Summary
Toxoplasma gondii is an apicomplexan parasite that secretes a large number of protein kinases and pseudokinases from its rhoptry organelles. Although some rhoptry kinases (ROPKs) act as virulence factors, many remain uncharacterised. In this study, predicted ROPKs were assessed for bradyzoite expression then pritoritised for a reverse genetic analysis in the type II strain Pru that is amenable to targeted disruption. Using CRISPR/Cas9 we engineered C-terminally epitope tagged ROP21 and ROP27 and demonstrated their localization to the PV and cyst matrix. ROP21 and ROP27 were not secreted from microneme, rhoptry, or dense granule organelles, but rather were located in small vesicles consistent with a constitutive pathway. Using CRISPR/Cas9, the genes for ROP21, ROP27, ROP28 and ROP30 were deleted individually and in combination, and the mutant parasites were assessed for growth and their ability to form tissue cysts in mice. All knockouts lines were normal for in vitro growth and bradyzoite differentiation but a combined Δrop21/Δrop17 knockout led to a 50% reduction in cyst burden in vivo. Our findings question the existing annotation of ROPKs based solely on bioinformatic techniques and yet highlight the importance of secreted kinases in determining the severity of chronic toxoplasmosis.
Introduction
Toxoplasma gondii is a widespread apicomplexan parasite that has the ability to cause severe disease in immunocompromised hosts (Hunter et al., 2012). It can establish a chronic infection that persists in intermediate hosts for the lifetime of the animal by forming tissue cysts (Pittman et al., 2015). Central to its lifestyle is the spatially and temporally coordinated secretion of proteins from specialized organelles: micronemes, rhoptries and dense granules (Santos et al., 2011, Mercier et al., 2015). Secreted proteins are required for adhesion, motility, invasion of the host cell, construction of the parasitophorous vacuole (PV), and manipulation of host cells, including defense from innate and adaptive immune responses (Hunter et al., 2012). Amongst the secretory compartments, rhoptries are highly specialized for the injection of proteins at the point of host cell invasion and contain structural components of the moving junction, and proteins that define the parasitophorous vacuole (PV) (Santos et al., 2011). Comprehensive proteomic analysis of the rhoptry organelles demonstrated they contain a large number of protein kinases and pseudokinases (Bradley et al., 2005). The role of rhoptry protein kinases (ROPKs) as virulence factors was first shown by genetic mapping approaches that identified key effectors responsible for the differences in modulating host gene expression (i.e. ROP16) and acute virulence (i.e. ROP18) between T. gondii strains (Saeij et al., 2006, Taylor et al., 2006, Saeij et al., 2007). The pseudokinase ROP5 was also identified through forward genetic approaches as a major strain-dependent virulence determinant (Behnke et al., 2011, Reese et al., 2011). ROP18 and ROP5 function by counteracting the recruitment of immune related GTPases (IRGs) to the membrane of the PV (PVM) (Fentress et al., 2010, Behnke et al., 2012). More recently, biochemical approaches have identified that ROP17 also interacts with ROP5 and targets IRGs (Etheridge et al., 2014). Together ROP17 and ROP18 synergise through ROP5 to prevent IRG accumulation on the PV, reducing parasite clearance and thus promoting survival.
The sequencing and assembly of the genomes of a diverse group of T. gondii strains revealed that polymorphic secretory pathogenesis factors, including ROPKs, comprise the most diverse gene families in the parasite (Lorenzi et al., 2016). Additionally, bioinformatic analyses identified a group of approximately 40-55 potential ROPKs, split equally into predicted active- and pseudo-kinases (Peixoto et al., 2010, Talevich et al., 2013). The ROPK family is defined by a high level of sequence divergence in the C-terminal kinase domain, an N-terminal extension to the kinase domain, and an N-terminal region that contains secretory signals such as signal peptides and transmembrane domains (Peixoto et al., 2010, Talevich et al., 2013). Gene expression profiling of the ROPK family highlighted that many of these genes are differentially regulated between the tachyzoite stage and the bradyzoite stage, and between the type strains of the parasite (Peixoto et al., 2010). Epitope tagging of selected putative ROPKs demonstrated that while many localize to the rhoptry, a subset do not conform to this pattern (Peixoto et al., 2010). Despite the lack of uniform targeting to the rhoptry, the genes retained their ROP nomenclature. The analysis of ROPK function in different parasite stages and strains has traditionally been hampered by the difficulty in performing reverse genetics in strains other the Type I RH strain and its derivatives. Hence, the biological role of many ROPKs in the lifecycle of the parasite has been incompletely defined.
Recently the development of a Type II background that is deficient in non-homologous end joining (NHEJ) opened the possibility to perform traditional gene knockout with long-homology flanked resistance cassettes (Fox et al., 2011). In combination with the application CRISPR/Cas9 the ability to perform rapid gene knockout, or targeted modifications, by supplying short-homology flanked repair templates has accelerated the pace of reverse genetic studies and for a range of genetic modifications (Shen et al., 2014a, Sidik et al., 2014). In this study we used CRISPR/Cas9 to localize and disrupt several putative bradyzoite ROPKs in a Type II strain of the parasite and examined their ability to undergo bradyzoite differentiation and establish chronic infection.
Results
Predicted ROPKs upregulated in chronic infection show atypical transcriptional profiles and gene models
The wealth of gene expression profiling data that exists for T. gondii allowed us to mine existing resources to select candidate genes that might play roles in chronic toxoplasmosis. Initially we selected approximately 20 predicted ROPKs that were suspected to possess protein kinase activity (i.e. containing a conserved catalytic triad of Lys, Asp, Asp (KDD) residues) (Peixoto et al., 2010) and analysed these further. The recent publication of transcriptomic data that compared infected mouse brains at acute and chronic infection time points allowed us search for predicted ROPKs that are upregulated in later stages of infection (Pittman et al., 2014). By comparing the FPKM (Fragments Per Kilobase of transcript per Million mapped reads) of each active ROPK gene in the chronic stage (28 days post infection (p.i.)) of infection versus the acute stage of infection (10 days p.i.) (Fig. 1A) a group of candidate genes was selected for evaluation. The candidate genes included ROP21 (TGME49_263220), ROP27 (TGME49_313330), ROP28 (TGME49_258370) and ROP30 (TGME49_227010). ROP18 (TGME49_205250) and the dense granule protein GRA4 (TGME49_310780) were selected as positive controls for genes known to affect infection burden in acute and chronic phases respectively (Taylor et al., 2006, Fox et al., 2011).
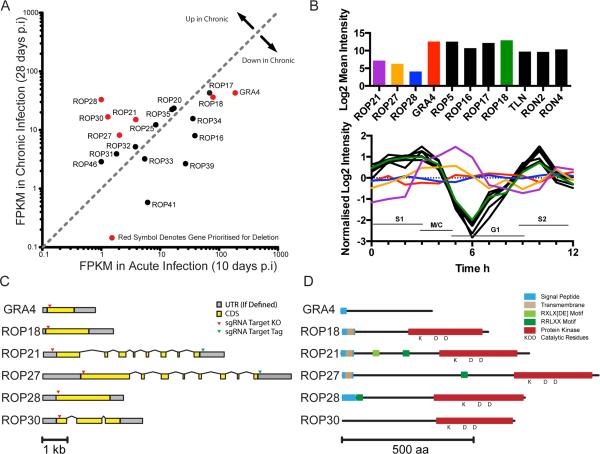
Figure 1.
