Abstract
Background
Scalp sensation and pain comprise the most common side effect of transcranial magnetic stimulation (TMS), which can reduce tolerability and complicate experimental blinding.
Objective
We explored whether changing the width of single TMS pulses affects the quality and tolerability of the resultant somatic sensation.
Methods
Using a controllable pulse parameter TMS device with a figure-8 coil, single monophasic magnetic pulses inducing electric field with initial phase width of 30, 60, and 120 µs were delivered in 23 healthy volunteers. Resting motor threshold of the right first dorsal interosseus was determined for each pulse width, as reported previously. Subsequently, pulses were delivered over the left dorsolateral prefrontal cortex at each of the three pulse widths at two amplitudes (100% and 120% of the pulse-width-specific motor threshold), with 20 repetitions per condition delivered in random order. After each pulse, subjects rated 0-to-10 visual analog scales for Discomfort, Sharpness, and Strength of the sensation.
Results
Briefer TMS pulses with amplitude normalized to the motor threshold were perceived as slightly more uncomfortable than longer pulses (with an average 0.89 points increase on the Discomfort scale for pulse width of 30 µs compared to 120 µs). The sensation of the briefer pulses was felt to be substantially sharper (2.95 point increase for 30 µs compared to 120 µs pulse width), but not stronger than longer pulses. As expected, higher amplitude pulses increased the perceived discomfort and strength, and, to a lesser degree the perceived sharpness.
Conclusions
Our findings contradict a previously published hypothesis that briefer TMS pulses are more tolerable. We discovered that the opposite is true, which merits further study as a means of enhancing tolerability in the context of repetitive TMS.
Keywords: Transcranial magnetic stimulation, cTMS, Pulse width, Sensation, Scalp, Tolerability
Introduction
Transcranial magnetic stimulation (TMS) and repetitive TMS are increasingly used as a safe and noninvasive tool to modulate brain function for research and therapeutic purposes. A significant drawback of TMS is, however, the unpleasant and potentially painful sensation experienced during pulse delivery [1–4]. Since the induced electric field drops off with distance from the TMS coil, the field in the tissue underlying the coil is stronger than at the cortical target. We have estimated that the electric field in the scalp is approximately twice as strong as in the underlying cortex for a conventional 70 mm figure-8 coil [5]. There are a number of possible causes of the somatic sensation from TMS at any specific scalp location. The trigeminal nerve is likely stimulated for anterior targets [2,6]. The electric field may activate nociceptors in the scalp, periosteum, and perhaps meninges directly underneath the coil [1]. Nociceptor Aδ fibers, which typically produce pain that is sharp, pricking, and temporally linked with the stimulus, are more likely to be recruited due to their shorter time constant and lower rheobase compared to C fibers, which tend to produce slow, dull, burning pain [6–8]. Similarly, sensory A-fibers related to mechanoreception, thermal reception, and muscle proprioception may be directly activated [1,6–8]. Further, sensation caused by directly induced muscle contraction can be relevant, particularly away from the vertex [2,8]. Another possible source of sensation is the mechanical vibration (tapping) generated by the electromagnetic forces within the coil, which can activate mechanoreceptors in the scalp [1,9]. Finally, the synchronous auditory stimulation via both bone and air conduction (partially attenuated by earplugs) may modulate the sensation [10]. Any combination of these factors can affect tolerability, and in addition, the sensation of TMS complicates the blinding of subjects to experimental conditions and requires sophisticated sham procedures to replicate the sensation [11,12].
Various approaches to reducing the scalp pain from TMS have been investigated or proposed. Topical anesthetics may reduce rTMS related scalp pain in some subjects, but the robustness of the effect and optimal application need further study [1,3]. In a small sample of healthy subjects, scalp injection of lidocaine or lidocaine and epinephrine reduced scalp pain and was more tolerable than the rTMS pain, although the lidocaine and epinephrine injection may result in subsequent hypersensitivity [1]. As well, introducing a thin foam pad between the coil and the scalp may slightly reduce scalp pain, [1] but it is not clear whether this effect is significant and whether it is due to dampening of the mechanical vibration produced by the coil or merely to reduction of the electric field strength in the scalp due to the extra spacing between the coil and the scalp introduced by the pad. At present, none of these methods have found widespread use.
Device design approaches to mitigate TMS induced scalp pain include injecting current through superficial electrodes to counter the TMS induced currents in the scalp, a small secondary surface coil suppressing the surface field, or increasing the size of the TMS coil [13]. Injecting current through scalp electrodes is impractical and may only shift spatially, but not reduce, the field maximum [13]. A secondary surface coil suppressing the surface field is available commercially [14] but it only reduces the peak electric field in the scalp by less than 13% [13]. Finally, increasing the coil size can substantially reduce the scalp field strength, but reduces the focality of the coil resulting in potentially wider spread suprathreshold stimulation both in the scalp and in the brain [13,15].
