Abstract
Background
Sherpas, a highlander population living in Khumbu region of Nepal, are well known for their superior climbing ability in Himalayas. However, the genetic basis of their adaptation to high‐altitude environments remains elusive.
Methods
We collected DNA samples of 582 Sherpas from Nepal and Tibetan Autonomous Region of China, and we measured their hemoglobin levels and degrees of blood oxygen saturation. We genotyped 29 EPAS1 SNPs, two EGLN1 SNPs and the TED polymorphism (3.4 kb deletion) in Sherpas. We also performed genetic association analysis among these sequence variants with phenotypic data.
Results
We found similar allele frequencies on the tested 32 variants of these genes in Sherpas and Tibetans. Sherpa individuals carrying the derived alleles of EPAS1 (rs113305133, rs116611511 and rs12467821), EGLN1 (rs186996510 and rs12097901) and TED have lower hemoglobin levels when compared with those wild‐type allele carriers. Most of the EPAS1 variants showing significant association with hemoglobin levels in Tibetans were replicated in Sherpas.
Conclusion
The shared sequence variants and hemoglobin trait between Sherpas and Tibetans indicate a shared genetic basis for high‐altitude adaptation, consistent with the proposal that Sherpas are in fact a recently derived population from Tibetans and they inherited adaptive variants for high‐altitude adaptation from their Tibetan ancestors.
Keywords: Genetic adaptation, high altitude, hypoxia, Sherpa, Tibetan
Introduction
Sherpas living in Khumbu region of Nepal are renowned for their superior capacity for climbing Himalayas. Most of the Sherpa people are involved in mountaineering field as climbers, porters, and trekking guides and have displayed extraordinary adaptive behavior at high altitude. Such distinctive traits seen in Sherpa staying permanently at high altitude are lower ventilatory response (Lahiri et al. 1967), larger spirometric values (Havryk et al. 2002), relatively lower hemoglobin concentrations (Adams and Shresta 1974; Beall and Reichsman 1984), higher arterial oxygen saturation (Hackett et al. 1980; Keyl et al. 2000), higher affinity of blood for oxygen (Morpurgo et al. 1976), higher heart rate (Pugh and Evans 1962), less psycho‐neurological symptoms (Garrido et al. 1996), and higher work economy (Bastien et al. 2005). These features suggested that Sherpas seem well adapted at Himalayas, therefore an ideal population for studying high‐altitude adaptation (HAA).
There have been extensive genetic studies in Tibetans, and mainly two genes (EPAS1, OMIM accession number: 603349 and EGLN1,OMIM accession number: 606425) of the HIF pathway were reported to undergone positive selection for high‐altitude adaptation. The EPAS1 gene encodes HIF‐2α and the EGLN1 gene encodes HIF prolyl 4‐hydroxylase 2 (PHD2). The adaptive mutations within these two genes may regulate the hemoglobin levels in high‐altitude natives as a strategy of adaptation (Beall et al. 2010; Bigham et al. 2010; Ge et al. 2012; Huerta‐Sánchez et al. 2014; Jeong et al. 2014; Lorenzo et al. 2014; Peng et al. 2011; Simonson 2010; Wang et al. 2011; Xiang et al. 2013; Xu et al. 2011; Yi et al. 2010). In addition, a recent study (Lou et al. 2015) identified a novel Tibetan‐enriched deletion (TED), where a 3.4‐kb deletion occurred at 80 kb downstream of EPAS1 in about 90% of Tibetans, but absent or extremely rare in other world populations including Han Chinese, implying its possible role in HAA. For Sherpas, previous studies have tested a limited number of sequence variants of several candidate genes likely involved in HAA, such as HIF‐1α (Suzuki et al. 2003), eNOS (Droma et al. 2006), ACE (Droma et al. 2008), EPAS1 (Hanaoka et al. 2012; Jeong et al. 2014), EGLN1, HYOU1, and HMBS (Jeong et al. 2014). Among these different candidate genes, we genotyped EPAS1, EGLN1, and TED in Sherpas for knowing their genetic cause of superior climbing ability in Himalayas. Previous studies (Bhandari et al. 2015) demonstrated that Sherpas is a recently(<1500 years ago) derived sublineage of Tibetans as reflected by the shared mitochondrial DNA (maternal) and Y chromosome (paternal) lineages between them. Here, we further tested EPAS1, EGLN1, and TED region in Sherpas to see if they share similar genetic variants on these genes with Tibetans or they have different patterns.
We collected 582 DNA samples from Sherpas staying permanently at highland villages of both Nepal and Tibetan Autonomous Region of China. Additionally, we measured hemoglobin level and degree of oxygen saturation level of 297 Nepalese Sherpas. We genotyped 31 genetic variants of the two key HAA genes (EPAS1 and EGLN1) as well as the 3.4 kb deletion locus (TED) in Sherpas. Our results indicated highly similar adaptive allele frequencies of all tested loci in Sherpas when compared with Tibetans. In contrast, there are sharp allelic divergences among these loci between the Himalayan populations (Sherpas and Tibetans) and lowland populations (Han Chinese, Europeans and Africans), supporting a shared genetic basis for HAA between Sherpas and Tibetans as proposed previously (Foll et al. 2014; Jeong et al. 2014; Jha et al. 2016).
