Abstract
Background
Although HF disproportionately affects older adults, little data exist regarding the prevalence of American College of Cardiology/American Heart Association heart failure (HF) stages among older individuals in the community. Additionally, the role of contemporary measures of longitudinal strain (LS) and diastolic dysfunction in defining HF stages is unclear.
Methods
HF stages were classified in 6,118 participants in the Atherosclerosis Risk in Communities study (age 67 – 91 years) at the fifth study visit as follows: stage A (asymptomatic with HF risk factors but no cardiac structural or functional abnormalities), B (asymptomatic with structural abnormalities, defined as left ventricular hypertrophy, dilation or dysfunction, or significant valvular disease), C1 (clinical HF without prior hospitalization), and C2 (clinical HF with prior hospitalization).
Results
Using the traditional definitions of HF stages, only 5% of examined participants were free of HF risk factors or structural heart disease (Stage 0), 52% were categorized as Stage A, 30% Stage B, 7% Stage C1, and 6% Stage C2. Worse HF stage was associated with a greater risk of incident HF hospitalization or death at a median follow-up of 608 days. LVEF was preserved in 77% and 65% in Stages C1 and C2 respectively. Incorporation of LS and diastolic dysfunction into the Stage B definition reclassified 14% of the sample from Stage A to B and improved the net reclassification index (p=0.028) and integrated discrimination index (p=0.016). Abnormal LV structure, systolic function (based on LVEF and LS), and diastolic function (based on e′, E/e′, and left atrial volume index) were each independently and additively associated with risk of incident HF hospitalization or death in Stage A and B participants.
Conclusions
The majority of older adults in the community are at risk for HF (Stages A or B), appreciably more compared to previous reports in younger community-based samples. LVEF is robustly preserved in at least two-thirds of older adults with prevalent HF (Stage C), highlighting the burden of HFpEF in the elderly. LV diastolic function and LS provide incremental prognostic value beyond conventional measures of LV structure and LVEF in identifying persons at risk for HF hospitalization or death.
Journal Subject Codes: Heart failure, epidemiology, echocardiography
Introduction
Heart failure (HF) is common, causes significant morbidity and mortality, and predominantly affects the elderly.1 The clinical syndrome of HF is characterized by symptoms of dyspnea and excise intolerance, and signs of pulmonary and systemic venous congestion, due to impairments in the filling or ejection of blood from the left ventricle (LV).2 The American College of Cardiology (ACC) and American Heart Association (AHA) HF staging system emphasizes identification of asymptomatic patients with clinical risk factors for HF without (Stage A) or with (Stage B) evidence of cardiac structural and functional abnormalities to facilitate preventive measures to halt progression to symptomatic HF, defined as Stage C (current or prior symptoms of HF) and D (refractory symptoms despite optimal medical therapy or specialized cardiac support).2 Despite recognition of the progressive course of HF and increasing focus on preventive strategies, the aging population and frequency of risk factors including hypertension,3 diabetes,4 and obesity5, contribute to an increasing pool of individuals at heightened risk for HF development. Findings from the Atherosclerosis Risk in Communities (ARIC) study demonstrate a cumulative lifetime incidence of clinical HF of 26% in the community.6 However, few data currently exist regarding the prevalence of HF stages among older adults in the community.
LV ejection fraction (LVEF) <50% and LV hypertrophy (LVH) are powerful risk factors for HF.7,8 Since the initial description of the HF Stages, Stage B has been defined as evidence of structural or functional cardiac abnormalities, and operationalized as the presence of reduced LVEF or wall motion abnormalities, LVH, and ventricular enlargement, in addition to significant valvular disease. However, LVEF is preserved in approximately half of HF overall and in the majority of HF in the elderly.9,10 The majority of patients with HF with preserved LVEF (HFpEF) in community based-studies do not have LVH,11 although abnormalities of LV diastolic function and novel measures of systolic function based on strain imaging are frequently impaired and predict adverse outcomes.12,13 Indeed, although increasingly described as important and prognostic in cardiac assessment, more contemporary measures of systolic function, such as longitudinal strain, and diastolic function based on e′, E/e′ and left atrial size, have not typically been incorporated in the Stage B definition. Therefore, the goals of this analysis were to: (1) define the distribution of HF stages in a large, elderly, primarily biracial community dwelling cohort; and (2) determine the impact of incorporating novel measures of LV diastolic and systolic function into ACC/AHA HF staging system with respect to participant prognosis.
Methods
Study Population
ARIC is a prospective epidemiologic cohort study, the design and methods of which have been previous described.14 Between 1987 and 1989, 15,792 middle-aged subjects were enrolled in 4 communities in the United States: Forsyth County, NC, Jackson, MS, suburban Minneapolis, MN, and Washington County, MD. Participants underwent four exam visits between 1987 and 1998. Between 2011 and 2013, 6,538 participants returned for a fifth study visit; these participants are the focus of the current analysis. HF stages were defined based on the presence of clinical HF risk factors, cardiac structural and functional abnormalities, and clinical HF as defined in Table 1. The study protocol was approved by institutional review boards at each field center, and all participants provided written informed consent.
Table 1.
Definition of ACC/AHA heart failure stages and classification criteria employed in this study.
| HF Stage | ACC/AHA Guideline Definition | Operational Definition in This Analysis |
|---|---|---|
| Stage 0 | Not meeting criteria for HF Stages A, B, C, or D |
None of the following clinical risk factors: prevalent cardiovascular disease (coronary artery disease, stroke, or peripheral arterial disease), hypertension, diabetes, obesity, metabolic syndrome, or chronic kidney disease None of the following cardiac structural or functional abnormalities: Abnormal LVEF, regional wall motion abnormality, LV enlargement based on LVEDV indexed to BSA, left ventricular hypertrophy based on LV mass indexed to height2.7, moderate or greater aortic stenosis, aortic regurgitation, mitral regurgitation, or mitral stenosis. |
| Stage A | At high risk for HF but without structural heart disease or symptoms of HF |
At least one of the following clinical risk factors: prevalent cardiovascular disease (coronary artery disease, stroke, or peripheral arterial disease), hypertension, diabetes, obesity, metabolic syndrome, or chronic kidney disease None of the following cardiac structural or functional abnormalities: Abnormal LVEF, regional wall motion abnormality, LV enlargement based on LVEDV indexed to BSA, left ventricular hypertrophy based on LV mass indexed to height2.7, moderate or greater aortic stenosis, aortic regurgitation, mitral regurgitation, or mitral stenosis. |
| Stage B | Structural heart disease but without signs or symptoms of HF | At least one of the following cardiac structural or functional abnormalities: Abnormal LVEF, regional wall motion abnormality, LV enlargement based on LVEDV indexed to BSA, left ventricular hypertrophy based on LV mass indexed to height2.7, moderate or greater aortic stenosis, aortic regurgitation, mitral regurgitation, or mitral stenosis. |
| Stage C1 | Structural heart disease with prior or current symptoms of HF | Prevalent HF not identified through a previous hospitalization, and instead based on self-report of HF or treatment for HF with at least one of the following: (1) subsequent confirmation of self-report by treating physician or the participant, or (2) an NT-proBNP at ARIC Visit 4 or 5 of at least 125 pg/ml |
| Stage C2 | Prevalent HF identified through a previous hospitalization based on (1) committee adjudicated HF hospitalization since 2005,13 or (2) hospitalization with an ICD code 428 prior to 20058 | |
| Stage D* | Refractory HF requiring specialized interventions | Left ventricular assist device or chronic inotropic therapy |
Metabolic syndrome was defined as the presence of at least 3 of the following 5 metrics assessed at Visit 5: waist circumference ≥102 cm in men and ≥88 cm in women, fasting triglycerides ≥150 mg/dL, systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or prevalent hypertension, and fasting glucose >100 mg/dL or prevalent diabetes.
Abnormal LVEF based on ARIC reference limits (<57.4% in women or <59.0% in men), regional wall motion abnormality; LV enlargement based on LVEDV indexed to BSA above ARIC reference limits (>51.9 ml/m2 in women or >60.2 ml/m2 in men); left ventricular hypertrophy based on ARIC reference limits for LV mass indexed to height2.7 (>41.5 g/m2.7 in women or >45.0 g/m2.7 in men); moderate or greater aortic stenosis defined as a peak transaortic velocity >3.0 m/sec; moderate or greater aortic regurgitation based on visual estimation by a staff echocardiographer; moderate or greater mitral regurgitation based on a mitral regurgitation jet area-to-left atrial area ratio of >0.20; moderate or greater mitral stenosis based on a mean antegrade transmitral gradient of at least 5 mmHg.
Stage D HF could not be distinguished from Stage C2 on the basis of symptoms, as HF symptom severity was not assessed at Visit 5, and was therefore defined on the basis of advanced HF therapies (LVAD or chronic inotropic therapy).