The device-based approaches described above aim to reshape the electric field spatial distribution to reduce the scalp sensation. Another potential device-based venue is to alter the pulse waveform characteristics so that sensation is modified while cortical effects are preserved. Specifically, the pulse width may affect the relative degree to which various neuronal types are recruited. For example, in peripheral nerves, the motor threshold is lower than the sensory threshold for brief pulses, whereas it is higher than the sensory threshold for longer stimuli [16]. It has been hypothesized that the ratio of cortical motor threshold to scalp sensory threshold may also be lower for brief pulses than for long stimuli, potentially leading to better tolerability of the former [17]. This hypothesis was supported by a simulation study of transcranial electrical stimulation that modeled the activation thresholds for motor cortex pyramidal axons and scalp Aδ nociceptor fibers [18]. Furthermore, briefer pulses decrease the coil energy [19,20] and the coil acoustic output, reducing the loudness [21] and possibly the mechanical tapping as well.
This question of whether pulse width affects discomfort from stimulation is relevant because there are differences in the pulse width across commercial TMS devices and there are now devices that allow adjustment of the pulse width. For example, among the FDA-approved TMS devices for the treatment of depression, there is a twofold range of pulse widths (185 to 370 µs biphasic pulse period) [22–25]. As well, some commercial TMS devices allow adjustment, albeit limited, of the pulse width [26,27]. Finally, we have developed a family of TMS devices with controllable pulse parameters (cTMS) that allow adjustment of the pulse width over a substantial range, potentially allowing optimization of this parameter [28–30].
In this study we used a cTMS device to explore the effect of pulse width and pulse amplitude on the sensation reported by subjects receiving single TMS pulses over the dorsolateral prefrontal cortex.
Material and methods
This study was part of a larger study that also characterized the corticospinal tract response to TMS with various pulse widths [20]. The general subject and methods information is provided in [20] and summarized below in addition to specific information about the TMS sensation investigation.
Subjects
This study was conducted at New York State Psychiatric Institute / Columbia University where it was approved by the Institutional Review Board. After consenting and screening [20], 23 healthy subjects took part in the TMS sensation study (age range = 19–49 years, mean ± SD = 28 ± 6.6 years; 16 female).
Experimental session
The study comprised a single TMS session. The subjects were seated in a chair, and their heads were supported by a head rest and stabilized between the TMS coil and a padded bracket countering the coil pressure. The subjects wore earplugs for hearing protection. The TMS session consisted of motor threshold determination and IO curve measurement reported previously [20], followed by single-pulse stimulation of the dorsolateral prefrontal cortex reported here, always administered in that order. To evaluate potential side effects, before and after the TMS session subjects were given a side effects checklist and a computerized five-item visual analog scale characterizing mood.
Transcranial magnetic stimulation
This study used a custom built cTMS device that generates monophasic magnetic pulses with independent control of the amplitude and width of the initial phase of the induced electric field (see Fig. 1) [20,28]. The cTMS device was connected to a commercial 70 mm figure-8 coil (P/N 9925-00, Magstim Co., Spring Gardens, Whitland, Carmarthenshire, UK). The device control and interface with the subject were implemented with an NI PCI-7831R control/acquisition board and custom code in LabVIEW (National Instruments, Austin, TX, USA) and MATLAB (The MathWorks, Inc., Natick, MA, USA). The coil position was maintained throughout the TMS session with the aid of a Brainsight computerized frameless stereotaxic system (Rogue Research Inc., Montreal, Canada).
Figure 1.
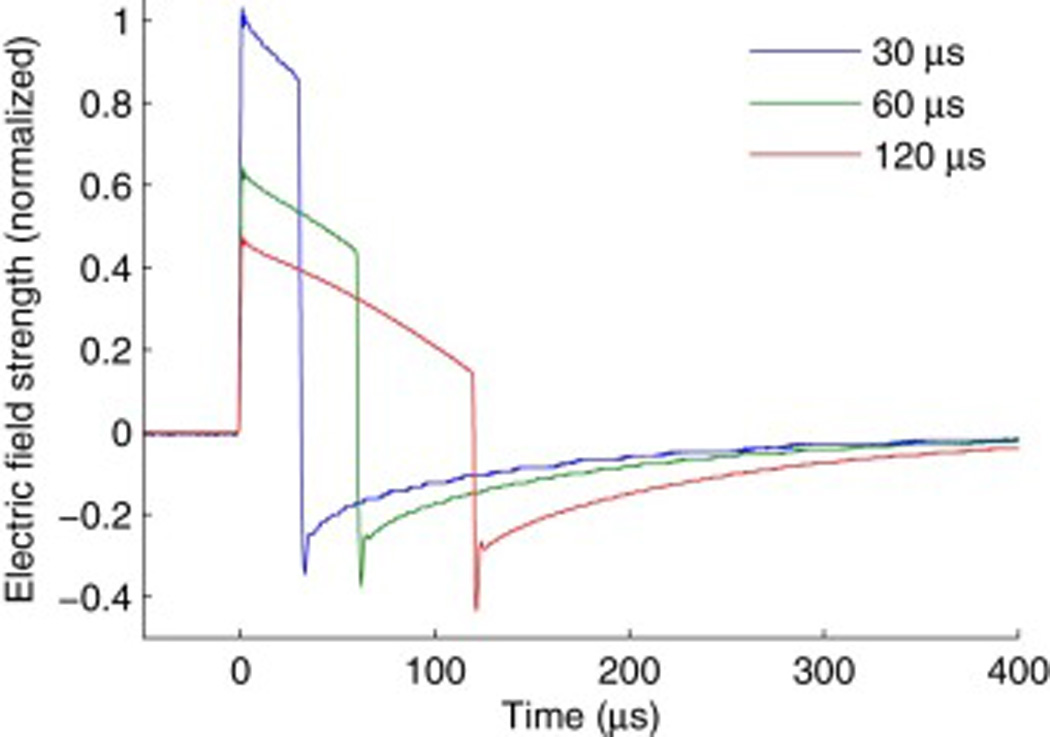
TMS electric field waveforms for pulse widths of 30, 60, and 120 µs. The waveforms were measured with a search coil placed under the TMS coil [20,28]. The pulse amplitude was scaled by the average motor threshold for the respective pulse width in order to illustrate the relative pulse amplitude delivered in the three pulse width conditions.
