Abstract
Inherited ataxias are an extremely heterogeneous group of disorders. Autosomal recessive spinocerebellar ataxia 20 (SCAR20) is a recently described disorder characterized by intellectual disability, ataxia, coarse facial features, progressive loss of Purkinje cells in the cerebellum and often hearing loss and skeletal abnormalities. Mutations in the gene SNX14, which plays an important role in autophagy, have been found to cause SCAR20. The unique clinical findings of progressive coarsening of facial features makes the clinical phenotype recognizable among the various hereditary ataxias. Here we report on a child with a novel missense mutation in the SNX14 gene that appears to be debilitating for protein conformation, function and review the previously reported cases from 15 families.
Keywords: Autosomal recessive spinocerebellar ataxia 20, hereditary ataxia, SNX14, autophagy, cerebellar atrophy
INTRODUCTION
Autosomal recessive spinocerebellar ataxias are a clinically and genetically heterogeneous group of early-onset disorders associated with cerebellar atrophy or hypoplasia, imbalance and uncoordinated gait. Multisource population based studies have estimated their prevalence at an average 3.3 per 100,000 (Ruano et al., 2014). The common forms of recessive childhood ataxias include Friedreich ataxia, ataxia-telangiectasia, and ataxia oculomotor apraxia. The uncommon forms constitute a highly heterogeneous group of disorders with early onset ataxia as the predominant feature. Intellectual disability is present in more than 60% of these patients (Poretti et al., 2014). The precise molecular etiology has been elucidated in only a small number of these conditions.
Autosomal recessive spinocerebellar ataxia 20 (SCAR20 [MIM 616354]) is a recently described disorder characterized by early onset cerebellar atrophy or hypoplasia, severe ataxia, neurodegeneration due to Purkinje cell loss, coarse facial features, hypotonia, developmental delay and severe intellectual disability caused by loss of function mutations in SNX14, encoding for a sorting nexin (Akizu et al., 2015; Sousa et al., 2013; Thomas et al., 2014). The distinct dysmorphism associated with this syndrome makes it a clinically recognizable disorder. SCAR20 is part of an emerging group of conditions with underlying defect in the degradation of intracellular proteins and organelles termed macroautophagy (Ebrahimi-Fakhari et al., 2016).
