) | P-Value |
|
| ||||
| Lp(a), nmol/L | 84.3 (49.5-134.7) | 55.7 (30.6-116.5) | 44.2 (12.0-98.0) | <0.001 |
| OxPL-apoB, RLU | 7066 (3884-11613) | 4482 (2893-9923) | 3836 (2459-9549) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 23.0 (20-26) | 24.0 (20-28) | 25.0 (19.75-32) | <0.001 |
| White | ||||
| AA (N = 474) | AG (N= 434) | GG (N = 98) | ||
|
|
||||
| Lp(a), nmol/L | 28.5 (11.7-80.1) | 23.8 (8.6-57.1) | 24.9 (4.2-108.2) | 0.025 |
| OxPL-apoB, RLU | 3104 (2250-5773) | 2958 (2229-4635) | 3127 (2134-8486) | 0.29 |
| Major apolipoprotein(a) isoform, #KIV repeats | 23.25 (18-29) | 25.0 20-28 | 24.25 (18-29) | 0.068 |
| Hispanic | ||||
|
|
||||
| AA (N = 192) | AG (N= 266) | GG (N = 126) | ||
|
|
||||
| Lp(a), nmol/L | 29.5 (13.4-78.5) | 19.6 (6.8-40.6) | 16.4 (7.8-36.8) | <0.001 |
| OxPL-apoB, RLU | 2898 (2172-3587) | 2739 (2049-3587) | 2520 (1850-3570) | 0.016 |
| Major apolipoprotein(a) isoform, #KIV repeats | 24.0 (19-28) | 28.0 (23-33) | 31.0 (25.38-33.63) | <0.001 |
| Black | ||||
| rs41272110 | TT (N = 1704) | TG (N= 78) | GG(N = 2) | P-Value |
|
| ||||
| Lp(a), nmol/L | 79.4 (43.9-132.8) | 59.3 (31.4-124.8) | 60.5 (-) | 0.33 |
| OxPL-apoB, RLU | 6509 (3521-11428) | 4769 (2960-10707) | 6317 (-1) | 0.37 |
| Major apolipoprotein(a) isoform, KIV repeats | 23.0 (20-26.5) | 21.5 (18-25) | 22.5 (-) | 0.071 |
| White | ||||
| TT (N = 746) | TG (N= 258) | GG(N = 14) | ||
|
|
||||
| Lp(a), nmol/L | 26.4 (7.3-76.6) | 27.2 (13.7-58.4) | 24.9 (13.4-106.7) | 0.51 |
| OxPL-apoB, RLU | 3097 (265-5576) | 2920 (2096-5036) | 2575 (2170-4421) | <0.001 |
| Major apolipoprotein(a) isoform, #KIV repeats | 26.0 (19-29.5) | 20.25 (18-25.63) | 19.0 (18-20) | 0.56 |
| Hispanic | ||||
|
|
||||
| TT (N = 490) | TG (N= 99) | GG(N = 4) | ||
|
|
||||
| Lp(a), nmol/L | 20.6 (7.1-47.7) | 23.5 (14.1-33.9) | 21.4 (5.4-35.0) | 0.47 |
| OxPL-apoB, RLU | 2774 (2056-3962) | 2519 (2050-3468) | 2566 (2264-3117) | 0.62 |
| Major apolipoprotein(a) isoform, #KIV repeats | 28.5 (23-33) | 20.0 (19-26) | 19.5(19-22) | <0.001 |
|
| ||||
| Black | ||||
| G+1/inKIV-8A | GG(N = 1673) | GA N(= 98) | AA(N = 4) | P-Value |
|
| ||||
| Lp(a), nmol/L | 78.9 (43.5-131.5) | 77.6(50.8-153.4) | 70.4 (30.8-91.2) | 0.90 |
| OxPL-apoB, RLU | 6481 (3520-11305) | 5845 (3036-13624) | 5956 (2972-14026) | 0.99 |
| Major apolipoprotein(a) isoform, #KIV repeats | 23.0 (20-26) | 22.75 (20-26) | 25.5 (19.5-31.5) | 0.60 |
| White | ||||
| GG (N = 962) | GA(N= 52) | AA(N = 3) | ||
|
|
||||
| Lp(a), nmol/L | 26.4 (10.1-68.9) | 32.3 (9.0-92.2) | 37.5 () | 0.66 |
| OxPL-apoB, RLU | 3020 (2239-5296) | 2984 (2262-5648) | 1812 (-) | 0.63 |
| Major apolipoprotein(a) isoform, #KIV repeats | 24.0 (19-29) | 23.5 (17.25-27.75) | 19.0 (-) | 0.30 |
| Hispanic | ||||
|
|
||||
| GG (N = 558) | GA (N= 28) | AA(N = 1) | ||
|
|
||||
| Lp(a), nmol/L | 21.1 (8.7-45.4) | 24.8 (7.5-70.3) | 5.1 (-) | 0.72 |
| OxPL-apoB, RLU | 2720 (2070-3730) | 2593 (2134-4741) | 1708 (-) | 0.71 |
| Major apolipoprotein(a) isoform, #KIV repeats | 27.0 (21-32) | 28.0 (19.5-31.0) | 34.0 (-) | 0.43 |
Values are given as median (IQR).
