Introduction
Ivacaftor offers tremendous clinical benefit to individuals with cystic fibrosis (CF) who have at least one G551D mutation (1). This orally administered drug is thought to increase CFTR channel opening, thus improving chloride ion flow and acting on the primary defect that leads to the clinical manifestations of CF (1, 2). However, despite the significant clinical observations, little is known about the drug’s metabolic actions, including its effects on fatty acid (FA) metabolism and related inflammation in CF patients.
Individuals with CF exhibit abnormalities in the metabolism of unsaturated fatty acids reflected in blood and tissue levels (3, 4). These abnormalities include increased metabolism of linoleic acid (18: 2 n-6; LA) to arachidonic acid (20:4 n-6; AA), due to increased expression and activity of Δ5- and Δ6-desaturase enzymes, which leads to low LA levels in the plasma and tissue (5, 6). In addition, there is a decrease in docosahexaenate (22:6 n-3; DHA), for which the mechanism is not fully understood. A prior study showed that the plasma LA × DHA product can be used to distinguish individuals with CF from healthy controls with good sensitivity and specificity, indicating that these observations are consistently observed in all individuals with CF (7).
There is evidence that the levels of specific fatty acids may correlate with clinical outcomes in CF (8, 9). This may be due to an imbalance of increased pro-inflammatory AA and decreased anti-inflammatory DHA and their metabolites in tissue contributing to more severe CF-related inflammation (10, 11). Arachidonic acid is metabolized to many downstream products, including prostaglandins and leukotrienes, and it may be possible to quantify some of the impact of fatty acid metabolism on inflammation by measurement of these products (12).
It is possible that at least some of the clinical improvement observed with ivacaftor is due to reduction in inflammation. The fatty acid metabolism abnormalities of CF, described above, predispose individuals to increased inflammatory eicosanoid production (3, 12–14). Thus, an improvement in FA metabolism could explain part of the clinical improvement observed with ivacaftor. To further explore this idea, it is necessary to not only analyze the plasma FA profile, but also its downstream inflammatory products. Urine prostaglandin E metabolite is a product of arachidonic acid and is therefore a marker of inflammation that directly relates to FA metabolism. Urine PGE-M has previously been shown to be elevated in the CF population in comparison to healthy controls as well as positively correlated to more severe CF genotypes, and thus is a reasonable biomarker for such an investigation (12).
This study tests the hypothesis that ivacaftor improves fatty acid metabolism in individuals with CF who have a G551D mutation. Using samples from a prospective observational trial (GOAL study) (15), plasma FA profiles and urine prostaglandin E metabolites (PGE-M) were analyzed before and after 6 months of ivacaftor therapy.
Methods
Study design
The GOAL study was a prospective, observational trial of the drug ivacaftor (VX-770, Vertex pharmaceuticals) orally administered to individuals with CF who had at least one G551D CFTR mutation. The study enrolled participants from February 2012 until January 2013 (15). Study visits were completed at baseline, 1 month, 3 months, and 6 months and at each of these visits biological samples (blood, sputum, and urine) were collected in addition to other clinical measures such as spirometry. After completion of the primary study analysis, remaining biological samples were stored in the CF Foundation Therapeutics Biorepository, and later these samples were made available to other researchers.
After report of the primary study outcomes, plasma and urine samples were requested from 40 individual participants in the GOAL study in order to measure the effect of ivacaftor on metabolism of fatty acids and their downstream inflammatory metabolites. We focused our analysis on the changes in the plasma fatty acid profile as well as changes in prostaglandin E, measured as its metabolite in the urine (PGE-M). We chose to evaluate samples at baseline and 6 month study visits to give the greatest length of time for potential impact of ivacaftor on fatty acid metabolism.
This analysis was approved by the CF Therapeutic Development Network (TDN) and a waiver for sample analysis was obtained from the Vanderbilt University Institutional Review Board.
Study Participants
Eligibility criteria for the GOAL study (15) included a minimum age of 6 years and a confirmed diagnosis of CF with at least one G551D CFTR mutation. Based on the power calculation (described below), we requested samples from 40 participants who were started on ivacaftor and had completed the 6-month follow-up visit. To sample a wide age range, we requested 20 samples from participants less than 18 years of age (pediatric) and 20 samples from participants greater than or equal to 18 years of age (adult). We also requested that the 40 samples come from participants with the greatest decrease in their sweat Cl as a marker of a good response to ivacaftor.