In the present study we describe a consanguineous Indian family with one child affected with SCAR20. Using exome sequencing we delineated a homozygous missense mutation in SNX14 coding region as its underlying molecular cause. We discuss our molecular and clinical findings in the light of the previous studies and review the currently known salient features of SNX14-linked spinocerebellar ataxia.
CLINICAL REPORT
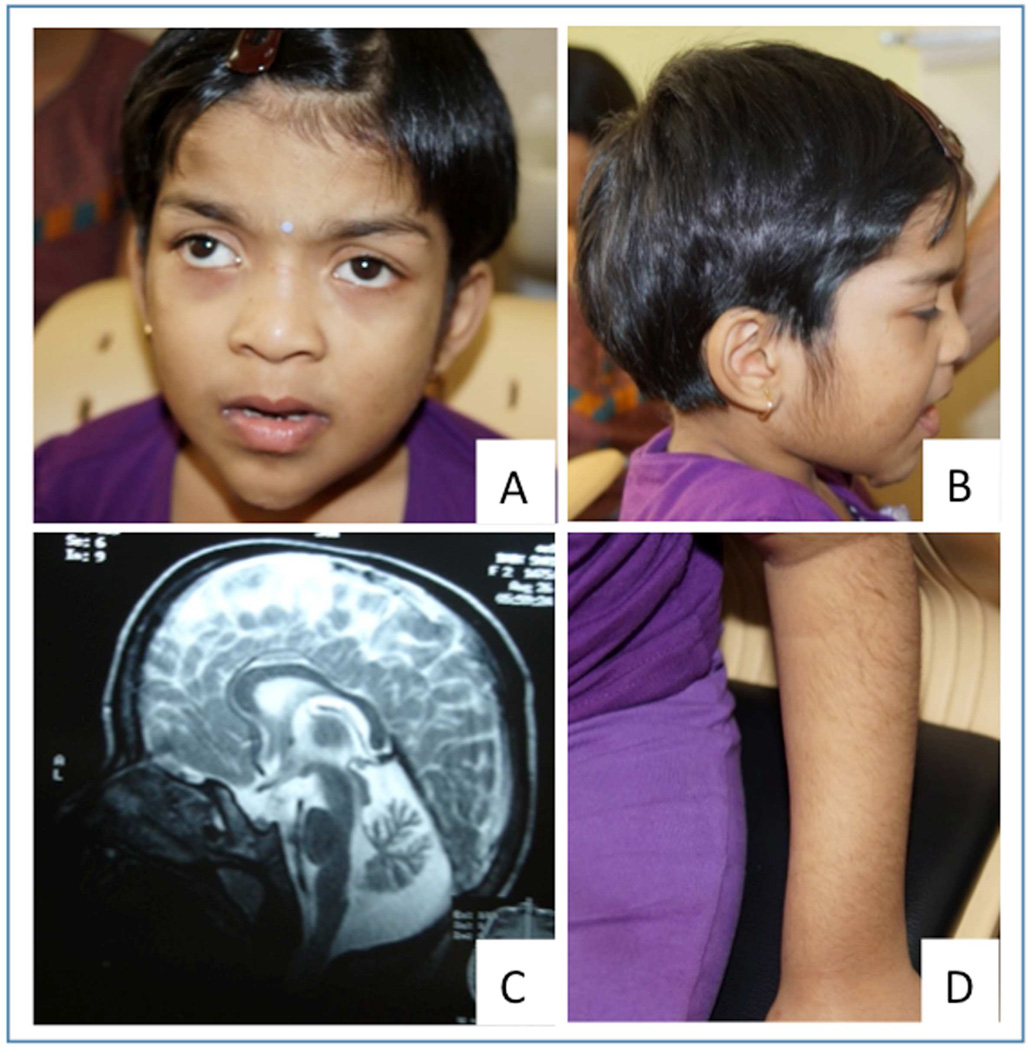
The proband was evaluated at 6 years and 9 months. She was born at term by lower segment cesarean section to a consanguineously married couple (Fig. 2A). She weighed 3.75 kg (normal) at birth. Global developmental delay was recognized in late infancy. She attained head control at 6 months of age and could crawl at 1 year and 3 months. She is able to walk with support and has frequent falls. There is no speech till date but is able to comprehend simple commands. There is no history of seizures in her. Behavioral abnormalities particularly aggression were noted in her. At the time of examination her weight was 21 kg (normal), height was 110 cm (normal) and head circumference was 52 cm (normal). Small forehead, thick eyebrows with lateral flaring, telecanthus, broad bridge and base of nose, long philtrum, thick and everted vermillion of the lower lip with small and narrow chin were noted (Fig. 1A). Dental caries were present. She had increased facial and body hair (Fig. 1B and 1D). Both gait and truncal ataxia were present. Tone and deep tendon reflexes were normal. There were no contractures or laxity. The child has normal hearing. Rest of the systemic examination was unremarkable. Her skeletal radiographs and karyotype did not show any abnormality. Magnetic resonance imaging of the brain revealed cerebellar atrophy with normal brain stem structures (Fig. 1C).
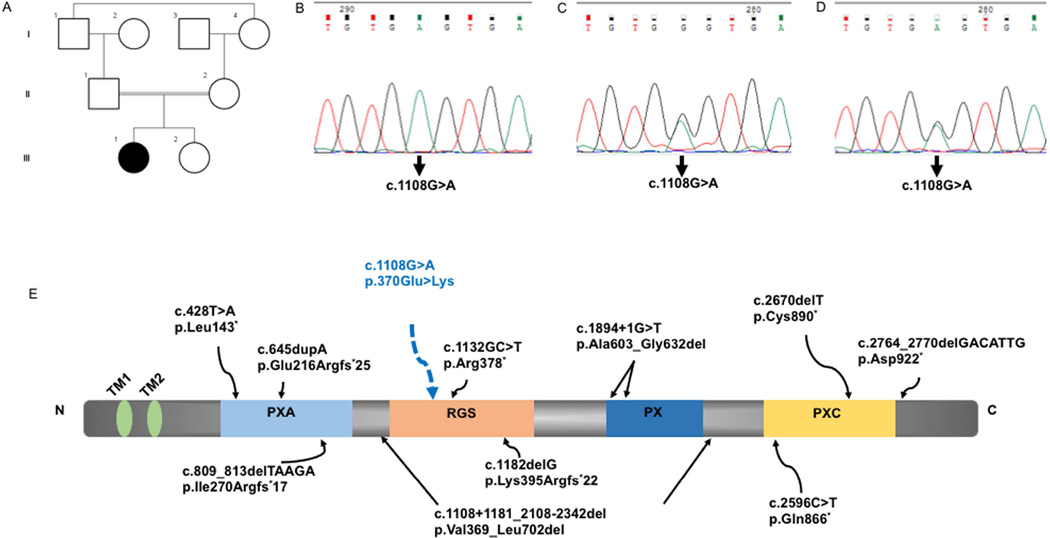
Fig. 2.