Ascertainment of Heart Failure Risk Factors
Since study inception, ARIC participants have undergone surveillance for cardiovascular events including incident hospitalized coronary heart disease events (definite or probable MI, or coronary revascularization) and stroke as previously described.15,16 Peripheral arterial disease was defined as an ankle-brachial index at Visit 5 of <0.9 in either leg.17 Hypertension was classified based on self-reported medication use or blood pressure ≥140/90 mmHg at any ARIC visit. Diabetes was defined based on self-report of a physician diagnosis of diabetes, anti-diabetic medication use, fasting glucose ≥126 mg/dL, or non-fasting glucose ≥200 mg/dL, at any ARIC visit. Body mass index (BMI) was assessed at Visit 5 and obesity defined as BMI ≥30 kg/m2. Metabolic syndrome was defined as the presence of at least 3 of the following 5 metrics assessed at Visit 5: waist circumference ≥102 cm in men and ≥88 cm in women, fasting triglycerides ≥150 mg/dL, systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or prevalent hypertension, and fasting glucose >100 mg/dL or prevalent diabetes.18 Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 using the CKD-Epi equation.19
Echocardiographic Assessment of Cardiac Structure and Function
Echocardiography in ARIC at Visit 5, including reproducibility metrics, has been previously described.20 Studies were acquired at Visit 5 by certified sonographers using uniform imaging equipment and acquisition protocol. Quantitative measures were performed by a dedicated Echocardiography Reading Center. LVEF was based on the modified Simpson’s method or, when volumes could not be accurately assessed, on the Teichholz’s method (n=27) or visual estimation by board certified echocardiographers at the Echocardiography Reading Center (n=166). LVMi was calculated from linear dimensions as recommended by the American Society of Echocardiography, and indexed to height2.7.21 Age-related changes in cardiac structure and function are well recognized, including smaller LV size, greater LVEF, and lower tissue Doppler relaxation velocities (TDI e′), even in older adults free of CV risk factors.22,23,24,25,26,27,28,29 Existing guildeline norms are based predominantly on data from younger populations and current guideline recommendations specifically cite the need for more data in the elderly.21,30 Therefore, for echocardiographic measures of structure and function, abnormal was based on sex-specific 95th percentile limits derived from a subgroup of 413 healthy ARIC participants without prevalent cardiovascular (CV) disease or risk factors. Prevalent CV disease was defined as coronary heart disease (CHD; includes myocardial infarction history or regional wall motion abnormality on echocardiography), prior HF hospitalization or HF self-report, atrial fibrillation, moderate or greater valvular disease. CV risk factors included hypertension, diabetes, Visit 5 body mass index (BMI) of >30 or <18.5 kg/m2, chronic kidney disease defined as an eGFR <60 ml/min/1.73 m2 at Visit 5, QRS duration ≥120 msec at Visit 5, and active smoking. As empiric estimates of distribution limits can vary substantially in small to moderate sized samples, we used quantile regression (STATA qreg) to define the 95th percentile limit of distribution in this healthy group. Regional wall motion abnormalities (RWMA) were identified by staff echocardiographers.
Abnormal LV structure and LVEF were used to classify Stage B HF and defined as follows: abnormal LVEF based on ARIC reference limits (<57.4% in women or <59.0% in men), regional wall motion abnormality; LV enlargement based on LVEDV indexed to BSA above ARIC reference limits (>51.9 ml/m2 in women or >60.2 ml/m2 in men); left ventricular hypertrophy based on ARIC reference limits for LV mass indexed to height2.7 (>41.5 g/m2.7 in women or >45.0 g/m2.7 in men); moderate or greater aortic stenosis defined as a peak transaortic velocity >3.0 m/sec; moderate or greater aortic regurgitation based on visual estimation by a staff echocardiographer; moderate or greater mitral regurgitation based on a mitral regurgitation jet area-to-left atrial area ratio of >0.20; and moderate or greater mitral stenosis based on a mean antegrade transmitral gradient of at least 5 mmHg. While the median LVEF in the healthy ARIC cohort was higher in women (66.8, IQR 63.8–69.5%) than in men (65.6, IQR 62.8–68.8%), the range was greater, leading to a lower value for normal LVEF in women based on the 95th percentile.
To test incorporation of more contemporary systolic function assessment into HF staging, LS was measured in the apical 4-chamber and 2-chamber views using the TomTec Cardiac Performance Analysis package, which has been validated against MRI and sono-micrometry31,32 as previously described.20 Abnormal LS and measures of diastolic function were also defined based on sex-specific 95th percentile limits derived from the ARIC healthy subgroup as follows: LS <15.2% and <14.7% in women and men respectively; TDI e′septal <4.1 cm/s and <4.3 cm/s respectively; E/e′septal >17.4 and >14.8 respectively; and LA volume indexed to BSA >32.4 ml/m2 and >34.2 ml/m2 respectively. These limits are generally concordant with guideline recommendations for LV mass indexed to height2.7, E/e′septal ratio, and LAVi (Supplemental Table 1). The limit employed for LVEF was higher, and for TDI e′septal was lower, compared to guideline recommendations but agreed well with reference values from other healthy populations of similar age33,34,35,36,37,38
Ascertainment of Prevalent (Stage C) and Incident HF Post-Visit 5
Prevalent HF in ARIC at Visit 5 was ascertained from multiple sources: physician adjudicated HF hospitalization occurring since 2005 as previously published;39 International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) 428 code for hospitalizations prior to 2005;15 or HF self-report at Visits 3–5 or on annual follow-up phone calls. In ARIC, the positive predictive value of ICD-9-CM 428 code for HF relative to physician adjudication is 0.77.39 In this analysis, HF Stage C2 was defined as HF identified through a prior hospitalization (an adjudicated HF hospitalization since 2005 or hospitalization with a HF ICD code prior to 2005). HF Stage C1 was defined as HF not identified through a prior hospitalization: self-report of HF or treatment for HF among those without a prior hospitalization with at least one of the following: (a) subsequent confirmation of self-report by treating physician or the participant, or (b) an NT-proBNP at Visit 4 or 5 of at least 125 pg/ml.40 Stage D HF could not be distinguished from Stage C2 on the basis of symtoms, as HF symptom severity was not assessed at Visit 5. Therefore, Stage D was defined based on therapy with a left ventricular assist device (LVAD) or chronic intravenous inotropes (milrinone or dobutamine), which were assess at Visit 5.
For incident HF and death post-Visit 5, incident HF was based on HF hospitalization or HF death according to ICD codes (code 410 in any position) obtained by ARIC surveillance of hospital discharges.15 Deaths were ascertained using the National Death Index.16
Cardiac Biomarker Assessment
Blood for cardiac biomarker measurement at Visit 5 was stored centrally at −80°C. Hs-TnT was measured using a highly sensitive assay (Elecsys Troponin T, Roche diagnostics, Indianapolis, IN). NT-proBNP was measured using electrochemiluminescent immunoassay (Roche Diagnostics) with a lower detection limit of ≤5 ng/mL.
Statistical Methods
Participants were first categorized based on HF stage using the standard criteria outlined in Table 1. Clinical and echocardiographic features were compared between categories using Wilcoxon rank sum test (continuous variables) and Chi-squared tests (categorical variables) for pair-wise between group comparisons. Prevalence of these HF stages was described in the sample overall and stratified by age category (65 – 70, 71 – 75, 76 – 80, >80 years old). Age-adjusted prevalence was presented by subgroups based on sex and race. Multivariable Cox proportional hazards models were used to assess the relationship of the HF stage at Visit 5 to incident HF hospitalization and mortality post-Visit 5.
We then assessed the impact of incorporating novel measures of systolic function (LS) and diastolic function (based on TDI e′, E/e′, and LAV/BSA). Among HF stage A and B participants, we assessed the associations of abnormal LV structure (defined based on LV mass indexed to height2.7, LVEDV/BSA, and ≥moderate valvular disease), systolic function (defined based on LVEF, RWMA, and LS), and diastolic function (defined based on TDI e′, E/e′, and LAV/BSA) – individually and in combination – with incident HF hospitalization or death using univariate and multivariable Cox proportional hazards models. We assessed the incremental prognostic value of LS and diastolic measures beyond conventional measures of LV structure and LVEF for incident HF hospitalization or death based on the continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) at 2 years using time-to-event data,41 and by comparing the C-statistic of predictive models with and without inclusion of the additional measures. We quantified the reclassification of participants from HF stage A to stage B when abnormalities of LS and diastolic function were included as stage B criteria. Finally, among HF stage A and stage B participants with HF risk factors, we characterized 5 cardiac phenotypes: (1) those with no abnormalities of LV structure (defined as abnormally high LV mass/height2.7, LVEDV/BSA, or ≥moderate valvular disease), LV systolic function (defined as abnormally low LVEF or LS), or LV diastolic function (defined as abnormally low TDI e′ or high E/e′ or LAV/BSA); (2) those with an abnormality of only one of these domains who were labeled as having isolated structural abnormality, isolated systolic abnormality, or isolated diastolic abnormality; and (3) those with abnormalities of more than one of these domains who were labeled as having combined abnormalities.
