Abstract
Purpose
Fibrosis accounts for approximately 50% of histological findings at post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND), and is associated with reported relapse rates of 10%–15%. We aimed to characterize patients with fibrosis at PC-RPLND, and identify predictors of adverse outcome within this group.
Materials and Methods
We reviewed the medical records of men who underwent PC-RPLND between 1989–2013 with histological findings of necrosis/fibrosis. With few exceptions, PC-RPLND after the year 1999 was performed with a bilateral template. Clinical, pathological and treatment related data were reported. Cox regression models were built to identify predictors of disease recurrence.
Results
The study cohort included 598 men at a median age of 32 years (IQR 25–38). Most patients (397/547, 73%) were classified as IGCCCG good risk, with no significant differences in risk classification before and after the year 1999 (p= 0.55). Median followup was 7.3 years (IQR 3.2–12.3). Five-year recurrence free and overall survival were 94% and 96%, respectively. 36 patients had a disease recurrence, most of which were distant or outside the RPLND template. Procedures performed after the year 1999 and the presence of embryonal cell carcinoma on primary histology were associated with improved recurrence free survival on multivariate analysis (p<0.01).
Conclusions
Disease recurrence in patients with fibrosis at PC-RPLND is an uncommon yet significant event, which is less likely to occur in patients treated after the year 1999, and those with embryonal carcinoma on primary histology.
Keywords: Fibrosis, Neoplasms, Germ Cell and Embryonal, Lymph node excision
Introduction
Testis cancer is the most commonly diagnosed cancer in young men, and is highly curable even in advanced stages using a multimodal treatment approach.1 The integration of post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND) for the treatment of patients with advanced non-seminomatous germ cell tumors (NSGCT) has resulted in survival rates of 70%–80%.2 Histological findings at RPLND after cisplatin based chemotherapy include necrosis/fibrosis, teratoma or persistent viable carcinoma.3–7 According to an initial report, each of these histologies appeared in approximately one third of the cases.3 However, in recent studies, necrosis/fibrosis comprised 41% – 49% of histological findings, teratomas 40% – 42%, and viable germ cell tumors (GCT) were apparent in only 11% – 17% of lesions.4–6 These rates are markedly different following second-line chemotherapy, with viable GCTs noted in 50% of the lesions, teratomas in 40%, and necrosis/fibrosis in 10%.7
Despite the presence of a presumably negative pathology, patients with necrosis/fibrosis undergoing PC-RPLND have relapse rates of 10%–15%.2 Furthermore, the outcome of patients with fibrosis improved when operated in recent rather than early years, consistent with the improvement in surgical technique.6 In addition, patients with elevated serum tumor markers who underwent PC-RPLND with evidence of fibrosis, had a normalization of their serum markers after surgery.8 Finally, a genetic analysis of stromal cells from fibrotic lesions after PC-RPLND found allelic loss in 85% of specimens, including loss of chromosome arm 12p, a hallmark finding in germ cell neoplasia, in 33% of cases. These genetic alterations suggest fibrotic lesions contain cells that are derived from tumor progenitor cells, challenging the concept that these cells are benign.9 Taken together, the findings suggest that a subset of patients may derive benefit from RPLND even in the presence of a seemingly negative pathology.
While previous studies have reported clinicopathological characteristics of patients with viable GCT and teratoma at post-chemotherapy RPLND,10–12 less is known regarding patients with findings of fibrosis. In the current study, we aimed to characterize patients with a finding of necrosis/fibrosis at PC-RPLND, and identify predictors of adverse outcome within this group of patients.
Materials and Methods
After obtaining Institutional Review Board approval, we queried our prospectively maintained surgical database, and identified 598 patients who underwent PC-RPLND after 1st or 2nd line chemotherapy, for both seminoma and NSGCT, between the years 1989 – 2013 with histological findings of necrosis/ fibrosis.