Study Assessments
Frozen plasma aliquots were shipped from the CF Therapeutics Biorepository and stored at −80°C until time of analysis. Each analysis used 50μL of plasma combined with 450μL of phosphate buffered saline (PBS) in addition to 10μg of heptadecanoic acid (17:0) as an internal standard. Fatty acids were extracted, methylated, identified and quantified by gas chromatography/mass spectroscopy (GC/MS) as previously described (6). Fatty acid levels were reported as the mole percent of each individual fatty acid. Frozen urine aliquots were also received and stored at −80°C until time of analysis. Urine PGE-M levels were quantified in the Vanderbilt Eicosanoid Core laboratory by methods previously described and all samples were run by the core at the same time (12).
Statistical Analysis
A power analysis, using previously published data (7), showed that an analysis of 35 samples was predicted to be adequate to demonstrate a 25% increase in the plasma LA × DHA product with 80% power at a significance level of p= 0.05. The plasma LA × DHA product has previously been shown to have good sensitivity and specificity in predicting CF(7). To account for potential technical failures, 40 samples were requested from the CF Foundation.
Statistical analysis was completed using GraphPad Prism 6 (La Jolla, CA). Individual plasma fatty acid levels at baseline and 6 months were evaluated using the non-parametric Wilcoxon signed rank test. Further statistical analysis by age was completed for all fatty acids that had a p-value ≤ 0.05 in the overall cohort. Because they have been previously reported as abnormal in individuals with CF, LA and DHA were also a major focus in this analysis. For the age-based analysis, the Wilcoxon signed rank test was used to compare fatty acid levels before and after ivacaftor treatment. To compare fatty acid levels between pediatric and adult participants at baseline and at the after ivacaftor time-point, a Mann-Whitney test was used. The Wilcoxon signed rank test was also used for the evaluation of urine PGE-M levels before and after ivacaftor. Statistical significance was set at a p-value of ≤ 0.05 for all testing.
Results
Participants
The demographics for the 40 GOAL participants in this study are displayed in table 1. The median age for the pediatric cohort was 9.6 years [7.7, 11.3], and the median age for the adult cohort was 26.3 years [23.4, 32.3]. A majority of individuals in both age cohorts had a ΔF508 CFTR mutation on the allele other than G551D. The adult cohort had a slight female predominance (80%). Consistent with the results observed in the primary analysis of the GOAL study (15), this subset of participants displayed a statistically significant increase in forced expiratory volume in one second (FEV1 % predicted) and body mass index (BMI) as well as a statistically significant decrease in sweat Cl measurements. However, when divided by age, the pediatric cohort showed a trend towards improvement in the median FEV1% predicted, that was not statistically significant.
Table 1.
Participant Demographics
| Participant Demographics (n=40) | Pediatric (< 18 years) n=20 | Adult (≥ 18 years) n=20 | |
|---|---|---|---|
|
| |||
| Age (years) | 9.6 [7.7, 11.3] | 26.3 [23.4, 32.3] | |
|
| |||
| Sex (female) | 50%, n=10 | 80%, n=16 | |
|
| |||
| Allele other than G551D that is ΔF508 | 95%, n=19 | 75%, n=15 | |
|
| |||
| FEV1 % predicted | Before Ivacaftor * | 104.8 [99.6, 120.5] | 57.4 [49, 90.6] |
|
| |||
| After Ivacaftor * | 112.4 [101.4, 121.7] | 78.9 [56.2, 91.8]† | |
|
| |||
| BMI | Before Ivacaftor * | 17.2 [15.9, 19.2] | 22.0 [19.3, 23] |
| 57.24% [38.01, 75.03] | |||
| 0.27 [−0.68, 0.85] | |||
|
| |||
| After Ivacaftor * | 17.7 [16.1, 20.7]†† | 22.9 [19.9, 24.4]† | |
| 61.14% [50.39, 86.10]† | |||
| 0.26 [−0.21, 1.05]† | |||
|
| |||
| Sweat Cl (mEq/L) | Before Ivacaftor | 106.3 [101.3, 110] | 105.3 [102.4, 109.8] |
|
| |||
| After Ivacaftor | 28.8 [22.5, 32.9]†† | 29.0 [26.8, 38.8]†† | |
Data expressed as median [IQR] where appropriate. For pediatric participants, BMI is expressed as raw value, as percentile (%), and z-score.
Statistical significance between age groups by Mann-Whitney (p<0.001).
Statistical significance before and after ivacaftor treatment within age groups by Wilcoxon test, † p=0.01,
p<0.001.
