Abstract
Background
Substance use is an important clinical issue in the older adult population. As older adults are susceptible to cognitive disorders, the intersection of the fields of substance use and cognitive neuroscience is an active area of research. Prior studies of alcohol use and cognitive performance are mixed, and inconsistencies may be due to under- or over-adjustment for confounders.
Aim
This manuscript adds to this literature by conducting a secondary analysis of self-reported lifetime history of alcohol use and cognitive performance in older adults (n = 133). We hypothesized that current alcohol users would have poorer cognitive performance compared to never/minimal and former alcohol users.
Methods
Older adult participants were classified into never/minimal alcohol users, former alcohol users, and current alcohol users. A neurocognitive battery included a global cognitive measure and individual measures of attention, memory, fluency, and executive function. A directed acyclic graph (DAG)-based approach was used to select variables to be included in the multiple linear regression models.
Results
Though unadjusted analyses showed some significant associations between alcohol use and cognitive performance, all associations between alcohol use and cognitive performance were eliminated after adjusting for age, education, sex, race and smoking pack years. Alcohol drink years were not significantly associated with cognitive performance among current and former alcohol users.
Discussion
These results suggest that lifetime alcohol use is not significantly associated with cognitive performance in older adults after adjustment for key confounders. Inconsistencies in prior studies may be due to uncontrolled confounding and/or unnecessary adjustment of mediators and/or colliders.
Keywords: alcohol, substance use, cognition, cognitive, older adult
INTRODUCTION
Substance use is an important clinical issue in the older adult population1–8. The fields of substance use and geriatrics intersect in many areas (conceptual model depicted in Figure 1; draw.io, Jgraph, version 5.5.6.0), such as drug-medication interactions9, substance-induced medical complications10, age-specific substance use treatments11, and psychiatric comorbidities12. Another key intersection of these fields is in the domain of cognition. As older adults are susceptible to cognitive disorders, the intersection of the fields of substance use and cognitive neuroscience is an active area of research13–19. The general consensus is that based on the effects of aging and substance use on cognitive ability20, there is a significant potential for cognitive ability to be affected among older adults who use substances compared to those who do not. Because the process of aging itself can negatively affect neurotransmitter systems and drug metabolism, substance use can increase drug-related complications in older adults20.
Figure 1.
Conceptual Model – Intersection of the Fields of Geriatrics and Substance Use.
This manuscript specifically focuses on alcohol use and cognitive performance in older adults. The research evidence of the association between alcohol use and cognitive performance in this population is mixed. Some reasons for the differences among studies include length of follow-up, how alcohol use is defined, which cognitive measures are used, sociodemographics and under- or over-adjustment for confounders. Some studies have shown that alcohol use is associated with either increased cognitive impairment21 or decreased cognitive impairment22. Other studies have shown that current alcohol users have better cognitive performance than former alcohol users or those who have never used alcohol23–28. Whether alcohol affects cognitive performance also depends on many other factors, such as sex29–34, dose of alcohol35–39 and length of abstinence40.
Most prior studies have focused on current alcohol use only and have not considered the full history of alcohol use and, in particular, have not differentiated between lifelong abstainers and former drinkers. Since there is no definitive consensus on the effects of social/non-hazardous alcohol use on cognitive performance in older adults, this manuscript adds to this literature by conducting a secondary analysis of self-reported lifetime history of alcohol use and cognitive performance in older adults using baseline data from the Mental Activity and eXercise (MAX) trial. We hypothesized that current alcohol users would have poorer cognitive performance compared to never/minimal and former alcohol users. We also hypothesized there would be a dose-dependent effect of alcohol use, such that increasing alcohol use over the lifetime would be associated with worse cognitive performance in late life. We used a directed acyclic graph (DAG)-based approach41 to select confounders to include in statistical models.
METHODS
Study Setting
Details of the original study have been published elsewhere42. The institutional review boards at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center approved this study. All participants provided written informed consent. Participants were recruited through direct mailing to the neighborhoods adjacent to the study site, in addition to advertisements, flyers, physician and friend referrals, and recruitment databases.
Inclusion criteria included being age 65 years or older, having a cognitive complaint (answering yes to the question “Do you feel that your memory or thinking skills have gotten worse recently?”), fluency in the English language, and not planning to travel for more than 1 week during the study period. Study participants with any history of an alcohol use disorder or heavy drinking (defined as ≥5 drinks/day for ≥5 days in a row OR ≥5 days/month) or a history of a substance use disorder or heavy drug use (defined as daily usage for ≥5 days in a row OR ≥5 days/month) were excluded. Other exclusion criteria included dementia (based on self-report, physician diagnosis, or scoring 18 or less on the modified Telephone Interview for Cognitive Status43), other neurological or major psychiatric disorders, limited life expectancy, or other factors that could limit ability to participate fully in the intervention.
Measures
Sociodemographic (e.g., age, race, income, occupation), medical (e.g., cardiovascular, diabetes), psychiatric (e.g., depression, sleep), alcohol, and smoking (e.g., never/former/current, pack-years) variables were collected through self-report. Sleep quality was assessed using seven questions from the Sleep Disorders Questionnaire on the 2005 to 2006 National Health and Nutrition Examination Survey (range 0–28, where higher scores reflect worse sleep quality)44. Depression was assessed using the Geriatric Depression Scale45. Study participants were asked at the baseline visit if they had more than 100 drinks in their lifetime. If yes, they were asked if they had any alcohol in the past 30 days, how old they were when they started drinking, how old they were when they had their most recent drink, the average number of drinks per day consumed when they drank, and the types of alcohol usually consumed (wine, beer or hard liquor).