For rs3798220, in Blacks, Lp(a) and OxPL-apoB levels were not significantly different among wild-type alleles, heterozygotes and homozygotes. In Whites, significant differences were noted in Lp(a), apolipoprotein(a) isoforms and OxPL-apoB (p<0.001 for all) with carriers of the C allele having highly elevated Lp(a) and OxPL-apoB levels and corresponding smaller isoforms. In Hispanics significant differences were noted in Lp(a), apolipoprotein(a) isoforms and OxPL-apoB (p<0.001 for all) but in contrast to Whites, an inverse association was present, with in carriers of the C allele having lower Lp(a), lower OxPL-apoB and larger isoforms sizes.
For rs10455872, Lp(a), OxPL-apoB, and apolipoprotein(a) isoforms were significantly different among wild-type alleles and heterozygotes and homozygotes among Blacks, Whites and Hispanics (p<0.001 in all 3 ethnic groups for each measure), with carriers of the G allele having highly elevated Lp(a) and OxPL-apoB levels and corresponding smaller isoforms.
For rs9457951, Lp(a), OxPL-apoB and apolipoprotein(a) isoforms were significantly different among wild-type alleles and heterozygotes and homozygotes among Blacks and Hispanics, but not Whites, with carriers of the G allele having highly elevated Lp(a) and OxPL-apoB levels. Blacks did not have corresponding smaller isoforms but Hispanics did.
For rs1801693, rs41272110, variable differences were noted Lp(a), OxPL-apoB and apolipoprotein(a) isoforms among ethnic groups and for G+1/inKIV-8A no differences were noted (Table 2).
Correlations Between LPA SNPs, Lp(a), OxPL-apoB and Size of the Major Apolipoprotein(a) Isoform
Table 3 displays the correlation between LPA SNPs and Lp(a), OxPL-apoB and size of the major apolipoprotein(a) isoform. rs3798220 was inversely associated with size of the major apolipoprotein(a) isoform in Whites, positively in Hispanics and not correlated in Blacks. rs10455872 was inversely associated with size of the major apolipoprotein(a) isoform in all 3 groups. rs9457951 was positively associated in Blacks, inversely in Hispanics and not correlated in Whites. Lp(a) was highly correlated with OxPL-apoB in Blacks (Spearman r=0.87, p<0.001), Whites (r=0.70, p<0.001) and Hispanics (r=0.63, p<0.001). The size of the major apolipoprotein(a) isoform was generally inversely associated with Lp(a) and OxPL-apoB. Finally, the 3 LPA SNPs did not correlate with each other in Blacks and Whites, but rs3798220 was negatively associated with rs10455872 in Hispanics.
Table 3. Spearman correlations of LPA single nucleotide polymorphisms with Lp(a), OxPL-apoB and size of the major apolipoprotein(a) isoform.
| rs10455872 | rs9457951 | rs1801693 | rs412722110 | G+1/inKIV-8A | Lp(a) | OxPL-apoB | Major apolipoprotein(a) Isoform | |
|---|---|---|---|---|---|---|---|---|
| Black | ||||||||
| rs3798220 | -0.02 | -0.07 | 0.22† | -0.005 | -0.03 | -0.05* | -0.05* | 0.03 |
| rs10455872 | - | -0.07 | -0.06* | -0.03 | 0.02 | -0.16† | 0.09† | -0.18† |
| rs9457951 | - | -0.08 | -0.07* | 0.002 | 0.15† | 0.21† | 0.09† | |
| rs1801693 | - | -0.04 | -0.005 | 0.17† | -0.15† | 0.09 | ||
| rs412722110 | - | -0.02 | -0.05* | -0.04 | -0.06* | |||
| G+1/inKIV-8A | - | -0.006 | -0.01 | -0.002 | ||||
| Lp(a) | - | 0.87† | -0.62† | |||||
| OxPL-apoB | -0.48† | |||||||
| White | ||||||||
| rs3798220 | -0.04 | -0.01 | 0.23† | -0.05 | 0.05 | 0.22† | 0.24† | -0.18† |
| rs10455872 | - | -0.03 | -0.18† | -0.11† | 0.02 | 0.48† | 0.42† | -0.50† |
| rs9457951 | - | -0.04 | 0.04 | 0.05 | 0.04 | 0.02 | 0.01 | |
| rs1801693 | - | -0.003 | -0.04 | 0.08† | -0.02 | 0.07 | ||
| rs412722110 | - | 0.003 | -0.05 | -0.04 | -0.22† | |||
| G+1/inKIV-8A | - | -0.02 | -0.02 | -0.04 | ||||
| Lp(a) | - | 0.70† | -0.56† | |||||
| OxPL-apoB | - | -0.47† | ||||||
| Hispanic | ||||||||
| rs3798220 | -0.18† | -0.08 | 0.64† | -0.10* | 0.06 | 0.17† | -0.16† | 0.37† |
| rs10455872 | - | -0.05 | -0.17† | -0.09* | -0.02 | 0.33† | 0.30† | -0.37† |
| rs9457951 | - | -0.14† | -0.04 | -0.002 | 0.18† | 0.16† | -0.13† | |
| rs1801693 | - | -0.16† | 0.02 | -0.17† | -0.12† | 0.34† | ||
| rs412722110 | - | 0.002 | 0.03 | -0.05 | -0.31† | |||
| G+1/inKIV-8A | - | 0.007 | -0.003 | -0.01 | ||||
| Lp(a) | - | 0.63† | -0.57† | |||||
| OxPL-apoB | -0.43† | |||||||
p<-05
p<0.001
For rs1801693, modest positive and inverse correlations were noted with Lp(a), OxPL-apoB, rs3798220 and rs10455872 depending on the ethnic group (Table 3).