Material and Methods
Ethical compliance
The protocols of this study were approved by the Internal Review Boards of Kunming Institute of Zoology, Chinese Academy of Sciences and Nepal Health Research Council, Kathmandu, Nepal. All participants provided written informed consent for this study.
DNA samples
DNA samples were extracted from blood of 582 unrelated Sherpa individuals staying permanently (>2800 m) in Khumbu region of Nepal and Zhangmu Town (bordering Nepal) of Tibetan Autonomous Region of China. Sherpa ethnicity was confirmed based on their self‐reported parents and grandparents origin. Besides blood sample collection, we also measured hemoglobin and arterial oxygen saturation level of 297 healthy adult Sherpas (126 males and 171 females) residing in highland villages of Khumbu region, Nepal. The arterial oxygen saturation (SaO2) was recorded using a hand‐held pulse oximeter (Nellcor NPB‐40, CA) after the individuals take a rest for 5–10 min. A HemoCueHb 201+ analyzer (Angelholm, Sweden) was used to measure hemoglobin of fingertip capillary blood.
Genotyping of EPAS1, EGLN1, and TED
Genotyping of selected EPAS1 (Genbank reference sequence: NC_000002.12) SNPs was done by partial sequencing method covering the respective genomic region of these SNPs. Primers were designed using Primer3 software and genotyping was performed using Sanger sequencing on an ABI 3730 sequencer (Applied Biosystems, Foster City, CA, USA). The LD map of EPAS1 was constructed using Haploview version 4.1(Barrett et al. 2005). Similarly, genotyping of two missense mutations (rs12097901G and rs186996510C) of EGLN1 (Genbank reference sequence: NC_000001.11) was done using SNaPshot method on an ABI 3730 sequencer (Applied Biosystems). The SNaPshot method was applied as described previously (Xiang et al. 2013). The 5.5 kb resequencing of EGLN1 in 50 Sherpa samples was also done using Sanger sequencing on an ABI 3730 sequencer (Applied Biosystems). In addition, genotyping of TED was done following the method described in the previous study (Lou et al. 2015).
Genetic association analysis
We collected hemoglobin and arterial oxygen saturation level data along with blood samples from 297 healthy adult Sherpas (126 men and 171 women) for genetic association analysis. The three different genotypes of TED were marked as a biallelic marker (zero copy, one copy, and two copies), and the association analysis was done like other SNPs genotype using linear regression with an additive genetic model in PLINK v1.07 (Purcell et al. 2007). We also tested Hardy–Weinberg equilibrium (HWE) for the 14 loci and no deviation was detected.
Haplotype network analysis
The 28 EPAS1 SNPs and 17 EGLN1 SNPs were used to construct haplotype network of Sherpas, Tibetans, and five other populations from the 1000 Human Genomes Projects. The haplotype reconstruction was done using PHASE program embedded in DnaSP Version 5 (Librado and Rozas 2009). The median joining network was constructed using NETWORK 4.6.1.0, Fluxus Engineering (Bandelt et al. 1999).
F ST calculation
The unbiased estimates of F ST was calculated using the method described previously (Weir and Cockerham 1984). We measured the genetic divergence of EPAS1 SNPs between Sherpas and other populations from the 1000 Genomes Project (CHB, JPT, CEU, and YRI).
Results
Sherpas share similar frequencies of the adaptive EPAS1 variants with Tibetans
EPAS1 is one of the key HAA genes identified in Tibetans (C. M. Beall et al. 2010; Huerta‐Sánchez et al. 2014; Peng et al. 2011). Previously, we conducted resequencing of the entire 94 kb gene region of EPAS1 in 50 Tibetans, and we observed many single‐nucleotide polymorphisms (SNPs) showing deep allelic divergence (F ST >0.45) between Tibetans and Han Chinese (Peng et al. 2011), suggesting that EPAS1 has undergone strong Darwinian positive selection at high altitude leading to the enrichment of adaptive sequence variants in Tibetans. Among the 82 deeply diverged SNPs (F ST >0.45), majority of them are located in three major linked blocks of EPAS1. Since SNPs lying in the same block are tightly linked (r 2 >0.8) to each other, we selected one or two SNPs from each block when performing genotyping of EPAS1 SNPs in Sherpas. In total, 29 out of the 82 SNPs were genotyped in 50 randomly selected Sherpa individuals from Nepal. As shown in Table 1, all 29 SNPs have high frequencies (~49–82%) of the derived alleles (presumably the adaptive alleles), highly similar with the reported allele frequencies in Tibetans (Peng et al. 2011), but deeply diverged from the lowland populations of the 1000 Genomes Project (Abecasis 2012)(CHB: Han Chinese in Beijing, JPT: Japanese in Tokyo, CEU: Utah residents with northern and western European ancestry and YRI: Yoruba in Ibadan, Nigeria).
Table 1.