FEV1 = forced expiratory volume in one second; BMI = body mass index; Cl = chloride
Urine PGE-M Analysis
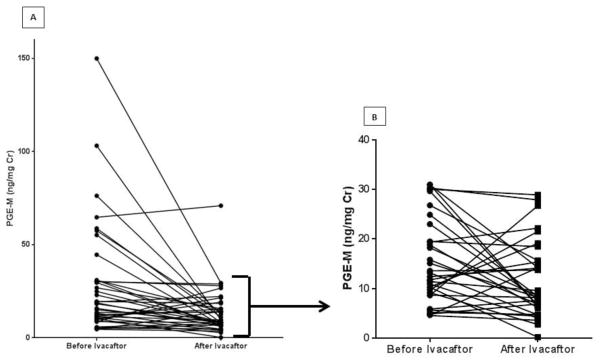
Urine PGE-M levels were significantly decreased after treatment with ivacaftor (Figure 1). In total, 29 of the 40 participants displayed a decrease in urine PGE-M level with ivacaftor treatment. Large ivacaftor-associated decreases (median decrease of 47 ng/mg Cr) in PGE-M levels were observed in 8 subjects who had markedly elevated PGE-M levels at baseline (> 44 ng/mg Cr). When a sensitivity analysis was performed excluding these 8 subjects from the cohort, the observed ivacaftor-associated decrease in PGE-M remained statistically significant.
Figure 1. Urine Prostaglandin E Metabolite (PGE-M) Analysis.
(A) The change in urine PGE-M for all 40 participants before and after treatment with ivacaftor; p< 0.001 by Wilcoxon signed rank test. (B) The change in urine PGE-M before and after ivacaftor with the elevated outliers (n=8) removed; p<0.05 by Wilcoxon signed rank test.
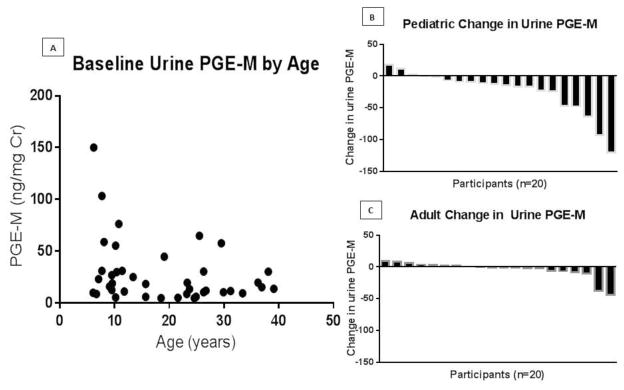
In comparison to adult participants, pediatric participants had higher urine PGE-M values at baseline and also exhibited the greatest decrease in urine PGE-M with ivacaftor treatment (Figure 3). There was no significant correlation between the change in urine PGE-M levels and FEV1% predicted or BMI (data not shown).
Fatty acid analysis
Twenty different fatty acids were measured in the plasma from each participant before and after 6 months of treatment with ivacaftor (Table 2). When analyzed as mole percent, the following fatty acids were significantly decreased after treatment with ivacaftor: palmitoleic acid (16:1), mead acid (20:3n-9), arachidonic acid (20:4n-6), and docosapentaenoic acid (22:5n-3). However, when fatty acids were analyzed by their absolute values (μM/L), the statistical significance was lost (Supplement Table 1). Eicosadienoic acid (20:2n-6) showed a small statistically significant increase by mole percent with treatment with ivacaftor, but also no statistically significant change by μM/L. There was no change in relative levels of LA (18:2n-6), DHA (22:6n-3), the LA × DHA product, or the triene/tetraene (T:T) ratio with ivacaftor treatment. Specifically, at baseline, 33 of 40 participants had an abnormally elevated T:T ratio (>0.4) and after ivacaftor treatment 36 participants had an elevated ratio. Reference ranges, obtained from previously reported data, for absolute values were also reported (supplement Table 1 & 2). Of note, in comparison to reference values, participants displayed elevated levels of palmitoleic acid (16:1), linoleic acid (18:2n-6), mead acid (20:3n-9), and arachidonic acid (20:4n-6); DHA (22:6n-3) levels were within the reference range (16).
Table 2.