We explored three pulse widths, 30, 60, and 120 µs. The resting motor threshold of the right first dorsal interosseous muscle was determined for each of these pulse widths [20]. Motor threshold was defined as the minimum pulse amplitude needed to evoke motor potentials of at least 50 µV peak-to-peak recorded via electromyography from the first dorsal interosseus muscle of the participant’s right hand in at least 5 out of 10 stimulations [31]. Fig. 1 shows the electric field waveforms for the three pulse widths with amplitudes scaled proportionally to the corresponding average motor thresholds. The initial phase of the induced electric field pulse (positive phase in Fig. 1) has posterior–anterior direction in cortex.
Subsequently, to study the TMS evoked sensation, single pulses were delivered to the left dorsolateral prefrontal cortex, as approximated by locating the F3 site of the 10–20 EEG system for each individual, with the coil tangent to the scalp and rotated 45° from the sagittal plane. For each pulse width, there were two pulse amplitude conditions: Low (100% motor threshold) and High (120% motor threshold). The left dorsolateral prefrontal cortex site and the pulse amplitude of 120% motor threshold were chosen since they comprise the FDA-approved TMS paradigm for depression [3,22]. The 100% motor threshold condition enabled characterization of the effect of pulse amplitude and represented the practice in clinical TMS to reduce or ramp up the pulse amplitude for patients who cannot tolerate the sensation [2,3]. To corroborate visually observed facial twitching during TMS, we monitored electromyography of the frontalis muscle in the forehead for seven of the subjects.
Sensation rating
In the sensation rating component of the study, the subjects were first allowed to experience, without rating, stimulation of the dorsolateral prefrontal cortex with increasing intensity corresponding to 80%, 100%, and 120% of motor threshold for each of the 3 pulse widths in increasing order. The subjects were shown the visual analog scales and were instructed that they will be given the whole range and they should think about rating the pulses using the whole scale. This “calibration” run was repeated twice. Then the subjects were asked to practice rating sample pulses at intensities and pulse widths chosen pseudo-randomly from the steps listed above. This practice run continued until the subjects were confident of their ratings and the experimenter determined that they were using the full range of each rating scale. This practice session was intended to diminish novelty effects and allow subjects to establish a reference scale for the sensation.
The subsequent experimental block involved six stimulation conditions corresponding to the three pulse widths (30, 60, and 120 µs) and two pulse amplitudes (Low and High, corresponding to 100% and 120% of motor threshold, respectively). The 80% motor threshold intensity was used only in the practice runs, but not in the experimental block. The pulses were delivered in pseudo-random order and the total number of repetitions per condition was constrained to 20.
The subjects rated the sensation from each pulse on visual analog scales that were displayed on a computer monitor. Three scales were presented—Discomfort, Sharpness, and Strength—that had levels from 0 to 10 with the extremes labeled respectively as “Least Uncomfortable → Most Uncomfortable”, “Dull → Sharp”, and “Weak → Strong”. The subjects selected their responses immediately following each stimulus, and the subsequent pulse was delivered after a random delay between 5 and 7 seconds. Ten subjects rated Discomfort in one block of pulses and Sharpness and Strength concurrently in a second block, with the order of the two blocks randomized. The remaining 13 subjects rated Discomfort and Sharpness concurrently in a single block, and did not rate Strength. Subjects were not asked to describe their sensations beyond these three dimensions: the study was not designed to differentiate potential sources of the sensation, such as induced electrical versus mechanical stimulation of the scalp, since the intention of this initial investigation was to quantify the holistic experience of TMS.
Six subjects had motor thresholds for one or more pulse widths that would have caused the High amplitude condition to be above the limit of the cTMS device. In these cases the pulse amplitudes for all conditions were scaled proportionately so that the highest amplitude did not exceed the device maximum. Thus, this adjustment did not affect the relative strength of the pulses across the conditions. In two of these subjects, however, the scaling procedure was applied incorrectly, and only some of the brief pulse width conditions were adjusted. Nevertheless, the data from these two subjects were included in the analysis, as the pulse amplitude was consistently larger for the High versus Low conditions and decreased monotonically with pulse width, maintaining qualitatively the intended relationships among the conditions. Furthermore, the actual absolute stimulation amplitude within each condition was included in the statistical analysis, accounting for these exceptions.