Family pedigree (A). Sanger sequencing in the proband showing homozygous c.1108G>A variation in SNX14 (B), heterozygous in mother (C) and father (D). SNX14 gene with the two transmembrane, four conserved domains with the mutation in proband in the RGS domain (E). The location of previously described pathogenic SNX14 variants are also indicated (E).
Fig. 1.
Subject at age 6 years and nine months. Note the telecanthus, strabismus, puffy eyelids, long and deep philtrum, broad bridge and base of nose, thick vermillions of upper and lower lip, pointed chin (A) and increased facial and body hair (B,D). Magnetic resonance imaging of brain shows cerebellar atrophy with normal brainstem structures (C)
Chromosomal microarray
Chromosomal microarray revealed no copy number changes. However, large regions of homozygosity were detected on chromosomes 1, 2, 3, 6, 7, 8, 9 and 10.
Exome sequencing
Genomic DNA was extracted from the whole blood using the standard phenol-chloroform method. Genomic capture was carried out with Illumina’s Nextera Rapid Capture Exome Kit. Massively parallel sequencing was done using the NextSeq500 Sequencer (Illumina, Inc., San Diego, CA, USA.) in combination with the NextSeq™ 500 High Output Kit (2 × 150 bp). Raw sequencing reads were subjected to quality control and aligned to the GRCh37 (hg19) build of the human reference genome using bwa software with mem algorithm. The primary alignment files based on GATK best practices recommendation were used for variant calling using three different variant callers (GATK HaplotypeCaller, freebayes and samtools). Variants were annotated using Annovar and in-house ad hoc bioinformatics tools. Alignments were visually verified with the Integrative Genomics Viewer v.2.3 and Alamut v.2.4.5 (Interactive Biosoftware, Rouen, France). Variant prioritization was performed without bias with a cascade of filtering steps. A homozygous variant p.E370K (c.1108G>A, NM_153816.5) was identified in the proband in exon 12 of SNX14. The variant information is submitted to ClinVar database in NCBI (Submission ID: SUB2086432). It is not present in heterozygous or homozygous state in The Exome Aggregation Consortium (ExAc), 1000 Genomes and Varsome. It is also absent in exomes of 139 unrelated individuals from local population. Further, functional in silico prediction tools SIFT, PolyPhen2 and Mutation Taster are consistent in predicting the damaging/disease causing nature of this variant as damaging, probably damaging and disease causing respectively. No other sequence variants of pathogenic significance were identified. The variant was validated by Sanger sequencing in the proband (Fig. 2B). Targeted testing of the parents using Sanger sequencing confirmed the variation to be heterozygous in them (Fig.2C, D) and absent in her unaffected sibling (data not shown). Informed consent was obtained from the family in accordance with the guidelines prescribed by the institutional ethics committee. Specific parental consent was obtained for the use of photographs, clinical and research findings for publication.