We classified participants into 3 groups of alcohol use: never/minimal users (≤100 drinks in lifetime), former users (>100 drinks in lifetime and no alcohol in past 30 days), and current users (>100 drinks in lifetime and alcohol in past 30 days). The rationale for this classification scheme was based on categories that are used for smoking, which define “never smokers” as an adult who has never smoked, or who has smoked <100 cigarettes in his/her lifetime46; we categorized those who used ≤100 drinks in lifetime as never/minimal. “Never/minimal” users in this study were not actively consuming alcohol. We also calculated the total number of “drink-years” over the lifetime as the age when they last drank minus the age when they first drank multiplied by the average drinks/day when consuming alcohol. Similar questions and procedures were used to determine lifetime smoking history, including definitions of non-smokers, former smokers, current smokers and lifetime pack-years consumed.
A detailed neurocognitive battery included the Modified Mini-Mental State examination (3MS) to assess global cognitive status (range, 0–100), and individual measures of attention, memory, fluency, and executive function. Specific measures were selected because they have good validity and reliability and are sensitive to cognitive decline in older adults. Measures included were verbal learning and memory (Rey Auditory Verbal Learning Test [RAVLT]), verbal fluency (letter and category), processing speed (Digit Symbol Substitution Test, Trail-Making Test part A [Trails A]), executive function/mental flexibility (Trail-Making Test part B [Trails B]), executive function/inhibition (Eriksen Flanker Test [EFT], congruent and incongruent reaction times), visuospatial function (Useful Field of View [UFOV], processing speed, divided attention, and selective attention). Z-scores were based on raw score transformations ([raw score – mean score] / standard deviation). A composite score for the neurocognitive measures was also calculated as previously described42. The current alcohol users group served as the standardization reference.
Statistical Analysis
We estimated and tested all statistical models using Stata/SE version 14.1 (College Station, TX; update level 7/20/2016). Parametric and non-parametric analyses were used as appropriate. When comparing alcohol use categories for baseline sociodemographic and clinical data, ANOVA was used for continuous variables, and Pearson’s chi-square or Fisher’s exact test were used for categorical variables. We initially considered p values < 0.05 as statistically significant. However, we used the False Discovery Rate (FDR) method in Stata/SE version 14.1 (author Roger Newson, King's College, London, UK) for multiple comparison correction of any significant analyses; specifically, we tried the liu1, liu2, simes, yekutieli, and krieger methods with the smileplot option.
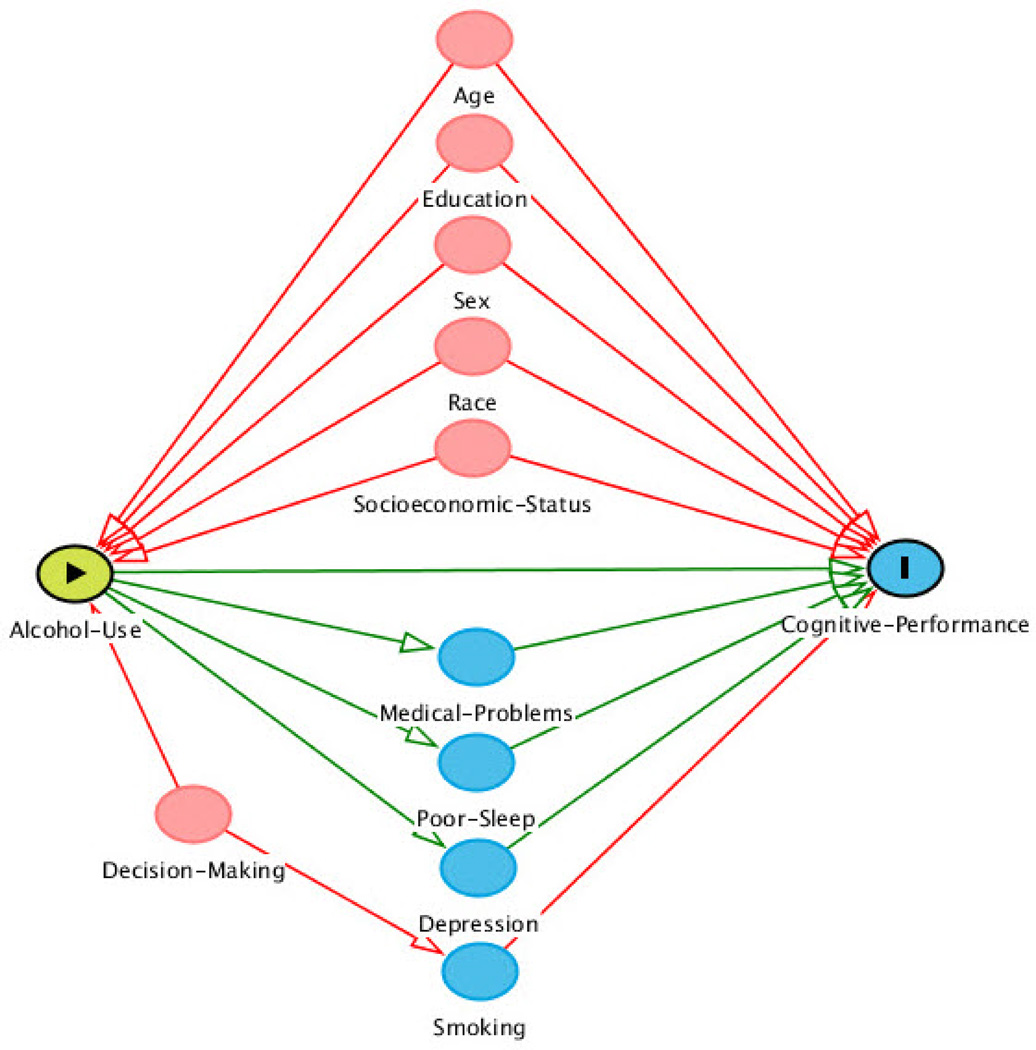
We conducted both unadjusted and adjusted analyses. Traditional methods of adjusting for confounding may miss or even introduce biases such as selection bias and collider bias, which can be minimized by using more modern graphical methods of adjustment47. We used a directed acyclic graph (DAG)-based approach to select the variables to be included in the regression models41, 47. Our DAG is drawn in Figure 2 using the website http://dagitty.net/48. The DAG-based approach allows for a graphical representation of causal effects among variables. Causation is indicated by an arrow that connects two variables. If there is no direct casual association between two variables, then the two variables are left unconnected. A DAG is called acyclic because no ordered sequence of arrows leads back to the variable from where the sequence began49. A confounding variable has arrows that are directed away from the variable, and adjusting for such variables closes the backdoor pathways between the primary predictor of interest (i.e., alcohol use) and the primary outcome of interest (i.e., cognitive performance)49.