Pre-treatment clinical and pathological information collected included patient age, histological subtypes of the primary tumor (seminoma, embryonal carcinoma, yolk sac tumor, teratoma, choriocarcinoma and other), International Germ Cell Cancer Collaborative Group (IGCCCG) risk category (good, intermediate, poor)13 and pre-chemotherapy node size as determined by the transverse diameter of the largest mass on axial imaging. All primary tumor specimens were reviewed by GU-pathologists at our institution in order to verify tumor histology. All patients underwent induction chemotherapy. The use of second line chemotherapy, post-chemotherapy node size and the presence of elevated serum tumor markers after completion of chemotherapy were noted. Information regarding the surgical treatment included the year the RPLND was performed and the template used for the resection (bilateral, unilateral or mass resection). PC-RPLND after the year 1999 was performed with a bilateral template. After the operation, patients were followed with serum tumor markers and periodical axial imaging. Disease recurrence was defined as the presence of imaging findings suggestive of a recurrence with or without elevated serum tumor markers, or elevated markers in the absence of a mass on followup imaging. Recurrence location was noted and categorized as loco-regional or distant. Loco-regional recurrences were further categorized as intra-template, within the boundaries of a standard bilateral template, and extra-template.
The primary study endpoint was disease recurrence. The probability of recurrence free survival (RFS) was analyzed using the Kaplan Meier method. Univariate Cox proportional hazards models were used to examine the association between primary histological subtype, IGCCCG risk category, the use of second line chemotherapy, post-chemotherapy node size, the year and template of RPLND and disease recurrence. A backward stepwise elimination process was used to select variables for the multivariate analysis. Multivariate Cox regression models were built to identify independent predictors of disease recurrence. All statistical analyses were two-sided. A P-value of less than 0.05 was considered statistically significant. SPSS Statistics for Windows, Version 23.0 (SPSS Inc., Chicago, IL) was used for all data analyses.
Results
The study cohort included 598 men at a median age of 32 years (IQR, 25–38). Clinical characteristics of the study cohort are reported in Table 1. The primary tumor site was the testis in 569 patients (95%) and retroperitoneum in 29 patients (5%). Tumor histology was available for 574 patients; 42 patients (7%) had pure seminoma, 462 patients (80%) had a NSGCT, and 70 patients (12%), with prior diagnosis of a germ cell tumor, did not have histological evidence of a viable germ cell tumor at orchiectomy, most of which had fibrosis or a burnt out scar. The most common histological subtype was embryonal carcinoma apparent in 404 patients (70%). Most patients (397/547, 73%) were classified as good risk according to the IGCCCG risk classification. No significant differences in IGCCCG risk classification were observed before and after the year 1999 (p= 0.55). A minority of patients received second line chemotherapy (46 patients, 8%), and most patients had normal serum tumor markers prior to RPLND (564 patients, 94%). Bilateral RPLND was performed in 419 patients (70%). After the year 1999, 371/382 patients (97%) underwent bilateral template RPLND while 11/382 patients (3%), underwent mass resection alone. Of these 11 patients, all except one had prior RPLND and were redo resections performed after receiving chemotherapy. One patient had seminoma with severe desmoplastic reaction and underwent resection of mass. Data regarding serum tumor marker status after surgery was available for 30/34 patients with elevated tumor markers prior to PC-RPLND, all of whom had normalization of their markers following surgery. Two patients with elevated serum tumor markers prior to PC-RPLND had a disease recurrence during followup.
Table 1.