Relationship of Lp(a), OxPL-apoB and Apolipoprotein(a) Isoforms to Time to MACE in All Subjects
Of the 2929 individuals with adjudicated MACE, there were 211 individuals (7.2% of total) with MACE events with 146 in Blacks, 52 in Whites and 13 in Hispanics over the median 9.5-year follow-up. A total of 336 (1.6 events per person with an event) events occurred, including 41 CV deaths, 61 MIs, 59 strokes, 19 TIAs, 25 cases of unstable angina, 65 PCIs, 33 CABGs, 12 carotid and 21 peripheral arterial revascularizations.
Table 4 shows the results of sequentially adjusted Cox regression analysis of Lp(a), OxPL-apoB and the major apolipoprotein(a) isoform with MACE in the entire group. Linear trends were significant for Lp(a), OxPL-apoB and apolipoprotein(a) isoforms across quartiles. In unadjusted analyses, the hazard ratios (HR) 95% confidence intervals (95% CI) quartiles 3 (1.87 (1.22-2.88), p=0.004) and 4 (2.44 (1.62-3.68), p<0.001) of Lp(a), quartile 4 (1.78 (1.22-2.59), p=0.003) of OxPL-apoB were significantly higher compared to quartile 1, and quartile 4 (0.60 (0.38-0.86), p=0.007) of the size of the major apolipoprotein(a) isoform had a lower HR compared to quartile 1. Adjustment for sex, HTN, diabetes, smoking, age in deciles, BMI, LDL per 25 mg/dL, HDL per 10 mg/dL and log2 triglycerides did not materially change the results for Lp(a), OxPL-apoB or size of the major apolipoprotein(a) isoform size. Addition of the 6 LPA SNPs to the model attenuated the risk slightly but significance was maintained for Lp(a), OxPL-apoB and size of the major apolipoprotein(a) isoform. For Lp(a), adding the size of the major apolipoprotein(a) isoform attenuated the risk further, and finally adding OxPL-apoB resulted in loss of significance. For OxPL-apoB and the size of the major apolipoprotein(a) isoform, similar findings were present with addition of Lp(a) fully attenuating risk. Removing arterial revascularization (PCI, CABG, carotid and peripheral arterial revascularization) for the MACE endpoint did not materially change the results.
Table 4.
Hazard Ratios for MACE According to Quartiles of Lp(a), OxPL-apoB and Size of the Major Apolipoprotein(a) Isoform with Sequential adjustment.