Figure 2.
Directed Acyclic Graph Approach to Variable Selection for Regression Models.
Alcohol use = predictor; Cognitive performance = outcome; red circles are covariates; blue circles are mediators
We conceptualized age, education, sex, race, socioeconomic status (i.e., income and occupation), and smoking to confound the association between alcohol use and cognitive performance; most of these variables were adjusted for in the regression models (see Results section for further comments on income and occupation). Whereas, we conceptualized medical problems (e.g., heart problems, diabetes), poor sleep and depression to partially mediate the association between alcohol use and cognitive performance; mediators were not adjusted for in the regression models, since adjustment for mediators would decrease the total effect of alcohol use on cognitive performance. When comparing alcohol use categories for cognitive performance data, multiple linear regression was used for all analyses.
Regression models were checked for normality, constant variance (homoscedasticity), outlying/high leverage/influential points and multicollinearity. Normality was assessed using Q-Q plots and kernel density plots of the residuals. Constant variance assessed by residual versus predictor (RVP) plots of continuous predictors and residual versus fitted (RVF) plots. Outlying/high leverage/influential points were assessed using boxplots to detect outlying values among the dfbetas. Multicollinearity was assessed analyzing the variance inflation factor values after each regression model. Normality, constant variance and a lack of multicollinearity were met for nearly all models (except as noted in the Results section), and no outlying/high leverage/influential points were found. When the primary predictor was continuous (e.g., alcohol drink years), linearity was also assessed using component plus residual (CPR) plots with the LOWESS smooth option. Linearity was met for nearly all models (except as noted in the Results section).
RESULTS
Table 1 presents baseline sociodemographic and clinical data by alcohol use categories (from n = 133: never/minimal 18.8%, former 17.3%, and current 63.9%). Current alcohol users had the highest level of education (mean 16.7 years, SD 2.1, p = 0.02) and were more likely to be non-Hispanic Caucasian (χ2(2) = 16.1, p < 0.001) and smokers (Fisher’s exact, p < 0.001). Never/minimal alcohol users were more likely to be female (χ2(2) = 6.0, p = 0.049). The three groups did not significantly differ on any other variables.
Table 1.
Baseline Sociodemographic and Clinical Data by Alcohol Use Categories.
| Never/ Minimal n = 25 |
Former n = 23 |
Current n = 85 |
Test Statistic and Significance |
|
|---|---|---|---|---|
| Unadjusted Mean (S.D.a) or % | ||||
| Age (years) | 74.1 (6.4) | 72.4 (5.6) | 73.3 (6.0) | F(2, 130) = 0.47, p = 0.62 |
| Education (years) | 15.4 (3.0) | 15.8 (2.2) | 16.7 (2.1) n = 83 |
F(2, 128) = 4.0, p = 0.02 |
| Female | 84% | 52.2% | 61.2% | χ2(2) = 6.0, p = 0.049 |
| Non-Hispanic Caucasian | 36% | 56.5% | 77.8% | χ2(2) = 16.1, p < 0.001 |
| Income – $20,000 and under | 20% | 4.4% | 10.6% | Fisher’s exact = 0.74 |
| Occupation – Management or Professional | 48% | 52.2% | 58.8% | Fisher’s exact = 0.43 |
| Hypertension | 64% | 43.5% | 54.1% | χ2(2) = 2.0, p = 0.36 |
| Congestive Heart Failure | 0% | 4.4% | 1.2% | Fisher’s exact = 0.35 |
| Heart Attack | 1.9% | 1.7% | 6.4% | Fisher’s exact = 0.71 |
| Diabetes | 3.8% | 3.5% | 12.8% | χ2(2) = 0.61, p = 0.74 |
| Total Sleep Score | 7.4 (6.3) | 8.2 (5.9) | 8.5 (5.3) | F(2, 130) = 0.38, p = 0.68 |
| Geriatric Depression Scale – total score | 1.6 (1.2) | 2.0 (1.7) | 1.7 (1.6) | F(2, 130) = 0.31, p = 0.74 |
| Never Smoker | 88% | 52.2% | 35.7% n = 84 |
Fisher’s exact < 0.001 |
| Former Smoker | 12% | 34.8% | 60.7% n = 84 |
|
| Current Smoker | 0% | 13% | 3.6% n = 84 |
|
| Smoking Pack Years | 5.4 (17.9) | 17.3 (28.6) n = 22 |
15.0 (25.2) n = 82 |
F(2, 126) = 1.8, p = 0.17 |
: S.D. = standard deviation
Table 2 presents cognitive performance data by alcohol use categories. In unadjusted analyses, never/minimal alcohol users significantly scored more poorly than current alcohol users on the 3MS (coefficient −3.55, 95% CI −5.57 to −1.53, p = 0.001) and the RAVLT total words learned (coefficient −0.62, 95% CI −1.05 to −0.17, p = 0.007); never/minimal alcohol users significantly scored higher on the Useful Field of View divided attention (which means worse performance) than current alcohol users (coefficient 0.64, 95% CI 0.18 to 1.09, p = 0.006); former alcohol users significantly scored more poorly than current alcohol users on verbal (letter) fluency (coefficient −0.57, 95% CI −1.03 to −0.11, p = 0.015). The above unadjusted analyses remained significant even after trying various FDR multiple comparison correction methods (lowest overall critical p-value = 0.0167 with the yekutieli method). Adjusted analyses were controlled for age, education, sex, race and smoking pack years; the three groups did not significantly differ on any cognitive variables.