Patient and disease characteristics of the study cohort (n=598)
| Age in years (Median, IQR) | 32 (25–38) | |
| Histological pattern subtype at orchiectomy (n=574) | Embryonal carcinoma | 404 (70%) |
| Seminoma | 220 (38%) | |
| Yolk sac tumor | 186 (32%) | |
| Teratoma | 165 (29%) | |
| Choriocarcinoma | 29 (5%) | |
| Other | 70 (12%) | |
| IGCCCG risk category (n=547) | Good | 397 (73%) |
| Intermediate | 62 (11%) | |
| Poor | 88 (16%) | |
| Pre-chemotherapy node size (n=434) | ≤2 cm | 106 (24%) |
| >2 cm and ≤5 cm | 197 (45%) | |
| >5 cm | 131 (30%) | |
| Second line chemotherapy | No | 552 (92%) |
| Yes | 46 (8%) | |
| Post-chemotherapy node size (n=438) | ≤1 cm | 137 (31%) |
| >1 cm and ≤2 cm | 122 (28%) | |
| >2 cm and ≤5 cm | 118 (27%) | |
| >5 cm | 61 (14%) | |
| Post-chemotherapy elevated AFP/hCG | No | 564 (94%) |
| Yes | 34 (6%) | |
| Year of RPLND | 1989–1998 | 216 (36%) |
| 1999–2013 | 382 (64%) | |
| Resection type | Bilateral template | 419 (70%) |
| Unilateral template | 131 (22%) | |
| Mass resection | 48 (8%) | |
IQR=interquartile range; IGCCCG= International Germ Cell Cancer Collaborative Group; AFP=alpha fetoprotein; hCG=human chorionic gonadotropin; RPLND=retroperitoneal lymph node dissection.
Median follow-up for patients without disease recurrence was 7.3 years (IQR, 3.2–12.3). Median time to disease recurrence was 5.5 months (IQR, 2–13.5). A total of 36 patients had a disease recurrence at 1 or more sites: 27 patients (75%) had a distant recurrence, and 14 patients (39%) had a loco-regional recurrence. Six patients with a loco-regional recurrence had a concurrent distant recurrence; therefore, only 8 patients (1.3% of the entire cohort) had a loco-regional recurrence alone. Among the 14 loco-regional recurrences, intra-template recurrences were observed in 2/3 patient who underwent mass resection, 6/7 patients who underwent unilateral template RPLND and 1/4 patients who underwent bilateral template RPLND (p=0.129). Elevated serum tumor markers were measured at the time of recurrence in 22/36 patient (61%). One patient had an elevation of serum tumor markers without evidence of recurrent disease on axial imaging. Recurrence histology was available for 21 patients: 9 patients (43%) had viable GCT, 8 patients (38%) had fibrosis, 3 patients (14%) had teratoma, and 1 patient (5%) had both viable GCT and teratoma.
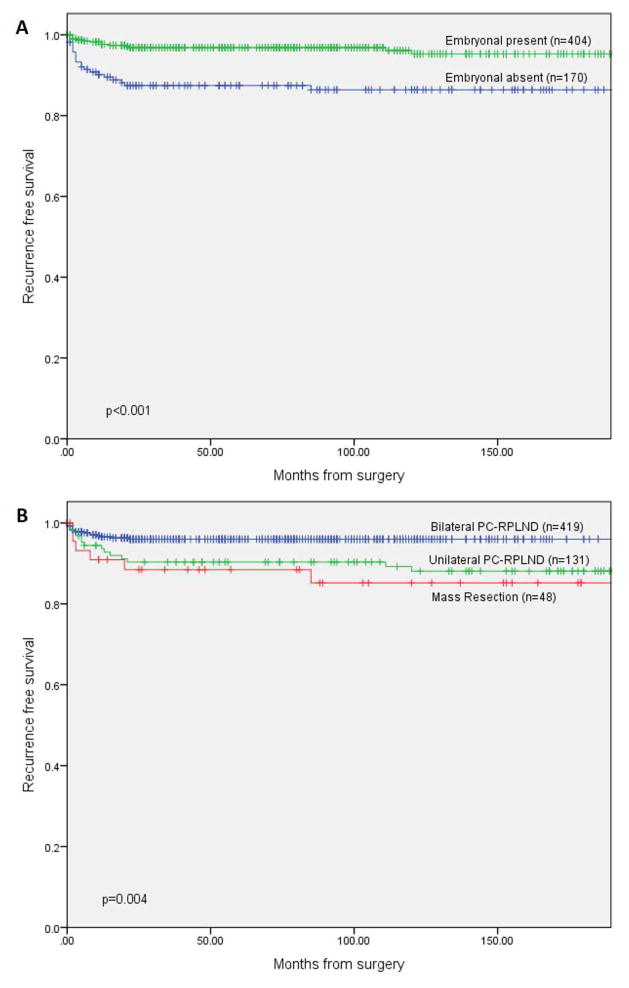
Five year RFS and overall survival (OS) of the entire cohort were 94% and 96%, respectively. On univariate analysis, intermediate or poor IGCCCG risk category, the use of second line chemotherapy and post-chemotherapy node size >1cm, were significantly associated with disease recurrence after PC-RPLND. The presence of embryonal carcinoma in the primary tumor, RPLND performed after the year 1999, and the use of a bilateral template were significantly associated with a lower rate of disease recurrence. The presence of a teratomatous element on the primary