Protein structure prediction
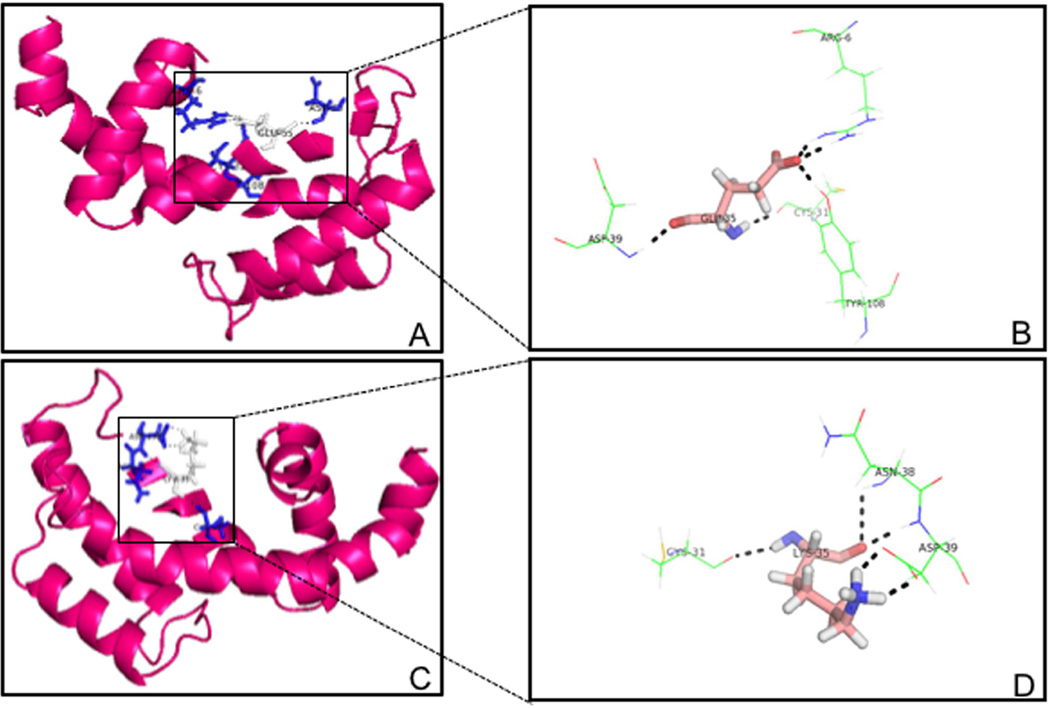
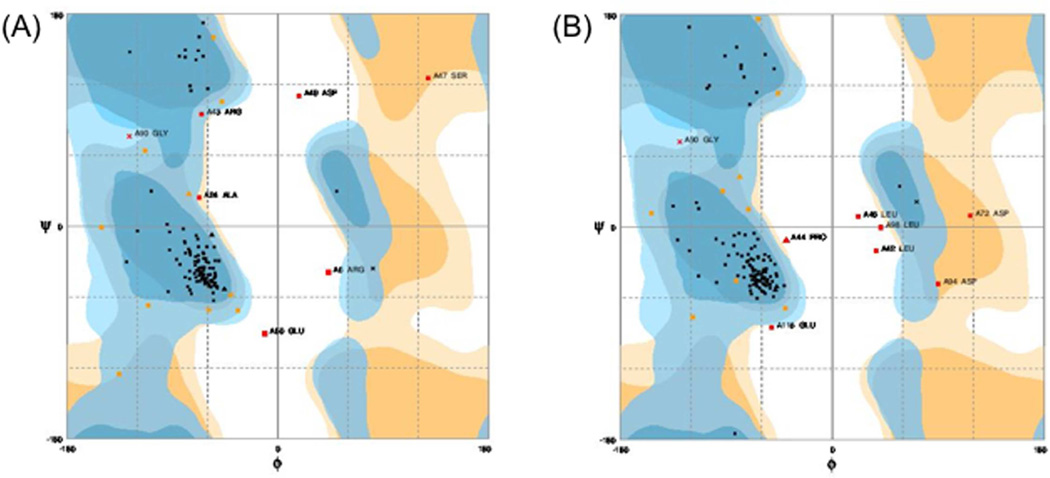
Due to the absence of a homologous three dimensional (3D) structure for the RGS domain of Snx14 in the RCSB Protein Data Bank, we have used I-TASSER (Iterative Threading ASSEmbly Refinement) server, an online platform for predicting protein structure and function. The best model for the wild-type RGS domain of Snx14 had a C score of 0.18, TM score of 0.74 ± 0.11 and RMSD score of 4.2±2.8 Å (Fig. 3). While the model for the mutant RGS domain (p.E370K) had a C score of 0.21, TM score of 0.74 ± 0.11 and RMSD score of 4.1±2.8 Å. The resultant molecular structure of the RGS domain of Snx14 (wild-type and mutant) and the hydrogen bonding interactions were visualized by PyMOL (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC). Substitution of the wild-type (Glutamic acid) at the 370 amino-acid position with the mutant (Lysine) residue appeared to result in the loss of long-distance hydrogen bonding interactions (Fig. 3). The ProSA (Protein Structure Analysis) web server was used for refinement and validation of protein structures (Wiederstein and Sippl, 2007). Further it was used for checking model structural quality with potential errors, and the resultant ??-scores were used to determine the overall quality of the model. This revealed that the mutant RGS domain was distinct from the wild-type as the Z score for the former was −7.01 and that of the latter was −6.06. Finally the Ramachandran plot assessment for the native and mutant RGS domain of Snx14 was carried out using RAMPAGE web-based server and is depicted in Fig. 4. In the plot, the native protein of phi/psi angles for 87% residues was in the most favored region, 7.6% residues were in the additionally allowed regions, while 5.3% residues were in the disallowed regions (Fig. 4A). In contrast, the analyses of the mutant RGS domain structure revealed that 87%, 6.9%, and 6.1% residues lie in the most favored, additionally allowed, and disallowed regions, respectively (Fig. 4B). These results indicated that the incorporated mutation p.E370K enforced shifting of residues towards the disallowed region from the additionally allowed regions in the mutant RGS domain. Taken together it appears that the mutation c. 1108G>A (p.E370K) in Snx14 has a likely debilitating effect on the RGS domain conformation and maybe detrimental to its function.
Fig. 3.
Predicted models of the wild-type and mutant RGS domain of Snx14. (A) The wild-type RGS domain structure depicting the native Glutamic acid residue and its predicted H-bonding interactions. Note while the Glu residue occurs at position 370 in the context of the entire Snx14 protein, it is depicted here as Glu35, as only the RGS domain of Snx14 has been modeled here. (B) A magnified view of the native Glu residue and interactions. We note that in the wild-type state the Glu35 residue is predicted to be interacting via H-bonds with