Comparison of allele frequencies of 29 EPAS1 SNPs between highland (Sherpa and Tibetans) and lowland populations (CHB, JPT, CEU, and YRI)
| Position Chr 2‐GRCh37 | SNP ID | Allele | Frequency of derived allele | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ancestral | Derived | Sherpa (50) | TIB (50) | CHB (97) | JPT (89) | CEU (85) | YRI (88) | ||
| 46550132 | rs116088026 | A | C | 0.51 | 0.58 | 0.00 | 0.00 | 0.00 | 0.06 |
| 46552202 | rs149594770 | T | A | 0.54 | 0.51 | 0.01 | 0.00 | 0.00 | 0.03 |
| 46552352 | rs140067727 | T | C | 0.49 | 0.51 | 0.01 | 0.00 | 0.00 | 0.02 |
| 46553044 | rs113305133 | A | G | 0.51 | 0.58 | 0.01 | 0.00 | 0.00 | 0.03 |
| 46565091 | rs12614710 | G | T | 0.52 | 0.42 | 0.07 | 0.04 | 0.44 | 0.05 |
| 46567916 | rs115321619 | G | A | 0.67 | 0.74 | 0.01 | 0.00 | 0.01 | 0.19 |
| 46568680 | rs73926263 | A | G | 0.64 | 0.73 | 0.01 | 0.00 | 0.00 | 0.07 |
| 46569017 | rs73926264 | A | G | 0.67 | 0.73 | 0.01 | 0.00 | 0.00 | 0.07 |
| 46569770 | rs73926265 | G | A | 0.73 | 0.73 | 0.01 | 0.00 | 0.00 | 0.08 |
| 46570342 | rs55981512 | G | A | 0.75 | 0.73 | 0.01 | 0.00 | 0.00 | 0.04 |
| 46571017 | rs149306391 | C | G | 0.75 | 0.73 | 0.00 | 0.00 | 0.00 | 0.00 |
| 46575388 | rs4953354 | A | G | 0.76 | 0.79 | 0.12 | 0.15 | 0.18 | 0.19 |
| 46576918 | rs76242811 | T | C | 0.75 | 0.75 | 0.01 | 0.00 | 0.00 | 0.10 |
| 46577251 | rs188801636 | T | C | 0.74 | 0.75 | 0.01 | 0.00 | 0.00 | 0.00 |
| 46577299 | rs6544889 | A | G | 0.82 | 0.81 | 0.14 | 0.10 | 0.54 | 0.18 |
| 46577797 | SNP155 | T | C | 0.76 | 0.75 | — | — | — | — |
| 46583581 | rs189807021 | G | A | 0.74 | 0.75 | 0.01 | 0.00 | 0.00 | 0.00 |
| 46588019 | rs150877473 | C | G | 0.74 | 0.75 | 0.01 | 0.00 | 0.00 | 0.00 |
| 46588331 | rs142826801 | G | C | 0.74 | 0.75 | 0.01 | 0.00 | 0.00 | 0.00 |
| 46589032 | rs74898705 | C | T | 0.74 | 0.75 | 0.01 | 0.00 | 0.03 | 0.17 |
| 46592807 | rs61151542 | C | T | 0.69 | 0.74 | 0.01 | 0.00 | 0.03 | 0.19 |
| 46594122 | rs141366568 | A | G | 0.69 | 0.74 | 0.01 | 0.00 | 0.00 | 0.00 |
| 46597756 | rs116062164 | A | C | 0.69 | 0.76 | 0.01 | 0.00 | 0.04 | 0.00 |
| 46598025 | rs141426873 | C | G | 0.69 | 0.74 | 0.01 | 0.00 | 0.00 | 0.00 |
| 46600030 | rs116611511 | A | G | 0.7 | 0.74 | 0.01 | 0.00 | 0.00 | 0.10 |
| 46600661 | rs58160876 | A | C | 0.7 | 0.74 | 0.01 | 0.00 | 0.00 | 0.10 |
| 46600894 | rs12467821 | T | C | 0.79 | 0.78 | 0.15 | 0.14 | 0.52 | 0.10 |
| 46609966 | rs11690951 | A | T | 0.68 | 0.73 | 0.20 | 0.22 | 0.52 | 0.06 |
| 46615955 | rs56161503 | G | A | 0.68 | 0.73 | 0.08 | 0.10 | 0.49 | 0.05 |
Furthermore, we constructed a haplotype network using these SNPs among Sherpas, Tibetans and other lowland populations (CHB, JPT, CEU, and YRI). It is clearly seen that Sherpas and Tibetans shared almost all EPAS1 haplotypes, which are extremely rare in Han Chinese, and absent in other world populations (Fig. 1A). Thus, Sherpas and Tibetans might share the same adaptive genetic variants of EPAS1, consistent with previous studies (Hanaoka et al. 2012; Jeong et al. 2014).
Figure 1.
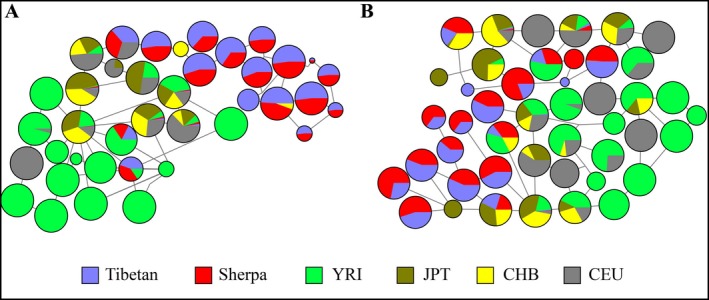
The haplotype networks of EPAS1 and EGLN1 among Sherpa and other populations (CHB, CEU, JPT, YRI, and Tibetans). A total of 28 and 17 SNPs were used in network construction for EPAS1 and EGLN1, respectively.