histology was not significantly associated with disease recurrence (Table 2). Five year RFS was 97% for patients with embryonal carcinoma in the initial tumor compared to 87% for those without. In addition, 5 year RFS was 96%, 90%, and 89% for patients who underwent bilateral RPLND, unilateral RPLND, and mass resection, respectively (Figure 1). Estimated 5-year recurrence free survival was 97.8% for patients with nodes ≤1cm compared to 92.3% for those with nodes >1cm (p=0.015). Embryonal carcinoma histology and year of RPLND remained significant predictors of outcome when included in a multivariate Cox-regression model (Table 2). In a similar multivariate analysis, performed on the subgroup of patients who received induction chemotherapy only (n=552), presence of embryonal carcinoma (HR=0.34, 95% CI 0.16–0.72, p=0.005) and surgery performed in recent years (HR=0.34, 95% CI 0.15–0.74, p=0.007) were significant predictors of a favorable outcome.
Table 2.
Univariate and multivariate Cox regression models evaluating predictors of disease recurrence
| Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Primary Embryonal | Absent (ref) | - | - | - | - |
| Present | 0.27 (0.14–0.53) | <0.001 | 0.29 (0.15–0.59) | <0.001 | |
| Primary Teratoma | Absent (ref) | - | - | - | - |
| Present | 0.86 (0.4–1.83) | 0.693 | - | - | |
| IGCCCG risk category | Good (ref) | - | - | - | - |
| Intermediate/ Poor | 2.24 (1.10–4.54) | 0.026 | - | - | |
| Second line chemotherapy | No (ref) | - | - | - | - |
| Yes | 3.62 (1.65–7.93) | 0.001 | - | - | |
| Post-chemo node size | ≤1 cm (ref) | - | - | - | - |
| >1 cm | 3.97 (1.19–13.14) | 0.024 | - | - | |
| Year of RPLND | 1989–1998 (ref) | - | - | - | - |
| 1999–2013 | 0.29 (0.15–0.59) | 0.001 | 0.33 (0.16–0.67) | 0.002 | |
| RPLND Template | Bilateral (ref) | - | - | - | - |
| Unilateral | 2.64 (1.29–5.43) | 0.008 | - | - | |
| Mass Resection | 3.39 (1.33–8.67) | 0.011 | - | - | |
HR=hazard ratio; CI=confidence interval; ref=reference; IGCCCG=International Germ Cell Cancer Collaborative Group; RPLND=retroperitoneal lymph node dissection.
Figure 1.
Kaplan-Meier curves of recurrence free survival after surgery stratified by (A) the presence of embryonal carcinoma on initial histology (n=574) and (B) post-chemotherapy retroperitoneal lymph node dissection templates (n=598)
Discussion
In the current study we evaluated 598 patients who underwent PC-RPLND with a histological finding of fibrosis. Most patients had a good IGCCCG risk category (73%), and did not require second line chemotherapy (92%) prior to surgery. The overall 5-year recurrence free survival was 94%. The presence of embryonal carcinoma in the primary histology was significantly associated with a better outcome on multivariate analysis (p<0.001). Similarly, patient treated after the year 1999 had a better outcome (p=0.002), which may be related, among other causes, to the widespread use of a bilateral surgical template during these years.