Plasma Fatty Acid Levels Before and After Ivacaftor
| Fatty Acid | Before Ivacaftor | After Ivacaftor | p-value |
|---|---|---|---|
| Tetradecanoic acid (14:0) | 1.10 [0.80, 0.67] | 1.26 [0.67, 1.72] | 0.40 |
| Hexadecanoic acid (16:0) | 32.84 [30.96, 35.20] | 33.58 [30.69, 35.85] | 0.18 |
| Palmitoleic acid (16:1) | 2.29 [1.47, 3.04] | 1.74 [1.44, 2.69] | 0.02 |
| Octadecanoic acid (18:0) | 10.73 [9.02, 11.56] | 10.88 [9.76, 12.10] | 0.22 |
| Oleic acid (18:1 n-9) | 11.10 [9.34, 12.59] | 10.05 [8.69, 11.55] | 0.20 |
| Vaccenic acid (18:1 n-7) | 11.48 [10.62, 12.57] | 11.44 [10.14, 12.93] | 0.95 |
| Linoleic acid (18:2 n-6; LA) | 21.37 [18.71, 24.80] | 21.61 [18.31, 25.23] | 0.62 |
| α-linoleic acid (18:3 n-3) | 0.21 [0.15, 0.28] | 0.18 [0.13, 0.31] | 0.29 |
| Gamma-linoleic acid (18:3 n-6) | 0.36 [0.24, 0.42] | 0.40 [0.20, 0.60] | 0.17 |
| Eicosanoic acid (20:0) | 0.04 [0.03, 0.06] | 0.04 [0.03, 0.07] | 0.84 |
| Eicosenoic acid (20:1 n-9) | 0.12 [0.08, 0.15] | 0.12 [0.10, 0.17] | 0.48 |
| Eicosadienoic acid (20:2 n-6) | 0.12 [0.1, 0.15] | 0.14 [0.11, 0.17] | 0.004 |
| Mead acid (20:3 n-9) | 0.31 [0.23, 0.39] | 0.25 [0.20, 0.34] | 0.006 |
| Dihomo-gamma-linoleic acid (20:3 n-6) | 1.09 [0.97, 1.34] | 1.13 [0.94, 1.22] | 0.66 |
| Arachidonic Acid (20:4 n-6; AA) | 4.90 [4.30, 5.68] | 4.62 [3.56, 5.96] | 0.04 |
| Eicosapentaenoic acid (20:5 n-3) | 0.18 [0.12, 0.25] | 0.17 [0.11, 0.28] | 0.55 |
| Adrenic acid (22:4 n-6) | 0.12 [0.10, 0.16] | 0.11 [0.08, 0.14] | 0.19 |
| Docosapentaenoic acid (22:5 n-6) | 0.07 [0.05, 0.10] | 0.08 [0.04, 0.10] | 0.85 |
| Docosapentaenoic acid (22:5 n-3) | 0.20 [0.16, 0.25] | 0.18 [0.13, 0.23] | 0.004 |
| Docosahexaenoic acid (22:6 n-3; DHA) | 0.37 [0.29, 0.51] | 0.38 [0.3, 0.47] | 0.87 |
| LA × DHA | 8.32 [5.93, 12.53] | 7.95 [6.04, 10.8] | 0.55 |
| Triene/Tetraene (20:3n-9/20:4n-6) | 0.06 [0.05, 0.07] | 0.06 [0.05, 0.06] | 0.16 |
Data in units of mole percent and expressed as median [IQR]. Statistical testing by Wilcoxon signed rank test with significance set at p <0.05.
An additional analysis of fatty acid levels by age group (pediatric vs. adult) was completed for fatty acids of clinical interest and fatty acids that showed a statistical change in the overall cohort. The analysis is displayed by mole percent in Table 3 and by μM/L in supplement table 2. Reference ranges are also provided in supplement Table 2. Palmitoleic acid (16:1) levels were significantly elevated in adults at baseline (by both mole percent and μM/L) and with ivacaftor treatment they displayed a significant decrease by mole percent, but not by μM/L. Pediatric participants had a higher LA levels in comparison to the adult participants at baseline, but this was only significant when analyzing by mole percent. There was no difference between age groups at either time point for arachidonic acid, but the pediatric participants did show a decrease with ivacaftor treatment when analyzed by mole percent. No differences were observed within age groups with treatment or between age groups at either time point for DHA. Statistically significant decreases in eicosadienoic acid, mead acid, and docosapentaenoic acid were seen with ivacaftor treatment in the pediatric group by mole percent, but when analyzed by μM/L no changes were observed. Eicosadienoic acid levels were statistically lower in pediatric participants at baseline (mole percent), but again the difference was not observed by μM/L.
Table 3.
