Abstract
Respiratory mucosa immunization is capable of eliciting both local and distal mucosal immune responses; it is a potentially powerful yet largely unused modality for vaccination against respiratory diseases. Targeting the lower versus upper airways by aerosol delivery alters the immunogenicity profile of a vaccine, although the full extent of this impact is not well characterized. We set out to define the cellular and humoral response profiles elicited by immunization via intranasal, small aerosol droplets, and large aerosol droplets. We compared responses following adenovirus-vectored vaccination by these routes in macaques, either for the generation of primary immune responses or for the boosting of previously primed systemic responses. Aerosol delivery (4 or 10µm diameter droplets, addressing lower or upper airways, respectively) generated the highest magnitude lung CD4 and CD8 T-cell responses, reaching 10–30% vaccine-specific levels in bronchoalveolar lavage cells. In contrast, intranasal delivery was less immunogenic with >10-fold lower peak lung T-cell responses. Systemic (blood) T-cell responses were only observed following 4µm aerosol (and parenteral) immunization, while all delivery routes elicited similar humoral responses. These data demonstrate distinct immune response profiles with each respiratory tract vaccination modality and suggest that small droplet aerosol offers several immunological advantages over other respiratory routes.
Keywords: Aerosol, nasal, vaccine delivery route, immunogenicity, rhesus macaque
Introduction
Many life-threatening respiratory diseases lack effective vaccines providing sterilizing immunity, including influenza, respiratory syncytia virus, and tuberculosis. Vaccines that elicit potent and durable immune responses in the respiratory mucosa have the potential to reduce the global burden of these and other airborne diseases. Immunization regimens delivered to the respiratory tract initiate immune responses in mucosa-associated inductive sites and draining lymph nodes, resulting in robust local cellular and humoral responses as well as distal genital responses via immune cell linkage between these sites [1–3]. A better understanding of how airway mucosal delivery modalities differ will inform multiple applications including vaccines, gene therapy, and drug delivery.
Airway vaccination approaches explored to date include instillation in the nasal cavity and aerosol delivery by nebulizer. These two approaches target different anatomic sites, which may impact the magnitude and quality of immune responses as well as safety profiles. The relative immunogenicity of different respiratory mucosal vaccine delivery routes has not been thoroughly investigated. Studies in animal models and humans suggest that upper and lower respiratory vaccinations elicit distinct immune profiles. For example, systemic and mucosal antibody responses are greatly enhanced in mice when intranasal viral-like particle (VLP) immunization is performed under anesthesia (akin to aerosol delivery) rather than a conscious state [4, 5]. This was attributed to greater antigen deposition and uptake observed in the lungs of anesthetized animals. Similarly, measles virus vaccine administered by nebulizer (aerosol) is more immunogenic and achieves higher levels of protection against measles challenge in rhesus macaques than vaccination targeting the upper respiratory tree [6, 7]. Results from human clinical trials with human papillomavirus VLP also indicate greater immunogenicity by lower airway vaccination compared to nasal administration [3]. Thus while several intranasal subunit and live attenuated vaccines are very effective in humans [8–10], there is mounting evidence that immunogenicity and efficacy may be further improved by aerosol delivery. Additional investigation of how immune responses differ when elicited by different respiratory mucosal vaccination routes is warranted, including a better understanding of the underlying immunogenicity mechanisms.
While intranasal immunization has been commonly studied in rodent models, the caveat to the interpretation of these data is that much of the instilled vaccine enters the lung, and thus induction occurs at both mucosa. The much larger physical separation of the nasal cavity from the bronchi in primates and man likely impacts to a much greater extent the immune profiles generated by separately addressing these two mucosal sites.