TED is highly prevalent in Sherpas
A 3.4‐kb deletion (TED) located 80 kb downstream of EPAS1 gene is highly prevalent in Tibetans (90%), but rare in Han Chinese and other world populations (3–7%), suggesting its potential role in HAA (Lou et al. 2015). We genotyped the TED locus in 582 Sherpas, and we found that 94% of them are TED carriers with 73% of them being TED homozygotes and 21% TED heterozygotes. The allele frequency of TED in Sherpas (83.5%) is similar with that in Tibetans (90%), consistent with the pattern seen for the EPAS1 sequence variants.
Prevalence of EGLN1 missense variants in Sherpas
Previous studies of EGLN1 have identified two missense mutations (rs12097901G>C, D4E and rs186996510G>C, S127C) with large allelic divergence between Tibetans and other world populations (Felipe R Lorenzo et al. 2014; Xiang et al. 2013). These two EGLN1 SNPs were characterized previously (Felipe R Lorenzo et al. 2014) as functional variants for Tibetan high‐altitude adaptation. We genotyped these two SNPs in 582 Sherpas, and it turned out that the adaptive alleles of these two SNPs are also prevalent in Sherpas (67% and 61%, respectively), similar with the reported frequencies in Tibetans (79% and 65–75%, respectively) (Felipe R Lorenzo et al. 2014; Xiang et al. 2013). Similarly, as seen for the EPAS1 variants, the EGLN1 adaptive alleles are also rare in Han Chinese and other world populations (0.5–2.3%)(Xiang et al. 2013), again suggesting shared adaptive variants between Sherpas and Tibetans.
We also resequenced a 5.5 kb fragment flanking the two EGLN1 SNPs in the 50 randomly selected Sherpa samples. A total of 17 SNPs in the 5.5 kb region were used to construct haplotype network, together with data from Tibetans (Xiang et al. 2013) and other world populations (Abecasis 2012)(CHB, JPT, CEU and YRI). The haplotype network analyses clearly indicate that Sherpas and Tibetans share distinct haplotypes of EGLN1, which are different from those haplotypes prevalent in other populations (Fig. 1B). Collectively, our data of EPAS1, TED, and EGLN1 all support that there might be shared genetic variants responsible for HAA between Sherpas and Tibetans.
Genetic association analysis of hemoglobin and blood oxygen saturation in Sherpas
To test if the sequence variants contribute to the known adaptive traits in Sherpas, we conducted association studies with hemoglobin concentration and blood oxygen saturation level in 297 Sherpas from Nepal (126 males and 171 females). A total of 14 variants (11 EPAS1 SNPs, two EGLN1 SNPs and TED) were genotyped among 297 Nepalese Sherpas for association analysis. As expected, six of the 11 EPAS1 SNPs showed significant association with hemoglobin concentration (P < 0.05, after Bonferroni correction) (Table 2). The significant SNPs reported previously in Tibetans (Beall et al. 2010) and Sherpa (Jeong et al. 2014) were replicated in our present Sherpa studies too. When males and females were analyzed separately, three EPAS1 SNPs (rs113305133, rs116611511, and rs12467821) showed significant association in males but none of these tested EPAS1 SNPs showed significant association in females (Table 2). Notably, Sherpa individuals carrying the adaptive alleles of the EPAS1 SNPs tend to have lower hemoglobin levels when compared with those wild‐type allele carriers (Fig. 2), consistent with the result in Tibetans (Beall et al. 2010). Interestingly, two EPAS1 SNPs (rs116611511 and rs12467821) showing significant association with hemoglobin in Sherpas are tightly linked with the previously proposed 2.5 kb EPAS1 motif of Denisovan introgression in Tibetans (Huerta‐Sánchez et al. 2014).
Table 2.