Statistical analysis
The visual analog scale ratings were averaged across the 20 repetitions for each condition within subject and these averages were analyzed with mixed effects models. Subject was treated as a random effect, enabling the inclusion of the Strength scale from a subgroup of subjects in the overall analysis. To account for the effect of absolute TMS pulse amplitude beyond the contributions of pulse width and amplitude relative to motor threshold, a z-score of the individual absolute pulse amplitude within each of the six experimental conditions was included as a factor in the analysis. Tukey HSD test and Student’s t-test were applied for pairwise comparisons. The statistical analyses were carried out in JMP 12 (SAS Institute Inc., Cary, NC, USA) and the data were plotted with MATLAB (The MathWorks Inc., Natick, MA, USA).
Results
Overall tolerability of procedure
Single pulse cTMS over the left dorsolateral prefrontal cortex was tolerated by all 23 subjects. As expected, stimulation of this site elicited visible twitching of facial muscles in all subjects [1,3]. This observation was confirmed by electromyography of the frontalis muscle in seven of the subjects.
Effect of pulse amplitude and width on sensation ratings
The ratings on the three visual analog scales versus pulse width and amplitude relative to motor threshold are summarized in Fig. 2. An omnibus mixed effects analysis of the ratings data incorporated random effect of Subject and fixed effects of Scale (Discomfort, Sharpness, Strength), Pulse Width (30, 60, 120 µs), Pulse Amplitude relative to motor threshold (Low, High), and Absolute Pulse Amplitude within condition (z-score). Scale was a significant effect (F2,280 = 3.39, p = 0.0352), although the mean ratings for each scale were close to the middle of the 10 point range: 5.00, 5.18, and 4.74 for Discomfort, Sharpness, and Strength, respectively. Overall, subjects increased their ratings with higher pulse amplitude both relative to motor threshold (F1,271= 496, p < 0.0001) as well as in absolute terms within condition (F1,35.7= 15.7, p = 0.0003), and decreased their ratings with increasing pulse width (F2,271= 33.1, p < 0.0001). The ratings increased with absolute pulse amplitude more strongly for Low than High relative amplitude (F2,271= 5.25, p = 0.0228).
Figure 2.
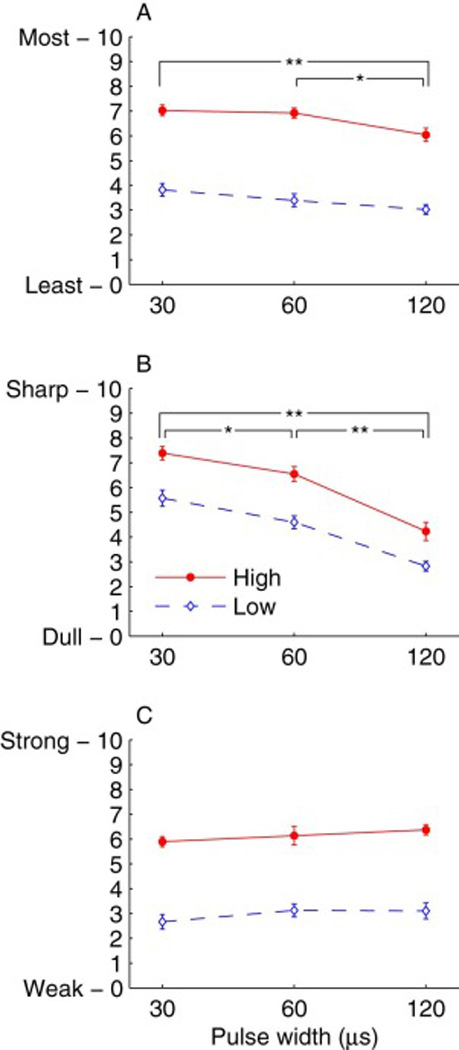
Visual analog scale ratings of Discomfort (A), Sharpness (B), and Strength (C) for single-pulse TMS of left dorsolateral prefrontal cortex with pulse widths of 30, 60, and 120 µs and Low and High pulse amplitudes (nominally 100% and 120% of motor threshold, respectively). Markers and error bars correspond to mean and standard error across subjects, respectively. Discomfort and Sharpness were rated by all 23 subjects, and Strength was rated by 10 of these subjects. Significant differences between the pulse width conditions are indicated by ** (p < 0.0001) or * (p < 0.01). Effect of pulse amplitude is significant (p < 0.0001) for all scales (not marked in plots).
There were, however, significant differences of how the ratings for each scale were affected by relative and absolute pulse amplitude (F2,271= 21.4, p < 0.0001; F2,276= 6.60, p = 0.0016) as well as by pulse width (F4,271= 22.6, p < 0.0001). These relationships were further explored with separate mixed effects models for each scale. While for all scales ratings increased significantly with pulse amplitude relative to motor threshold (p’s < 0.0001), the mean rating difference between High and Low amplitude was 3.25 and 3.17 points for Discomfort and Strength, respectively, but only 1.72 points for Sharpness. On the other hand, pulse width had the strongest effect on Sharpness (F2,103= 68.7, p < 0.0001). All three pulse widths were perceived to have distinct relative sharpness (p’s < 0.002), with the 30 µs and 120 µs pulses being the most and least sharp, respectively, differing on average by 2.95 points (see Fig. 2B). The effect of pulse width on Discomfort followed the same trend but was much weaker (F2,101= 12.1, p < 0.0001; see Fig. 2A). The discomfort from the 30 µs and 60 µs pulses was indistinguishable (p = 0.325), but both were perceived as less comfortable than the 120 µs pulses (p’s < 0.004). The differences in absolute terms were small, however, with the average rating of the 30 µs pulses higher than the 120 µs pulses by less than one point (0.892). Finally, longer pulses were perceived on average as slightly stronger that shorter pulses (0.435 point increase for 120 µs compared to 30 µs pulse width), but this effect was not significant (F2,39= 2.33, p = 0.111; see Fig. 2C).