Fibrosis/necrosis is found in 40%–50% of residual masses in patients with advanced germ cell tumors treated with PC-RPLND.14 Several authors consider the finding of fibrosis/necrosis a benign finding that may not require extensive surgery; therefore, efforts were made to establish prognostic models capable of predicting the presence of fibrosis/necrosis on PC-RPLND.15, 16 However, since these models hold a false-positive rate of approximately 20% with regard to the correct detection of fibrosis and necrosis, they may be unsuitable for routine clinical use.17 Furthermore, several studies have suggested the nature of fibrosis may not be benign. Cheng et al. performed genetic analyses on the dissection specimens of 27 patients who were found to have fibrosis or necrosis at PC-RPLND. Stromal cells in 88% of these specimens displayed allelic loss at multiple DNA loci and/ or chromosome arm 12p abnormalities, suggesting these cells are of neoplastic origin and may be the source of secondary sarcomas.9 In addition, normalization of serum tumor markers in patients undergoing PC-RPLND with histological finding of fibrosis/necrosis, suggests surgery may be beneficial in this setting.8 Thus, PC-RPLND in a high volume center remains necessary for histological diagnosis, staging and treatment of residual masses after platinum based chemotherapy regardless of the final pathology.18
Patients with viable tumor at the time of post-chemotherapy RPLND are at an increased risk for disease progression, with an estimated 5 year RFS rate of 50%. An intermediate or poor IGCCCG risk classification was associated with a decreased RFS in these patients.10 Five year RFS of patients with residual teratoma at PC-RPLND was 81%–83%. Significant predictors of an adverse outcome included residual mass size, IGCCCG risk classification, elevate post-chemotherapy alpha fetoprotein levels, and mediastinal involvement at presentation.11, 12 In the current study, the 5 year RFS for patients with fibrosis at PC-RPLND was 94%, substantially higher than that of viable GCT and teratoma. The presence of embryonal carcinoma histology and an RPLND performed in recent years were associated with an improvement in RFS.
Previous studies have supported the use of unilateral modified templates in well selected patients for whom the location of the retroperitoneal mass corresponded with the primary landing zone of the tumor bearing testicle and the residual mass diameter measured ≤5 cm.19, 20 In these studies, recurrence rates were low with only one reported recurrence occurring within the boundaries of the surgical template.19, 20 In the current study, “pure” loco-regional recurrences were uncommon, observed in 1.3% of the entire cohort at a median follow-up of 12 months (IQR, 5–19.25). However, among 14 patients with loco-regional recurrences, intra-template recurrences were found in 6 of 7 patients who underwent unilateral template RPLND, 2 of 3 patients who underwent mass resection, and 1 of 4 patients treated with a full bilateral template. While differences in inclusion criteria may explain the relatively higher intra-template recurrence rates in patients undergoing unilateral resection, if supported by studies with larger cohorts, these findings may justify performing a full bilateral RPLND in the setting of fibrosis.
In a previous study from our center 15 months’ relapse rates were evaluated in a cohort of patients with advanced NSGCT who underwent PC-RPLND between the years 1989–2002. When comparing patients treated between the years 1989–1997 and 1998–2002 a significant improvement in outcome was seen in the latter years. This decrease in the probability of relapse was apparent in patients with fibrosis or teratoma, however patients with persistent viable GCT did not show an improvement in RFS rates during the years of the study.6 In the current study, focused on patients with fibrosis on PC-RPLND, a similar improvement in outcome was observed when comparing patients who were operated on between the years 1989–1998 and the years 1999–2013 suggesting the improvement in outcome was sustained in recent years. Carver et al. reported that the improvement in outcome was related in part to stage migration but was also significantly related to the use of complete RPLND (unilateral or bilateral) rather than resection of the residual mass alone.6 Our current findings suggest that the use of bilateral template RPLND rather than unilateral template or mass resection alone may be associated with a better outcome; however, larger studies are required to support the importance of adequate surgery in this setting. These findings are consistent with the current National Comprehensive Cancer Network guidelines suggesting a full bilateral template RPLND should be performed in all cases of RPLND after chemotherapy.18
The presence of embryonal carcinoma histology in the primary tumor was significantly associated with an improved recurrence free survival on multivariate analysis in the current study. Previous studies evaluating patients with clinical stage I NSGCT found that a high percentage of embryonal carcinoma in the primary tumor predicted the presence of occult retroperitoneal disease and a higher pathological stage.21, 22 These seemingly inconsistent findings are likely the result of the different population studied, and the high sensitivity of embryonal carcinoma to cisplatin based chemotherapy. Multiple in vitro studies performed on embryonal carcinoma cell lines have shown these cells are highly sensitive to DNA-damaging agents, especially cisplatin. The high rate of embryonal carcinoma component in mixed germ cell tumors may explain the general high chemosensitivity and curability of these tumors in the clinical setting.23, 24 Therefore, patients with an embryonal carcinoma component in their primary tumor may have responded better to cisplatin based chemotherapy leading to a lower recurrence rate after PC-RPLND. Further studies are required to evaluate the reproducibility of this finding within other cohorts of patients undergoing PC-RPLND and identify causes for this association.