distant residues such as Arg and Tyr. (C) The predicted mutant RGS domain structure, (p.E370K) where a Lys residue occurs in place of Glu, at position 370 with regards to the whole Snx14 protein and position 35 in the context of the RGS domain alone. (D) A magnified view of the mutant Lys residue and its interactions. We note that the occurrence of a mutant Lys residue at this position appears to significantly alter the native interactions seen in this context, such that all long distance H-bonding interactions appear to be absent.
Fig. 4. The Ramachandran plot of Snx14 wild-type and mutant RGS domain models.
Plots for (A) wild-type and (B) mutant Snx14 RGS domain. Note that the most favored regions, additionally allowed regions, and disallowed regions are indicated in dark blue, light blue and white respectively.
DISCUSSION
The definitive clinical diagnosis of childhood onset ataxias, cerebellar atrophy and intellectual disability is challenging. Often it is aided by the presence of associated features in these disorders. SCAR20 is a recently described progressive disorder with a recognizable phenotype caused by mutations in SNX14 gene. We discuss the phenotypic features in our patient ascertained through whole exome sequencing and review the features in 29 patients from 15 families reported earlier (Table S1) [Akizu et al., 2015; Sousa et al., 2013; Thomas et al., 2014]. We also discuss briefly the underlying pathophysiology of this disorder resulting in progressive cerebellar dysfunction, ataxia and coarsening of facial features.
Subjects diagnosed with SCAR20 are asymptomatic at birth. The common age of presentation for the previous reported cases is early childhood. Few children have been diagnosed as early as late infancy owing due to severe developmental delay. The maximum age of the individuals reported with this disorder is 32 years. Distinct and characteristic facial dysmorphism is the predominant clue to the clinical diagnosis in this disorder. Coarse facial features have been noted in all individuals reported till date. The characteristic facial features include increased facial hair, small forehead with frontal bossing, puffy eyelids, telecanthus, wide and depressed nasal bridge, wide base of nose, full cheeks, long and deep philtrum, often thick vermillion borders and a pointed chin.
Severe motor delay and cognitive delay is consistently seen in all the cases. Two-thirds of these children had complete lack of speech at presentation. Hypotonia was evident in most. One third individuals had significant hearing loss. Skeletal abnormalities were observed in more than half and included spinal deformities, pectus carinatum, brachydactyly and camptodactyly. Cerebellar atrophy/hypoplasia has been reported in all but two cases. Imaging was unavailable in one of the patient. Associated pontine thinning/hypoplasia has been seen in only 3 cases. Retrocerebellar cysts have been reported in seven individuals with this disorder.