Bioinformatic Profiling of ROPKs. Expression and transcript data were analyzed to identify candidate ROPKs for further study. A. RNAseq data (Pittman et al., 2014) compares ROPK expression levels in acute and chronic infections of C57bl/6 mice with ME49 parasites. Genes upregulated in chronic phase and/or selected for knockout are marked in red. B. Cell cycle microarray data (Behnke et al., 2010) of asynchronous RH tachyzoites (upper) plotted as mean expression levels, and synchronized RH tachyzoites (lower) plotted as expression level change during the cell cycle. Dotted lines S1, M/C, G1 and S2 mark cell cycle stages. (Note: data were not available for ROP30). C. Predicted gene models for selected ROPKs and GRA4 control. UTRs are marked in grey and exons are marked in yellow. Arrowheads denote the location of sgRNA target sites used in CRISPR/Cas9 knockout and tagging strategies. D. Predicted domain architecture of selected proteins, based on PFAM motifs, TMHMM, and motif search in CLC Genomics Workbench. Conserved residues predicted in active protein kinases are marked with KDD.
Existing data deposited in ToxoDB was assessed for further descriptive information for these genes (Gajria et al., 2007). Genes for rhoptry proteins are typically expressed in a characteristic cell cycle profile (Behnke et al., 2010) so the microarray expression profiles of ROP21, ROP27 and ROP28 were compared against a sample of defined rhoptry genes, as well as GRA4 (Fig. 1B). Expression levels for ROP21, ROP27 and ROP28 were generally lower in asynchronous cells compared to other rhoptry genes, as well as GRA4. More strikingly was that synchronous, cell cycle expression profiles for ROP21, ROP27 and ROP28 were not consistent with typical rhoptry profile genes (Fig. 1B). Instead, ROP27 and ROP28 displayed a stable profile across the cell cycle, similar to GRA4, whereas ROP21 exhibited a profile peaking at 5 h. Furthermore, the predicted gene models (Fig. 1C) for the candidates were not consistent with other ROPK gene models. ROPKs are typically expressed from single exon genes (Lorenzi et al., 2016), as exemplified by ROP18 in Fig. 1 C (GRA4 also happens to consist of a single exon CDS). The gene models for ROP21 and ROP27 were similar as they consist of multi-exon arrangements (8 exons each) with a larger first exon and smaller subsequent exons. ROP27 possess a larger first exon than ROP21, and the regions encoding the protein kinase domain are well conserved (Talevich et al., 2013). ROP28 had a single exon model predicted and ROP30 was predicted to contain 3 exons. These gene models were used to define the amino acid sequence for the predicted protein (Fig. 1 D). All the predicted proteins apart from ROP30 contained an N-terminal region consistent with a secreted protein containing regions annotated as a signal peptide or transmembrane domain. The previously annotated C-terminal kinase domains for each protein contained residues (KDD) and motifs consistent with them being active protein kinases rather than pseudokinases (Hanks et al., 1995, Peixoto et al., 2010). Despite being predicted as ROPK proteins it was also possible to identify motifs that can be processed by other secretory pathway systems. These included the HT/PEXEL and TEXEL motifs (RXLXD/E and RRLXX respectively) (Hsiao et al., 2013, Coffey et al., 2015). Although the PEXEL motif was initially defined in Plasmodium falciparum (Hiller et al., 2004, Marti et al., 2004), T. gondii utilizes similar motifs in many of the secreted dense granule (GRA) proteins. A similar TEXEL motif is processed by the Golgi localized ASP5 protease (Coffey et al., 2015, Curt-Varesano et al., 2015, Hammoudi et al., 2015). By motif searching in CLC Genomics software the following potential HT/PEXEL and TEXEL sites were identified in ROP21: RQLLE111-115 and RRLAG250-254, ROP27: RRLKT443-447, and ROP28: RRLLR55-59.
Ectopically expressed ROP21::YFP is secreted into the PV during intracellular growth
Due to its unusual gene model, transcriptional profile and multiple PEXEL-like motifs, we selected ROP21 for further characterisation. ROP21 has been previously suggested to localize to the PV and host cell cytoplasm, although the published images did not test co-localisation with secretory organelles such as rhoptries, dense granules, or micronemes (Peixoto et al., 2010). ROP21 cDNA was cloned into a constitutive expression vector that incorporates a C-terminal YFP tag (Fig. 2A). As ROP21 contains the motif (RRLAG250-254) that is cleavable by the Golgi resident, aspartic protease ASP5 (Shea et al., 2007), the ROP21::YFP expression plasmid was transfected into both wild type RH and RHΔku80Δasp5 parasites to investigate if processing occurs. Western blot analysis of parasite lysates demonstrated that ectopic ROP21::YFP was expressed in both backgrounds (Fig. 2B) with no YFP signal detected in parental controls. In the RH background the expression of ROP21::YFP was detected at both the predicted molecular weight of 100.7 kDa and a second, smaller, band of approximately 75 kDa (Fig. 2B). This pattern is consistent with cleavage of ROP21::YFP at the internal RRLAG250-254 motif by ASP5. Concordant with this, expression of ROP21::YFP in Δasp5 parasites yielded a single band with a molecular weight of 100 kDa (Fig. 2B). Together these results suggest that that ROP21 is processed in an ASP5-dependant manner, likely at the RRLAG motif. Proteins that have been characterized as being processed by ASP5 have typically been associated with dense granule secretion pathways (Coffey et al., 2015, Curt-Varesano et al., 2015, Hammoudi et al., 2015). As such we used live-cell, spinning disc confocal video microscopy to examine patterns of localisation of ROP21::YFP in tachyzoite stage parasites (Fig. 2C and Supplemental Videos 1 and 2). In wild type RH parasites, ROP21::YFP fluorescence was detected in the PV surrounding the growing tachyzoites, and as puncta inside the parasites (Fig. 2C and Supplemental Videos 1). Rapid intracellular trafficking of YFP-positive puncta inside parasites was observed by video microscopy (Supplemental Videos 1 and 2), but was not consistent with the club-shaped, apical structure that is characteristic of the rhoptries. Despite the evidence above for ROP21 processing, the secretion of ROP21::YFP to the PV was not dependent on ASP5 and the pattern of YFP fluorescence was the same in both wild type and Δasp5 backgrounds (Fig. 2C and Supplemental Video S2). We did not observe ROP21::YFP within the host cell cytoplasm, in contrast to previous immunofluorescence analysis that suggested ROP21 was secreted into the host cytoplasm (Peixoto et al., 2010).
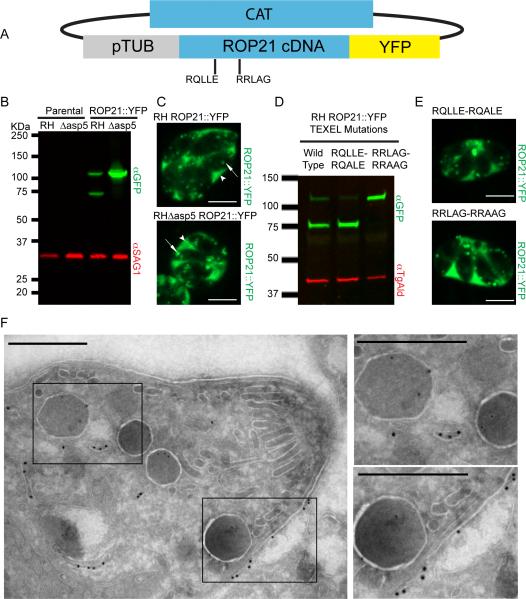
Figure 2.