The effect of the individual variation in absolute TMS pulse amplitude, which is proportional to the individual motor threshold, is illustrated in Fig. 3. The group average and standard deviation of the motor thresholds for the 30, 60, and 120 µs pulse widths were respectively 75.3 ± 9.9, 46.9 ± 6.2, and 34.7 ± 5.2 as percentage of the device maximum amplitude. Larger absolute pulse amplitude was significantly correlated with higher Discomfort (F1,26.8= 19.1, p = 0.0002), beyond the contribution of pulse amplitude relative to motor threshold described above. Over the range of individual variability in TMS amplitude, this effect spanned nearly 2 points on the Discomfort scale. There was no main effect of absolute pulse amplitude on Sharpness and Strength (p’s > 0.2). For Strength, however, there was a significant interaction of absolute pulse amplitude with amplitude relative to motor threshold (F1,38.8= 11.5, p = 0.0016). Specifically, Strength and absolute pulse amplitude were significantly positively correlated in the Low but not High amplitude condition (see Fig. 3).
Figure 3.
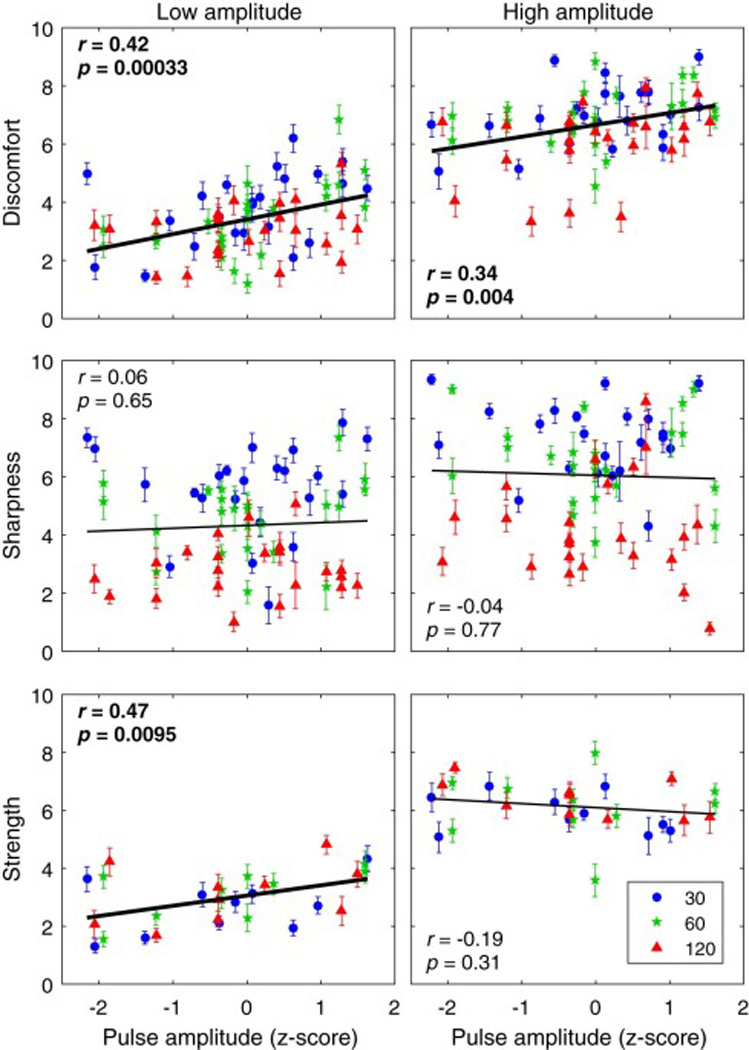
Individual visual analog scale ratings versus individual absolute TMS pulse amplitude. Columns correspond to Low (left) and High (right) pulse amplitude conditions (nominally 100% and 120% of motor threshold, respectively). Markers and error bars correspond to individual means and standard errors, respectively. Different marker shapes and colors correspond to the three pulse width conditions indicated in the legend in units of µs. The x-axis is individual TMS amplitude given as z-score within each of the six experimental conditions. Linear regression lines and corresponding correlation coefficients are given for the data within each plot, with significant results in bold.
Relationship of sensation ratings with post-TMS side effects
In the side effects questionnaire administered after the TMS session, two subjects reported scalp pain and two others reported headache probably or possibly resulting from TMS. While the occurrence of these side effects was uncommon, precluding more sophisticated analysis, we compared with t-tests the average ratings on the Discomfort and Sharpness scales between the subjects who reported these side effects and those who did not. There was no significant difference for either the Discomfort (t5.64 = 0.0626, p = 0.952) or Sharpness (t3.61 = 0.735, p = 0.507) scales, indicating, on an exploratory basis, that the visual analog scales administered during the pulse administration did not predict the occurrence of side effects potentially related to the TMS sensation.