For time to event analyses, two multivariable models were constructed. The first adjusted for age, sex, race, and ARIC Field Center. The second additionally adjusted for history of hypertension, diabetes, atrial fibrillation, chronic kidney disease, obesity, prior myocardial infarction, and prior stroke. The proportional hazards assumption was tested for all analyses, and there was no evidence of violation of the proportional hazards assumption.
To assess the impact of potential bias due to Visit 5 non-attendance, we performed a sensitivity analysis using inverse probability of attrition weighting.42,43 Visit 5 non-attendance was modeled among participants alive at the initiation of Visit 5 using the following covariates from Visit 1: age, gender, race, study center, systolic and diastolic blood pressure, heart rate, body mass index, smoking and drinking status, diabetes, hypertension, and chronic kidney disease. The resulting calculated weights were incorporated into multivariable models for HF stage estimates. Analyses were performed using STATA 14. NRI and IDI analyses were performed using R version 3.2.0. Two-sided P-values of less than 0.05 were considered significant.
Results
Of the 15,792 participants enrolled in the ARIC cohort at study inception, 10,742 (68%) were alive at the initiation of Visit 5 and 6,538 participants (62% of those alive) attended. Both clinical and echocardiographic assessments necessary to determine HF stage were available in 6,118 participants.
Prevalence of HF Stages
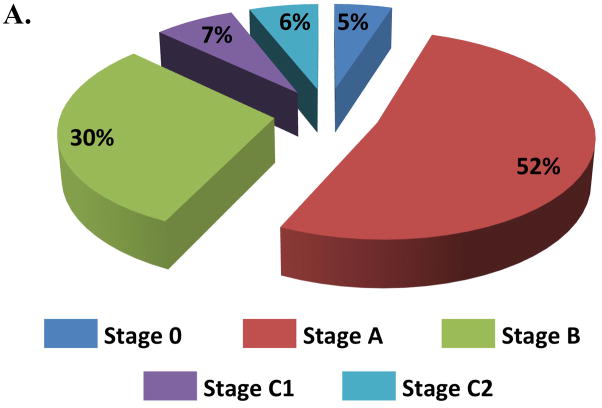
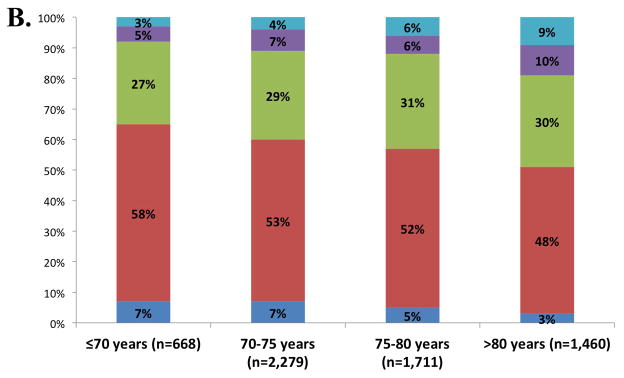
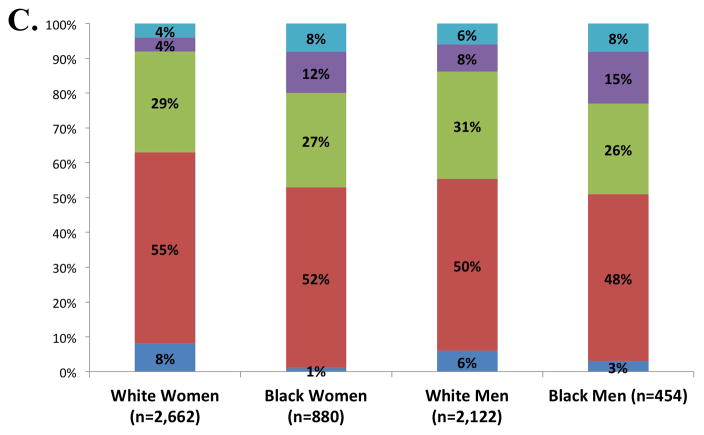
Five percent of participants were free of both clinical HF risk factors and structural heart disease (Stage 0), with the majority of ARIC participants (52%) classified as Stage A HF (Figure 1, panel A). The prevalences of Stage B and Stage C HF were 30% and 13% respectively. One participant had an LVAD (<0.1%) and no participants were receiving continuous intravenous inotropic therapy. The prevalences of Stages C1 and C2 HF were higher in older compared to younger participants, men compared to women, and blacks compared to whites (Figure 1, panels B and C). However, across all subgroups, Stage A was the most prevalent HF stage. Worse HF stage was characterized by higher levels of NT-proBNP and high sensitivity troponin T (Table 2). At a median follow-up of 608 days (25th to 75th percentile range 469–761 days), 194 participants died or experienced a HF hospitalization. In multivariable adjusted analysis, worse HF stage was associated with a higher risk of death and the composite of death or HF hospitalization in a graded fashion (Figure 2; see Supplemental table 2 for results after additional adjustment for hypertension, diabetes, chronic kidney disease, obesity, prior stroke, myocardial infarction, and atrial fibrillation).
Figure 1.
Prevalence of heart failure stages. (A) Prevalence in the study population overall; (B) prevalence among age categories; and (C) age-adjusted prevalence among subgroups defined by sex and race. One participant was classified as Stage D on the basis of having an LVAD (prevalence <0.1%).
Table 2.
Participant characteristics by heart failure stage in the ARIC study, 2011–2013.
| Overall (n=6,118) | Stage 0 (n=308) | Stage A (n=3,192) | Stage B (n=1,801) | Stage C1 (n=450) | Stage C2 (n=366) | Criteria | |
|---|---|---|---|---|---|---|---|
| Age (years) | 75.3 [71.7, 79.7] | 73.8 [71.1, 77.5] | 75.0 [71.6, 79.4]*†‡ | 75.4 [72.0, 79.8]†§¶ | 75.7 [72.0, 81.1]# | 78.2 [73.5, 82.0] | |
| Male | 42% | 35% | 40%*†‡ | 43%§ | 53% | 48% | |
| Black | 22% | 8% | 22%†‡ | 20%§¶ | 37%# | 28% | |
| Field Center | |||||||
| Forsyth County | 23% | 29% | 24%*†‡ | 21% | 20% | 17% | |
| Jackson | 20% | 8% | 20%*†‡ | 17%§¶ | 34%# | 26% | |
| Minneapolis | 30% | 41% | 30%†‡ | 31%§¶ | 24% | 25% | |
| Washington County | 27% | 22% | 26%*‡ | 31%§ | 22%# | 32% | |
| HF Risk Factors | |||||||
| Hypertension | 83% | 0 | 86%*†‡ | 88%§¶ | 96% | 97% | |
| Diabetes | 38% | 0 | 35%*†‡ | 41%§¶ | 52%# | 61% | |
| Obesity | 34% | 0 | 29%*†‡ | 46% | 41% | 46% | |
| Metabolic Syndrome | 60% | 0 | 60%*†‡ | 66%¶ | 67% | 72% | |
| CKD | 28% | 0 | 27%†‡ | 28%§¶ | 39%# | 54% | |
| Ever smoker | 62% | 56% | 60%‡ | 63%¶ | 64% | 69% | |
| Current smoking | 6% | 6% | 6% | 6% | 6% | 6% | |
| Prevalent CVD | |||||||
| CAD | 17% | 0 | 10%*†‡ | 16%§¶ | 49% | 54% | |
| Prior MI | 8% | 0 | 3%*†‡ | 7%§¶ | 30% | 33% | |
| PAD | 6% | 0 | 5%*†‡ | 7%¶ | 10% | 16% | |
| Stroke | 4% | 0 | 3%†‡ | 3%§¶ | 8%# | 13% | |
| Atrial Fibrillation | 7% | 0 | 4%*†‡ | 7%§¶ | 11%# | 37% | |
| Physical Exam | |||||||
| BMI (kg/m2) | 27.9 [24.9, 31.6] | 24.5 [23.0, 26.4] | 27.4 [24.6, 30.5]*†‡ | 29.4 [25.9, 33.4]§ | 28.7 [25.2, 32.9]# | 29.4 [26.2, 34.0] | |