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-Value Q1 vs. Q4 |
|---|---|---|---|---|---|
| Lp(a) | 1.00 | 1.21 (0.76-1.92) | 1.87 (1.22-2.88) | 2.44 (1.62-3.68) | <0.001 |
| Plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 1.30 (0.80-2.12) | 1.80 (1.13-2.87) | 2.35 (1.50-3.69) | <0.001 |
| Plus LPA SNPs | 1.00 | 1.28 (0.76-2.13) | 1.75 (1.07-2.88) | 2.43 (1.48-3.97) | <0.001 |
| Plus log2 apolipoprotein(a) major isoform | 1.00 | 1.23 (0.73-2.06) | 1.59 (0.94-2.68) | 2.06 (1.17-3.62) | 0.012 |
| Plus log2 OxPL-apoB | 1.00 | 1.23 (0.73-2.08) | 1.46 (0.79-2.69) | 1.69 (0.75-3.82) | 0.21 |
| OxPL-apoB | 1.00 | 0.79 (0.50-1.24) | 1.37 (0.92-2.03) | 1.78 (1.22-2.59) | 0.003 |
| Plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.86 (0.54-1.36) | 1.32 (0.87-2.00) | 1.89 (1.26-2.84) | 0.003 |
| Plus LPA SNPs | 1.00 | 0.85 (0.52-1.38) | 1.31 (0.84-2.04) | 1.80 (1.21-2.87) | 0.005 |
| Plus log2 apolipoprotein(a) major isoforms | 1.00 | 0.84 (0.51-1.36) | 1.17 (0.74-1.84) | 1.58 (0.99-2.51) | 0.056 |
| Plus log2 Lp(a) | 1.00 | 0.69 (0.42-1.15) | 0.74 (0.46-1.36) | 0.90 (0.48-1.69) | 0.73 |
| Major apolipoprotein(a) isoform size | 1.00 | 1.03 (0.72-1.47) | 0.97 (0.67-1.40) | 0.60 (0.38-0.86) | 0.007 |
| Plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.79 (0.55-1.14) | 0.77 (0.53-1.13) | 0.52 (0.34-0.80) | 0.003 |
| Plus LPA SNPs | 1.00 | 0.83 (0.55-1.24) | 0.76 (0.50-1.17) | 0.49 (0.30-0.78) | 0.003 |
| Plus log2 OxPL-apoB | 1.00 | 0.84 (0.56-1.27) | 0.94 (0.59-1.45) | 0.62 (0.37-1.05) | 0.076 |
| Plus log2 Lp(a) | 1.00 | 0.99 (0.64-1.52) | 1.05 (0.66-1.66) | 0.79 (0.65-1.37) | 0.40 |
Quartiles for Lp(a): Q1<19.6, Q2 >19.6 - <49.9, Q3 >49.9 - <110.5, Q4 >110.5 nmol/L. Quartiles for major isoform size: Q1<19.5, Q2 >19.5 - <24.0, Q3 >24.0 - <28.0, Q4 >28.0 Kringle IV repeats. Quartiles for OxPL-apoB: Q1<2564, Q2 >2564 - <4025, Q3 >4025 - <8734, Q4 >8734 RLU.
Figure 1A-C displays the cumulative event-free survival curves for Lp(a) over the median 9.5-year follow-up, OxPL-apoB and size of the major apolipoprotein(a) isoform after adjustment for sex, HTN, DM, current smoking, BMI, age in deciles, LDL-C per increase of 25 mg/dl, log2 triglycerides, HDL-C per increase of 10 mg/dL, and by carrier status of rs3798220, rs15455872 and rs9457951, rs1801693, rs412722110 and G+1/inKIV-8A. Separation of MACE events was evident across all 4 quartiles.
Figure 1.
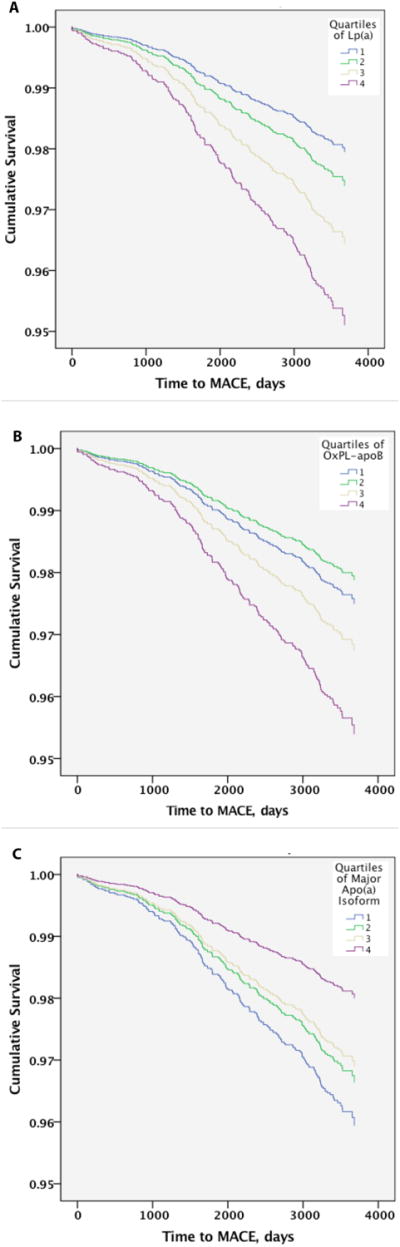
Relationship of Lp(a) (A), OxPL-apoB (B) and size of the major apolipoprotein(a) isoform (C) to time to MACE by multivariable adjusted Cox regression analysis.