The limitations of the current study include its retrospective nature and the possibility of a selection and referral bias. In addition, the study period extended over 25 years, during which several changes in treatment policy may have occurred, influencing the current results. The relatively small number of events limited our ability to include all predictive variables in the multivariable model. Finally, as the current study is a single center study, our findings may not be generalizable to additional cohorts.
Conclusions
Disease recurrence in patients with fibrosis at PC-RPLND is an uncommon yet significant event, occurring more often in patients treated before the year 1999, a large percent of whom were treated with unilateral RPLND or mass resection.
The data suggests that the presence of embryonal carcinoma in the primary tumor was associated with a lower relapse rate in patients with fibrosis at PC-RPLND possibly due to an increased sensitivity to chemotherapy. The use of bilateral template was associated with a better outcome on univariate analysis however further studies are required to validate these findings when adjusting for additional factors.
Acknowledgments
Funding
The Capri Foundation and The Sidney Kimmel Center for Prostate and Urologic Cancers.
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations and Acronyms
- PC-RPLND
post-chemotherapy retroperitoneal lymph node dissection
- NSGCT
non-seminomatous germ cell tumors
- RPLND
retroperitoneal lymph node dissection
- GCT
germ cell tumors
- IGCCCG
International Germ Cell Cancer Collaborative Group
- RFS
recurrence free survival
Footnotes
Conflict of Interest and Disclosure Statement
All authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevenson SM, Lowrance WT. Epidemiology and Diagnosis of Testis Cancer. Urol Clin North Am. 2015;42:269. doi: 10.1016/j.ucl.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson AJ, Sheinfeld J. The role of retroperitoneal lymph node dissection in the management of testicular cancer. Urol Oncol. 2004;22:225. doi: 10.1016/j.urolonc.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Donohue JP, Einhorn LH, Williams SD. Cytoreductive surgery for metastatic testis cancer: considerations of timing and extent. J Urol. 1980;123:876. doi: 10.1016/s0022-5347(17)56173-x. [DOI] [PubMed] [Google Scholar]
- 4.Steyerberg EW, Keizer HJ, Fossa SD, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol. 1995;13:1177. doi: 10.1200/JCO.1995.13.5.1177. [DOI] [PubMed] [Google Scholar]
- 5.Spiess PE, Brown GA, Pisters LL, et al. Viable malignant germ cell tumor in the postchemotherapy retroperitoneal lymph node dissection specimen: can it be predicted using clinical parameters? Cancer. 2006;107:1503. doi: 10.1002/cncr.22181. [DOI] [PubMed] [Google Scholar]
- 6.Carver BS, Serio AM, Bajorin D, et al. Improved clinical outcome in recent years for men with metastatic nonseminomatous germ cell tumors. J Clin Oncol. 2007;25:5603. doi: 10.1200/JCO.2007.13.6283. [DOI] [PubMed] [Google Scholar]
- 7.Fox EP, Weathers TD, Williams SD, et al. Outcome analysis for patients with persistent nonteratomatous germ cell tumor in postchemotherapy retroperitoneal lymph node dissections. J Clin Oncol. 1993;11:1294. doi: 10.1200/JCO.1993.11.7.1294. [DOI] [PubMed] [Google Scholar]
- 8.Beck SD, Foster RS, Bihrle R, et al. Outcome analysis for patients with elevated serum tumor markers at postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol. 2005;23:6149. doi: 10.1200/JCO.2005.11.684. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Zhang S, Wang M, et al. Molecular genetic evidence supporting the neoplastic nature of stromal cells in ‘fibrosis’ after chemotherapy for testicular germ cell tumours. J Pathol. 2007;213:65. doi: 10.1002/path.2202. [DOI] [PubMed] [Google Scholar]