Plasma Fatty Acid Levels Before and After Ivacaftor by Participant Age
| Fatty Acid | Before Ivacaftor | After Ivacaftor | |
|---|---|---|---|
| Palmitoleic acid (16:1) | Pediatric | 1.64 [1.03, 2.50] | 1.53 [1.12, 2.03] |
| Adult | 2.83 [1.78, 3.86]** | 2.41 [1.67, 3.42]**† | |
| Linoleic acid (18:2 n-6; LA) | Pediatric | 24.41 [19.72, 26.4] | 22.8 [18.53, 26.78] |
| Adult | 20.46 [18.23, 22.88]** | 21.13 [18.31, 23.73] | |
| Eicosadienoic acid (20:2 n-6) | Pediatric | 0.11 [0.09, 0.13] | 0.14 [0.11, 0.16]†† |
| Adult | 0.14 [0.11, 0.18]* | 0.15 [0.13, 0.18] | |
| Mead acid (20:3 n-9) | Pediatric | 0.30 [0.24, 0.35] | 0.24 [0.21, 0.31]† |
| Adult | 0.32 [0.21, 0.41] | 0.26 [0.2, 0.36] | |
| Arachidonic acid (20:4 n-6) | Pediatric | 4.97 [4.44, 6.47] | 4.62 [3.56, 6]†† |
| Adult | 4.82 [4.05, 5.49] | 4.59 [3.3, 5.94] | |
| Docosapentaenoic acid (22:5 n-3) | Pediatric | 0.23 [0.2, 0.27] | 0.18 [0.14, 0.23]†† |
| Adult | 0.18 [0.13, 0.23] | 0.17 [0.12, 0.23] | |
| Docosahexaenoic acid (22:6 n-3; DHA) | Pediatric | 0.34 [0.29, 0.5] | 0.36 [0.26, 0.43] |
| Adult | 0.39 [0.3, 0.51] | 0.44 [0.31, 0.57] | |
| LA × DHA | Pediatric | 8.17 [5.37, 12.59] | 7.72 [5.78, 9.34] |
| Adult | 8.32 [6.03, 12.2] | 9.44 [6.04, 10.86] | |
| Triene/Tetraene (T:T) | Pediatric | 0.06 [0.05, 0.06] | 0.05 [0.05, 0.06] |
| Adult | 0.07 [0.04, 0.08] | 0.06 [0.04, 0.07] | |
Data in units of mole percent and expressed as median [IQR]. Statistical testing by Wilcoxon signed rank test with significance set at p <0.05.
The changes in fatty acid levels were compared to changes in measured clinical parameters. There was a weak positive correlation (r=0.47, [0.18, 0.69]; p<0.01) between the change in FEV1% predicted and the change in arachidonic acid levels with ivacaftor treatment in the overall cohort (supplement figure 1). No other significant correlations between change in fatty acid levels and change in either FEV1 or BMI were noted.
Discussion
In this pilot study, we analyzed biological samples from 40 participants in the GOAL study to investigate the effects of the drug ivacaftor on fatty acid metabolism and subsequent downstream inflammatory metabolites. There was a significant decrease in urine PGE-M levels with ivacaftor treatment. While some changes in fatty acid levels were noted with ivacaftor therapy, especially when analyzed by age and in units of mole percent, the fatty acid profile was not corrected to levels consistent with healthy controls, specifically in regards to clinically relevant fatty acids such as LA and DHA (3).
There are now multiple documented reports of the clinical benefits of ivacaftor. This drug has not only been shown to improve FEV1, but also improve nutritional status as measured by BMI as well as improvement in other CF disease manifestations such as sinus disease(1, 15, 17–19). However, despite these demonstrations of significant clinical improvement, relatively little is known about the mechanism of action. Ivacaftor is thought to induce CFTR channel opening through a nonconventional ATP-independent mechanism (2, 20) and consequently ivacaftor may fail to resolve downstream signaling of defective CFTR. There is evidence that CF fatty acid abnormalities are related to defective signaling from CFTR (5, 21) and thus the lack of clinically significant fatty acid changes observed in this study may be a reflection of ivacaftor’s mechanism. However, the robust decrease in urine PGE-M may be representative of improvement in the airway surface layer and subsequent improvement in airway clearance (15) that leads to decreased inflammation. Urine PGE-M is unique in that there have not been many other inflammatory markers that have shown a decrease with ivacaftor treatment (15). While it is possible that some of the change in urine PGE-M could be attributed to other treatments or increased inflammation at baseline, the uniqueness of this marker warrants further investigation for future CF therapeutic studies.