| SBP (mmHg) | 129 [118, 141] | 120 [112, 127] | 129 [118, 140]* | 131 [119, 143]¶ | 129 [117, 142] | 128 [113, 143] | |
| DBP (mmHg) | 66 [59, 74] | 62 [58, 69] | 67 [60, 74]†‡ | 67 [59, 74]§ ¶ | 65 [58, 73]# | 62 [55, 70] | |
| HR (bpm) | 61 [55, 68] | 60 [54, 66] | 62 [56, 69]*‡ | 61 [54, 68] ¶ | 61 [55, 69]# | 64 [59, 72] | |
| Laboratory Values | |||||||
| HbA1c (%) | 5.7 [5.5, 6.1] | 5.5 [5.3, 5.7] | 5.7 [5.5, 6.1]*†‡ | 5.8 [5.5, 6.2]¶ | 5.9 [5.5, 6.3]# | 6.0 [5.6, 6.7] | |
| eGFR (ml/min per 1.73 m2) | 70.8 [58.2, 83.0] | 77.7 [70.0, 85.3] | 71.3 [59.2, 83.1]†‡ | 71.2 [58.1, 83.3]§ ¶ | 65.6 [54.0, 81.0]# | 57.9 [43.6, 73.3] | |
| LDL (mg/dL) | 101 [79, 125] | 119 [98, 140] | 103 [82, 127]*†‡ | 99 [77, 123]§ ¶ | 90 [71, 115] | 85 [65, 110] | |
| HDL (mg/dL) | 50 [42, 60] | 59 [52, 69] | 51 [43, 61]*†‡ | 49 [41, 58]§ ¶ | 46 [39, 54] | 46 [39, 56] | |
| hsCRP | 2.0 [1.0, 4.2] | 1.2 [0.7, 2.3] | 1.9 [0.9, 4.0]*†‡ | 2.1 [1.0, 4.5]¶ | 2.5 [1.1, 4.8]# | 3.2 [1.6, 6.8] | |
| Echo: LV Structure | |||||||
| Wall thickness (cm) | 0.97 [0.89, 1.07] | 0.88 [0.83, 0.94] | 0.93 [0.88, 1.00]*†‡ | 1.04 [0.95, 1.14]§ | 1.02 [0.92, 1.12]# | 1.05 [0.94, 1.17] | |
| EDV/BSA (ml/m2) | 41.7 [35.8, 49.0] | 41.3 [35.6, 46.4] | 39.6 [34.5, 45.4]*†‡ | 45.8 [38.2, 54.6]¶ | 44.3 [37.5, 53.0]# | 47.4 [39.1, 60.7] | |
| LV enlargement | 10% | 0 | 0 | 24%§¶ | 17%# | 32% | EDV/BSA >51.9 in women; >60.2 in men |
| Mass/height2.7 (g/m2.7) | 36.3 [30.9, 43.7] | 30.3 [26.7, 33.7] | 33.3 [29.0, 37.2]*†‡ | 45.8 [39.4, 51.0]§ ¶ | 41.1 [34.3, 49.0]# | 46.4 [37.6, 57.4] | |
| LVH | 27% | 0 | 0 | 68%§¶ | 41%# | 59% | Mass/ht2.7 >41.5 in women; >45.0 in men |
| Significant valve disease | 4% | 0 | 0 | 10%¶ | 5%# | 15% | |
| Echo: LV Systolic Function | |||||||
| LVEF (%) | 65.6 [61.8, 69.2] | 66.7 [64.2, 69.5] | 66.7 [63.7, 69.9]*†‡ | 63.9 [58.5, 68.1]¶ | 64.0 [58.8, 68.1]# | 61.2 [53.4, 66.1] | |
| Abnormal LVEF | 11% | 0 | 0 | 25%¶ | 23%# | 35% | <57.4 in women; <59.0 in men |
| LVEF <50% | 3% | 0 | 0 | 5%§¶ | 9%# | 20% | |
| RWMA | 1.8% | 0 | 0 | 3.0%§¶ | 5.6% | 9.0% | |
| LS (%) | −18.2 [−19.7, −16.4] | −19.0 [−20.4, −17.5] | −18.5 [−19.9, −17.0]*†‡ | −17.6 [−19.4, −15.7]§ ¶ | −17.4 [−19.1, −15.3]# | −15.9 [−18.3, −13.5] | |
| Abnormal LS | 13% | 3% | 7%*†‡ | 18%§¶ | 22%# | 39% | <15.2 in women; <14.7 in men |
| Echo: LV Diastolic Function | |||||||
| TDI e′ (cm/s) | 5.5 [4.7, 6.5] | 6.2 [5.3, 7.4] | 5.7 [4.8, 6.6]*†‡ | 5.2 [4.4, 6.2] ¶ | 5.2 [4.3, 6.1] | 5.2 [4.1, 6.1] | |
| Abnormal e′ | 14% | 4% | 10%*†‡ | 19%¶ | 21% | 26% | TDI e′septal <4.1 in women; <4.3 in men |
| E/e′ ratio | 11.7 [9.5, 14.5] | 10.2 [8.5, 12.6] | 11.3 [9.3, 13.7]*†‡ | 12.0 [9.8, 15.2]§ ¶ | 12.6 [10.1, 15.9]# | 14.6 [11.1, 18.7] | |
| Abnormal E/e′ | 16% | 4% | 11%*†‡ | 18%§¶ | 26%# | 40% | E/e′septal >17.4 in women; >14.8 in men |
| LAVi (ml/m2) | 24.4 [20.0, 29.7] | 21.5 [18.3, 25.3] | 22.9 [18.9, 27.3]*†‡ | 26.5 [21.7, 32.5] ¶ | 27.1 [22.0, 33.2]# | 30.9 [25.1, 41.3] | |
| Abnormal LAVi | 16% | 4% | 8%*†‡ | 23%¶ | 24%# | 42% | >32.4 in women; >34.2 in men |
| Cardiac Biomarkers | |||||||
| NT-proBNP (ng/L) | 134 [69, 269] | 84 [53, 156] | 108 [58, 200]*†‡ | 156 [78, 337]§ ¶ | 232 [128, 473]# | 504 [215, 1370] | |
| hs-TnT (ng/L) | 11 [7, 16] | 7 [5, 10] | 10 [7, 15]*†‡ | 11 [8, 17]§ ¶ | 14 [9, 22]# | 18 [11, 33] | |
Values presented are n (%) for categorical variables and median [interquartile range] for continuous variables. Italics indicate that the variable was a criterion for Stage B assignment.
p <0.05 for A vs B;
p <0.05 for A vs C1;
p <0.05 for A vs C2;
p <0.05 for B vs C1;
p <0.05 for B vs C2;
p <0.05 for C1 vs C2.
CKD – chronic kidney disease; CAD - coronary artery disease, MI- myocardial infarction, PAD – peripheral arterial disease, RWMA – regional wall motion abnormality, LVH – LV hypertrophy, BMI – body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, HR – heart rate, HbA1c – hemoglobin A1c; eGFR – estimated glomerular filtration rate; EDV – LV end-diastolic volume, LS – longitudinal strain; TDI – tissue Doppler imaging; LAVi – left atrial volume index
Figure 2.
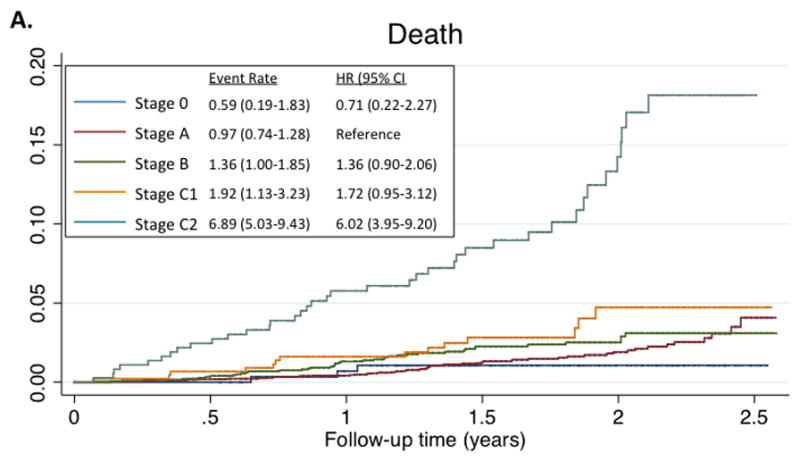
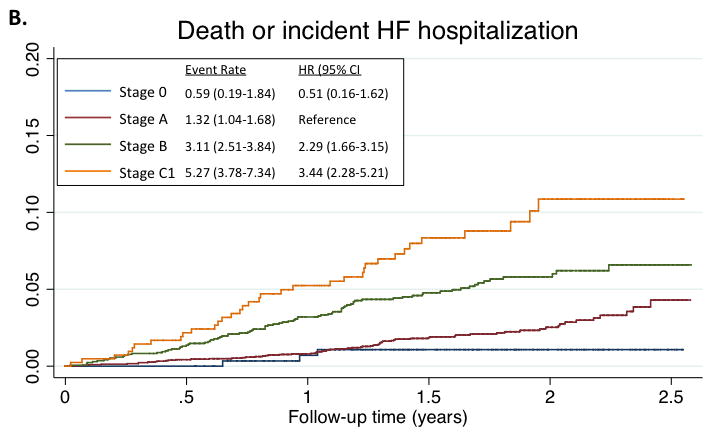
Kaplan-Meyer survival curves for death (panel A), and the composite of death or HF hospitalization (panel B), by HF Stage. Median follow-up time for the composite endpoint was 608 days (25th to 75th percentile range 469–761 days). Total number of events was 194. For the composite endpoint, estimates for HF Stage C2 are not provided as all participants in this stage had, by definition, experienced a previous HF hospitalization. Event rate is expressed per 100 person-years. Hazard ratios are adjusted for age, sex, race, and Field Center.