Discussion
In this study we used a cTMS device to explore the sensation experienced by subjects receiving single pulse TMS over the dorsolateral prefrontal cortex. This was the first application of cTMS over a non-motor cortical area. The study indicated that pulse width modulates the sensation of TMS even when the pulse amplitude is scaled with the individual motor threshold for each pulse width, presumably normalizing the cortical effect of the stimulus. Specifically, briefer pulses produced, on average, a significantly sharper and more uncomfortable sensation than longer pulses. However, while the effect on sharpness was pronounced—on average nearly 3 out of 10 points maximum—the difference in discomfort was small—less than 1 point over the range of tested pulse widths.
On the other hand, there was no significant effect of pulse width on the perceived strength of the pulses. As well, an exploratory analysis found no evidence that the Discomfort or Sharpness ratings predicted whether the subject reported side effects such as headache or scalp pain related to the TMS session.
The three visual analog scales—Discomfort, Sharpness, and Strength—appear to capture distinct albeit overlapping aspects of the TMS pulse sensation. Given the drops in ratings with increasing pulse width seen in the Sharpness and Discomfort results but not in Strength ratings, and the observation that Discomfort and Strength were substantially more affected by the pulse amplitude than Sharpness, it could be speculated that Discomfort was driven by a combination of the perceived sharpness and strength of the pulse.
Discomfort ratings increased not only with TMS amplitude relative to motor threshold but also with the individual absolute TMS amplitude. This finding is consistent with the fact that electric field exposure in the scalp is proportional to the absolute pulse amplitude. The results for absolute pulse amplitude should be interpreted with caution, however, since the instructions given to the study participants encouraged relative rather than absolute rating of the sensation.
The central finding that briefer pulses are less tolerable than longer pulses runs counter to hypothesis of the opposite effect based on longer strength–duration time constant of scalp nociceptors compared to cortical neurons [17,18]. Several considerations could help explain this discrepancy. Geddes assumed membrane time constants of 50 µs and 300 µs for motor cortex and scalp sensory receptors, respectively [17]. This sensory time constant is consistent with Aδ fibers [7], which matches the assumption of Suihko [18]. The assumed motor cortex time constant, however, is significantly lower than the 196 µs estimate derived with the TMS pulses used in this study [20]. Furthermore, fiber types with shorter membrane time constants than Aδ, such as Aα and Aβ, may be involved in the stimulation of the scalp including muscles [6,7]. Collectively these considerations argue for less clear separation between the membrane time constant for motor cortex and various relevant nerve fibers in the scalp than assumed by Geddes [17]. Indeed, the lack of effect of pulse width on the perceived strength of stimulation supports a similar time constant for this aspect of sensation as for motor cortex activation. Apparently, the perception of strength was not affected by the reduction of coil energy [20] and acoustic output [21] for briefer pulses, suggesting that these contributions may be insignificant. On the other hand, the different audible pitch of the pulses, which is higher for briefer widths [32], may have modulated the perception of stimulation, resulting in sharper sensation associated with briefer pulses. Alternatively or in conjunction, the sharper sensation may reflect the decreasing relative threshold of Aδ fibers compared to C fibers as the pulse width is reduced, due to larger membrane time constant of C fibers [7,8]. Finally, there is the possibility that the various pulse widths differentially affected the left dorsolateral prefrontal cortex target [33,34] and consequently produced different degrees of modulation of pain perception circuits in the brain [4,35,36].
This study, however, does not allow us to dissect mechanisms of the observed effects since it was not designed to differentiate the various contributions such as direct stimulation of nociceptor and receptor fibers, scalp muscle contraction, coil vibration, auditory perception, and direct cortical effects. In future studies, separating these contributions may allow mechanistic interpretations and inform the development of approaches to effectively reduce the sensation. This could be done by manipulating the influence of the various factors (e.g., by using mechanically damping foam spacers, applying topical anesthetics to the scalp, delivering masking noise with earphones, comparing with electrical scalp stimulation), rating additional dimensions of the sensation, as well as electrophysiological recordings from the scalp. Future studies may also explore even briefer pulses where the mechano-acoustic emission of the coil is reduced further [10,32] and it is unclear if the tendency for increased discomfort would continue.
A limitation of this study is that only the sensation associated with single pulses was investigated. The sensation resulting from repetitive TMS was not studied, but is more important in practice since repetitive TMS is generally less tolerable than single pulse TMS [1,2]. It is possible that the pulse characteristics such as pulse width affect the perception of TMS in different ways for single pulses and for pulse trains. In future studies, the pulse characteristics of repetitive TMS pulse trains could be modified using cTMS devices that allow high-frequency trains [29,30]. Moreover, this study did not explore the differences in sensation between conventional sinusoidal pulses and cTMS near-rectangular pulses. Among the investigated cTMS pulses, the one with pulse width of 60 µs is the closest approximation in terms of duration to conventional sinusoidal monophasic magnetic pulses [20,28]. The relationship of sensation and pulse width may also change for conventional biphasic magnetic pulses which induce a more symmetrically bidirectional electric field, potentially affecting differential neural recruitment [8]. Neither did we study the effect of coil design and placement, which may affect scalp and auditory sensation by virtue of different electric field spatial distribution in the scalp and different mechano-acoustic output. For example, more focal coils stimulate a smaller portion of the scalp but at a higher intensity than less focal coils [5,13,15]. Finally, stimulation over prefrontal cortex tends to be less tolerable than other common sites such as motor cortex [1,3].