The objective of the present study was to directly compare immunogenicity of several airway vaccination routes targeting different regions of the upper and lower respiratory tract in rhesus macaques. We previously demonstrated that smaller aerosol droplets (4 µm diameter) elicit greater systemic cellular and humoral responses than larger, 10–11 µm droplets, despite similar responses in the respiratory tract [11]. We directly compared the immunogenicity of vaccine delivery via the following four routes: intranasal, 4 µm aerosol (AE 4µm), 10 µm aerosol (AE 10µm), and parenteral. We tested the ability of these delivery routes to elicit responses alone (i.e., priming) and following a systemic DNA prime (i.e., heterologous boosting). We find that the latter greatly enhances immunogenicity over mucosal delivery alone and is likely to be employed in any mucosal vaccination approach [12–14]. Our studies reveal distinct profiles of mucosal and systemic T cell responses across the vaccine delivery routes and highlight the variability of immune induction within the respiratory mucosa.
Methods
Animals and immunizations
Colony-bred Indian-origin female rhesus macaques were immunized with three plasmid DNA priming immunizations four weeks apart, consisting of 4 mg each of codon-optimized SIVmac239 env (gp145ΔCFI; Althea Technologies, CA). Immunogens were expressed within the vector pVR1012 under the control of cytomegalovirus immediate-early enhancer, promoter, and first intron. Delivery was intramuscular in the anterior quadriceps by Biojector. Recombinant E1/E3/E4-deleted rAd5 constructs and virus stocks were generated as previously described [15–17]. rAd5 expressing GagPolSIV (1×1010) and EnvSIV (gp140; 1×1010) was administered eight weeks following the last DNA prime by one of four routes: intranasal (IN), aerosol (AE) 4 µm diameter droplets, AE 10 µm, or intramuscular (IM). IM was performed by needle and syringe in the right quadriceps; AE by e-Flow® Nebulizer System (PARI Pharma, Germany); and IN by instillation in the nasal cavity. Particle size distributions generated by the e-Flow® were determined by the Aerodynamic Particle Sizer® 3321 spectrometer (TSI, MN). For tracking studies, Fluoresbrite® Polychromatic Red 0.5 µm microspheres (Polysciences, Inc. Cat no. 19507-5) were delivered by AE at 2.5×1011 or 4×1011 total particles. Animals were sacrificed 18 hours after bead delivery for tissue collection and analysis of cellular bead uptake by flow cytometry. Single cell suspensions were generated from respiratory tract tissue using a gentleMACS™ Dissociator.
Ethics Statement
All in vivo procedures were carried out in accordance to institutional, local, state, and national guidelines and laws governing research in animals including the Animal Welfare Act. Animal protocols and procedures were reviewed and approved by the Animal Care and Use Committee (ACUC) of both the Vaccine Research Center as well as the Institutional Animal Care and Use Committee of Bioqual, Inc. where non-human primates were housed for the duration of the study. Bioqual Inc., and the NIH are both accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and are in full compliance with the Animal Welfare Act and Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Antibody measurements
SIV Gag- and Env-specific humoral IgG responses were evaluated by a standardized binding antibody multiplex array as previously described [18, 19]. Positive and negative monkey sera controls were used in each assay and the midpoint titer (EC50) of each sample was calculated using a 4 parameter logistic curve fit. Positive responses to the vaccine were assessed as three-fold over pre-immune values and at least 100 MFI. Rectal, nasal, and vaginal secretions were sampled by a modified wick method using Weck-Cel Spears (Windsor Biomedical, Newton, NH) as previously described [20]. The antigen-specific IgA or IgG fluorescence intensity was divided by the concentration of total IgA or IgG for each sample to obtain specific activity. Adenoviral neutralizing titers were performed by the NIAID Vaccine Immune T-Cell and Antibody Laboratory based on a published assay [21].
Cellular immune responses
BAL and peripheral blood were collected longitudinally from animals following immunization. Single cell suspensions were stimulated with overlapping peptide pools of SIV Env, Gag, or Pol at 2.0 µg/ml for 16 hours as previously described. Following stimulation, cells were labeled with cell surface markers (CD4-Alexa700APC, CD8-QDot655, CD3-Cy7APC) and ViViD (to discriminate live/dead cells). Intracellular cytokine staining was performed on fixed and permeabilized (BD Cytofix/Cytoperm, Becton Dickenson) samples with IFNγ-FITC, TNFα-Cy7PE, and IL-2-PE. Samples were analyzed on an LSR II (Becton Dickenson) and analyzed using FlowJo software (Tree Star, Inc.). Statistical analysis and display of multicomponent distributions was performed with SPICE v4.