Cardiac Structure and Function in Persons at Risk for Heart Failure (Stages A and B)
Among Stage A participants, despite the absence of overt structural heart disease or hypertrophy, greater risk factor burden was associated with greater wall thickness and mass, smaller LV size, worse longitudinal systolic function (LS), worse early diastolic relaxation (TDI e′), higher filling pressure (E/e′ ratio), and higher levels of high sensitivity troponin T (Table 3). Number of risk factors was not related to LVEF.
Table 3.
Measures of cardiac structure and function among ARIC participants with Stage A HF at Visit 5 stratified by the number of HF risk factors present.
| Overall (n=3,192) | 1 Risk Factor (n=868) | 2 Risk Factors (n=908) | 3 Risk Factors (n=789) | 4 Risk Factors (n=570) | Unadjusted P value | Adjusted P value | |
|---|---|---|---|---|---|---|---|
| LV structure | |||||||
| Wall thickness (cm) | 0.93 [0.88, 1.00] | 0.91 [0.85, 0.97] | 0.93 [0.87, 0.99] | 0.95 [0.89, 1.02] | 0.96 [0.90, 1.04] | <0.0001 | <0.0001 |
| Mass/height2.7 (g/m2.7) | 33.3 [29.0, 37.2] | 31.5 [27.4, 35.9] | 32.9 [28.9, 36.8] | 33.8 [29.7, 37.5] | 35.3 [31.4, 38.8] | <0.0001 | <0.0001 |
| EDV/BSA (ml/m2) | 39.6 [34.5, 45.4] | 40.9 [36.0, 46.6] | 39.3 [34.7, 44.9] | 39.0 [33.9, 44.7] | 38.4 [33.3, 44.4] | <0.0001 | <0.0001 |
| Systolic Function | |||||||
| LVEF (%) | 66.7 [63.7, 69.9] | 66.6 [63.7, 69.9] | 66.9 [63.7, 69.9] | 66.6 [64.0, 69.9] | 66.7 [63.4, 70.2] | 0.53 | 0.18 |
| LS (%) | −18.5 [−19.9, −17.0] | −18.7 [−20.1, −17.2] | −18.6 [−19.9, −17.1] | −18.5 [−19.8, −16.9] | −18.1 [−19.7, −16.5] | 0.0001 | <0.0001 |
| Diastolic Function | |||||||
| TDI e′ (cm/s) | 5.7 [4.8, 6.6] | 5.8 [5.0, 6.9] | 5.7 [4.8, 6.6] | 5.6 [4.8, 6.4] | 5.6 [4.7, 6.5] | <0.0001 | <0.0001 |
| E/e′ ratio | 11.3 [9.3, 13.7] | 10.9 [9.0, 13.1] | 11.3 [9.3, 13.6] | 11.5 [9.5, 14.0] | 11.9 [9.6, 14.8] | <0.0001 | <0.0001 |
| LAVi (ml/m2) | 22.9 [18.9, 27.3] | 23.1 [19.3, 27.6] | 22.6 [18.8, 27.1] | 22.9 [19.1, 27.1] | 22.9 [18.9, 27.5] | 0.49 | 0.27 |
| Soluble biomarkers | |||||||
| NT-proBNP (ng/L) | 108 [58, 200] | 110 [63, 195] | 111 [60, 209] | 101 [54, 182] | 109 [53, 221] | 0.087 | 0.63 |
| Hs-TnT (ng/L) | 10 [7, 15] | 9 [7, 13] | 9 [7, 14] | 10 [7, 15] | 12 [8, 18] | <0.0001 | <0.0001 |
Values presented are median [interquartile range]. P values are for trend across risk factor categories. Adjusted P value is adjusted for age, sex, race, and Field Center. EDV – end-diastolic volume, BSA – body surface area, LVEF – left ventricular ejection fraction, LS – LV longitudinal strain, LAVi – left atrial volume index, hs-TnT – high sensitivity troponin-T
Among the 1,801 Stage B participants, LVEF was reduced in 25%, LVH was present in 68%, LV enlargement was present in 24%, and moderate or greater left-sided valve disease was present in 9% (Table 2). Among these Stage B participants, men were more likely than women to have reduced LVEF (42 vs 14% respectively, p<0.001) and a regional wall motion abnormality (5 vs 2% respectively, p<0.001), but less likely to have LVH (54 vs 79% respectively, p<0.001).
Structural Heart Disease and LVEF in Stage C Heart Failure
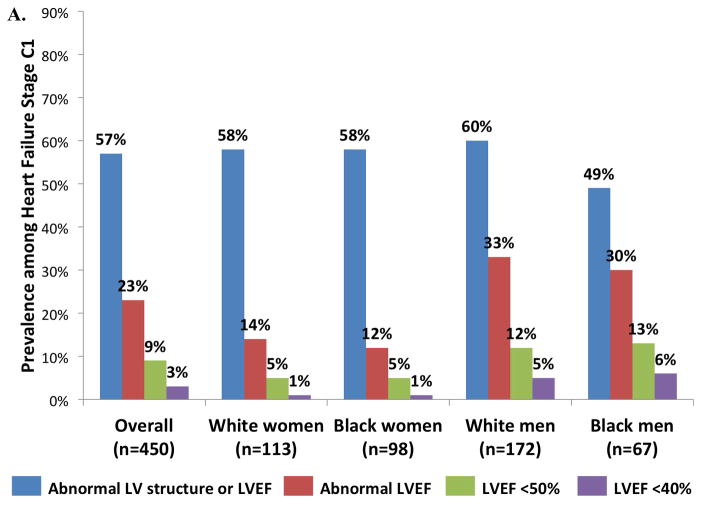
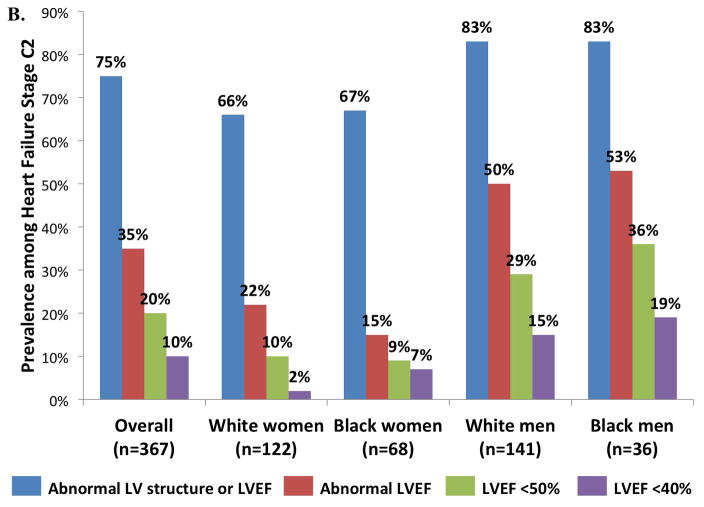
Among participants with Stage C2 HF, 75% had abnormalities of LV structure (hypertrophy, enlargement, regional wall motion abnormality, or ≥moderate valvular disease) or LVEF (Figure 3, panel B). LVEF was below the ARIC-based reference value in 35%, and was <50% in 20% and <40% in 10%. Compared to women, men had a higher prevalence of structural abnormality or a reduced LVEF (Figure 4B, all p ≤0.001), but not of diastolic dysfunction. Abnormal LV structure or LVEF and abnormal LVEF alone were less common in Stage C1 compared to Stage C2 HF (57 versus 75% and 23 versus 35% respectively; Figure 3, panel A).
Figure 3.
Prevalence of cardiac structural abnormalities and abnormal LVEF among (A) Stage C1 and (B) C2 heart failure participants in the study population overall, and separately in subgroups defined by sex and race.