Association analysis of 14 variants (11 EPAS1 SNPs, 2 EGLN1 SNPs, and TED) with hemoglobin and blood oxygen saturation in Nepalese Sherpas
| Sample | Gene | SNP | Effect allele | Hemoglobin level | Degree of blood oxygen saturation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BETA | SE | P | Corrected P | BETA | SE | P | Corrected P | ||||
| Male N = 126 | EPAS1 | rs149594770 | A | 0.629 | 0.257 | 0.016 | 0.204 | −0.0022 | 0.0032 | 0.490 | / |
| EPAS1 | rs140067727 | C | −0.671 | 0.275 | 0.016 | 0.214 | 0.0001 | 0.0030 | 0.985 | / | |
| EPAS1 | rs113305133 | G | −0.901 | 0.244 | 3.30E‐04 | 0.004 | −0.0020 | 0.0030 | 0.518 | / | |
| EPAS1 | rs149306391 | G | 0.617 | 0.260 | 0.019 | 0.252 | 0.0026 | 0.0033 | 0.426 | / | |
| EPAS1 | rs4953354 | G | 0.468 | 0.283 | 0.101 | / | −0.0001 | 0.0035 | 0.983 | / | |
| EPAS1 | rs188801636 | C | 0.610 | 0.243 | 0.013 | 0.172 | 0.0007 | 0.0030 | 0.819 | / | |
| EPAS1 | rs6544889 | G | 0.599 | 0.239 | 0.013 | 0.174 | −0.0002 | 0.0029 | 0.947 | / | |
| EPAS1 | SNP155 | C | 0.196 | 0.193 | 0.311 | / | −0.0001 | 0.0023 | 0.959 | / | |
| EPAS1 | rs116611511 | G | 0.722 | 0.233 | 0.002 | 0.032 | 0.0003 | 0.0029 | 0.929 | / | |
| EPAS1 | rs58160876 | C | 0.665 | 0.239 | 0.006 | 0.081 | 0.0003 | 0.0029 | 0.920 | / | |
| EPAS1 | rs12467821 | C | 0.806 | 0.252 | 0.002 | 0.023 | −0.0006 | 0.0031 | 0.854 | / | |
| TED | Deletion | Zero copy | 0.930 | 0.262 | 0.001 | 0.007 | 0.0012 | 0.0033 | 0.710 | / | |
| EGLN1 | rs186996510 | C | −0.173 | 0.251 | 0.491 | 0.982 | 0.0015 | 0.0030 | 0.617 | / | |
| EGLN1 | rs12097901 | G | −0.154 | 0.252 | 0.543 | / | 0.0002 | 0.0030 | 0.959 | / | |
| Female N = 171 | EPAS1 | rs149594770 | A | 0.023 | 0.213 | 0.916 | / | −0.0023 | 0.0022 | 0.302 | / |
| EPAS1 | rs140067727 | C | 0.083 | 0.233 | 0.723 | / | 0.0040 | 0.0022 | 0.072 | 0.930 | |
| EPAS1 | rs113305133 | G | −0.028 | 0.217 | 0.896 | / | 0.0048 | 0.0022 | 0.030 | 0.388 | |
| EPAS1 | rs149306391 | G | 0.652 | 0.244 | 0.008 | 0.106 | −0.0015 | 0.0026 | 0.565 | / | |
| EPAS1 | rs4953354 | G | −0.128 | 0.248 | 0.607 | / | −0.0012 | 0.0026 | 0.654 | / | |
| EPAS1 | rs188801636 | C | 0.457 | 0.237 | 0.055 | 0.718 | 0.0005 | 0.0025 | 0.848 | / | |
| EPAS1 | rs6544889 | G | 0.359 | 0.241 | 0.138 | / | −0.0009 | 0.0025 | 0.720 | / | |
| EPAS1 | SNP155 | C | 0.246 | 0.176 | 0.165 | / | −0.0013 | 0.0018 | 0.478 | / | |
| EPAS1 | rs116611511 | G | 0.286 | 0.235 | 0.227 | / | 0.0005 | 0.0024 | 0.835 | / | |
| EPAS1 | rs58160876 | C | 0.350 | 0.234 | 0.136 | / | 0.0005 | 0.0024 | 0.832 | / | |
| EPAS1 | rs12467821 | C | 0.406 | 0.247 | 0.103 | / | 0.0003 | 0.0026 | 0.900 | / | |
| TED | Deletion | Zero copy | 1.004 | 0.276 | 3.63E‐04 | 0.005 | −0.0010 | 0.0029 | 0.727 | / | |
| EGLN1 | rs186996510 | C | 0.323 | 0.192 | 0.094 | 0.189 | 0.0002 | 0.0021 | 0.924 | / | |
| EGLN1 | rs12097901 | G | 0.333 | 0.208 | 0.111 | 0.222 | −0.0010 | 0.0024 | 0.656 | / | |
| All N = 297 | EPAS1 | rs149594770 | A | 0.293 | 0.183 | 0.110 | / | −0.0023 | 0.0018 | 0.207 | / |
| EPAS1 | rs140067727 | C | −0.271 | 0.197 | 0.170 | / | 0.0024 | 0.0018 | 0.198 | / | |
| EPAS1 | rs113305133 | G | −0.430 | 0.182 | 0.019 | 0.242 | 0.0020 | 0.0018 | 0.275 | / | |
| EPAS1 | rs149306391 | G | 0.722 | 0.198 | 3.16E‐04 | 0.004 | 0.0002 | 0.0020 | 0.932 | / | |
| EPAS1 | rs4953354 | G | 0.211 | 0.208 | 0.312 | / | −0.0009 | 0.0021 | 0.654 | / | |
| EPAS1 | rs188801636 | C | 0.674 | 0.185 | 3.25E‐04 | 0.004 | 0.0001 | 0.0019 | 0.956 | / | |
| EPAS1 | rs6544889 | G | 0.630 | 0.185 | 0.001 | 0.010 | −0.0010 | 0.0019 | 0.589 | / | |
| EPAS1 | SNP155 | C | 0.338 | 0.142 | 0.018 | 0.235 | −0.0011 | 0.0014 | 0.443 | / | |
| EPAS1 | rs116611511 | G | 0.643 | 0.182 | 4.66E‐04 | 0.006 | −0.0001 | 0.0019 | 0.966 | / | |
| EPAS1 | rs58160876 | C | 0.640 | 0.183 | 0.001 | 0.007 | 0.0000 | 0.0019 | 0.982 | / | |
| EPAS1 | rs12467821 | C | 0.750 | 0.194 | 1.31E‐04 | 0.002 | −0.0006 | 0.0020 | 0.761 | / | |
| TED | Deletion | Zero copy | 1.032 | 0.209 | 1.32E‐06 | 1.71E‐05 | 0.0000 | 0.0022 | 0.983 | / | |
| EGLN1 | rs186996510 | C | 0.051 | 0.166 | 0.759 | / | 0.0008 | 0.0018 | 0.629 | / | |
| EGLN1 | rs12097901 | G | 0.029 | 0.174 | 0.866 | / | −0.0003 | 0.0019 | 0.872 | / | |
Bold values indicated those p values <0.05.