Table 2.
Cognitive Performance Data by Alcohol Use Categories.
| Never/ Minimal n = 25 |
Former n = 23 |
Current n = 85 |
Adjusted Coefficienta for comparator group (standard error), Significance and Standardized Beta |
|
|---|---|---|---|---|
| Unadjusted Mean (S.D.) | ||||
| 3MS total score | 91.8 (6.63) | 95.8 (3.50) | 95.4 (3.92) | Never/Minimal: −1.60 (1.11), p = 0.14, β = −0.13 Former: 1.17 (1.02), p = 0.26, β = 0.09 |
| Composite z score at baseline |
0.02 (0.24) n = 23 |
−0.02 (0.27) n = 22 |
0.003 (0.31) n = 77 |
Never/Minimal: 0.07 (0.07), p = 0.30, β = 0.11 Former: 0.06 (0.07), p = 0.41, β = 0.08 |
| Verbal (letter) fluency z-score | −0.13 (1.08) | −0.40 (0.87) | 0.18 (0.99) | Never/Minimal: 0.15 (0.24), p = 0.52, β = 0.06 Former: −0.27 (0.23), p = 0.24, β = −0.10 |
| Category fluency z-score | −0.20 (1.24) | −0.10 (0.91) | 0.07 (0.95) | Never/Minimal: 0.08 (0.23), p = 0.72, β = 0.03 Former: −0.005 (0.23), p = 0.98, β = −0.002 |
| RAVLT delayed recall z-score | −0.13 (0.95) | 0.16 (0.83) | 0.06 (1.03) n = 83 |
Never/Minimal: −0.004 (0.22), p = 0.99, β = −0.02 Former: 0.24 (0.22), p = 0.27, β = 0.10 |
| RAVLT total words learned z-score |
−0.48 (1.07) | 0.23 (0.87) | 0.12 (0.98) n = 83 |
Never/Minimal: −0.27 (0.21), p = 0.21, β = −0.11 Former: 0.27 (0.20), p = 0.18, β = 0.10 |
| Digit Symbol Substitution z-score | 0.03 (1.08) | 0.01 (1.08) | 0.02 (0.97) n = 83 |
Never/Minimal: 0.23 (0.24), p = 0.34, β = 0.09 Former: 0.15 (0.23), p = 0.52, β = 0.06 |
| Trails A z-score | 0.11 (1.03) | 0.06 (0.90) n = 22 |
−0.06 (1.02) n = 82 |
Never/Minimal: −0.06 (0.24), p = 0.82, β = −0.02 Former: 0.002 (0.24), p = 0.99, β = 0.001 |
| Trails B z-score | 0.32 (1.23) | 0.13 (1.16) | −0.12 (0.88) n = 83 |
Never/Minimal: 0.11 (0.24), p = 0.65, β = 0.04 Former: 0.12 (0.23), p = 0.60, β = 0.05 |
| Eriksen Flanker – congruent z-score |
0.05 (0.93) | −0.28 (1.31) | 0.02 (0.84) n = 83 |
Never/Minimal: 0.08 (0.24), p = 0.74, β = 0.03 Former: −0.20 (0.23), p = 0.38, β = −0.08 |
| Eriksen Flanker – incongruent z-score |
−0.06 (0.97) | −0.17 (0.94) | 0.003 (0.90) n = 83 |
Never/Minimal: 0.002 (0.24), p = 0.99, β = 0.001 Former: −0.06 (0.23), p = 0.79, β = −0.03 |
| Useful Field of View – processing z-score |
0.19 (1.27) n = 24 |
−0.02 (1.21) | −0.04 (0.86) n = 81 |
Never/Minimal: −0.005 (0.26), p = 0.98, β = −0.002 Former: −0.07 (0.25), p = 0.79, β = −0.02 |
| Useful Field of View – divided attention z-score |
0.47 (1.21) n = 24 |
0.10 (1.21) | −0.16 (0.84) n = 81 |
Never/Minimal: 0.42 (0.24), p = 0.08, β = 0.16 Former: 0.16 (0.23), p = 0.48, β = 0.06 |
| Useful Field of View – selective attention z-score |
0.11 (0.88) n = 23 |
0.27 (1.24) | −0.10 (0.96) n = 78 |
Never/Minimal: 0.01 (0.24), p = 0.96, β = 0.005 Former: 0.31 (0.23), p = 0.19, β = 0.12 |
: Controlled for age, education, sex, race and smoking pack years with “current drinkers” serving as the standardization reference.