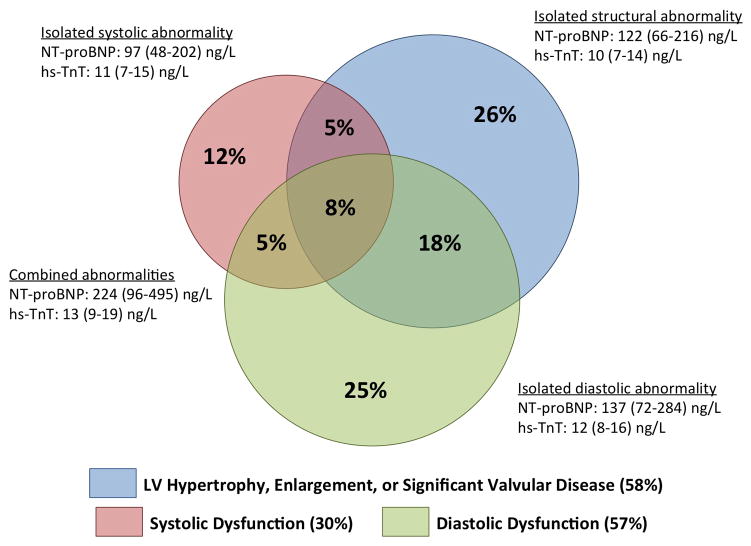
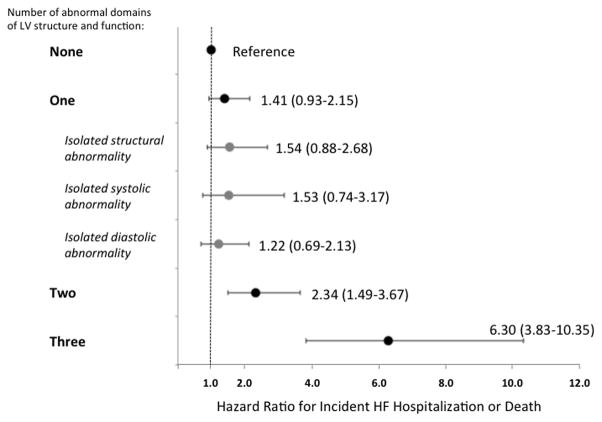
Figure 4.
Prevalence and prognostic relevance of abnormalities of LV structure, systolic, and diastolic function among elderly persons in the community. Panel A. Venn diagram demonstrating the prevalence of abnormalities of cardiac structure and function among participants with Stage B heart failure defined using abnormal LV strain and diastolic measures in addition to abnormal LVEF, LVH, LV enlargement, and valvular disease. Values for NT-proBNP and hs-TnT are median and interquaritile range. For biomarker levels, P for all between group comparisons <0.05 except for hs-TnT in isolated structural abnormality vs isolated systolic abnormality (p=0.14). Panel B. Hazard ratio for incident HF hospitalization or death associated with abnormal LV structure, systolic function, and diastolic function among HF Stage A and B participants relative to those with clinical risk factors but no abnormalities. Multivariable models are adjusted for age, sex, race, and ARIC Field Center. See Supplemental Table 2 for results with additional adjustment for hypertension, diabetes, chronic kidney disease, obesity, prior stroke, myocardial infarction, and atrial fibrillation.
Impact of Novel Measures of LV Function on HF Stages
Among Stage A and B participants at risk for clinical HF, diastolic function (based on TDI e′, E/e′ ratio, and LAV/BSA) was abnormal in 30%. Systolic function was abnormal by LVEF in 9% and by LS in 10%, while the LVEF was <50% in only 2% and was <40% in 0.4%. Notably, only 3% demonstrated both abnormal LVEF and LS. Abnormalities of LV structure, diastolic function, and systolic function (based on either LVEF or LS) were each independently and additively associated with the risk of incident HF hospitalization or death among Stage A and B participants at risk for HF (Table 4). Furthermore, among HF Stage A and B participants, incorporating information on LS and diastolic dysfunction provided incremental prognostic information beyond conventional measures of LV structure and LVEF based on the continuous NRI (12.1% [95% CI 1.8–20.4%], p=0.028) and IDI (0.3% [95% CI 0.0–1.5%], p=0.016), although the improvement in C-statistic was not statistically significant (C-statistic 0.70 with conventional measures alone versus 0.71 additionally including LS and diastolic measures; p=0.19).
Table 4.
Association of abnormal LV structure, systolic dysfunction, or diastolic dysfunction with risk of incident HF hospitalization or death among those with HF risk factors (Stages A and B)
| Structural abnormality | Systolic dysfunction | Diastolic dysfunction | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Unadjusted | 2.41 (1.75–3.32) | <0.001 | 2.15 (1.52–3.06) | <0.001 | 2.49 (1.81–3.43) | <0.001 |
| Unadjusted + other LV domains* | 1.91 (1.37–2.67) | <0.001 | 1.73 (1.21–2.49) | 0.003 | 2.04 (1.46–2.85) | <0.001 |
| Adjusted (model 1) | 2.42 (1.75–3.34) | <0.001 | 1.91 (1.34–2.73) | <0.001 | 2.12 (1.53–2.93) | <0.001 |
| Adjusted (model 1) + other LV domains* | 2.00 (1.43–2.80) | <0.001 | 1.55 (1.07–2.24) | 0.02 | 1.75 (1.24–2.46) | 0.001 |
| Adjusted (model 2) | 2.22 (1.56–3.16) | <0.001 | 1.77 (1.20–2.60) | 0.004 | 1.96 (1.37–2.79) | <0.001 |
| Adjusted (model 2) + other LV domains* | 1.88 (1.31–2.72) | 0.001 | 1.53 (1.03–2.25) | 0.033 | 1.64 (1.14–2.37) | 0.008 |
Model 1: adjusted for age, sex, race, and field center; Model 2: adjusted for age, sex, race, ARIC Field Center, hypertension, diabetes, chronic kidney disease, prior stroke, prior myocardial infarction, obesity, and atrial fibrillation;
indicates models containing abnormal LV structure, systolic dysfunction, and diastolic dysfunction as predictors variables
Abnormal LV structure was defined as LV enlargement (LVEDV/BSA >51.9 ml/m2 in women or >60.2 ml/m2 in men) or LVH (LV mass/ height2.7 >41.5 g/m2.7 in women or >45.0 g/m2.7 in men); systolic dysfunction was defined as abnormal LVEF (<57.4% in women or <59.0% in men) or abnormal LS (<15.2% in women or <14.7% in men); diastolic dysfunction was defined as abnormal TDI e′septal (<4.1 cm/s in women or <4.3 cm/s in men), E/e′septal (>17.4 in women or >14.8 in men), or LAVi (LAV/BSA >32.4 ml/m2 in women or >34.2 ml/m2 in men).
Incorporating diastolic measures and LS into the definition of Stage B HF resulted in reclassification of 14% of the study population from Stage A to Stage B, with a drop in prevalence of Stage A HF from 52 to 38% and an increase in prevalence of Stage B HF from 30 to 44% (Supplemental Figure 1). Similar to findings using conventional criteria alone (Figure 2), worse HF stage when defined using diastolic measures and LS in the Stage B definition was also associated with a higher risk of death or the composite of death or HF hospitalization in a graded fashion (Supplemental Figure 2). Participants reclassified to Stage B had a hs-TnT level equivalent to existing Stage B participants (12 [8–17] vs 12 [8–17] ng/L respectively, p=0.29), and significantly higher than non-reclassified Stage A participants (9 [7–14] ng/L, p<0.0001; Supplemental Table 3). NT-proBNP levels in the reclassified participants were significantly higher than the non-reclassified Stage A participants (133 [68–288] vs 101 [56–180] respectively, p<0.0001) but lower than existing Stage B participants (158 [78–344, p=0.0003). The rate of death or incident HF hospitalization during the follow-up period was 12.1 (9.0–16.2) per 1,000 person-years in non-reclassified Stage A, 16.3 (10.8–24.5) in reclassified participants, and 31.1 (25.1–38.4) in existing Stage B participants (p for trend <0.001; Supplemental Table 3). No statistical difference was noted in the event rates between the reclassified participants and non-reclassified Stage A participants (p=0.26), possibly related to limited power given the small number of events in the reclassified group (n=23).
Together abnormalities of LV structure, systolic function, and diastolic function identified four phenotypes: isolated structural abnormality, isolated systolic abnormality, isolated diastolic abnormality, and combined abnormalities. Cardiac biomarkers differed significantly between these groups (Figure 4, panel A), with the highest NT-proBNP and hs-TnT level noted among those with combined abnormalities. When compared to those without abnormalities of structure or function, a greater number of abnormalities in these domains was associated with higher risk of incident HF hospitalization or death (Figure 4, panel B). However, among the large number of participants with only one abnormality, no significant difference was noted in the risk associated with isolated structural abnormality, isolated systolic abnormality, or isolated diastolic abnormality (Figure 4, panel B; Supplemental Table 4).