Relationship of Lp(a), OxPL-apoB and Size of the Major Apolipoprotein (a) Isoform to Time to MACE by Ethnicity
In Blacks, adjustment for sex, HTN, diabetes, smoking, age in deciles, BMI, LDL per 25 mg/dL, HDL per 10 mg/dL and log2 triglycerides, Lp(a) was a positive predictor (HR 3.27 (1.27-7.11), p=0.012) and the size of the major apolipoprotein(a) isoform and inverse predictor (HR 0.49 (0.27-0.88), p=0.17) of time to MACE (Table 5). In Whites, the size of the major apolipoprotein(a) isoform was an inverse predictor (HR 0.49 (0.27-0.88), p=0.017), whereas strong trends were noted for Lp(a) and OxPL-apoB. In Hispanics, OxPL-apoB (HR 13.4 (1.89-94,3), p=0.009) was a predictor of time to MACE, with Lp(a) a strong trend. The interaction tests between ethnicity and Lp(a), OxPL-apoB and the size of the major apolipoprotein(a) isoform were negative (p>0.05 for all).
Table 5.
Hazard Ratios for MACE by Ethnicity According to Quartiles of Lp(a), OxPL-apoB and Size of the Major Apolipoprotein(a) Isoform with Sequential adjustment.
| Black | |||||
|---|---|---|---|---|---|
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-Value Q1 vs. Q4 |
| Lp(a) plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 1.80 (0.73-4.43) | 2.37 (0.99-5.63) | 3.27 (1.27-7.11) | 0.012 |
| OxPL-apoB plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.86 (0.44-1.68) | 1.13 (0.63-2.02) | 1.54 (0.87-2.72) | 0.140 |
| Major apolipoprotein(a) isoform size plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.81 (0.52-1.25) | 0.76 (0.48-1.20) | 0.49 (0.27-0.88) | 0.017 |
| White | |||||
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-Value Q1 vs. Q4 |
| Lp(a) plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.88 (0.42-1.88) | 1.31 (0.56-3.03) | 1.83 (0.88-3.80) | 0.105 |
| OxPL-apoB plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.54 (0.24-1.23) | 0.75 (0.34-1.63) | 0.57 (0.28-1.17) | 0.127 |
| Major apolipoprotein(a) isoform size plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.81 (0.52-1.25) | 0.76 (0.48-1.20) | 0.49 (0.27-0.88) | 0.017 |
| Hispanic | |||||
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-Value Q1 vs. Q4 |
| Lp(a) plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 3.44 (0.55-21.7) | 2.29 (0.38-13.8) | 5.43 (0.79-37.2) | 0.085 |
| OxPL-apoB plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 1.66 (0.31-8.82) | 1.07 (0.10-11.2) | 13.4 (1.89-94.3) | 0.009 |
| Major apolipoprotein(a) isoform size plus sex, HTN, diabetes, smoking, age in deciles, BMI, LDL-C per 25 mg/dL, HDL-C per 10 mg/dL, log2 TG | 1.00 | 0.38 (0.04-3.80) | 0.68 (0.11-4.03) | 0.35 (0.06-1.91) | 0.23 |
Quartiles for Lp(a): Q1<19.6, Q2 >19.6 - <49.9, Q3 >49.9 - <110.5, Q4 >110.5 nmol/L. Quartiles for major isoform size: Q1<19.5, Q2 >19.5 - <24.0, Q3 >24.0 - <28.0, Q4 >28.0 Kringle IV repeats. Quartiles for OxPL-apoB: Q1<2564, Q2 >2564 - <4025, Q3 >4025 - <8734, Q4 >8734 RLU
Discussion
This study demonstrates that the prevalence and association of LPA SNPs with size of apolipoprotein(a) isoforms, Lp(a) and OxPL-apoB levels is highly variable and ethnicity-specific. Depending on their association with small or large isoforms, LPA SNPs may be associated with either higher or lower Lp(a) and OxPL-apoB levels in different ethnic groups. Importantly, irrespective of and independent of the SNP association, elevated plasma levels of Lp(a) or OxPL-apoB were independent predictors of MACE in the overall group and across ethnic groups. Smaller apolipoprotein(a) isoform sizes were predictive of incident MACE, but this relationship was abrogated by LPA SNPs or Lp(a)/OxPL-apoB levels. Finally, adding OxPL-apoB levels, which primarily reflects OxPL on Lp(a),17,18 to the multivariable analysis abrogated the risk, consistent with the notion that much of the CVD risk of Lp(a) is driven by its content of pro-inflammatory OxPL.16,19-26
This study provides clarity the role of the LPA gene and elevated Lp(a) levels in predicting CVD risk by demonstrating that elevated circulating Lp(a) levels is the key variable in predicting CVD risk irrespective of ethnicity. Most of the prior data on LPA and Lp(a) have been generated in subjects of European descent. There has been significant controversy whether elevated Lp(a) levels are similarly associated with CVD risk in Blacks, who tend to have the highest average Lp(a) levels.7,27 Interestingly, they tend to have larger isoforms that are associate with such levels, rather than small isoforms as has been shown in subjects of European descent.28,29 Importantly, because they key LPA SNPs and apolipoprotein(a) isoform sizes were measured in this study, their relative contributions alone as well as in conjunction with Lp(a)-mediated risk were additionally determined.