Table 3 presents cognitive performance data by alcohol drink years. For former users, the mean number of drink years was 27.3 (standard deviation 44.7, range 2 to 198). For current users, the mean number of drink years was 35.5 (standard deviation 35.4, range 4 to 192). In unadjusted analyses, drink years was significantly associated with verbal (letter) fluency in current alcohol users (coefficient 0.006, 95% CI 0.0004 to 0.012, p = 0.036), and drink years was significantly associated with Useful Field of View divided attention in former alcohol users (coefficient 0.012, 95% CI 0.0009 to 0.023, p = 0.035). The above unadjusted analyses remained significant even after trying various FDR multiple comparison correction methods (lowest overall critical p-value = 0.0476 with the krieger method). Adjusted analyses were again controlled for age, education, sex, race and smoking pack years; the three groups again did not significantly differ on any cognitive variables.
Table 3.
Cognitive Performance Data by Alcohol Drink Years.
| All | Former | Current | |
|---|---|---|---|
| Adjusted Coefficienta (standard error), Significance and Standardized Beta | |||
| 3MS total score | 0.01 (0.01), p = 0.21, β = 0.10 n = 127 |
0.03 (0.02), p = 0.23, β = 0.36 n = 22 |
0.009 (0.01), p = 0.46, β = 0.08 n = 80 |
| Composite z score at baseline | −0.0001 (0.0008), p = 0.88, β = −0.01 n = 117 |
−0.001 (0.001), p = 0.39, β = −0.21 n = 21 |
−0.00004 (0.001), p = 0.97, β = −0.005 n = 73 |
| Verbal (letter) fluency z-score | 0.003 (0.002), p = 0.28, β = 0.09 n = 127 |
−0.001 (0.005), p = 0.85, β = −0.05 n = 22 |
0.005 (0.003), p = 0.08, β = 0.20 n = 80 |
| Category fluency z-score | −0.001 (0.002), p = 0.64, β = −0.04 n = 127 |
0.006 (0.006), p = 0.37, β = 0.28 n = 22 |
−0.002 (0.003), p = 0.49, β = −0.07 n = 80 |
| RAVLT delayed recall z-score | 0.0005 (0.002), p = 0.83, β = 0.02 n = 126 |
−0.004 (0.005), p = 0.49, β = −0.19 n = 22 |
0.001 (0.003), p = 0.67, β = 0.05 n = 79 |
| RAVLT total words learned z-score | 0.0002 (0.002), p = 0.92, β = 0.008 n = 126 |
0.0004 (0.005), p = 0.93, β = 0.02 n = 22 |
−0.003 (0.003), p = 0.32, β = −0.10 n = 79 |
| Digit Symbol Substitution z-score | −0.001 (0.002), p = 0.72, β = −0.03 n = 126 |
0.001 (0.008), p = 0.88, β = 0.04 n = 22 |
0.0008 (0.003), p = 0.80, β = 0.03 n = 79 |
| Trails A z-score | −0.001 (0.002), p = 0.68, β = −0.04 n = 124 |
−0.003 (0.005), p = 0.63, β = −0.13 n = 21 |
−0.002 (0.003), p = 0.47, β = −0.08 n = 78 |
| Trails B z-score | −0.001 (0.002), p = 0.66, β = −0.04 n = 126 |
−0.01 (0.007), p = 0.11, β = −0.40 n = 22 |
0.0007 (0.003), p = 0.79, β = 0.03 n = 79 |
| Eriksen Flanker – congruent z-score | 0.0003 (0.002), p = 0.91, β = 0.01 n = 126 |
−0.005 (0.01), p = 0.66, β = −0.15 n = 22 |
0.001 (0.003), p = 0.59, β = 0.06 n = 79 |
| Eriksen Flanker – incongruent z-score | −0.0005 (0.002), p = 0.84, β = −0.02 n = 126 |
−0.01 (0.006), p = 0.13, β = −0.46 n = 22 |
0.0004 (0.003), p = 0.88, β = 0.02 n = 79 |
| Useful Field of View – processing z-score | 0.004 (0.003), p = 0.17, β = 0.12 n = 123 |
−0.003 (0.008), p = 0.72, β = −0.10 n = 22 |
0.004 (0.003), p = 0.17, β = 0.14 n = 77 |
| Useful Field of View – divided attention z-score | 0.0003 (0.003), p = 0.90, β = 0.01 n = 123 |
0.01 (0.007), p = 0.16, β = 0.38 n = 22 |
−0.003 (0.003), p = 0.22, β = −0.13 n = 77 |
| Useful Field of View – selective attention z-score | 0.003 (0.003), p = 0.34, β = 0.08 n = 119 |
0.007 (0.008), p = 0.42, β = 0.22 n = 22 |
−0.0004 (0.003), p = 0.91, β = −0.01 n = 74 |
: Controlled for age, education, sex, race and smoking pack years
For the Useful Field of View processing z-score in Tables 2 and 3, the Q-Q plot and kernel density plot of the residuals showed a skewed distribution; a bootstrapping procedure using percentile-based confidence intervals (1000 replications specified) did not significantly change the coefficient estimates. For the 3MS total score, Composite z-score at baseline, Verbal (letter) fluency z-score, Category fluency z-score, RAVLT delayed recall z-score, RAVLT total words learned z-score, Digit Symbol Substitution z-score, Trails A z-score, Eriksen Flanker congruent z-score, Eriksen Flanker incongruent z-score, and Useful Field of View processing z-score in Table 3, the CPR plots appeared to show a slight departure from linearity. Restricted cubic splines were created, but statistically significant departures from linearity were not found.