Results
Localization of aerosol droplet uptake
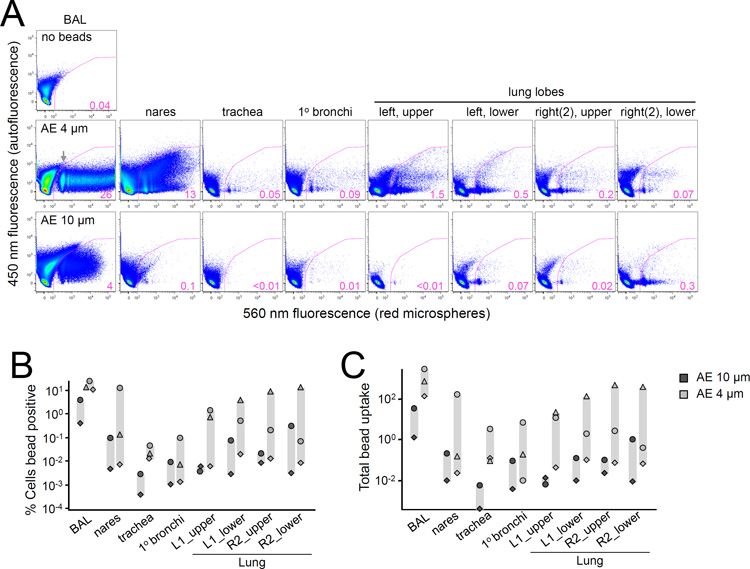
We hypothesized that small aerosol (AE) droplets penetrate deep into the lower respiratory tract while larger droplets are restricted to the head airways, based on previous observations in humans and non-human primates [22–25]. Using the PARI e-Flow nebulizer, we performed tracking studies to measure the deposition of differentially sized aerosol droplets in rhesus macaques. Fluorescent polystyrene microspheres were delivered by nebulizer-generated 4 or 10µm diameter droplets. Aerosol droplet sizes in this range previously exhibited differential deposition properties and infection transmission rates [22, 25]. Cells isolated from upper and lower respiratory tract tissue were examined for bead uptake by flow cytometry 18h after delivery. Cells positive for microspheres were readily detected in BAL following AE delivery of both droplet sizes, ranging from 0.4–26% of all cells (Figure 1A–B). Strikingly, the fluorescence profiles revealed discrete cell populations containing anywhere from one to hundreds of microspheres. Across the five animals studied, trends indicated greater microsphere uptake following 4µm AE in most tissues, particularly in BAL, trachea, and lung lobes (Figure 1B). Cellular uptake of the aerosolized beads was further quantitated to account for multiple microspheres per cell by calculating the total number of beads taken up by all positive cells (Figure 1C). This analysis yielded similar results, confirming that 4µm AE delivers material more efficiently than 10µm AE to cells residing throughout the respiratory mucosa.
Figure 1.
Differential uptake of aerosolized micro-beads with 4 and 10 µm aerosol droplets. Macaques received fluorescent particles by either 4 or 10 µm nebulizers and were sacrificed 18h later for bead uptake in respiratory tract tissues. (A) Flow cytometric analysis of ungated single cells isolated from the indicated tissue is shown for two representative animals; 4µm (middle), 10µm (bottom), and negative control BAL from an unexposed animal (top). Cellular uptake of red fluorescent microspheres is depicted by the gated population, with percent positive indicated. Fluorescence at 450 nm is plotted as a negative control, although some spill over or increased autofluorescence is observed at this wavelength. Gray arrow indicates cell population containing a single microsphere. (B) The percentage of cells positive for fluorescent microspheres by flow cytometric analysis is plotted for five animals (n=3, AE 4µm; n=2, AE 10µm) across the indicated tissues. Symbols represent individual animals. (C) Total microsphere uptake is plotted as in (B). Uptake was calculated as follows: (fraction cells containing ≥1 microsphere×mean G560 fluorescence of all positive cells) / (mean G560 fluorescence of cells containing one microsphere).