Ectopic Expression and Localisation of ROP21. A. Ectopic expression of ROP21::YFP was performed by cloning ROP21 cDNA in frame with a C-terminal YFP tag, driven by the tubulin promoter; selection was provided by the chloramphenicol acetyltransferase (CAT) gene. B. Western blot of stable clones expressing the ROP21::YFP fusion in RH wild type and RHΔasp5 background vs. parental controls. Rabbit anti-GFP was used to detect YFP and mouse anti-SAG1 was used as a loading control. C. Single frames from spinning-disc confocal video microscopy movies (Supplemental video S1 and S2) that demonstrate ROP21::YFP trafficking and secretion. Scale bar denotes 5 μm, arrow heads denote accumulation of ROP21::YFP in the PV, arrows denote intracellular vesicular/granular structures containing ROP21::YFP. D. Western blot of parasites transiently expressing ROP21::YFP and ROP21::YFP with mutated TEXEL motifs. Mouse anti-GFP was used to detect YFP and rabbit anti-TgAld was used as a loading control. E. Spinning-disc confocal microscopy images of parasites expressing ROP21::YFP containing mutated TEXEL motifs. F. Immunoelectron microscopy of RH ROP21::YFP tachyzoites. Scale bar denotes 500 nm, 18 nm gold nanoparticles mark detectable ROP21::YFP, 12 nm gold particles mark detectable GRA2. Boxed regions correspond to zoomed regions presented to right of main image.
As ROP21 contained two potential processing sites for ASP5 cleavage we sought to confirm which motif was the target for processing. Using the ROP21::YFP expression plasmid as a template, site-directed mutagenesis was performed to individually edit the RQLLE111-115 motif to RQALE111-115 and the RRLAG250-254 to RRAAG250-254. These proteins were transiently expressed in wild type RH parasites and processing was determined by western blot analysis (Fig. 2D). Mutation of the RQLLE111-115 motif to RQALE111-115 had no effect on processing, with a banding pattern identical to wild type ROP21::YFP being observed (Fig. 2D). However, mutation of RRLAG250-254 to RRAAG250-254 abolished processing of the ROP21::YFP protein (Fig. 2D), as was observed when the wild type protein was expressed in Δasp5 parasites (Fig. 2B). Export of the protein to the lumen of the PV was not impeded by this lack of processing, as both mutant versions of ROP21::YFP showed similar localization patterns (Fig. 2E) to wild type ROP21::YFP (Fig. 2C).
To further define the distribution of ROP21, we used immunoelectron microscopy to examine localization at the ultra structural level (Fig. 2F). Immunogold labeling was readily detected in the lumen of the PV and labeling within parasite cells was frequently detected in endomembranous structures (Fig. 2F). However, we rarely observed immunogold labeling localizing to mature, electron dense granules (Fig. 2F). Co-staining of samples with anti-GRA2 was used to identify mature dense granules (Fig. 2F).
CRISPR/Cas9 mediated endogenously tagged ROP21 and ROP27 localise to the PV and cyst matrix
In order to circumvent any potential mislocalisation caused by the bulky YFP tag or overexpression by the tubulin promoter of the ectopic expression plasmid we also examined the localization of ROP21 and ROP27 by epitope tagging at the endogenous locus (Fig. 3A). ROP21::3xHA was readily detectable in tachyzoites with a band at the predicted size of 86 kDa and a smaller band in the region of 51.4 kDa, replicating the processing event observed for ROP21::YFP (Fig. 3 B). No anti-HA signal was detected in the parental control samples (Fig. 2B). Because ROP21 is predicted to be upregulated in chronic stages of the parasite lifecycle, lysate from in vitro bradyzoites was probed by western blot. The sample of 72h in vitro bradyzoites expressed the processed form of ROP21::HA, consistent with the tachyzoites; however, the amount of the full-length form was reduced (Fig. 3B).
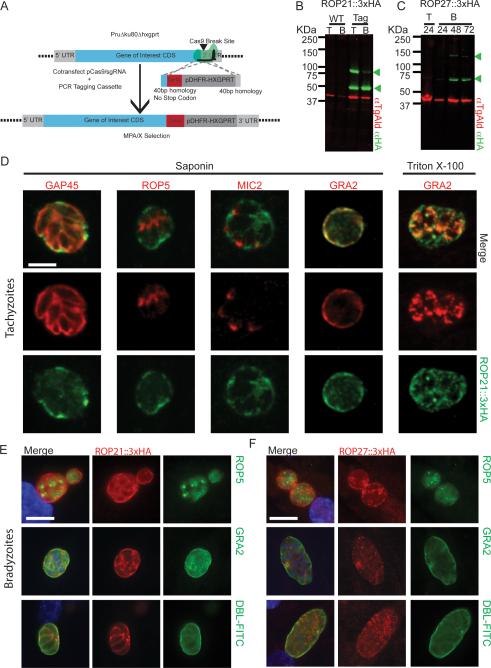
Figure 3.
Endogenous Epitope Tagging and Localisation of ROP21 and ROP27. A. Scheme depicting CRISPR/Cas9-mediated endogenous tagging, where a short-homology flanked cassette provides a repair template for homologous recombination following CRISPR/Cas9 mediated double strand break in PruΔku80Δhxgprt parasites. B. Western blot of parental PruΔku80Δhxgprt parasites and a clone engineered to express ROP21::3xHA. Lysates from both tachyzoites (T) and in vitro derived cysts/bradyzoites (B) were probed for HA signal. Arrowheads mark full length and processed products. C. Western blot of PruΔku80Δhxgprt parasites expressing ROP27::3xHA during the course of tachyzoites (T) growth and in vitro differentiation to bradyzoites (B). Arrowheads mark full length and processed products. D. Immunofluorescence analysis of tachyzoites expressing ROP21::3xHA with co-labeling of GAP45, MIC2, GRA2, and ROP5. E. Immunofluorescence analysis of ROP21::3xHA localization within in vitro cysts, co-labeled for ROP5, GRA2 or cyst wall (DBL-FITC). Scale bar denotes 5 μm F. Immunofluorescence analysis of ROP27::3xHA expressing in vitro cysts, co-labeled for ROP5, GRA2 or cyst wall (DBL-FITC). Scale bar denotes 20 μm.
In ROP27::3xHA tagged parasites there was no detectable signal in tachyzoites; however, upon differentiation to bradyzoites a weak band corresponding to the predicted 110.3 kDa of the full length protein and a prominent band at 63 kDa were detected (Fig. 3C). These bands correspond to predicted sizes of the predicted full-length tagged protein and a processed form of the protein, likely at the RRLKT443-437 motif, suggesting that ROP27 may also be processed by ASP5.
Following epitope tagging of the ROP21 and ROP27 proteins their cellular localization was assessed by immunofluorescence analysis. (IFA). In saponin permeabilised tachyzoites, ROP21::3xHA staining was detected in the lumen of the PV, consistent with the localization of ROP21::YFP in live parasites (Fig. 3D). ROP21::3xHA did not co-localise with ROP5 or MIC2 (Fig. 3D). Saponin permeabilised tachyzoites showed ROP21::3xHA co-localised with GRA2 in the lumen of the PV, but intracellular stores were not clearly visible (Fig. 3D). In 0.1% Triton X-100 permeabilised cells, intracellular dense granules containing GRA2 were clearly visible; however, there was only partial co-localisation with ROP21::3xHA (Fig. 3D).
As the ROP21 and ROP27 genes are predicted to be upregulated in chronic infection we performed the IFA on in vitro cysts to determine their localization. When the parasites were differentiated to in vitro cysts (72 h) the ROP21::3xHA signal was strongly detectable in the matrix of the cysts surrounding the parasites (Fig. 3E). There was no co-localisation with ROP5 inside bradyzoites but ROP21::3xHA did co-localise with secreted GRA2 in the cyst matrix (Fig. 3E). The ROP21::3xHA signal was retained within the cyst wall (Dolichos biflorus lectin (DBL)-FITC labeling) and could be seen packing around the parasite bodies (Fig. 3E).