Regarding confounding variables, smoking was alternatively controlled for as a categorical variable (never/former/current). However, whether smoking was included continuously as pack years or categorically as never/former/current made no significant difference in any adjusted analysis; we kept smoking as a continuous variable in all adjusted analyses. Regarding income and occupation, though we initially conceptualized these to be confounding variables, income and occupation caused multicollinearity issues (variance inflation factor values > 10; tolerance values < 0.1) in some analyses in Table 3. Whether we included or excluded income and occupation made no significant difference in any adjusted analysis; we removed these variables from all adjusted analyses in Tables 2 and 3. Regarding education, education could be viewed as a mediator for early-life cognitive ability50 instead of a confounding variable; however, whether we included or excluded education made no significant difference in any adjusted analysis (after correcting for multiple comparisons). We kept education in the final adjusted analyses in order to stay consistent with publications that use some of the same neurocognitive tests used in this study and adjust for education (e.g., Trails A and B51).
DISCUSSION
In this secondary analysis of older adults, we found that never/minimal alcohol users, former alcohol users, and current alcohol users did not significantly differ on any cognitive performance measures after adjusting for age, education, sex, race and smoking pack years. The number of alcohol drink years was not significantly associated with cognitive performance among current alcohol users and former alcohol users.
Interestingly, the unadjusted analyses showed some significant associations between different types of alcohol use and cognitive performance. Never/minimal alcohol users performed worse on some cognitive measures compared to current alcohol users. These unadjusted associations show that this dataset had enough power to detect significant associations. These unadjusted associations are also consistent with several prior studies that showed worse cognitive performance among those who do not drink alcohol or have stopped drinking alcohol (e.g.,23–26, 28, 30, 31, 39), compared to those who currently drink alcohol. However, these unadjusted associations were eliminated after controlling for the variables that we presented in Figure 2. This shows the importance of carefully adjusting for confounders and not adjusting for mediators or colliders in analyses by using a more modern graphical method of adjustment, such as a DAG-based approach. Significant associations between alcohol use and cognitive performance in previous studies may have been due to uncontrolled confounding and/or unnecessary adjustment of mediators and/or colliders.
This manuscript has several strengths. First, a detailed neurocognitive battery allowed for the analysis of various cognitive domains. Second, while the DAG-based approach is by no means the definitive approach to variable selection in a regression model, and other clinical researchers might differently conceptualize the variables that we included in the regression models, we used this approach as a reference with which to compare future publications on this topic. Other approaches for variable selection include, but are not limited to, forward selection, backward selection, and stepwise selection49. By using a DAG, at minimum, our goal was to avoid including colliders (which can falsely induce an association) or mediators (which can ameliorate an association) in the regression models49. To our knowledge, a DAG-based approach to variable selection has not been previously used in the literature of alcohol use and cognitive performance in older adults. Previous studies that have not used a DAG-based approach may have unknowingly included colliders or mediators, and this may contribute to inconsistency in the literature. Third, we were able to capture a lifetime history of alcohol use with the metric of alcohol drink years. Finally, we analyzed the cognitive data not only by alcohol use categories, but we were able to assess dose response by analyzing alcohol drink years.
This manuscript undoubtedly has limitations. First, the research questions asked in this secondary analysis were not considered when the study was originally designed. In addition, the participants recruited were generally higher educated, and this may limit external generalizability to groups with lower education. Second, for an observational study, the sample size is small. Though violations of regression model assumptions were checked, the significantly smaller number of never/minimal and former drinkers will still be more susceptible to sampling error. A larger sample size would also allow for more advanced statistical modeling techniques, such as latent variable modeling for the neurocognitive measures or structural equation modeling. A larger sample, which would result in larger statistical power, might have also allowed the discovery of some significant associations between alcohol use and cognitive performance. Third, due to the small sample size, the regression models did not explicitly examine for interactions among the predictors. There may have been interactions that served as significant moderators. Finally, while this sample is unlikely to have other substance use, urine toxicology may have helped confirm a lack of other substance use during the study period.
CONCLUSIONS
In summary, this secondary analysis of older adults found that alcohol use was not associated with cognitive performance after adjustment for key confounders using a DAG-based approach. Future directions include larger sample sizes to examine smaller effect sizes, including individuals with alcohol use disorders, selecting different variables based on an updated DAG, conducting other variable selection procedures and confirming lack of other substance use with urine toxicology.
Acknowledgments
None.
Source of Funding: Dr. Kalapatapu is currently funded by K23DA034883. The Mental Activity and eXercise (MAX) Trial was funded by the National Institutes of Health (K01AG024069) and the Alzheimer’s Association (IIRG-06-27306). This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR991872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Contributors: Dr. Kalapatapu & Dr. Barnes completed the background literature search. Dr. Kalapatapu completed the statistical analyses with guidance from Dr. Barnes. Dr. Kalapatapu wrote the 1st draft of the manuscript. All authors have approved the final manuscript.