Figure 2.
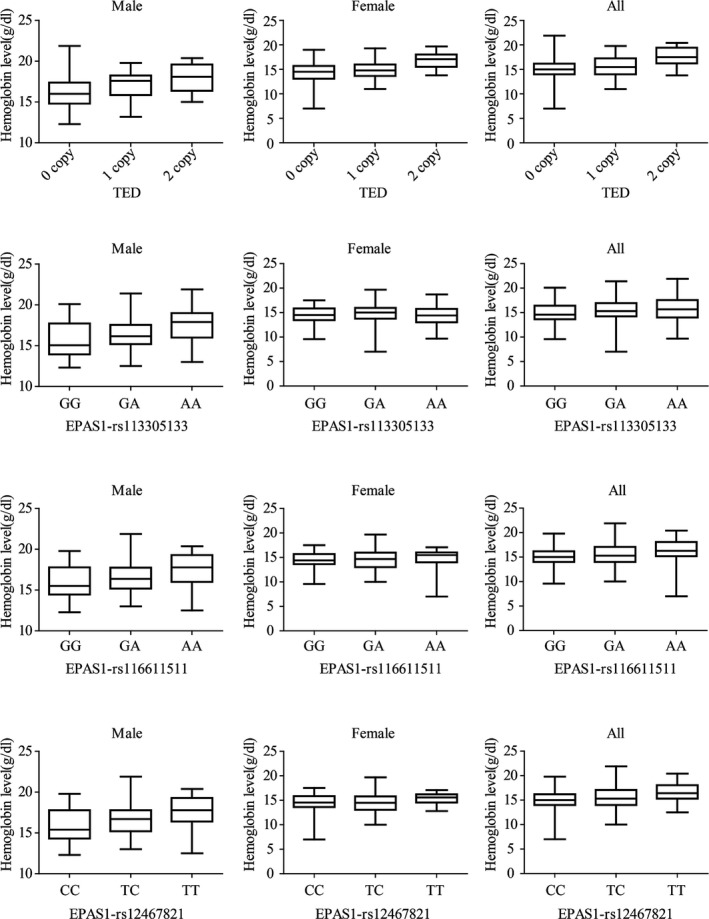
The box plot showing comparison of hemoglobin levels of different genotypes of TED and three EPAS1 SNPs (rs113305133, rs116611511, and rs12467821) in Sherpas.
Furthermore, TED showed significant association with hemoglobin in both males and females, and it stands the most significant variant in the combined sample (P = 1.71 × 10−5, after Bonferroni correction) (Table 2). Similar with the EPAS1 SNPs, the TED carriers have lower hemoglobin levels than the nondeletion carriers (Fig. 2). Taken together, these data suggest that EPAS1 and TED might have contributed to hemoglobin regulation in Sherpas by keeping a relatively low hemoglobin concentration at high altitude. We did not observe significant association of the tested variants (Table 2) with blood oxygen saturation, implying that there might be different genetic mechanisms of regulating hemoglobin and blood oxygen saturation.
Discussion
EPAS1 and EGLN1 are two top candidate genes reported in previous studies for HAA in Tibetans (Beall et al. 2010; Lorenzo 2010; Lorenzo et al. 2014; Peng et al. 2011; Scheinfeldt and Tishkoff 2013; Simonson 2010; Xiang et al. 2013; Xu et al. 2011; Yi et al. 2010). Additionally, a recent study (Lou et al. 2015) identified TED with high frequency in Tibetans, but rare or absent in lowland populations though whether TED is functionally related with EPAS1 is unknown because it is located 80 kb downstream of EPAS1. We previously showed that Sherpas are likely a recently derived population from Tibetans (<1500 years ago) based on their shared mtDNA and Y chromosome lineages (Bhandari et al. 2015). Hence, we propose that they might share similar genes/mutations for HAA, which was confirmed by our analysis of 32 variants of the top candidate HAA polymorphisms (EPAS1, TED, and EGLN1). Our comparative studies between these two highland populations (Sherpas and Tibetans) demonstrated sharing of similar genetic variants in EPAS1, EGLN1, and TED, a further support for the biological significance of these HAA genes for future functional studies.
The observed significant association of EPAS1 SNPs and TED with hemoglobin levels suggests the functional importance of EPAS1 for high‐altitude adaptation because a relatively low hemoglobin level can prevent onset of polycythemia that impairs tissue blood flow and oxygen delivery (Beall 2007). Our association studies did not find any significant EPAS1 SNPs in females when analyzed separately in males and females. It might be due to either the differences in gender‐specific variants responsible for maintaining hemoglobin concentration in males and females or our limited phenotypic data on pregnancy, menstruation, and breast feeding for association studies which may affects hemoglobin levels in females.