SNX14 belongs to a large family of sorting nexin (SNX) proteins and the PXARGS-PX-PXC subfamily and plays a vital role in cellular trafficking, signaling and development (Worby and Dixon, 2002). It generates two transcripts consisting of either 29 exons encoding a 946 amino acid isoform a (RefSeq NM_153816.2) or 26 exons, lacking exons 14, 23, and 24, encoding a shorter protein of 893 amino acids, isoform b (RefSeq NM_020468.3). SNX14 isoforms contain two putative transmembrane domains and four conserved domains: the PX (phosphoinositide binding, Phox homology), RGS (regulator of G protein signaling) and domains of unknown function PXA (PX-associated domain A) and PXC (PX-associated domain C) (Fig. 2C) (Mas et al., 2014). The PX module in SNX14 enables it to bind specific phosphatidylinositol moieties and mediate differential intracellular trafficking (Krauss and Haucke, 2007; Mas et al., 2014). The RGS domain in SNX14 is predicted to function as a GTPase activating (GAP) module, and potentially attenuates G protein–coupled receptor (GPCR) signaling (Jean-Baptiste et al., 2006; Ross and Wilkie, 2000).
SNX14 protein has been localized to the neuronal lineages during embryonic development in mice and zebrafish, while in adult mice tissues, highest transcript levels are noted in the cerebellum and hippocampus [Akizu et al., 2015; Carroll et al., 2001]. SNX14 mRNA is expressed in all human fetal and adult tissues, but at greater levels in the cerebellum increasing steadily from embryonic to postnatal stages [Akizu et al., 2015; Thomas et al., 2014]. In human neural precursor cells (NPCs) SNX14 protein localized predominantly to the lysosomes (Akizu et al., 2015). In induced pluripotent stem cell (iPSC)- derived neuronal cells from patients with SNX14 mutations, lysosomes were found to be increased in size with impaired autophagosome clearance (Akizu et al., 2015), thus mimicking the pathogenesis of several lysosomal storage diseases (Ebrahimi-Fakhari et al., 2016). Knockdown of snx14 in a zebrafish model confirmed progressive Purkinje cell loss and neuronal cell death secondary to dysfunction in autophagy. This defect was rescued with coexpression of human SNX14 [Akizu et al., 2015].
Eleven mutations, all highly deleterious (nonsense, splicing and frameshift), across all the four conserved domains have been described in the SNX14 gene (Fig. 2C). The pathogenic variant in our subject, c.1108G>A occurs within an exon that codes for both SNX14 transcripts, resulting in a missense variation, p.E370K in the RGS domain that is predicted to be pathogenic via in silico analysis tools (Fig. 2B). This was consistent with our findings based on the predicted protein structures the of native and mutant RGS domain. The phenotype, prediction of functional consequences and segregation of the variant suggest the mutation is likely to be disease causing.
Overall SNX14 appears to function in the autophagic degradation pathway in neuronal cell types, and the disease phenotype observed in SNX14 linked spinocerebellar ataxia results from abolished or impaired SNX14 activity. There is progressive neurodegeneration and lysosomal dysfunction manifesting as progressive ataxia, cerebellar atrophy and coarsening of facial features in this disorder. Recently defects in genes in the autophagy pathway have been recognized as a distinct class of inborn errors of metabolism manifesting as progressive multisystem disorders with predominant central nervous system involvement and neurodegeneration (Ebrahimi-Fakhari et al., 2016). These disorders include EPG5-related Vici syndrome, beta-propeller protein-associated neurodegeneration due to mutations in WDR45, three forms of hereditary spastic paraplegia, SPG11, SPG15 and SPG49 caused by SPG11, ZFYVE26 and TECPR2 mutations and SNX14-associated autosomal-recessive cerebellar ataxia and intellectual disability syndrome.
This report summarizes the phenotypes and SNX14 alleles underlying autosomal recessive spinocerebellar ataxia 20. Progressive coarse facial features and cerebellar atrophy are likely to point towards the diagnosis in a child with intellectual disability and ataxia. This report also illustrates the importance of exome sequencing in such genetically heterogeneous conditions.