Declaration of Interests: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
References
- 1.Wu L-T, Blazer DG. Substance use disorders and psychiatric comorbidity in mid and later life: a review. International Journal of Epidemiology. 2014;43(2):304–317. doi: 10.1093/ije/dyt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalapatapu RK, Paris P, Neugroschl JA. Alcohol Use Disorders in Geriatrics. The International Journal of Psychiatry in Medicine. 2010;40(3):321–337. doi: 10.2190/PM.40.3.g. [DOI] [PubMed] [Google Scholar]
- 3.Kalapatapu RK, Sullivan MA. Prescription use disorders in older adults. Am J Addict. 2010;19(6):515–522. doi: 10.1111/j.1521-0391.2010.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen D, Engel RJ, Hunsaker AE, Engel Y, Detlefsen EG, Reynolds CF., 3rd Just say know: an examination of substance use disorders among older adults in gerontological and substance abuse journals. Soc Work Public Health. 2013;28(3–4):377–387. doi: 10.1080/19371918.2013.774668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Searby A, Maude P, McGrath I. Prevalence of co-occurring alcohol and other drug use in an Australian older adult mental health service. Int J Ment Health Nurs. 2016 doi: 10.1111/inm.12215. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead NE, Trenz RC, Keen Ln, Rose J, Latimer WW. Younger versus older African Americans: patterns and prevalence of recent illicit drug use. J Ethn Subst Abuse. 2014;13(2):126–138. doi: 10.1080/15332640.2014.883581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bommersbach TJ, Lapid MI, Rummans TA, Morse RM. Geriatric alcohol use disorder: a review for primary care physicians. Mayo Clin Proc. 2015;90(5):659–666. doi: 10.1016/j.mayocp.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Draper B, Ridley N, Johnco C, Withall A, Sim W, Freeman M, Contini E, Lintzeris N. Screening for alcohol and substance use for older people in geriatric hospital and community health settings. Int Psychogeriatr. 2015;27(1):157–166. doi: 10.1017/S1041610214002014. [DOI] [PubMed] [Google Scholar]
- 9.NIDA. Drug Abuse in the 21st Century - Science Meeting Summaries & Special Reports. 2016 [Google Scholar]
- 10.Hurt RD, Finlayson RE, Morse RM, Davis LJ., Jr Alcoholism in elderly persons: medical aspects and prognosis of 216 inpatients. Mayo Clin Proc. 1988;63(8):753–760. doi: 10.1016/s0025-6196(12)62354-4. [DOI] [PubMed] [Google Scholar]
- 11.Oslin DW. Evidence-based treatment of geriatric substance abuse. Psychiatr Clin North Am. 2005;28(4):897–911. ix. doi: 10.1016/j.psc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- 13.Kalapatapu RK, Delucchi KL, Wang S, Harbison JD, Nelson EE, Kramer JH. Substance use history in behavioral-variant frontotemporal dementia versus primary progressive aphasia. J Addict Dis. 2015:1–6. doi: 10.1080/10550887.2015.1102026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalapatapu RK, Lewis DF, Vinogradov S, Batki SL, Winhusen T. Relationship of age to impulsivity and decision making: a baseline secondary analysis of a behavioral treatment study in stimulant use disorders. J Addict Dis. 2013;32(2):206–216. doi: 10.1080/10550887.2013.795471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgenstern J, Naqvi NH, Debellis R, Breiter HC. The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychol Addict Behav. 2013;27(2):336–350. doi: 10.1037/a0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durazzo TC, Pennington DL, Schmidt TP, Mon A, Abe C, Meyerhoff DJ. Neurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smoking. Alcohol Clin Exp Res. 2013;37(10):1794–1803. doi: 10.1111/acer.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadet JL, Bisagno V. The primacy of cognition in the manifestations of substance use disorders. Front Neurol. 2013;4(189) doi: 10.3389/fneur.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadardi JS, Cox WM, Rahmani A. Neuroscience of attentional processes for addiction medicine: from brain mechanisms to practical considerations. Prog Brain Res. 2016;223(77–89) doi: 10.1016/bs.pbr.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Franken IH, van de Wetering BJ. Bridging the gap between the neurocognitive lab and the addiction clinic. Addict Behav. 2015;44(108–114) doi: 10.1016/j.addbeh.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Dowling GJ, Weiss SR, Condon TP. Drugs of abuse and the aging brain. Neuropsychopharmacology. 2008;33(2):209–218. doi: 10.1038/sj.npp.1301412. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar-Navarro SG, Reyes-Guerrero J, Borgues G. Cognitive impairment and alcohol and cigarette consumption in Mexican adults older than 65 years. Salud Publica Mex. 2007;49(Suppl 4):S467–S474. doi: 10.1590/s0036-36342007001000005. [DOI] [PubMed] [Google Scholar]
- 22.Zuccala G, Onder G, Pedone C, Cesari M, Landi F, Bernabei R, Cocchi A. Dose-related impact of alcohol consumption on cognitive function in advanced age: results of a multicenter survey. Alcohol Clin Exp Res. 2001;25(12):1743–1748. [PubMed] [Google Scholar]
- 23.Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Alcohol consumption and cognitive impairment in older men: a mendelian randomization study. Neurology. 2014;82(12):1038–1044. doi: 10.1212/WNL.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bond GE, Burr R, McCurry SM, Rice MM, Borenstein AR, Kukull WA, Teri L, Bowen JD, McCormick WC, Larson EB. Alcohol, gender, and cognitive performance: a longitudinal study comparing older Japanese and non-Hispanic white Americans. J Aging Health. 2004;16(5):615–640. doi: 10.1177/0898264304268587. [DOI] [PubMed] [Google Scholar]