IFA was performed on ROP27::3xHA tachyzoites but no detectable HA signal was observed (not shown), consistent with the western blot analysis (Fig. 3C). Following 72 h of in vitro differentiation to bradyzoites, ROP27::3xHA was detectable in the matrix of DBL positive cysts surrounding the parasites (Fig. 3F), demonstrating it is a stage specific protein. The ROP27::3xHA signal (Fig. 3F) was less intense than ROP21::3xHA (Fig. 3E) and more granular in nature. ROP27::3xHA did not co-localise with ROP5 signal but it did partially co-localise with GRA2 in the matrix of the cyst (Fig. 3F).
CRISPR/Cas9 mediated gene knockouts
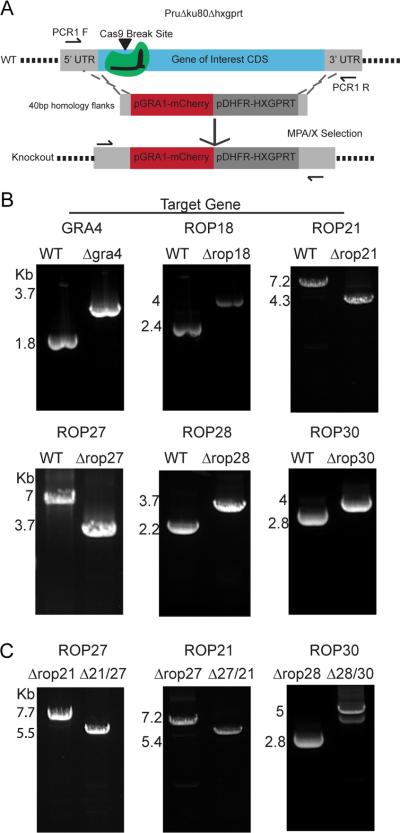
In order to perform reverse genetic analyses for selected predicted ROPK genes we used CRISPR/Cas9 to delete their coding sequences. sgRNAs were selected to target the 5’ region of the CDS for ROP18, ROP27, ROP28, ROP30, and GRA4 genes (marked on Fig. 1C). ROP21 was targeted with a sgRNA homologous to the 5’ UTR region, close to the start codon of the CDS (marked on Fig. 1C). A short-homology flanked cassette, containing mCherry and HXGPRT markers, replaced the CDS. Stable, isogenic clones were generated and identified by PCR (Fig. 4B). The generation of single knockouts was rapidly achieved leading to Δgra4, Δrop18, Δrop21, Δrop28 and Δrop30 parasite strains, demonstrating they were not essential for tachyzoites. Due to many biological systems having some level of redundancy, we repeated the CRISPR/Cas9 approach to generate double knockouts for pairs of genes by replacing the second gene with a DHFR-TS resistant cassette and selecting with pyrimethamine. This dual targeting approach allowed the generation of Δrop27/Δrop21 and Δrop28/Δrop30 parasites (Fig. 4C). In lieu of complementing the Δrop27/Δrop21 parasites, a reciprocal double knockout of Δrop21/Δrop27 that utilized different sgRNAs was generated in parallel (Fig. 4C). All of the single and double knockouts were tested by in vitro plaque assay to identify any gross defects in the lytic growth cycle (Fig. S1A). Based on plaque formation the mutants were not attenuated compared to the parental strain (Fig. S1A). In case there were subtle phenotypes that would not be identified by individual growth assays, competition assays were performed that pitted each double knockout against the PruΔku80Δhxgprt background (Fig. S1B). The competition assay did not identify any reduction in the proportion of knockout parasites in the population over time (Fig. S1B) demonstrating that, at least in vitro, the mutants grew normally as tachyzoites.
Figure 4.
Complete Knockout of Selected Genes. A. Scheme of CRISPR/Cas9-mediated gene knockout using short-homology flanked resistance cassette. PCR primer positions are indicated by arrows. B. Agarose gel electrophoresis of PCR1 products for single gene knockout parasites. PCR1 was conducted on parental PruΔku80Δhxgprt wild type and the corresponding knockout parasite for the CDS of the gene of interest. PCR was conducted on clonal parasite lines, changes in PCR amplicon sizes were consistent with predicted sizes for wild type loci and those replaced by the resistance cassettes C. Agarose gel electrophoresis of PCR1 products for double knockout parasites. PCR was conducted across the CDS region of the gene of interest in a single gene knockout parental strain and the corresponding double knockouts.
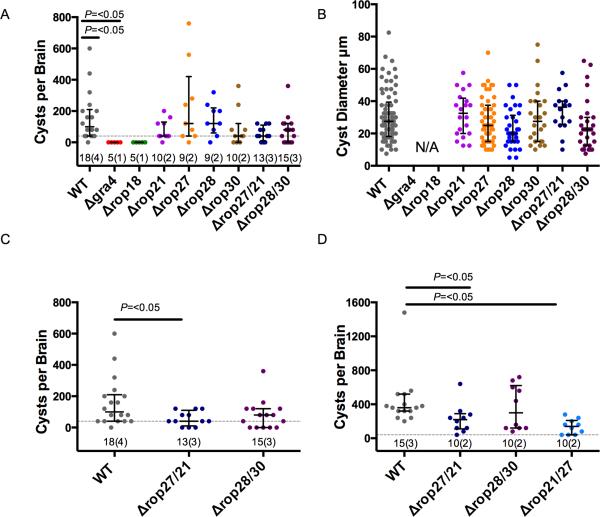
To examine the process of in vitro differentiation, the size and number of DBL-FITC positive cysts was monitored after 72 h of induction (Fig. S1C). No significant defect was observed (Fig. S1C) suggesting the parasites remained competent in forming tissue cysts. However, we reasoned that the knockout parasites might be attenuated in a chronic mouse infection model as they encounter additional immune pressure in vivo. Initially we performed this in C57BL/6J mice, infecting by intraperitoneal (IP) injection with 200 tachyzoites and allowing the infection to proceed for 30 days. All of the single and double knockout parasites, apart from Δgra4 and Δrop18 mutants, caused significant weight loss similar to that seen in the mice infected with wild type parasites (Fig. S2B and S2C). Mice infected with Δrop18 parasites retained a stable weight, consistent with this parasite line being attenuated in the acute phase (Taylor et al., 2006). After 30 days the mice were humanely sacrificed, brains dissected and cyst numbers were enumerated by microscopy after DBL-FITC staining. Mice infected with Δgra4 or Δrop18 parasites did not have a detectable level of cysts in the brain, consistent with previous data and their attenuation in the acute phase of infection (Taylor et al., 2006, Fox et al., 2011), this was significantly different to the wild type infected mice (Fig. 5A). The single and double ROPK knockout cell lines trended to have a lower cyst burden although this difference was not deemed statistically significant when considered as part of a large multiple strain comparison (Fig. 5A). While enumerating the cyst burden of C57BL/6J mice, the diameter of each cyst was measured (Fig. 5B), no difference in the size of cysts formed by knockout parasites compared to wild type was identified. Likewise, there was no significant defect in the oral infectivity of these cysts to naïve CD1 mice (Fig. S2C).
Figure 5.