Among participants with Stage C2 HF, beyond traditional measures of LV structure and LVEF, LS was abnormal in 39% (59% of whom also had an abnormal LVEF) and abnormalities of diastolic measures were present in 67%. Including these novel measures, an abnormality of LV structure and/or function was identifiable in 91% of Stage C2 participants. Abnormal LV structure or LVEF (57%), LS (22%), and diastolic function (48%) identified an LV abnormality in 75% of Stage C1 participants.
Discussion
Our analysis of HF stages among 6,118 participants in the community-based ARIC cohort aged 66 to 90 years has three major findings. First, the vast majority of this elderly cohort was at risk for symptomatic HF (i.e. 82% were Stages A or B), with only 5% of participants totally free of clinical risk factors or abnormalities of cardiac structure or function. Worse HF stage was associated with greater risk of death or incident HF hospitalization in a graded fashion. Within Stage A HF, a broad spectrum of risk factor burden, alterations in cardiac structure and function, and biomarker levels was observed. Second, among Stage A and B participants, abnormal LV structure, systolic function, and diastolic function were independently and additively associated with incident HF hospitalization or death. Diastolic measures and LS provided incremental prognostic value beyond conventional measures of LV structure and LVEF. Incorporating LS and diastolic function into the Stage B definition increased the prevalence of Stage B HF from 30% to 44% of the sample, and appreciably increased the proportion of Stage C participants with an identifiable abnormality of LV structure or function. Third, the large majority of participants with clinical HF (Stages C1 and C2) in this elderly cohort had a robustly normal LVEF (77% and 65%, respectively, with LVEF ≥57.4% in women or 59.0% in men).
The construct of the HF stages emphasizes the continuum of risk for the HF syndrome, and helps providers identify and optimally manage patients at particularly high risk for developing signs and symptoms of HF.2 To our knowledge, ours is the only study to characterize HF stages in an elderly, biracial community-based sample. The distribution of HF stages differs substantially from previous reports in younger, predominantly white cohorts.44,45 Among 2,029 residents of Olmsted County, MN, approximately two-thirds of whom were ≤65 years of age, 32% of participants had neither HF risk factors or structural heart disease (Stage 0), while only 22% were classified as Stage A.44 Similarly, among 739 participants in a Portuguese population health survey with a mean age of 62 years, 19% of men and 26% of women were Stage 0, while prevalence of Stage A HF was 54% and 44% respectively.45 The most prominent difference we observed from these prior studies in younger samples was a markedly higher prevalence of Stage A HF (52%) and lower prevalence of Stage 0 (5%). Even within the age-range represented in our study sample, we observed a decrease in the prevalence of Stage 0 and increase in prevalence of Stages B, C1, and C2 with older age. This age-associated growth in clinical risk factors and abnormal cardiac structure and function helps explain the appreciable increase in the incidence and prevalence of clinical HF in the elderly.46
A unique strength of our study is the use of age-appropriate cut-offs to define abnormal cardiac structure and function. Using these cutpoints, which included an LVEF <57.4% in women or <59.0% in men, we classified 30% of participants as Stage B HF. This prevalence is comparable to that noted in the Portuguese sample,45 although they used a lower LVEF cutpoint, and to the younger Olmsted county cohort.44 Importantly, however, HFpEF accounts for the majority of HF among elderly persons in the community,10 and the majority of these have neither LVH nor LV enlargement.11 Diastolic dysfunction is important in the pathophysiology of HFpEF, and echocardiographic measures of diastolic function including TDI e′, E/e′ ratio, and LAVi, have been associated with a heightened risk for incident HF.47,48,49 More recently, subtle abnormalities of LV systolic strain despite preserved LVEF have also been associated with greater risk of mortality and incident HF in the community.50,51 Consistent with these data, in our study both abnormal diastolic function and systolic function – based on LVEF and LS – were predictive of incident HF hospitalization or death independent of LV structural abnormalities and of each other. An isolated abnormality of any one of these was associated with a similar risk. Furthermore, among Stage A and B participants at risk for clinical HF, LS and diastolic measures provided incremental prognostic value beyond conventional measures of LV structure and LVEF. Incorporating abnormalities in these novel imaging-based measures of HF risk into the definition of Stage B HF resulted in 14% of the ARIC sample being reclassified as Stage B. Reclassified participants demonstrated levels of hs-TnT and NT-proBNP, prognostic biomarkers of incident HF,52 significantly higher than non-reclassified Stage A participants. In addition, beyond LV structure and LVEF, consideration of diastolic measures and LS appreciably increased the prevalence of an identifiable cardiac abnormality in Stage C1 (from 57% to 75%) and C2 (from 75% to 91%) HF. Together, these findings argue for the incorporation of these novel imaging measures of HF risk into the ACC/AHA HF staging system and definition of Stage B HF.
Among stage B participants, the overlap between diastolic dysfunction, systolic dysfunction, and LV structural abnormalities was modest, with isolated diastolic dysfunction in 25%, isolated systolic dysfunction in 12%, and abnormal structure in the absence of abnormal function in 26% (Figure 4A). This pattern is in marked contrast to that observed in patients with established HFpEF, in whom the large majority demonstrate abnormalities in at least 2 – and often 3 – of these domains.53 Furthermore, in our study, the risk of incident HF hospitalization or death increased in a graded fashion with greater number of abnormal domains (structure, systolic, diastolic; Figure 4B). While only cross-sectional echocardiographic data are available, these findings suggest that the development of clinical HF is characterized by the progressive accumulation of abnormalities in multiple domains – LV structure, systolic function, and diastolic function – occurring largely despite preserved LVEF. The high prevalence of abnormal diastolic function and LS in Stage C1 and C2 participants in our study further supports this hypothesis. This also suggests that regular assessment of diastolic indices and LS, in addition to conventional measures of LV structure and LVEF, can identify elderly persons at heightened risk for progression to symptomatic (Stage C) HF, with those demonstrating abnormalities in more than one domain of LV performance at the highest risk. Improvements in cardiovascular health factors and behaviors from mid- to late-life have been associated with better measures of diastolic function and LS in late life.40 In addition, diastolic measures and LS appear modifiable with pharmacotherapy.53,54,55 Therefore, elderly persons with abnormalities in one or more domains of LV performance may represent an optimal population in which to study lifestyle and pharmacologic interventions to prevent the development of clinical HF.
Clinical HF (Stage C) was prevalent in 13% of our study population, considerably higher than that in the Portuguese sample but similar to the prevalence reported in the Olmsted County study.19,20 Direct comparisons between studies are difficult due to differences in HF ascertainment and definition. Of note, when considering participants with evidence of more advanced – and definitive – HF (Stage C2), the prevalence in our study was considerably higher than the younger Olmsted County sample.44 The large majority of participants with symptomatic HF (Stage C) had a preserved LVEF. The low prevalence of abnormal LVEF among both Stage C1 and C2 participants (23% and 35%, respectively) and the rarity of an LVEF <50% (9% and 20% respectively) is in marked contrast to findings from the younger Olmsted population sample in whom the prevalence of an LVEF <50% among Stage C2 participants was 52%.44 However, the Cardiovascular Health Study (aged 66–103 year), which studied a population of similar age to ARIC at Visit 5, found that 80% of HF cases had an LVEF >45%, similar to our findings.10 The low prevalence of reduced LVEF among Stage C participants in our cohort suggests that alterations in myocardial function not captured by LVEF may have relatively greater contributions to HF risk and pathogenesis in the elderly, in particular abnormal diastolic function and LS. Survivor bias may also contribute, as mortality rates in HFrEF appear higher than for HFpEF.56,57 Ascertainment bias due to differential Visit 5 attendance (lower for those with HF with reduced LVEF than HF with preserved LVEF) is also a possibility that cannot be addressed from our data. However, participants alive at start of Visit 5 with a hospitalization ICD9 HF code were only modestly less likely to attend Visit 5 (prevalence 15% among non-attendees versus 13% among attendees, p=0.02), arguing against a large impact of ascertainment bias. Additionally, our sensitivity analysis using inverse probability attrition weighting did not result in appreciable changes in prevalence estimates (Supplemental Table 5).