The 2.5 kb EPAS1 motif initially identified in Tibetans was proposed to emerge as the result of Denisovan introgression, potentially responsible for high‐altitude adaptation (Huerta‐Sánchez et al. 2014). But it is still difficult to pinpoint the causal mutation(s) since multiple SNPs in EPAS1 have large allelic divergence between Tibetans/Sherpas and lowland populations (e.g., Han Chinese), and show significant association with hemoglobin levels. Furthermore, in Sherpas the deletion polymorphism (TED), 80 kb downstream of EPAS1 showed a stronger signal of association with hemoglobin than the EPAS1 SNPs. But TED is not caused by Denisovan introgression since it is absent in the Denisovan genome (Lou et al. 2015). Hence, whether Tibetans and Sherpas acquired their adaptive ability of living in Himalayas through Denisovan introgression need to be tested in future studies.
In conclusion, we observed shared adaptive variants of the key HAA genes (EPAS1, EGLN1 and TED) between Sherpas and Tibetans. The superior capacity of Sherpas for mountain climbing as a reflection of adaptation to high altitude was inherited from their Tibetan ancestors who have been living in the Himalayas for a long time.
Competing Financial Interests
The authors declare no competing financial interests.
Web Resources
1000 Genomes Projects, http://www.1000genomes.org/; UCSC Genome Browser, http://genome.ucsc.edu/; Primer3, http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi.
Acknowledgments
We thank all the Sherpa volunteers for their participation in this research. We also thank Dr. Ashwani Kumar Sinha for helping us during sample collection from the remote Sherpa villages in Nepal. This research was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB13010000), the National 973 Program of China (2012CB518202), the National Natural Science Foundation of China (91231203, 31371269 and 31460287), the State Key Laboratory of Genetic Resources and Evolution (GREKF13‐04, GREKF12‐04), and the Zhufeng Scholar Program of Tibetan University.
Contributor Information
Xuebin Qi, Email: qixuebin@mail.kiz.ac.cn.
Bing Su, Email: sub@mail.kiz.ac.cn.
References
- Abecasis, G. R. 2012. An integrated map of genetic variation from 1092 human genomes. Nature 491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, W. H. , and Shresta S. M.. 1974. Hemoglobin levels, vitamin B12, and folate status in a Himalayan village. Am. J. Clin. Nutr. 27:217–219. [DOI] [PubMed] [Google Scholar]
- Bandelt, H.‐J. , Forster P., and Röhl A.. 1999. Median‐joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16:37–48. [DOI] [PubMed] [Google Scholar]
- Barrett, J. C. , Fry B., Maller J. D. M. J., and Daly M. J.. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. [DOI] [PubMed] [Google Scholar]
- Bastien, G. . J. , Schepens B., Willems P. A., and Heglund N. C.. 2005. Energetics of load carrying in Nepalese porters. Science 308:1755. [DOI] [PubMed] [Google Scholar]
- Beall, C. M. 2007. Two routes to functional adaptation: Tibetan and Andean high‐altitude natives. Proc. Natl Acad. Sci. 104(suppl 1):8655–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall, C. M. , and Reichsman A. B.. 1984. Hemoglobin levels in a Himalayan high altitude population. Am. J. Phys. Anthropol. 63:301–306. [DOI] [PubMed] [Google Scholar]
- Beall, C. M. , Cavalleri G. L., Deng L., Elston R. C., Gao Y., Knight J., et al. 2010. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl Acad. Sci. 107:11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari, S. , Zhang X., Cui C., Bianba, Liao S., Peng Y., et al. 2015. Genetic evidence of a recent Tibetan ancestry to Sherpas in the Himalayan region. Sci. Rep., 5:16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham, A. , Bauchet M., Pinto D., Mao X., Akey J. M., Mei R., et al. 2010. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 6:e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droma, Y. , Hanaoka M., Basnyat B., Arjyal A., Neupane P., A. Pandit , et al. 2006. Genetic contribution of the endothelial nitric oxide synthase gene to high altitude adaptation in sherpas. High Alt. Med. Biol. 7:209–220. [DOI] [PubMed] [Google Scholar]
- Droma, Y. , Hanaoka M., Basnyat B., Arjyal A., Neupane P., A. Pandit , et al. 2008. Adaptation to high altitude in Sherpas: association with the insertion/deletion polymorphism in the Angiotensin‐converting enzyme gene. Wilderness Environ. Med. 19:22–29. [DOI] [PubMed] [Google Scholar]
- Foll, M. , Gaggiotti O. E., Daub J. T., Vatsiou A., and Excoffier L.. 2014. Widespread signals of convergent adaptation to high altitude in Asia and America. Am. J. Hum. Genet. 95:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, E. , Segura R., Capdevila A., Pujol J., Javierre C., and Ventura J. L.. 1996. Are Himalayan Sherpas better protected against brain damage associated with extreme altitude climbs? Clin. Sci. 90:81. [DOI] [PubMed] [Google Scholar]
- Ge, R.‐L. , Simonson T. S., Cooksey R. C., Tanna U., Qin G., Huff C. D., et al. 2012. Metabolic insight into mechanisms of high‐altitude adaptation in Tibetans. Mol. Genet. Metab. 106:244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, P. H. , Reeves J. T., Reeves C. D., Grover R. F., and Rennie D.. 1980. Control of breathing in Sherpas at low and high altitude. J. Appl. Physiol. 49:374–379. [DOI] [PubMed] [Google Scholar]