- 25.Virtaa JJ, Jarvenpaa T, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, Kaprio J. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939–948. doi: 10.3233/JAD-2010-100870. [DOI] [PubMed] [Google Scholar]
- 26.Lindeman RD, Wayne SJ, Baumgartner RN, Garry PJ. Cognitive function in drinkers compared to abstainers in the New Mexico elder health survey. J Gerontol A Biol Sci Med Sci. 2005;60(8):1065–1070. doi: 10.1093/gerona/60.8.1065. [DOI] [PubMed] [Google Scholar]
- 27.Hendrie HC, Gao S, Hall KS, Hui SL, Unverzagt FW. The relationship between alcohol consumption, cognitive performance, and daily functioning in an urban sample of older black Americans. J Am Geriatr Soc. 1996;44(10):1158–1165. doi: 10.1111/j.1532-5415.1996.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 28.Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65(8):1210–1217. doi: 10.1212/01.wnl.0000180520.35181.24. [DOI] [PubMed] [Google Scholar]
- 29.Edelstein SL, Kritz-Silverstein D, Barrett-Connor E. Prospective association of smoking and alcohol use with cognitive function in an elderly cohort. J Womens Health. 1998;7(10):1271–1281. doi: 10.1089/jwh.1998.7.1271. [DOI] [PubMed] [Google Scholar]
- 30.Horvat P, Richards M, Kubinova R, Pajak A, Malyutina S, Shishkin S, Pikhart H, Peasey A, Marmot MG, Singh-Manoux A, Bobak M. Alcohol consumption, drinking patterns, and cognitive function in older Eastern European adults. Neurology. 2015;84(3):287–295. doi: 10.1212/WNL.0000000000001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Touvier M, Hercberg S, Galan P. Alcohol consumption in midlife and cognitive performance assessed 13 years later in the SU.VI.MAX 2 cohort. PLoS One. 2012;7(12):e52311. doi: 10.1371/journal.pone.0052311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leroi I, Sheppard JM, Lyketsos CG. Cognitive function after 11.5 years of alcohol use: relation to alcohol use. Am J Epidemiol. 2002;156(8):747–752. doi: 10.1093/aje/kwf107. [DOI] [PubMed] [Google Scholar]
- 33.Vincze G, Almos P, Boda K, Dome P, Bodi N, Szlavik G, Magloczki E, Pakaski M, Janka Z, Kalman J. Risk factors of cognitive decline in residential care in Hungary. Int J Geriatr Psychiatry. 2007;22(12):1208–1216. doi: 10.1002/gps.1815. [DOI] [PubMed] [Google Scholar]
- 34.Lopes MA, Furtado EF, Ferrioli E, Litvoc J, Bottino CM. Prevalence of alcohol-related problems in an elderly population and their association with cognitive impairment and dementia. Alcohol Clin Exp Res. 2010;34(4):726–733. doi: 10.1111/j.1530-0277.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 35.Schinka JA, Belanger H, Mortimer JA, Borenstein Graves A. Effects of the use of alcohol and cigarettes on cognition in elderly African American adults. J Int Neuropsychol Soc. 2003;9(5):690–697. doi: 10.1017/S1355617703950028. [DOI] [PubMed] [Google Scholar]
- 36.Christian JC, Reed T, Carmelli D, Page WF, Norton JA, Jr, Breitner JC. Self-reported alcohol intake and cognition in aging twins. J Stud Alcohol. 1995;56(4):414–416. doi: 10.15288/jsa.1995.56.414. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie SJ, Bates TC, Corley J, McNeill G, Davies G, Liewald DC, Starr JM, Deary IJ. Alcohol consumption and lifetime change in cognitive ability: a gene × environment interaction study. Age (Dordr) 2014;36(3):9638. doi: 10.1007/s11357-014-9638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross AL, Rebok GW, Ford DE, Chu AY, Gallo JJ, Liang KY, Meoni LA, Shihab HM, Wang NY, Klag MJ. Alcohol consumption and domain-specific cognitive function in older adults: longitudinal data from the Johns Hopkins Precursors Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(1):39–47. doi: 10.1093/geronb/gbq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galanis DJ, Joseph C, Masaki KH, Petrovitch H, Ross GW, White L. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: the Honolulu-Asia Aging Study. Am J Public Health. 2000;90(8):1254–1259. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res. 2000;24(10):1510–1516. [PubMed] [Google Scholar]
- 41.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 42.Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173(9):797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Cognitive and Behavioral Neurology. 1988;1(2):111–118. [Google Scholar]
- 44.Pa J, Goodson W, Bloch A, King AC, Yaffe K, Barnes DE. Effect of exercise and cognitive activity on self-reported sleep quality in community-dwelling older adults with cognitive complaints: a randomized controlled trial. J Am Geriatr Soc. 2014;62(12):2319–2326. doi: 10.1111/jgs.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 46.Survey NHI. Adult Tobacco Use Information: Centers for Disease Control and Prevention. 2015 [Google Scholar]
- 47.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8(70) doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 49.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models. Springer; 2012. [Google Scholar]
- 50.Corley J, Jia X, Brett CE, Gow AJ, Starr JM, Kyle JA, McNeill G, Deary IJ. Alcohol intake and cognitive abilities in old age: the Lothian Birth Cohort 1936 study. Neuropsychology. 2011;25(2):166–175. doi: 10.1037/a0021571. [DOI] [PubMed] [Google Scholar]
- 51.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]