Consistent with the role of Lp(a) and OxPL-apoB in predicting future MACE, it can be concluded that small isoforms are primarily important to CVD risk prediction in that they contribute to higher Lp(a)/OxPL-apoB levels via a shorter synthesis time in the hepatocyte.30 Interestingly, this was noted seen in Whites and Hispanics and not Blacks, consistent with the fact that isoforms size explains <50% of circulating Lp(a) levels.8
These data further demonstrate that LPA SNPs are likely to be markers rather than mediators of elevated Lp(a) and/or OxPL-apoB and their association with Lp(a)/OxPL-apoB appears to be due to their random co-segregation with apolipoprotein(a) isoform sizes. Similarly, LPA SNPs appear to be tagging SNPs for isoform size but not necessarily small isoforms. For example, SNP rs3798220 was present in 42.76% of Hispanics, subjects, but only in 4.27% of Whites, yet was associated with large isoforms and lower Lp(a) levels in Hispanics but very small isoforms and higher Lp(a) levels in Whites. For clinical translatability and to address the clinical question on what is the optimal “Lp(a)” measure, these data suggest that circulating Lp(a) levels are the main variable in quantitating cardiovascular risk, rather than LPA SNPs or apolipoprotein(a) isoform size. Interestingly, in prior studies, OxPL-apoB was either a similar or a better reflector of Lp(a)-mediated risk, consistent with the fact that the presence of OxPL on Lp(a) is variable and may reflect the flux of OxPL particles in different pathophysiological situations as well as the OxPL carrying capacity of Lp(a) as well as Lp(a) particle number.22,23,25,31 For example, it was previously shown in DHS that high Lp(a) concentrations that are also associated small apo(a) isoforms (i.e. higher particle number) had the highest correlation with OxPL-apoB, irrespective of ethnic group status.7
Although the issue of ethnicity and Lp(a) risk has been controversial due to inconsistent results in individuals of African descent, this study adds to the growing data that ethnicity by itself is not the relevant variable, but the Lp(a) level within the ethnicity. Elevated Lp(a) in individuals of African descent, who have higher Lp(a), but also differences in the distribution of kringle repeats than Whites, is a risk factor as suggested by both the ARIC study with 20-yr follow-up,27 as well as the recent results from the MESA study.32
Data in LPA SNPs is also highly variable according to ethnic status. For example, data derived primarily from European populations show that rs3798220 is a relatively infrequent SNP present in 2-4% of community subjects.8,33-36 However, the frequency of this SNP is increased in subjects with elevated Lp(a) levels and 24% of Europeans who are in the >95th percentile for elevated Lp(a) (i.e. >139 mg/dL) carry this SNP.36 rs3798220 is also more prevalent in individuals with cardiovascular disease, such as in individuals undergoing apheresis for elevated Lp(a) (>60 mg/dL) for recurrent cardiovascular events having an allele frequency of 26.2%.37 Additionally, this SNP also associated with increasing levels of OxPL-apoB that are associated with increased cardiovascular risk.38,39 In European populations this SNP is associated with small apolipoprotein(a) isoforms, usually <22 KIV repeats, and markedly elevated Lp(a) levels, particularly in homozygotes. Interestingly, this SNP is not present at all in Africans,40 is present in <10% in Asian Indians but is more prevalent (11.6%) in East Asians such as Chinese in Hong Kong.41,42 LPA SNP rs10455872 is present in 7-15% of the general population in Europeans.8,43,44 Its prevalence increases with increasing Lp(a) levels and is present in 47% of northern Europeans with Lp(a) levels >95th percentile. rs10455872 was prevalent in 37.2% of individuals undergoing apheresis with progressive coronary artery disease.37 Less is known about this SNP in non-European populations, but it was not associated with cardiovascular disease or small isoforms in Japan in a genome wide association study or other similar East Asian populations.42 The prevalence of rs9457951 is primarily associated with Black subjects but it only explains approximately 5% of the contribution to Lp(a) levels.9
Limitations of this study include the fact that the Hispanic group was smallest in size and had few events, therefore the role of the LPA gene, Lp(a) and OxPL-apoB levels may be underpowered for the MACE endpoint.
In conclusion, the relationship of the LPA gene to MACE is best explained by elevated plasma Lp(a) or OxPL-apoB levels and not by LPA SNPs or size of apolipoprotein(a) isoforms. Elevated Lp(a) levels, irrespective of ethnicity, are a predictor of future MACE. Studies with potent and specific Lp(a) lowering drugs are underway to test the hypothesis that lowering Lp(a) will reduce CVD risk in subjects with elevated Lp(a).12
Supplementary Material
Clinical Perspective.