- Hanaoka, M. , Droma Y., Basnyat B., Ito M., Kobayashi N., Katsuyama Y., et al. 2012. Genetic variants in EPAS1 contribute to adaptation to high‐altitude hypoxia in Sherpas. PLoS ONE 7:e50566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havryk, A. P. , Gilbert M., and Burgess K. R.. 2002. Spirometry values in Himalayan high altitude residents (Sherpas). Respir. Physiol. Neurobiol. 132:223–232. [DOI] [PubMed] [Google Scholar]
- Huerta‐Sánchez, E. , Jin X., Bianba Z., Peter B. M., Vinckenbosch N., et al. 2014. Altitude adaptation in Tibetans caused by introgression of Denisovan‐like DNA. Nature 512:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, C. , Alkorta‐Aranburu G., Basnyat B., Neupane M., Witonsky D. B., Pritchard J. K., et al. 2014. Admixture facilitates genetic adaptations to high altitude in Tibet. Nat. Commun. 5:3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, A. R. , Zhou D., Brown C. D., Kreitman M., Haddad G. G., and White K. P.. 2016. Shared genetic signals of hypoxia adaptation in Drosophila and in high‐altitude human populations. Mol. Biol. Evol. 33:501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyl, C. , Schneider A., Greene R. E., Passino C., Spadacini G., Bandinelli G., et al. 2000. Effects of breathing control on cardiocirculatory modulation in Caucasian lowlanders and Himalayan Sherpas. Eur. J. Appl. Physiol. 83:481–486. [DOI] [PubMed] [Google Scholar]
- Lahiri, S. , Milledge J. S., Chattopadhyay H. P., Bhattacharyya A. K., and Sinha A. K.. 1967. Respiration and heart rate of Sherpa highlanders during exercise. J. Appl. Physiol. 23:545–554. [DOI] [PubMed] [Google Scholar]
- Librado, P. , and Rozas J.. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. [DOI] [PubMed] [Google Scholar]
- Lorenzo, F. R. 2010. Novel PHD2 mutation associated with Tibetan genetic adaptation to high altitude hypoxia. ASH 52nd Annual Meeting.
- Lorenzo, F. R. , Huff C., Myllymäki M., Olenchock B., Swierczek S., Tashi T., et al. 2014. A genetic mechanism for Tibetan high‐altitude adaptation. Nat. Genet. 46:951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, H. , Lu Y., Lu D., Fu R., Wang X., Feng Q., et al. 2015. A 3.4‐kb copy‐number deletion near epas1 is significantly enriched in high‐altitude Tibetans but absent from the denisovan sequence. Am. J. Hum. Genet. 97:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpurgo, G. , Arese P., Bosia A., Pescarmona G. P., Luzzana M., and Modiano G.. 1976. Sherpas living permanently at high altitutde: a new pattern of adaptation. Proc. Natl Acad. Sci. 73:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. , Yang Z., Zhang H., Cui C., Qi X., Luo X., et al. 2011. Genetic variations in Tibetan populations and high‐altitude adaptation at the Himalayas. Mol. Biol. Evol. 28:1075–1081. [DOI] [PubMed] [Google Scholar]
- Pugh, L. G. , and Evans C.. 1962. Physiological and medical aspects of the Himalayan Scientific and Mountaineering Expedition. BMJ 2:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, S. , Neale B., Todd‐Brown K., Thomas L., Ferreira M. A. R., Bender D., et al. 2007. PLINK: a tool set for whole‐genome association and population‐based linkage analyses. Am. J. Hum. Genet. 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinfeldt, L. B. , and Tishkoff S. A.. 2013. Recent human adaptation: genomic approaches, interpretation and insights. Nat. Rev. Genet. 14:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson, T. S. , Yang Y., Huff C. D., Yun H., Qin G., Witherspoon D. J., et al. 2010. Genetic evidence for high‐altitude adaptation in Tibet. Science 329:72–75. [DOI] [PubMed] [Google Scholar]
- Suzuki, K. , Kizaki T., Hitomi Y., Nukita M., Kimoto K., Miyazawa N., et al. 2003. Genetic variation in hypoxia‐inducible factor 1α and its possible association with high altitude adaptation in Sherpas. Med. Hypotheses 61:385–389. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Zhang Y. B., Zhang F., Lin H., Wang X., Wan N., et al. 2011. On the origin of Tibetans and their genetic basis in adapting high‐altitude environments. PLoS ONE 6:e17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, B. S. , and Cockerham C. C.. 1984. Estimating F‐statistics for the analysis of population structure. Evolution 38:1358–1370. [DOI] [PubMed] [Google Scholar]
- Xiang, K. , Ouzhuluobu, Peng Y., Yang Z., Zhang X., Cui C., et al. 2013. Identification of a Tibetan‐specific mutation in the hypoxic gene EGLN1 and its contribution to high‐altitude adaptation. Mol. Biol. Evol. 30:1889–1898. [DOI] [PubMed] [Google Scholar]
- Xu, S. , Li S., Yang Y., Tan J., Lou H., Jin W., et al. 2011. A genome‐wide search for signals of high‐altitude adaptation in Tibetans. Mol. Biol. Evol. 28:1003–1011. [DOI] [PubMed] [Google Scholar]
- Yi, X. , Liang Y., Huerta‐Sanchez E., Jin X., Cuo Z. X. P., et al. 2010. Sequencing of 50 human exomes reveals adaptation to high altitude. Science, 329:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