Analysis of Knockout Parasites. A. Brain cyst burdens of C57BL/6J mice at 30 days post infection. Lines and error bars denote the median and interquartile range, data were analysed using Kruskal-Wallis test with Dunn's multiple comparison correction. Dotted line indicates the limit of detection in this assay and the numbering below each group denotes total number of animals analysed followed by the number of experiments performed in parentheses (numbering is similar in C, and D). B. Diameter of cysts from C57BL/6J mice at 30 days post infection. Error bars denote the median and interquartile range of samples. C. Brain cyst burden of C57BL/6J at 30 days post infection showing data from A. for double-knockouts only. Data were analysed by pairwise Mann-Whitney U test, error bars denote median and interquartile range. D. Brain cyst burden of CBA/CaJ mice at 30 days post infection with double-knockout parasites. Lines and error bars denote median and interquartile range, data were analysed by pairwise Mann-Whitney U test.
When we separately compared the cyst burden data in C57BL/6J (Fig. 5C), for the wild type and the double mutant strains the difference between the Δrop27/rop21 was statistically significant (Fig. 5C). Because of the low cyst burden in C57BL/6J mice we sought improve the power of our analysis by experiments using CBA/CaJ mice, which exhibit higher cyst burdens during chronic infection. We tested the original Δrop27/rop21 line, the reciprocal Δrop21/Δrop27 line, and the Δrop28/Δrop30 in this model (Fig. 5D). The median cysts per brain value more than tripled from 100 in C57BL/6J mice to 360 in CBA/CaJ mice. CBA/CaJ mice infected with Δrop28/Δrop30 parasites had similar cyst burdens to the wild type infected mice (Fig. 5C), commensurate with the results from C57BL/6J (Fig. 5C). Importantly, the cyst burdens of the Δrop27/Δrop21 and Δrop21/Δrop27 infected CBA/CaJ mice were again lower than the wild type infected CBA/CaJ mice (61% and 38% of wild type respectively) and this was statistically significant (Fig. 5D). This result reinforces the phenotype previously observed in C57BL/6J mice (Fig. 5A) that parasites deficient in ROP21 and ROP27 are attenuated at forming brain cysts.
Discussion
In this study we aimed to identify the role of putative ROPKs that are upregulated in bradyzoite stages of chronic toxoplasma infections. Bioinformatic analysis of candidate bradyzoite ROPKs revealed they have features suggestive of secreted protein kinases, but not consistent with canonical ROPKs. We confirmed that ROP21 and ROP27 were secreted into the PV and/or cyst matrix although they were not localized to the rhoptries, nor to dense granules, but rather they were found in small vesicular structures, suggesting they are constitutively secreted. Although ROP21 is processed in an ASP5-dependant manner, cleavage was not required for its export to the PV. Using CRISPR/Cas9 we knocked out four predicted bradyzoite ROPKs individually and in combination. These genes were not essential for growth or fitness in vitro but a Δrop21/rop27 combined knockout resulted in a 50% reduction of cyst burden in vivo. Our findings highlight that some annotated ROP-kinases are in fact secreted by other pathways, and that they play an important role in the establishment or formation of the chronic tissue cyst stage.
Based on existing transcriptomic data we identified potential ROPKs that are upregulated in chronic infection. Upon assessing the bioinformatics data available for these proteins it was clear they contained features suggestive of secreted protein kinases but had multi-exon gene models and transcriptional profiles not consistent with canonical ROPKs. To gain insight on the localization and trafficking of these proteins, ROP21 and ROP27 were visualised by live cell imaging and/or epitope tagging followed by IFA. Both ROP21 and ROP27 proteins were secreted into the PV and cyst matrix as shown by live cell video microscopy (ROP21), IFA (ROP21 and ROP27), and immuno-EM (ROP21). The localization of ROP21 by immunoelectron microscopy suggested it was secreted by a constitutive mechanism, and not one of the regulated secretory systems (micronemes, rhoptries or dense granules). The evidence for subpopulations of secretory organelles in T. gondii is beginning to mount, and the presence of another, non-dense granule, constitutive secretion system has been proposed (Kremer et al., 2013, Mercier et al., 2015). Using immunoelectron microscopy, ROP21 was localized to small vesicles that were not coincident with mature dense granules, suggesting that ROP21 is primarily secreted by constitutive secretory vesicles, analogous to GRA-like proteins (Mercier et al., 2015). Based on differences in their localization, we suggest that the annotation of ROPK should be revisited, and reserved for validated rhoptry protein kinases.
Although ROP21 showed evidence of being processed by ASP5 at the RRLAG250-254 motif, secretion of the protein to the PV was not dependent on processing. ASP5 has been shown to process several dense granule proteins (GRA19 and GRA20), but also is not required for their export to the PV (Coffey et al., 2015, Curt-Varesano et al., 2015, Hammoudi et al., 2015). GRA proteins such as GRA16 and GRA24 (which do not contain PEXEL-like motifs) that translocate out of the PV to occupy the host nucleus are blocked in the PV due to the disruption of MYR1 processing in Δasp5 parasites (Coffeyet al., 2015, Curt-Varesano et al., 2015, Hammoudi et al., 2015, Franco et al., 2016). These findings demonstrate that improper cleavage of some secreted proteins can impact the export and hence function of other proteins. We also did not find that absence of ASP5 processing had any impact of the distribution of ROP21 in the PVM, unlike previously described genes granule proteins GRA19 and GRA20 that associate to the PVM in a manner that depends on the TEXEL processing motif (Hsiao et al., 2013). Although we do not currently have any evidence that processing is important for the function of ROP21 and ROP27 this possibility could be tested in future work using the mutated version of ROP21::YFP that cannot be cleaved by ASP5.
We identified a double knockout of ROP21/ROP27 that did not possess a defect in tachyzoite growth or fitness in vitro, and yet was attenuated by 50% in its ability to form brain cysts in vivo. Although we did not appreciate a significant difference for single knockouts, during the preparation of this manuscript, a separate study reported significant reductions in the cyst burden for knockouts of ROP21 and ROP27, using a different statistical test (Fox et al., 2016). In contrast to previously described Δgra4 and Δgra6 mutants (Fox et al., 2011), parasites deleted for ROP21 and ROP27 did not appear to be attenuated in vivo based on mouse weight loss during the acute phase of the infection. The reduced cysts burden in the rop21/Δrop27 mutant may be due to reduced numbers or parasites that cross the blood brain barrier to establish CNS infection, or alternatively they may be less able to establish a cyst or to maintain it over the duration of infection. Regardless of the exact mechanism, our findings suggest that ROP21 and ROP27 together play a role in the establishment or formation of tissue cysts during chronic infection. The cyst matrix is a highly complex environment composed of distinctive layers, vesicular and tubular structures (Lemgruber et al., 2011). The regulation of these structures and their cargo remains poorly investigated and the role of protein kinases in controlling the development of these structures remain largely unexplored. It is possible that ROP21 and ROP27 play a role in modulating other components of the cyst matrix or wall, such as CST1, which is a phosphoprotein that is important in cyst formation (Treeck et al., 2011).