To our knowledge, urine PGE-M values have not been previously reported in the pediatric CF population. In our cohort, pediatric participants had the highest baseline urine PGE-M levels and with ivacaftor treatment exhibited the greatest decrease. Despite having higher PGE-M levels, the pediatric participants had a higher FEV1% predicted at baseline in comparison to the adult participants. In addition, while the adult participants observed a significant increase in FEV1% predicted, the pediatric cohort only observed a trend towards an increase in FEV1% predicted. Taking these observations together, it may be interesting to consider a potential role for urine PGE-M as a biomarker for CF therapeutic studies, especially when more traditional efficacy measures such as FEV1 are sometimes less helpful in the pediatric population.
At baseline, we observed fatty acid levels that were similar to what has been previously reported, including low levels of DHA as well as elevated levels of mead acid and palmitoleic acid (3, 21, 22). Additionally, despite a decrease in mead acid levels in the overall cohort, we did not observe a significant decrease in the triene: tetraene (T:T) ratio because there was also a decrease in arachidonic acid levels. An elevated T:T ratio is used clinically as a marker of essential fatty acid deficiency and it has previously been reported as being elevated in individuals with CF (22, 23); though, the etiology for fatty acid changes in individuals with CF is likely different than that of individuals with dietary essential fatty acid deficiency as for individuals with CF the fatty acid changes are likely related to up-regulation of enzymes of fatty acid metabolism by dysfunctional CFTR (5).
Considering the overall fatty acid profiles, we did not observe statistically significant changes when the fatty acids were analyzed in units of μM/L as we did when fatty acids were analyzed in units of mole percent. This observation raises questions regarding the best units to report fatty acid levels and it also questions the overall significance of the observed fatty acid changes when analyzed by mole percent. It also gives further evidence that ivacaftor is likely not causing robust changes in the fatty acid profile of these participants. However, ivacaftor decreases inflammation in individuals with CF, as demonstrated by the urine PGE-M results, and this decrease in inflammation could have some effect on fatty acid levels and account for some of the observations, including mead acid and arachidonic acid, though further research is needed in this regard.
We also observed linoleic acid levels that were intermediate between what has been previously reported for individuals with CF and healthy controls, when analyzed by mole percent (3). In particular, we observed that at baseline pediatric participants had significantly greater levels of linoleic acid than the adult participants. Several recent studies have also reported higher levels of plasma LA levels in pediatric patients with CF than were originally reported for adults with CF (9, 22, 24). In addition, when analyzed by μMol/L, elevated levels of LA were observed for both pediatric and adult participants in comparison to a healthy control reference range (16). There could be several factors that influence this observation, including trends in diet modification and a clinical practice that seeks a higher BMI for pediatric patients with CF as it has been associated with better lung function (25). In any case, the observation of higher baseline plasma LA levels in CF patients in this GOAL subset is interesting and warrants further follow-up.
There were several limitations to this study. First, this study investigated biological samples from a small subset of participants from a previous prospective observational trial. Fatty acid levels can be affected by diet and by the fasted state of the participant at time of measurement, but due to the observational design of the GOAL study we were not able to control for this in our analysis. Our investigation was well powered to detect a change in the LA × DHA product, but this change was not observed. In exploratory analyses, we split the cohort by 18 years of age to investigate the widest age range as possible and we found some interesting differences in fatty acid levels and urine PGE-M levels between the two age populations. However, this study was not powered to detect age related changes in fatty acid metabolism. Due to the pilot nature of this study, we were only able to investigate one inflammatory metabolite related to fatty acid metabolism. We chose urine PGE-M because it has previously shown to be elevated in the CF population and to be correlated with severity of CF genotype (12). Finally, measuring fatty acids in the plasma alone, as opposed to also measuring in the tissue, may not reflect the true changes in overall fatty acid levels (26–28).
In summary, the purpose of this study was to investigate the effects of ivacaftor on fatty acid metabolism in individuals with CF who have a G551D mutation. While in this cohort, ivacaftor failed to demonstrate a robust improvement in the plasma fatty acid profile, we did observe a significant decrease in a prostaglandin E metabolite. After further investigation, this metabolite could be useful as a CF biomarker, especially in the pediatric population where there is such a need for alternative measures of therapeutic efficacy. Finally, this study also observed age differences in the plasma fatty acid profile that may be explained by several clinical factors including diet. It will be important to determine if these age differences are seen in larger cohorts and if they have any long-term clinical significance.
Figure 2. Urine Prostaglandin E Metabolite (PGE-M) Levels by Age.