-
What is new?
The prevalence of LPA snps and apolipoprotein(a) isoforms are very different across ethnic groups.
LPA snps that are associated with elevated Lp(a) in Whites are associated with low Lp(a) in Hispanics, mainly due to differences in apolipoprotein(a) isoforms size.
After multivariable adjustment Lp(a) and OxPL-apoB are both predictors of MACE in a multi-ethnic cohort.
LPA snps and apolipoprotein(a) isoforms do not add predictive value to models when Lp(a) or OxPL-apoB is included.
-
What are the clinical implications?
Elevated Lp(a) and OxPL-apoB are predictors of MACE across racial groups.
The data suggest that much of Lp(a)-mediated MACE is driven by OxPL.
LPA snps and isoforms did not have clinical utility in this study and further research is needed to assess whether they add additional clinical value in specific patient populations.
Acknowledgments
Funding Sources: NIH R01-HL119828, P01-HL088093, P01-HL055798, R01-HL093767 R01-HL086599 (ST, JLW).
Footnotes
Disclosures: ST and JLW are co-inventors and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies. ST has a dual appointment at UCSD and Ionis Pharmaceuticals. JLW is a consultant for Ionis Pharmaceuticals, Intercept, CymaBay and Prometheus. The other co-authors have no conflicts of interest.
References
- 1.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64:851–60. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F, Utermann G. Lipoprotein(a): Resurrected by genetics. J Int Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 3.Capoulade R, Chan KL, Yeang C, Mathieu P, Bosse Y, Dumesnil JG, Tam JW, Teo KK, Mahmut A, Yang X, Witztum JL, Arsenault BJ, Despres JP, Pibarot P, Tsimikas S. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–46. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Thanassoulis G. Lipoprotein (a) in calcific aortic valve disease: from genomics to novel drug target for aortic stenosis. J Lipid Res. 2016;57:917–24. doi: 10.1194/jlr.R051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016;57:1111–25. doi: 10.1194/jlr.R051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcovina SM, Hobbs HH, Albers JJ. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: basis for a standardized isoform nomenclature. Clin Chem. 1996;42:436–9. [PubMed] [Google Scholar]
- 7.Tsimikas S, Clopton P, Brilakis ES, Marcovina SM, Khera A, Miller ER, de Lemos JA, Witztum JL. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119:1711–9. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M the PC. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 9.Deo RC, Wilson JG, Xing C, Lawson K, Kao WH, Reich D, Tandon A, Akylbekova E, Patterson N, Mosley TH, Jr, Boerwinkle E, Taylor HA., Jr Single-nucleotide polymorphisms in LPA explain most of the ancestry-specific variation in Lp(a) levels in African Americans. PLoS One. 2011;6:e14581. doi: 10.1371/journal.pone.0014581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–88. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet D, Kereiakes DJ, McKenney JM, Roth EM, Hanotin C, Gipe D, Du Y, Ferrand AC, Ginsberg HN, Stein EA. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials) Am J Cardiol. 2014;114:711–5. doi: 10.1016/j.amjcard.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 12.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, Crooke RM, Witztum JL. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–83. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 13.Guerra R, Yu Z, Marcovina S, Peshock R, Cohen JC, Hobbs HH. Lipoprotein(a) and apolipoprotein(a) isoforms: no association with coronary artery calcification in the Dallas Heart Study. Circulation. 2005;111:1471–9. doi: 10.1161/01.CIR.0000159263.50305.BD. [DOI] [PubMed] [Google Scholar]
- 14.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein B-100 (OxPL/apoB) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoia ML, Pai JK, Lee JH, Taleb A, Joosten MM, Mittleman MA, Yang X, Witztum JL, Rimm EB, Tsimikas S, Mukamal KJ. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013;61:2169–79. doi: 10.1016/j.jacc.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byun YS, Lee JH, Arsenault BJ, Yang X, Bao W, DeMicco D, Laskey R, Witztum JL, Tsimikas S Investigators TNTT. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J Am Coll Cardiol. 2015;65:1286–95. doi: 10.1016/j.jacc.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, Yang X, Dennis EA, Witztum JL, Koschinsky ML, Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein(a) and lipoprotein(a) J Lipid Res. 2013;54:2815–30. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–9. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Scipione CA, Sayegh SE, Romagnuolo R, Tsimikas S, Marcovina SM, Boffa MB, Koschinsky ML. Mechanistic insights into Lp(a)-induced IL-8 expression: a role for oxidized phospholipid modification of apo(a) J Lipid Res. 2015;56:2273–85. doi: 10.1194/jlr.M060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesner P, Tafelmeier M, Chittka D, Choi SH, Zhang L, Byun YS, Almazan F, Yang X, Iqbal N, Chowdhury P, Maisel A, Witztum JL, Handel TM, Tsimikas S, Miller YI. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J Lipid Res. 2013;54:1877–83. doi: 10.1194/jlr.M036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsimikas S, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Sandhu MS, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Boekholdt SM. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–55. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 23.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–29. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 24.Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Current opinion in cardiology. 2016;31:440–50. doi: 10.1097/HCO.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsimikas S, Duff GW, Berger PB, Rogus J, Huttner K, Clopton P, Brilakis E, Kornman KS, Witztum JL. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a) J Am Coll Cardiol. 2014;63:1724–34. doi: 10.1016/j.jacc.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LA, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ES. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation. 2016;134:611–24. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in Black and White subjects: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–9. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, Berglund L. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and White men. Arterioscler Thromb Vasc Biol. 2000;20:2619–2624. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 29.Rubin J, Kim HJ, Pearson TA, Holleran S, Ramakrishnan R, Berglund L. Apo[a] size and PNR explain African American-Caucasian differences in allele-specific apo[a] levels for small but not large apo[a] J Lipid Res. 2006;47:982–9. doi: 10.1194/jlr.M500359-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a) J Lipid Res. 2016;57:1339–59. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 32.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luke MM, Kane JP, Liu DM, Rowland CM, Shiffman D, Cassano J, Catanese JJ, Pullinger CR, Leong DU, Arellano AR, Tong CH, Movsesyan I, Naya-Vigne J, Noordhof C, Feric NT, Malloy MJ, Topol EJ, Koschinsky ML, Devlin JJ, Ellis SG. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler Thromb Vasc Biol. 2007;27:2030–6. doi: 10.1161/ATVBAHA.107.141291. [DOI] [PubMed] [Google Scholar]
- 34.Chasman DI, Shiffman D, Zee RY, Louie JZ, Luke MM, Rowland CM, Catanese JJ, Buring JE, Devlin JJ, Ridker PM. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–6. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Luke MM, Shiffman D, Devlin JJ. Genetic variants in the apolipoprotein(a) gene and coronary heart disease. Circ Cardiovasc Genet. 2011;4:565–73. doi: 10.1161/CIRCGENETICS.111.959601. [DOI] [PubMed] [Google Scholar]
- 36.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61:1146–56. doi: 10.1016/j.jacc.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Maerz W, Lehmacher W, Heibges A, Klingel R. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: Prospective observational multicenter study. Circulation. 2013;128:2567–76. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]
- 38.Rao F, Schork AJ, Maihofer AX, Nievergelt CM, Marcovina SM, Miller ER, Witztum JL, O'Connor DT, Tsimikas S. Heritability of biomarkers of oxidized lipoproteins: Twin pair study. Arterioscler Thromb Vasc Biol. 2015;35:1704–11. doi: 10.1161/ATVBAHA.115.305306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arai K, Luke MM, Koschinsky ML, Miller ER, Pullinger CR, Witztum JL, Kane JP, Tsimikas S. The I4399M variant of apolipoprotein(a) is associated with increased oxidized phospholipids on apolipoprotein B-100 particles. Atherosclerosis. 2010;209:498–503. doi: 10.1016/j.atherosclerosis.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 40.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikainen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, Konig IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimaki T, Loos RJ, Melander O, Metspalu A, Marz W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M Consortium CAD. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–30. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalifa M, Noureen A, Ertelthalner K, Bandegi AR, Delport R, Firdaus WJ, Geethanjali FS, Luthra K, Makemaharn O, Pang RW, Salem AH, Sasaki J, Schiefenhoevel W, Lingenhel A, Kronenberg F, Utermann G, Schmidt K. Lack of association of rs3798220 with small apolipoprotein(a) isoforms and high lipoprotein(a) levels in East and Southeast Asians. Atherosclerosis. 2015;242:521–8. doi: 10.1016/j.atherosclerosis.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Li ZG, Li G, Zhou YL, Chen ZJ, Yang JQ, Zhang Y, Sun S, Zhong SL. Lack of association between lipoprotein(a) genetic variants and subsequent cardiovascular events in Chinese Han patients with coronary artery disease after percutaneous coronary intervention. Lipids in health and disease. 2013;12:127. doi: 10.1186/1476-511X-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopewell JC, Clarke R, Parish S, Armitage J, Lathrop M, Hager J, Collins R Heart Protection Study Collaborative G. Lipoprotein(a) genetic variants associated with coronary and peripheral vascular disease but not with stroke risk in the Heart Protection Study. Circ Cardiovasc Genet. 2011;4:68–73. doi: 10.1161/CIRCGENETICS.110.958371. [DOI] [PubMed] [Google Scholar]
- 44.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–7. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