Women had a lower prevalence of Stage C HF compared to men. Among participants with Stages C1 and C2 HF, women demonstrated a significantly lower prevalence of abnormal LVEF regardless of the cutpoint used. These findings are concordant with findings from the Cardiovascular Health Study, which found that HFpEF accounted for a significantly higher proportion of HF cases in women (67%) compared to men (42%).10 These sex-based differences in Stage C HF were mirrored in Stage B, where the prevalence of systolic dysfunction in women was less than half that in men. Compared to white participants, black participants had a higher prevalence of Stage C HF, while no race-based differences in the prevalence of abnormal LVEF or LVH were noted in Stages C1 and C2. Similarly, no prominent differences in the prevalence of Stage B HF by race were observed.
This analysis has several limitations. While reference limits for LS in our study are similar to those from the Framingham Heart Study and a prior large meta-analysis,58 LS values may vary based on measurement platform, and therefore the limits applied in this analysis may not be generalizable to LS values measured using other strain measurement platforms. Selection bias due to visit non-attendance may influence our estimates of the prevalence of HF stages, as 62% of ARIC participants who were alive at the start of Visit 5 attended the visit. However, a sensitivity analysis using inverse probability attrition weighting (Supplemental Tables 5–7) demonstrated consistent findings with the primary analysis, suggesting that the influence of such bias on our findings may be small. We were unable to fully quantify the prevalence of Stage D HF, as data on HF symptom severity were not available. However, only one participant was receiving advanced HF therapy (LVAD). The clinical diagnosis of HF among many participants with Stage C1 HF is less certain than Stage C2 participants, as in many Stage C1 participants the classification was based on serial self-report. However, the incidence of death or HF hospitalization in Stage C1 participants was higher than Stage B participants and similar to that observed in HF patients without prior HF hospitalization enrolled in HFpEF clinical trials.59,60 The use of an objective physiologic biomarker (NT-proBNP) and/or requirement for at least 1 serial HF report should also improve the specificity. Additionally, for Stage C2 participants, those identified solely from hospitalizations prior to 2005 were based on ICD code and not adjudicated. However, ICD-based ascertainment demonstrates an acceptable positive predictive value for HF when compared to adjudication in the ARIC study.22 Nonetheless, misclassification of HF cases is a potential limitation.
Conclusions
In this large community-based sample of older adults, HF risk factors are present in the vast majority of elderly persons in the community (82%), significantly higher than estimates from younger samples, with a spectrum of risk factor burden and alterations in cardiac structure and function among Stage A and B participants. Abnormalities of diastolic function and LS identify participants at particularly heightened risk for incident HF hospitalization or death, and potentially should be considered in the HF staging system. At least two-thirds of older adults with clinical HF (Stage C) have a robustly preserved LVEF, but demonstrate a high prevalence of diastolic dysfunction and abnormal LS. These findings help define the scope of the HF epidemic in the elderly, particularly the burden of HFpEF, and highlight the importance of primordial and primary prevention strategies to prevent the development of HF Stages A and B.
Supplementary Material
Clinical Perspective.
What is new?
In an elderly community-based cohort, 82% are AHA/ACC heart failure (HF) stage A or B (i.e. have risk factors for clinical HF). Worse HF stage is associated with greater risk of incident HF hospitalization or death in a graded fashion.
Abnormal LV structure, systolic (LVEF, longitudinal strain), and diastolic function are each independently and additively associated with incident HF or death. Longitudinal strain and diastolic dysfunction provide incremental prognostic value beyond LV structure and LVEF.
LVEF is preserved in at least two-thirds of older adults with clinical HF, in whom prevalence of diastolic dysfunction and abnormal longitudinal strain is high.
What are the clinical implications?
Our findings suggest that the development of clinical HF is characterized by the progressive accumulation of abnormalities in multiple domains – LV structure, systolic function, and diastolic function – occurring largely despite preserved LVEF.
Regular assessment of diastolic indices and longitudinal strain, in addition to conventional measures of LV structure and LVEF, can identify elderly persons at heightened risk for progression to symptomatic HF. Elderly persons with abnormalities in ≥1 domain of LV performance may represent an optimal population in whom to test interventions to prevent the development of clinical HF.
Acknowledgments
The authors wish to thank the staff and participants of the ARIC study for their important contributions. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding Source: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The work for this manuscript was also supported by NHLBI grant K08HL116792 (A.M.S.) and AHA grant 14CRP20380422 (A.M.S.).
Footnotes
Disclosures: Dr Shah reports receiving research support from Novartis, Gilead, Actelion, and Myocardia.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 3.Herz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 4.Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997–2003. Am J Prev Med. 2006;30:371–7. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association’s Life’s Simple 7: Avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–6. doi: 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moyé LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 9.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients ≥65 years of age. Am J Cardiol. 2001;87:413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 11.Lam CSP, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function in Heart Failure with Preserved Ejection Fraction: Baseline Findings from the Echocardiographic Study of the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function and Prognosis in Heart Failure With Preserved Ejection Fraction: Findings From the Echocardiographic Study of the Treatment Of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–51. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 16.White A, Folsom A, Chambless L, Sharret R, Yang K, Conwill D, Higgins M, Dale Williams O, Tyroler HA the ARIC Investigators. ScienceDirect - Journal of Clinical Epidemiology : Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: Methods and initial two years’ experience. Journal of Clinical Epidemiology. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 17.2011 Writing Group Members; 2005 Writing Committee Members; ACCF/AHA Task Force Members. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124:2020–45. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and Design of a Multicenter Echocardiographic Study to Assess the Relationship between Cardiac Structure and Function and Heart Failure Risk in a Biracial Cohort of Community Dwelling Elderly Persons: The Atherosclerosis Risk in Communities (ARIC) Study. Circ Cardiovasc Img. 2014;7:173–81. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part ii (maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman DW, Sheikh KH, Beere PA, Philips JL, Higginbotham MB. Age-related alterations of doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol. 1991;18:1243–1250. doi: 10.1016/0735-1097(91)90542-h. [DOI] [PubMed] [Google Scholar]
- 24.Gardin JM, Arnold AM, Bild DE, Smith VE, Lima JA, Klopfenstein HS, Kitzman DW. Left ventricular diastolic filling in the elderly: The cardiovascular health study. Am J Cardiol. 1998;82:345–351. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- 25.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: The cardiovascular health study. Circ Cardiovasc Imaging. 2009;2:282–289. doi: 10.1161/CIRCIMAGING.108.826602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 26.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: Clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: Longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klain AL, Lancellotti P, Marino P, Oh JK, Popescu A, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Amundsen BH, Helle-Velle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 32.Pirat B, Khoury DS, Hartley CJ, et al. A novel feature-tracking echocardiographic method for the quantification of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol. 2008;51:651–9. doi: 10.1016/j.jacc.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De La Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging. 2014;15:680–90. doi: 10.1093/ehjci/jet284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, Gonzalez A, Herregods MC, Fagard RH, Diez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–12. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 35.De Sutter J, De Backer J, de Veire NV, Velghe A, De Buyzere M, Gillebert TC. Effects of age, gender, and left ventricular mass on septal mitral annulus velocity (E′) and the ratio of transmitral early peak velocity to E′ (E/E′) Am J Cardiol. 2005;95:1020–3. doi: 10.1016/j.amjcard.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3:6114–622. doi: 10.1161/CIRCIMAGING.109.926022. [DOI] [PubMed] [Google Scholar]
- 37.Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Oliva MJ, Hagendorff A, Hristova K, Lopez T, Magne J, Martinez C, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Bernard A, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging. 2015;16:1031–41. doi: 10.1093/ehjci/jev083. [DOI] [PubMed] [Google Scholar]
- 38.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr. 2010;11:51–6. doi: 10.1093/ejechocard/jep164. [DOI] [PubMed] [Google Scholar]
- 39.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: A comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health duing adult life and cardiovasculare structure and function among the elderly. Circulation. 2015;132:1979–89. doi: 10.1161/CIRCULATIONAHA.115.017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, Mendes de Leon CF. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–0128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, Heiss G, Knopman DS, Patel MD, Penman AD, Power MC, Seines OA, Schneider AL, Wagenknecht LE, Windham BG, Wruck LM, Mosley TH. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stage: Application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 45.Azevedo A, Bettencourt P, Dias P, Abreu-Lima C, Hense H-W, Barros H. Population based study on the prevalence of the stages of heart failure. Heart. 2006;92:1161–3. doi: 10.1136/hrt.2005.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 47.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 48.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–63. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) Am J Cardiol. 2006;97:83–9. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 50.Choi EY, Rosen BD, Fernandes VRS, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida ALC, Wu CO, Gomes AS, Bluemke DA, Lima JAC. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–61. doi: 10.1093/eurheartj/eht133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo C, Jin Z, Elkind MSV, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–1309. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. The prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–14. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 55.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The ALDO-DHF randomized controlled trial. JAMA. 2013;209:781–91. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 56.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJ. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–56. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 57.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 58.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJV, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 60.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.