(A) Baseline urine PGE-M by age. (B) The individual participant change in urine PGE-M after 6 months of ivacaftor treatment in the pediatric population (age < 18 years). (C) Displays the individual participant change in urine PGE-M after 6 months of ivacaftor treatment in the adult population (age ≥ 18 years).
Highlights.
Ivacaftor decreases urine prostaglandin-E metabolite (PGE-M) in CF participants.
Pediatric CF participants had higher baseline urine PGE-M in comparison to adults.
Ivacaftor failed to correct plasma fatty acid levels to that of healthy controls.
Differences in plasma fatty acid levels were observed by participant age.
After further investigation, urine PGE-M may be a useful CF biomarker.
Acknowledgments
Funding: This project was supported by T32 GM07569 in clinical pharmacology and the Vanderbilt University CTSA grant UL1 RR024975.
We would like to thank the Cystic Fibrosis Foundation for contributing the samples for this study as well as Steven M. Rowe, M.D. M.S.P.H as the sponsor-investigator for the GOAL study along with the GOAL investigators of the CF TDN.
We would also like to thank Ginger Milne, Ph.D., and other members of the Vanderbilt Eicosanoid Core Laboratory for their help in running the urine PGE-M samples.
The work was presented as a poster abstract at the 2015 North American Cystic Fibrosis Conference.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY & REFERENCES CITED
- 1.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. The New England journal of medicine. 2011 Nov 3;365(18):1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadida S, Van Goor F, Zhou J, Arumugam V, McCartney J, Hazlewood A, et al. Discovery of N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (VX-770, ivacaftor), a potent and orally bioavailable CFTR potentiator. Journal of medicinal chemistry. 2014 Dec 11;57(23):9776–95. doi: 10.1021/jm5012808. [DOI] [PubMed] [Google Scholar]
- 3.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. The New England journal of medicine. 2004 Feb 5;350(6):560–9. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 4.Al-Turkmani MR, Andersson C, Alturkmani R, Katrangi W, Cluette-Brown JE, Freedman SD, et al. A mechanism accounting for the low cellular level of linoleic acid in cystic fibrosis and its reversal by DHA. Journal of lipid research. 2008 Sep;49(9):1946–54. doi: 10.1194/jlr.M800035-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Njoroge SW, Seegmiller AC, Katrangi W, Laposata M. Increased Delta5- and Delta6-desaturase, cyclooxygenase-2, and lipoxygenase-5 expression and activity are associated with fatty acid and eicosanoid changes in cystic fibrosis. Biochimica et biophysica acta. 2011 Jul-Aug;1811(7–8):431–40. doi: 10.1016/j.bbalip.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Njoroge SW, Laposata M, Katrangi W, Seegmiller AC. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. Journal of lipid research. 2012 Feb;53(2):257–65. doi: 10.1194/jlr.M018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batal I, Ericsoussi MB, Cluette-Brown JE, O’Sullivan BP, Freedman SD, Savaille JE, et al. Potential utility of plasma fatty acid analysis in the diagnosis of cystic fibrosis. Clinical chemistry. 2007 Jan;53(1):78–84. doi: 10.1373/clinchem.2006.077008. [DOI] [PubMed] [Google Scholar]
- 8.Strandvik B, Gronowitz E, Enlund F, Martinsson T, Wahlstrom J. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. The Journal of pediatrics. 2001 Nov;139(5):650–5. doi: 10.1067/mpd.2001.118890. [DOI] [PubMed] [Google Scholar]
- 9.Maqbool A, Schall JI, Garcia-Espana JF, Zemel BS, Strandvik B, Stallings VA. Serum linoleic acid status as a clinical indicator of essential fatty acid status in children with cystic fibrosis. Journal of pediatric gastroenterology and nutrition. 2008 Nov;47(5):635–44. doi: 10.1097/MPG.0b013e31817fb76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Biervliet S, Vanbillemont G, Van Biervliet JP, Declercq D, Robberecht E, Christophe A. Relation between fatty acid composition and clinical status or genotype in cystic fibrosis patients. Annals of nutrition & metabolism. 2007;51(6):541–9. doi: 10.1159/000114208. [DOI] [PubMed] [Google Scholar]