- 10.Spiess PE, Tannir NM, Tu SM, et al. Viable germ cell tumor at postchemotherapy retroperitoneal lymph node dissection: can we predict patients at risk of disease progression? Cancer. 2007;110:2700. doi: 10.1002/cncr.23104. [DOI] [PubMed] [Google Scholar]
- 11.Carver BS, Shayegan B, Serio A, et al. Long-term clinical outcome after postchemotherapy retroperitoneal lymph node dissection in men with residual teratoma. J Clin Oncol. 2007;25:1033. doi: 10.1200/JCO.2005.05.4791. [DOI] [PubMed] [Google Scholar]
- 12.Svatek RS, Spiess PE, Sundi D, et al. Long-term outcome for men with teratoma found at postchemotherapy retroperitoneal lymph node dissection. Cancer. 2009;115:1310. doi: 10.1002/cncr.24145. [DOI] [PubMed] [Google Scholar]
- 13.International Germ Cell Consensus Classification. a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 14.Chery L, Dash A. The Role of Postchemotherapy Surgery in Germ Cell Tumors. Urol Clin North Am. 2015;42:331. doi: 10.1016/j.ucl.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Albers P, Weissbach L, Krege S, et al. Prediction of necrosis after chemotherapy of advanced germ cell tumors: results of a prospective multicenter trial of the German Testicular Cancer Study Group. J Urol. 2004;171:1835. doi: 10.1097/01.ju.0000119121.36427.09. [DOI] [PubMed] [Google Scholar]
- 16.Vergouwe Y, Steyerberg EW, Foster RS, et al. Predicting retroperitoneal histology in postchemotherapy testicular germ cell cancer: a model update and multicentre validation with more than 1000 patients. Eur Urol. 2007;51:424. doi: 10.1016/j.eururo.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Heidenreich A. Residual tumour resection following inductive chemotherapy in advanced testicular cancer. Eur Urol. 2007;51:299. doi: 10.1016/j.eururo.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Jonasch E, Agarwal N, et al. Testicular Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015;13:772. doi: 10.6004/jnccn.2015.0092. [DOI] [PubMed] [Google Scholar]
- 19.Beck SD, Foster RS, Bihrle R, et al. Is full bilateral retroperitoneal lymph node dissection always necessary for postchemotherapy residual tumor? Cancer. 2007;110:1235. doi: 10.1002/cncr.22898. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich A, Pfister D, Witthuhn R, et al. Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol. 2009;55:217. doi: 10.1016/j.eururo.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich A, Sesterhenn IA, Mostofi FK, et al. Prognostic risk factors that identify patients with clinical stage I nonseminomatous germ cell tumors at low risk and high risk for metastasis. Cancer. 1998;83:1002. [PubMed] [Google Scholar]
- 22.Moul JW, McCarthy WF, Fernandez EB, et al. Percentage of embryonal carcinoma and of vascular invasion predicts pathological stage in clinical stage I nonseminomatous testicular cancer. Cancer Res. 1994;54:362. [PubMed] [Google Scholar]
- 23.Mueller T, Mueller LP, Luetzkendorf J, et al. Loss of Oct-3/4 expression in embryonal carcinoma cells is associated with induction of cisplatin resistance. Tumour Biol. 2006;27:71. doi: 10.1159/000092324. [DOI] [PubMed] [Google Scholar]
- 24.Pera MF, Friedlos F, Mills J, et al. Inherent sensitivity of cultured human embryonal carcinoma cells to adducts of cis-diamminedichloroplatinum(II) on DNA. Cancer Res. 1987;47:6810. [PubMed] [Google Scholar]