The phenotype of the double Δrop21/rop27 mutant is in contrast to that of bradyzoite pseudokinase 1 (BPK1) mutants: Δbpk1 parasites exhibit normal cyst burdens but tissue cysts are highly attenuated at establishing oral infections in new hosts (Buchholz et al., 2011). BPK1 is not found in rhoptries, and although it is not known how it is released, it also accumulates in the cyst wall of chronic stages (Buchholz et al., 2011). When BPK1 was deleted from PruΔhxgprt parasites, the resultant strain generated reduced (although not statistically significant) cyst burdens in CBA/J mice after 8 weeks of infection. The Δbpk1 mutant produced significantly smaller cysts that were highly defective in their ability to orally infect mice, suggesting there was a defect in growth, maintenance or stability of the cysts during the course of chronic infection. In contrast, our Δrop27/Δrop21 cell line was attenuated at establishing normal cyst burdens but the cysts were still able to initiate new infections, suggesting the bradyzoites contained within are healthy. It would be interesting to test comparable knockouts in a strain that is normally capable of making oocysts for their ability to infect a feline host in a future study. Unfortunately, the PruΔku80 strain is not capable of completing the entire life cycle (Wang et al., 2015) (Sibley laboratory, unpublished data), despite the ease of generating gene knockouts in this background. This possibility is especially pertinent as most canonical ROP proteins are downregulated during the life stages in, or derived from, the cat (Fritz et al., 2012, Behnke et al., 2014, Hehl et al., 2015). Several studies have detected putative ROPK transcripts that display up regulation in merozoites and oocysts. ROP21 and ROP30 transcripts were detected as upregulated in merozoites (Behnke et al., 2014) and ROP21, ROP27 and ROP28 are detected as expressed in oocysts and sporozoites (Fritz et al., 2012). Utilizing dual-CRISPR approaches in other cat-competent strains T. gondii, such as ME49, would in the future allow for testing the role of bradyzoite upregulated genes in the definitive feline host.
In summary, we have identified that the predicted ROPKs, ROP21 and ROP27, do not conform to the classification a true rhoptry proteins. They are secreted into the PV and cyst matrix surrounding the parasites by constitutive secretory vesicles where they perform an as yet undefined role in cyst formation or maintenance in vivo. The reduction in cyst burden for the double knockout line to ~50% of wild type levels suggest that lack of these genes would be expected to be highly disadvantageous over evolutionary time frames. Orthologs of ROP21 and ROP27 are found in other cyst forming coccidians including Hammondia hammondi, Neospora caninum, and Sarcocystis neurona, as well as Eimeria tenella, suggesting these are widely conserved (Talevich et al., 2013). Hence they may play a broader role in adaptation to intracellular survival in coccidian parasites.
Experimental Procedures
Bioinformatics analyses
Genomic and transcriptomic data pertaining to the T. gondii genome was obtained from http://ToxoDB.org (Gajria et al., 2007, Lorenzi et al., 2016). The TgME49 V9.0 genome was loaded into CLC Genomics Workbench (Qiagen) for sequence viewing and manipulation, PFAM and TMHMM, and SignalP plugins were installed to identify these features. Genes of interest were assessed for appropriate Cas9 target sites using the service ECRISP (www.e-crisp.org/E-CRISP/) (Heigwer et al., 2014). The design of sgRNAs was performed using the DNA sequence from ME49 strain of parasite but were also compared to genomic reads of the Pru strain genome mapped to the ME49 reference genome to ensure there were no SNPs in target regions. Lists of oligonucleotide PCR primers (Table S1) and plasmids (Table S2) used in this study are provided in the supplementary materials. Data representation and statistical analysis was performed in Prism 6 (Graphpad).
Parasites and cell culture
PruΔku80Δhxgprt parasites that are defective in non-homologous end joining were used as the background for gene knockouts and epitope tagging (Fox et al., 2011). Wild type RH and RHΔku80Δasp5 (Curt-Varesano et al., 2015) strains were used for ectopic expression of ROP21::YFP. Parasites were grown in monolayers of human foreskin fibroblasts (HFFs) as described previously (Long et al., 2016). All strains and host cell lines were mycoplasma negative as determined using the eMyco plus kit (Intron Biotechnology).
Spinning disc confocal video microscopy
Full-length ROP21 cDNA was amplified from TgME49 SMART cDNA and cloned into linear pTub-YFPYFP-CAT (Gubbels et al., 2003) (digested with BglII and AvrII to remove the first YFP copy) using NEBuilder HIFI Assembly mix (NEB). The validated plasmid was electroporated into RH and RHΔku80Δasp5 (Curt-Varesano et al., 2015) using 10 μg per transfection, and after 24 h chloramphenicol selection was applied at 20 μM for wild type RH and 10 μM for the Δasp5 background. After establishment of stable pools, parasites were cloned by limiting dilution. Expression of the protein was confirmed by western blotting using rabbit anti-GFP polyclonal serum (Molecular Probes) and mouse anti-SAG1 DG52 (Burg et al., 1988) as a loading control. For live cell imaging, parasites were seeded into HFFs growing in glass-bottomed culture dishes (MatTek, Ashland, MA) and allowed to replicate for 24-30 h. Infected cell monolayers were then imaged using a Zeiss AxioObserver Z1 imaging system and Zen Software (Drewry et al., 2015) equipped with a Cell Observer SD spinning disc confocal system with 488nm (50mW) and 561 nM (50mW) lasers (Zeiss) and an Evolve 512 EMCCD Camera (Photometrics).
TEXEL Mutations
pTUB-ROP21YFP-CAT was used as a template in Q5 Mutagenesis (NEB) reactions to engineer point mutations at the two potential ASP5 processing sites and these were confirmed by sequencing. Fifteen micrograms of each plasmid were used to transfect RH T. gondii that were seeded into T25 flasks of HFFs and incubated for 36 hours. Monolayers were dissociated using trypsin (Life Technologies) and suspended in 3% FCS PBS with additional 5mM EDTA, filtered using a 70 μm cell strainer (Falcon). Samples were analyzed using a Sony SY3200 “Synergy” and YFP positive events were sorted into 500 μl D10. These were recovered by centrifugation at 1000 × g for 10 min, and then 15,000 cells per sample were analyzed by western blotting as above, with the exception that YFP was detected by α-GFP mouse monoclonal JL8 (Living Colors, Clontech), loading control was rabbit anti-T. gondii aldolase (TgAld) (Starnes et al., 2006).
Endogenous epitope tagging
To endogenously tag ROP21 and ROP27 with a 3xHA epitope tag, CRISPR/Cas9 plasmids were engineered to target a site downstream of the stop codon of each CDS. The sgRNA CRISPR/Cas9 plasmids were co-transfected into cells with a short-homology-flanked, PCR-derived repair template encoding the 3’ 40 bp portion of the CDS (without the stop codon) fused to a 3xHA tag followed by a DHFR-TS 3’ UTR, a HXGPRT minigene and finally 40 bp homology to the 3’ UTR of the gene, downstream of the CRISPR/Cas9 cut site. The ratio of CRISPR/Cas9 plasmid to resistance cassette was 10 μg to 0.5-1 μg, respectively. Parasites pools were selected with MPA/Xanthine (25 μg/ml and 50 μg/ml respectively), and then cloned by limiting dilution. Correct tagging was confirmed using PCR (primer sequences are provided in Table S1) and then western blotting against lysates of both intracellular tachyzoites and bradyzoites using mouse α-HA.11 16B12 (Biolegend) and rabbit anti-TgAld as a loading control (Starnes et al., 2006).
Immuno-fluorescence microscopy
HFF monolayers were seeded onto circular coverslips and grown until confluent. Parasites were allowed to invade and grow for 24 h (for tachyzoites) or were switched into differentiation media after 4 h and allowed to develop for 72 h. At the appropriate time point, coverslips were washed in PBS and fixed in 4% formaldehyde PBS (Fisher) for 10 min at room temperature. Permeabilisation was conducted with 0.05% saponin, 5% goat serum in PBS, and washing and staining steps were carried out with 0.01% saponin, 1% goat serum in PBS. Alternatively, cells were permeabilized after fixation with 0.1% Triton X-100 (Fisher) for 10 min at room temperature and washed 3 times with PBS. Samples were blocked with 10% goat serum (Gibco) for 20 min prior to labeling. Primary antibodies used were mouse α-HA.11 16B12 (Biolegend), rabbit α-GAP45 (Plattner et al., 2008), rabbit α-MIC2 (Carruthers et al., 2000), rabbit α-GRA2 (Charron et al., 2002) and rabbit α-ROP5 (Behnke et al., 2011). Antibodies were detected with Alexa-fluor conjugated secondaries (Molecular Probes) and counterstained with Hoechst 33258 prior to mounting in Prolong Gold Antifade Reagent (Molecular Probes). Imaging was performed on Zeiss Axioskop 2MOT Plus epifluorescence microscope equipped with a 100x oil immersion objective (N.A. 1.4), and AxioCam MRM, using Axiovision for image capture of in vitro cyst samples. Tachyzoites were imaged using the Zeiss AxioObserver Z1 imaging system and spinning disc confocal system described above.