- 11.Robroeks CM, Rosias PP, van Vliet D, Jobsis Q, Yntema JB, Brackel HJ, et al. Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2008 Nov;19(7):652–9. doi: 10.1111/j.1399-3038.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 12.Jabr S, Gartner S, Milne GL, Roca-Ferrer J, Casas J, Moreno A, et al. Quantification of major urinary metabolites of PGE2 and PGD2 in cystic fibrosis: Correlation with disease severity. Prostaglandins, leukotrienes, and essential fatty acids. 2013 Aug;89(2–3):121–6. doi: 10.1016/j.plefa.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr(−/−) mice. Proceedings of the National Academy of Sciences of the United States of America. 1999 Nov 23;96(24):13995–4000. doi: 10.1073/pnas.96.24.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucidi V, Ciabattoni G, Bella S, Barnes PJ, Montuschi P. Exhaled 8-isoprostane and prostaglandin E(2) in patients with stable and unstable cystic fibrosis. Free radical biology & medicine. 2008 Sep 15;45(6):913–9. doi: 10.1016/j.freeradbiomed.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. American journal of respiratory and critical care medicine. 2014 Jul 15;190(2):175–84. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8–c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Molecular genetics and metabolism. 2001 May;73(1):38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 17.Borowitz D, Lubarsky B, Wilschanski M, Munck A, Gelfond D, Bodewes F, et al. Nutritional Status Improved in Cystic Fibrosis Patients with the G551D Mutation After Treatment with Ivacaftor. Digestive diseases and sciences. 2015 Aug 7; doi: 10.1007/s10620-015-3834-2. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh SI, Long FR, McCoy KS, Johnson T, Ryan-Wenger NA, Hayes D., Jr Ivacaftor improves appearance of sinus disease on computerised tomography in cystic fibrosis patients with G551D mutation. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2015 Feb;40(1):16–21. doi: 10.1111/coa.12310. [DOI] [PubMed] [Google Scholar]
- 19.McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST) The lancet Respiratory medicine. 2014 Nov;2(11):902–10. doi: 10.1016/S2213-2600(14)70218-8. [DOI] [PubMed] [Google Scholar]
- 20.Eckford PD, Li C, Ramjeesingh M, Bear CE. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. The Journal of biological chemistry. 2012 Oct 26;287(44):36639–49. doi: 10.1074/jbc.M112.393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomsen KF, Laposata M, Njoroge SW, Umunakwe OC, Katrangi W, Seegmiller AC. Increased elongase 6 and Delta9-desaturase activity are associated with n-7 and n-9 fatty acid changes in cystic fibrosis. Lipids. 2011 Aug;46(8):669–77. doi: 10.1007/s11745-011-3563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldamiz-Echevarria L, Prieto JA, Andrade F, Elorz J, Sojo A, Lage S, et al. Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatric research. 2009 Nov;66(5):585–9. doi: 10.1203/PDR.0b013e3181b4e8d3. [DOI] [PubMed] [Google Scholar]
- 23.Maqbool A, Schall JI, Gallagher PR, Zemel BS, Strandvik B, Stallings VA. Relation between dietary fat intake type and serum fatty acid status in children with cystic fibrosis. Journal of pediatric gastroenterology and nutrition. 2012 Nov;55(5):605–11. doi: 10.1097/MPG.0b013e3182618f33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alicandro G, Faelli N, Gagliardini R, Santini B, Magazzu G, Biffi A, et al. A randomized placebo-controlled study on high-dose oral algal docosahexaenoic acid supplementation in children with cystic fibrosis. Prostaglandins, leukotrienes, and essential fatty acids. 2013 Feb;88(2):163–9. doi: 10.1016/j.plefa.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Sanders DB, Fink A, Mayer-Hamblett N, Schechter MS, Sawicki GS, Rosenfeld M, et al. Early Life Growth Trajectories in Cystic Fibrosis are Associated with Pulmonary Function at Age 6 Years. The Journal of pediatrics. 2015 Sep 2; doi: 10.1016/j.jpeds.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jumpsen JA, Brown NE, Thomson AB, Paul Man SF, Goh YK, Ma D, et al. Fatty acids in blood and intestine following docosahexaenoic acid supplementation in adults with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2006 May;5(2):77–84. doi: 10.1016/j.jcf.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Al-Turkmani MR, Freedman SD, Laposata M. Fatty acid alterations and n-3 fatty acid supplementation in cystic fibrosis. Prostaglandins, leukotrienes, and essential fatty acids. 2007 Nov-Dec;77(5–6):309–18. doi: 10.1016/j.plefa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Seegmiller AC. Abnormal unsaturated fatty acid metabolism in cystic fibrosis: biochemical mechanisms and clinical implications. International journal of molecular sciences. 2014;15(9):16083–99. doi: 10.3390/ijms150916083. [DOI] [PMC free article] [PubMed] [Google Scholar]