Conclusions
Contrary to a previously published hypothesis, briefer TMS pulses with amplitude normalized to the respective motor threshold were perceived as slightly more uncomfortable than longer pulses. The sensation of the briefer pulses was further classified as substantially sharper but not stronger than longer pulses. These results demonstrate for the first time that pulse width affects the sensation of TMS and could potentially be used to reduce scalp discomfort. However, the significance of this finding for repetitive TMS protocols has to be determined in future studies.
Highlights.
Effect of pulse width on scalp sensation was studied with cTMS.
Briefer TMS pulses are slightly more uncomfortable than longer pulses.
Briefer TMS pulses are felt as substantially sharper than longer pulses.
TMS pulse width does not affect significantly perceived strength of stimulation.
Higher TMS pulse amplitude increases perceived discomfort, strength, and sharpness.
Acknowledgments
This study was supported by NY State Office of Science, Technology and Academic Research (NYSTAR) Faculty Development Award C040071. The cTMS device was developed with support from NIH R21EB006855 and Columbia Technology Ventures Seed Fund. The authors would like to thank Dr. Tor D. Wager and Dr. Stefan M. Goetz for the helpful discussions, Dr. L. Gregory Appelbaum for help with data processing, and Mr. David L. K. Murphy for technical assistance. Part of this work was presented in preliminary form as abstract at the Annual Meeting of the American College of Neuropsychopharmacology in 2008 (Peterchev AV, Luber B, Westin GG, Wager TD, Lisanby SH. First human study of controllable pulse shape transcranial magnetic stimulation (cTMS): Effect of pulse width on corticospinal response and scalp sensation).
A. V. Peterchev is inventor on patents and patent applications on TMS technology including cTMS; has received research and travel support as well as patent royalties from Rogue Research for cTMS technology, research and travel support as well as equipment loan from Tal Medical, patent application support from Magstim related to TMS technology, and TMS equipment loans from MagVenture. S. H. Lisanby has served as Principal Investigator or co-investigator on industry-sponsored research grants to Duke (Brainsway, NeoSync, Nexstim); equipment loans to Duke (Magstim, MagVenture); is co-inventor on a patent and patent applications on TMS technology; has been supported by grants from NIH (R01MH091083, U01MH084241), Stanley Medical Research Institute, US Air Force, and Brain and Behavior Foundation; and has no consultancies, speakers bureau memberships, board affiliations, or equity holdings in related industries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
B. Luber and G. G. Westin have no relevant disclosures.
References
- 1.Borckardt JJ, Smith AR, Hutcheson K, Johnson K, Nahas Z, Anderson B, et al. Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. J ECT. 2006;22:259–264. doi: 10.1097/01.yct.0000244248.40662.9a. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trevino K, McClintock SM, Husain MM. The use of topical lidocaine to reduce pain during repetitive transcranial magnetic stimulation for the treatment of depression. J ECT. 2011;27:44–47. doi: 10.1097/YCT.0b013e3181f5581c. [DOI] [PubMed] [Google Scholar]
- 4.Borckardt JJ, Nahas ZH, Teal J, Lisanby SH, McDonald WM, Avery D, et al. The Painfulness of Active, but not Sham, Transcranial Magnetic Stimulation Decreases Rapidly Over Time: Results From the Double-Blind Phase of the OPT-TMS Trial. Brain Stimul. 2013 doi: 10.1016/j.brs.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng ZD, Peterchev AV. Transcranial magnetic stimulation coil with electronically switchable active and sham modes. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1993–1996. doi: 10.1109/IEMBS.2011.6090561. [DOI] [PubMed] [Google Scholar]
- 6.Gardner EP, Martin JH, Jessell TM. Chapter 22: The bodily senses. In: Kandell ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th. New York: McGraw-Hill; 2000. [Google Scholar]
- 7.Li CL, Bak A. Excitability characteristics of the A- and C-fibers in a peripheral nerve. Exp Neurol. 1976;50:67–79. doi: 10.1016/0014-4886(76)90236-3. [DOI] [PubMed] [Google Scholar]
- 8.Reilly JP, Diamant AM. Electrostimulation: Theory, Applications, and Computational Model. Boston, MA: Artech; 2011. Chapter 6: Model application to C-fibers and the heart. [Google Scholar]
- 9.Sommer J, Jansen A, Drager B, Steinstrater B, Breitenstein C, Deppe M, et al. Transcranial magnetic stimulation - a sandwich coil design for a better sham. Clin Neurophysiol. 2006;117:440–446. doi: 10.1016/j.clinph.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Goetz SM, Lisanby SH, Murphy DL, Price RJ, O'Grady G, Peterchev AV. Impulse noise of transcranial magnetic stimulation: measurement, safety, and auditory neuromodulation. Brain Stimul. 2015;8:161–163. doi: 10.1016/j.brs.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, et al. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2007;118:709–716. doi: 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Arana AB, Borckardt JJ, Ricci R, Anderson B, Li X, Linder KJ, et al. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: Does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimulat. 2008;1:44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey KR, Riehl M. Suppressing the surface field during transcranial magnetic stimulation. IEEE Trans Biomed Eng. 2006;53:190–194. doi: 10.1109/TBME.2005.862545. [DOI] [PubMed] [Google Scholar]