Immunoelectron microscopy
For immunolocalization at the ultrastructural level, infected cells were fixed in 4% paraformaldehyde/0.05% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100 mM PIPES/0.5 mM MgCl2, pH 7.2 for 1 h at 4°C. Samples were then embedded in 10% gelatin and infiltrated overnight with 2.3 M sucrose/20% polyvinyl pyrrolidone in PIPES/MgCl2 at 4°C. Samples were trimmed, frozen in liquid nitrogen, and sectioned with a Leica Ultracut UCT cryo-ultramicrotome (Leica Microsystems Inc., Bannockburn, IL). 50 nm sections were blocked with 5% FBS/5% NGS for 30 min and subsequently incubated with rabbit anti-GFP A11122 (Life Technologies, Eugene OR) and mouse anti-GRA2 Tg17 followed by secondary anti-rabbit antibody conjugated to 18 nm colloidal gold (Jackson ImmunoResearch Laboratories, Inc., West Grove PA) and goat anti-mouse conjugated to 12 nm colloidal gold (Jackson). Sections were washed in PIPES buffer followed by a water rinse, and stained with 0.3% uranyl acetate/2% methyl cellulose. Samples were viewed with a JEOL 1200EX transmission electron microscope (JEOL USA Inc., Peabody, MA) equipped with an AMT 8 megapixel digital camera (Advanced Microscopy Techniques, Woburn, MA). All labeling experiments were conducted in parallel with controls omitting the primary antibody. These controls were consistently negative at the concentration of colloidal gold conjugated secondary antibodies used in these studies.
Single gene knockout by CRISPR/Cas9
To generate CRISPR/Cas9 expression vectors targeted to genes of interest, sgRNAs were engineered into pSag1-Cas9-UPRT (Shen et al., 2014b) using the Q5 Mutagenesis Kit (NEB). To accomplish this, gene specific primers were used with a common reverse primer (Shen et al., 2014b) to generate site-directed mutants that were confirmed by sequencing (GeneWiz). Resistance cassettes were amplified from a plasmid containing a loxP-GRA1 5’-mCherry-SAG1 3’ – DHFR 5’ - HXGPRT DHFR3’ - loxP cassette using Q5 DNA Polymerase (NEB). The mCherry reporter was under the control of a GRA1 promoter and the HXGPRT gene was under the control of the DHFRTS promoter. Amplicons were flanked with 40 bp homology to the UTRs of the gene of interest and purified by gel extraction (Qiagen). Freshly egressed tachyzoites were harvested, filtered and CRISPR/Cas9 plasmids were co-transfected with the appropriate resistance cassette at a ratio of 10 μg to 1μg. Parasites were selected with MPA/Xanthine (25 μg/ml and 50 μg/ml respectively) treatment until a stable pool arose, and were then cloned by limiting dilution. Clones were screened by PCR for replacement of the gene of interest coding sequence by the resistance cassette (Primers detailed in Table S1).
Double gene knockout by CRISPR/Cas9
Parasites that were single knockouts for ROP21 or ROP27 were co-transfected with a CRISPR/Cas9 plasmid to target the reciprocal gene using a corresponding homology flanked resistance cassette. In this instance the resistance cassette used was loxPDHFR5’-DHFR-TS::GFP-loxP-DHFR3’. Double knockout parasites were selected with 3 μM pyrimethamine until a stable pool arose and cloned by limiting dilution and screened by PCR.
Plaque assays
Tachyzoite plaque assays were conducted in HFF monolayers and visualized with crystal violet staining as previously described (Long et al., 2016).
Competition assays
Mixed parasite populations were monitored over multiple passages to determine the fitness of knockout parasites against the parental PruΔku80Δhxgprt strain. Equal number of wild type and mutant parasites were inoculated into HFF monolayers in T25 cm2 flasks and passaged on a 48h growth cycle. At intervals, parasites were analysed using a FACSCanto flow cytometer (BD Bioscience). Total parasite events were gated using FSC-H/SSC-H and these were then divided into mCherry positive (knockout) and negative (wild type) populations by detecting fluorescence in the PerCP-Cy5 channel.
In vitro differentiation
In vitro bradyzoites were generated by infection of HFFs for 4 h at 37°C 5% CO2 in D10 prior to switching to pH 8.2 RPMI 1640 and transferring to an ambient CO2 concentration at 37°C, as described previously (Weiss et al., 1995).
In vivo infections
Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved facility at Washington University School of Medicine. All animal studies were conducted in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, and protocols were approved by the Institutional Animal Care and Use Committee at the School of Medicine, Washington University in St. Louis.
For cyst burden experiments 8-10 week old female C57BL/6J mice (The Jackson Laboratory) or CBA/CaJ mice (The Jackson Laboratory) were infected with 200 tachyzoites by intraperitoneal injection (IP). Infections were allowed to progress for 30 days with mice being weighed and monitored daily. Brain cyst burden was determined at the experimental endpoint by lectin staining and microscopy as previously described (Wang et al., 2015, Long et al., 2016), cyst diameter was measured using a calibrated eyepiece reticule and a 40x objective. If the cyst was asymmetric then the diameter of the widest section was measured. The remaining brain portions were adjusted to 10 cyst per 200 μl and were fed by oral gavage to 8-10 week, female CD1 mice (Charles River). Following a 30-day period these mice were euthanized, and blood was collected for serological analysis. ELISA to determine seroconversion status was performed as previously described (Khan et al., 2014).
Supplementary Material
Acknowledgements
We wish to thank Kevin Brown and Shaojun Long for sharing plasmids, Keliang Tang for SMART cDNA, and other Sibley lab members for helpful discussions and advice. We are grateful to Wandy Beatty, Microbiology Imaging Facility, for performing the immunoelectron microscopy analyses. Δasp5 mutant parasites were kindly provided by Mohamed-Ali Hakimi. PruΔku80 strain parasites were kindly provided by David Bzik. We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Mo., for the use of the Siteman Flow Cytometry Core, which provided Sony Synergy FACS service.
Funding Information
Supported in part by NIH grants AI082423 and AI118426. The funders did not have any role in the study design, data collection and interpretation, or decision to publish the work. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
Conflicts
The authors declare that they have no conflicts of interest to declare.