Supplementary Material
Acknowledgments
We thank the family who cooperated with the evaluation of the child and consented for participation in this study. The US National Institutes of Health funded the project titled “Genetic Diagnosis of Heritable Neurodevelopmental Disorders in India: Investigating the Use of Whole Exome Sequencing and Genetic Counseling to Address the High Burden of Neurodevelopmental Disorders” (1R21NS094047-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akizu N, Cantagrel V, Zaki MS, Al-Gazali L, Wang X, Rosti RO, Dikoglu E, Gelot AB, Rosti B, Vaux KK, Scott EM, Silhavy JL, Schroth J, Copeland B, Schaffer AE, Gordts PL, Esko JD, Buschman MD, Field SJ, Napolitano G, Abdel-Salam GM, Ozgul RK, Sagiroglu MS, Azam M, Ismail S, Aglan M, Selim L, Mahmoud IG, Abdel-Hadi S, Badawy AE, Sadek AA, Mojahedi F, Kayserili H, Masri A, Bastaki L, Temtamy S, Muller U, Desguerre I, Casanova JL, Dursun A, Gunel M, Gabriel SB, de Lonlay P, Gleeson JG. Biallelic mutations in SNX14 cause a syndromic form of cerebellar atrophy and lysosome-autophagosome dysfunction. Nat Genet. 2015;47(5):528–534. doi: 10.1038/ng.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Saffari A, Wahlster L, Lu J, Byrne S, Hoffmann GF, Jungbluth H, Sahin M. Congenital disorders of autophagy: an emerging novel class of inborn errors of neuro-metabolism. Brain. 2016;139(Pt 2):317–337. doi: 10.1093/brain/awv371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Baptiste G, Yang Z, Greenwood MT. Regulatory mechanisms involved in modulating RGS function. Cell Mol Life Sci. 2006;63(17):1969–1985. doi: 10.1007/s00018-006-6066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Haucke V. Phosphoinositides: regulators of membrane traffic and protein function. FEBS Lett. 2007;581(11):2105–2111. doi: 10.1016/j.febslet.2007.01.089. [DOI] [PubMed] [Google Scholar]
- Mas C, Norwood SJ, Bugarcic A, Kinna G, Leneva N, Kovtun O, Ghai R, Ona Yanez LE, Davis JL, Teasdale RD, Collins BM. Structural basis for different phosphoinositide specificities of the PX domains of sorting nexins regulating G-protein signaling. J Biol Chem. 2014;289(41):28554–28568. doi: 10.1074/jbc.M114.595959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A, Boltshauser E, Doherty D. Cerebellar hypoplasia: differential diagnosis and diagnostic approach. Am J Med Genet C Semin Med Genet. 2014;166C(2):211–226. doi: 10.1002/ajmg.c.31398. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174–183. doi: 10.1159/000358801. [DOI] [PubMed] [Google Scholar]
- Sousa SB, Venancio M, Chanudet E, Palmer R, Ramos L, Beales PL, Moore GE, Saraiva JM, Hennekam RC. Intellectual disability, unusual facial morphology and hand anomalies in sibs. Am J Med Genet A. 2013;161A(10):2401–2406. doi: 10.1002/ajmg.a.36124. [DOI] [PubMed] [Google Scholar]
- The PyMOL Molecular Graphics System, Version, 1.2r3pre. Schrödinger, LLC: [Google Scholar]
- Thomas AC, Williams H, Seto-Salvia N, Bacchelli C, Jenkins D, O'Sullivan M, Mengrelis K, Ishida M, Ocaka L, Chanudet E, James C, Lescai F, Anderson G, Morrogh D, Ryten M, Duncan AJ, Pai YJ, Saraiva JM, Ramos F, Farren B, Saunders D, Vernay B, Gissen P, Straatmaan-Iwanowska A, Baas F, Wood NW, Hersheson J, Houlden H, Hurst J, Scott R, Bitner-Glindzicz M, Moore GE, Sousa SB, Stanier P. Mutations in SNX14 cause a distinctive autosomal-recessive cerebellar ataxia and intellectual disability syndrome. Am J Hum Genet. 2014;95(5):611–621. doi: 10.1016/j.ajhg.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35(Web Server):W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3(12):919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.