- 14.Neuronetics Inc. NeuroStar TMS Therapy(R) System Components: SenStar(R) Treatment Link. [accessed 31.05.2016];2016 http://www.neuronetics.com/products-services/senstar-treatment-link/ [Google Scholar]
- 15.Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol. 2014;125:1202–1212. doi: 10.1016/j.clinph.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panizza M, Nilsson J, Roth BJ, Basser PJ, Hallett M. Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1992;85:22–29. doi: 10.1016/0168-5597(92)90097-u. [DOI] [PubMed] [Google Scholar]
- 17.Geddes LA. Optimal stimulus duration for extracranial cortical stimulation. Neurosurgery. 1987;20:94–99. doi: 10.1097/00006123-198701000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Suihko V. Modelling the response of scalp sensory receptors to transcranial electrical stimulation. Med Biol Eng Comput. 2002;40:395–401. doi: 10.1007/BF02345071. [DOI] [PubMed] [Google Scholar]
- 19.Barker AT, Garnham CW, Freeston IL. Magnetic nerve stimulation: the effect of waveform on efficiency, determination of neural membrane time constants and the measurement of stimulator output. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:227–237. [PubMed] [Google Scholar]
- 20.Peterchev AV, Goetz SM, Westin GG, Luber B, Lisanby SH. Pulse width dependence of motor threshold and input-output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2013;124:1364–1372. doi: 10.1016/j.clinph.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetz SM, Murphy DL, Peterchev AV. Transcranial magnetic stimulation device with reduced acoustic noise. IEEE Mag Lett. 2014;5:1500104. [Google Scholar]
- 22.U. S. Food and Drug Administration. NeuroStar TMS Therapy System: 510(k) Number K083538. [accessed 31.05.16];2008 http://www.accessdata.fda.gov/cdrh_docs/pdf8/K083538.pdf.
- 23.U. S. Food and Drug Administration. Brainsway Deep TMS System: 510(k) Number K122288. [accessed 31.05.16];2013 http://www.accessdata.fda.gov/cdrh_docs/pdf12/k122288.pdf.
- 24.U. S Food and Drug Administration. MagVita TMS Therapy System: 510(k) Number K150641. [accessed 31.05.16];2015 http://www.accessdata.fda.gov/cdrh_docs/pdf15/K150641.pdf.
- 25.U. S. Food and Drug Administration. Rapid Therapy System: 510(k) Number K143531. [accessed 31.05.16];2015 http://www.accessdata.fda.gov/cdrh_docs/pdf14/K143531.pdf.
- 26.MagVenture A/S. MagPro X100 with Option, Technical data. Farum, Denmark: MagVenture A/S; 2007. Oct, [Google Scholar]
- 27.Rothkegel H, Sommer M, Paulus W, Lang N. Impact of pulse duration in single pulse TMS. Clin Neurophysiol. 2010;121:1915–1921. doi: 10.1016/j.clinph.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Peterchev AV, Jalinous R, Lisanby SH. A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS) IEEE Trans Biomed Eng. 2008;55:257–266. doi: 10.1109/TBME.2007.900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterchev AV, Murphy DL, Lisanby SH. A repetitive transcranial magnetic stimulator with controllable pulse parameters. J Neural Eng. 2011;8(036016):13. doi: 10.1088/1741-2560/8/3/036016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterchev AV, D'Ostilio K, Rothwell JC, Murphy DL. Controllable pulse parameter transcranial magnetic stimulator with enhanced circuit topology and pulse shaping. J Neural Eng. 2014;11:056023. doi: 10.1088/1741-2560/11/5/056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 32.Peterchev AV, Murphy DL, Goetz SM. Quiet transcranial magnetic stimulation: Status and future directions. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:226–229. doi: 10.1109/EMBC.2015.7318341. [DOI] [PubMed] [Google Scholar]
- 33.Goetz SM, Luber B, Lisanby SH, Murphy DL, Kozyrkov IC, Grill WM, et al. Enhancement of Neuromodulation with Novel Pulse Shapes Generated by Controllable Pulse Parameter Transcranial Magnetic Stimulation. Brain Stimul. 2016;9:39–47. doi: 10.1016/j.brs.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Ostilio K, Goetz SM, Hannah R, Ciocca M, Chieffo R, Chen JC, et al. Effect of coil orientation on strength-duration time constant and I-wave activation with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2016;127:675–683. doi: 10.1016/j.clinph.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin L, Borckardt JJ, Reeves ST, Frohman H, Beam W, Nahas Z, et al. A pilot functional MRI study of the effects of prefrontal rTMS on pain perception. Pain Med. 2013;14:999–1009. doi: 10.1111/pme.12129. [DOI] [PubMed] [Google Scholar]
- 36.Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Nahas Z, et al. Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag. 2007;12:287–290. doi: 10.1155/2007/741897. [DOI] [PMC free article] [PubMed] [Google Scholar]