References
- Behnke M, Wooten JC, Lehmann M, Radke J, Lucas O, Nawas J, et al. Coordinated progression through two subtranscriptions underlies the tachyzoite cycle of Toxoplasma gondii. Plos One. 2010;5:e12354. doi: 10.1371/journal.pone.0012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Fentress SJ, Mashayekhi M, Li LL, Taylor GA, L.D., S. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Path. 2012;8:e1002992. doi: 10.1371/journal.ppat.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseuodokinases. Proc Natl Acad Sci (USA) 2011;108:9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Zhang TP, Dubey JP, Sibley LD. Toxoplasma gondii merozoite gene expression analysis with comparison to the life cycle discloses a unique expression state during enteric development. BMC Genomics. 2014;15:350. doi: 10.1186/1471-2164-15-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for hostparasite interactions in T. gondii. J. Biol. Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- Buchholz KR, Fritz HM, Chen X, Durbin-Johnson B, Rocke DM, Ferguson DJ, et al. Identification of tissue cyst wall components by transcriptome analysis of in vivo and in vitro Toxoplasma gondii bradyzoites. Eukaryot Cell. 2011;10:1637–1647. doi: 10.1128/EC.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg JL, Perlman D, Kasper LH, Ware PL, Boothroyd JC. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- Carruthers VB, Sherman GD, Sibley LD. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 2000;275:14346–14353. doi: 10.1074/jbc.275.19.14346. [DOI] [PubMed] [Google Scholar]
- Charron AJ, Sibley LD. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J Cell Sci. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- Coffey MJ, Sleebs BE, Uboldi AD, Garnham A, Franco M, Marino ND, et al. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife. 2015;4 doi: 10.7554/eLife.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt-Varesano A, Braun L, Ranquet C, Hakimi MA, Bougdour A. The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell Microbiol. 2015 doi: 10.1111/cmi.12498. [DOI] [PubMed] [Google Scholar]
- Drewry LL, Sibley LD. Toxoplasma Actin Is Required for Efficient Host Cell Invasion. MBio. 2015;6:e00557. doi: 10.1128/mBio.00557-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge RD, Alagan A, Tang K, Turk BE, Sibley LD. ROP18 and ROP17 kinase complexes synergize to control acute virulence of Toxoplasma in the mouse. Cell Host Microbe. 2014;15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress SJ, Behnke MS, Dunay IR, Moashayekhi M, Rommereim LM, Fox BA, et al. Phosphorylation of immunity-related GTPases by a parasite secretory kinase promotes macrophage survival and virulence. Cell Host Microbe. 2010;16:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, Mercier C, et al. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot Cell. 2011;10:1193–1206. doi: 10.1128/EC.00297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Rommereim LM, Guevara RB, Falla A, Hortua Triana MA, Sun Y, Bzik DJ. The Toxoplasma gondii Rhoptry Kinome Is Essential for Chronic Infection. MBio. 2016;7 doi: 10.1128/mBio.00193-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Panas MW, Marino ND, Lee MC, Buchholz KR, Kelly FD, et al. A Novel Secreted Protein, MYR1, Is Central to Toxoplasma's Manipulation of Host Cells. MBio. 2016;7 doi: 10.1128/mBio.02231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HM, Buchholz KR, Chen X, Durbin-Johnson B, Rocke DM, Conrad PA, Boothroyd JC. Transcriptomic analysis of toxoplasma development reveals many novel functions and structures specific to sporozoites and oocysts. PLoS One. 2012;7:e29998. doi: 10.1371/journal.pone.0029998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajria B, Bahl A, Brestelli J, Dommer J, Fischer S, Gao X, et al. ToxoDB: an integrated Toxoplasma gondii database resource. Nucl. Acids Res. 2007;36:D553–556. doi: 10.1093/nar/gkm981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chem. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoudi PM, Jacot D, Mueller C, Di Cristina M, Dogga SK, Marq JB, et al. Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS Pathog. 2015;11:e1005211. doi: 10.1371/journal.ppat.1005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB Journal. 1995;9:576–596. [PubMed] [Google Scholar]
- Hehl AB, Basso WU, Lippuner C, Ramakrishnan C, Okoniewski M, Walker RA, et al. Asexual expansion of Toxoplasma gondii merozoites is distinct from tachyzoites and entails expression of non-overlapping gene families to attach, invade, and replicate within feline enterocytes. BMC Genomics. 2015;16:66. doi: 10.1186/s12864-015-1225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigwer F, Kerr G, Boutros M. E-CRISP: fast CRISPR target site identification. Nat Methods. 2014;11:122–123. doi: 10.1038/nmeth.2812. [DOI] [PubMed] [Google Scholar]
- Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- Hsiao CH, Luisa Hiller N, Haldar K, Knoll LJ. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic. 2013;14:519–531. doi: 10.1111/tra.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Ajzenberg D, Mercier A, Demar M, Simon S, Darde ML, et al. Geographic separation of domestic and wild strains of Toxoplasma gondii in French Guiana correlates with a monomorphic version of chromosome1a. Plos Negl. Trop. Dis. 2014;8:e3182. doi: 10.1371/journal.pntd.0003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, Mahler S, et al. An overexpression screen of Toxoplasma gondii Rab-GTPases reveals distinct transport routes to the micronemes. PLoS Pathog. 2013;9:e1003213. doi: 10.1371/journal.ppat.1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemgruber L, Lupetti P, Martins-Duarte ES, De Souza W, Vommaro RC. The organization of the wall filaments and characterization of the matrix structures of Toxoplasma gondii cyst form. Cell Microbiol. 2011;13:1920–1932. doi: 10.1111/j.1462-5822.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- Long S, Wang Q, Sibley LD. Analysis of non-canonical calcium dependent protein kinases in Toxoplasma gondii by targeted gene deletion using CRISPR/Cas9. Infect Immun. 2016 doi: 10.1128/IAI.01173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun. 2016;7:10147. doi: 10.1038/ncomms10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- Mercier C, Cesbron-Delauw MF. Toxoplasma secretory granules: one population or more? Trends Parasitol. 2015;31:60–71. doi: 10.1016/j.pt.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, et al. Integrative genomics approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe. 2010;8:208–218. doi: 10.1016/j.chom.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman KJ, Aliota MT, Knoll LJ. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genomics. 2014;15:806. doi: 10.1186/1471-2164-15-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman KJ, Knoll LJ. Long-Term Relationships: the Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol Mol Biol Rev. 2015;79:387–401. doi: 10.1128/MMBR.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011;108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JPJ, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JPJ, Coller S, Boyle JP, Jerome ME, White ME, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Soldati-Favre D. Invasion factors are coupled to key signalling events leading to the establishment of infection in apicomplexan parasites. Cell Microbiol. 2011;13:787–796. doi: 10.1111/j.1462-5822.2011.01585.x. [DOI] [PubMed] [Google Scholar]
- Shea M, Jakle U, Liu Q, Berry C, Joiner KA, Soldati-Favre D. A family of aspartic proteases and a novel, dynamic and cell-cycle-dependent protease localization in the secretory pathway of Toxoplasma gondii. Traffic. 2007;8:1018–1034. doi: 10.1111/j.1600-0854.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio. 2014a;5:e01114–01114. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. mBio. 2014b;135(3):e01114–14. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One. 2014;9:e100450. doi: 10.1371/journal.pone.0100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes GL, Jewett TJ, Carruthers VB, Sibley LD. Two separate, conserved acidic amino acid domains within the Toxoplasma gondii MIC2 cytoplasmic tail are required for parasite survival. J. Biol. Chem. 2006;281:30745–30754. doi: 10.1074/jbc.M606523200. [DOI] [PubMed] [Google Scholar]
- Talevich E, Kannan N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol Biol. 2013;13:117. doi: 10.1186/1471-2148-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serinethreonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites' boundaries. Cell Host Microbe. 2011;10:410–419. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZT, Harmon S, O'Malley KL, Sibley LD. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infect Immun. 2015;83:1039–1047. doi: 10.1128/IAI.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Laplace D, Takvorian PM, Tanowitz HB, Cali A, Wittner M. A cell culure system for study of the development of Toxoplasma gondii bradyzoites. Journal of Eukaryotic Microbiology. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.