Mucosal (BAL) T cell responses
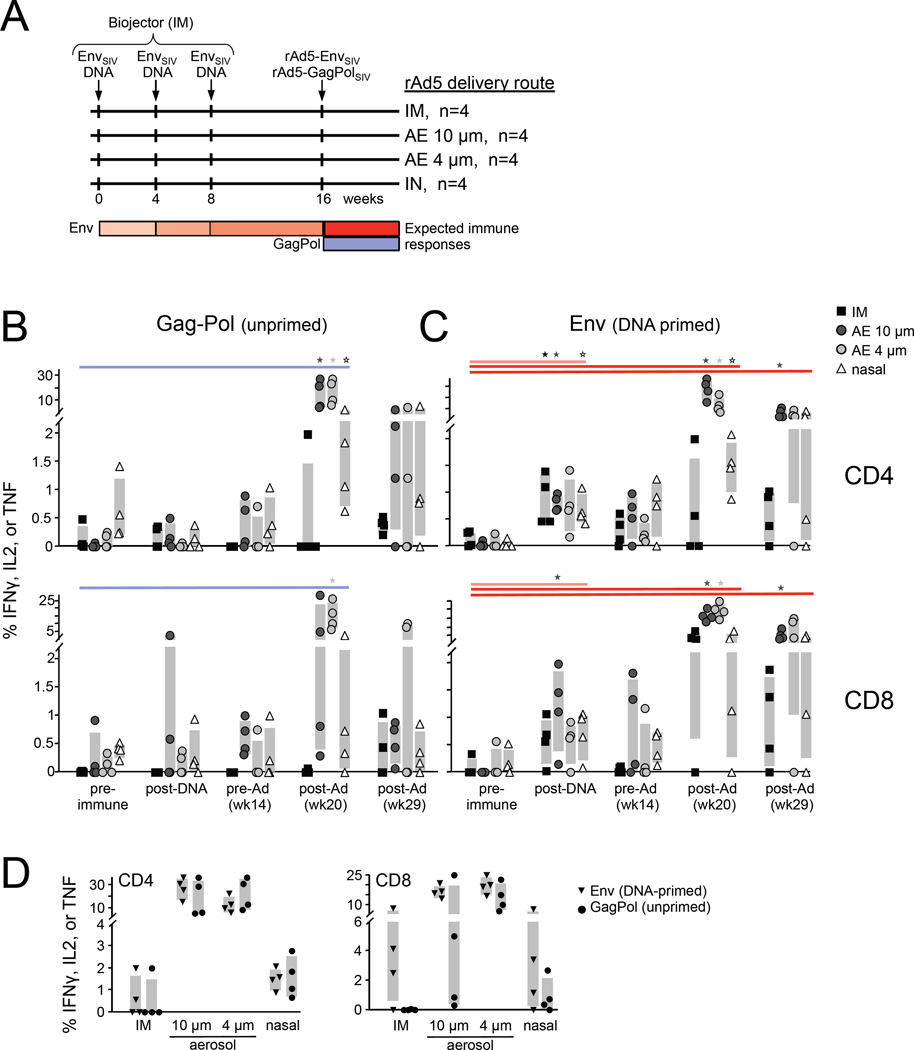
To compare humoral and cellular immune responses induced by AE droplets and intranasal (IN) and intramuscular (IM) vaccine delivery routes, we immunized rhesus macaques with rAd5 encoding GagPolSIV via each route (Figure 2A). Mucosal tissue and blood sampling was performed at regular intervals following immunization to measure antigen-specific T-cells and antibodies across multiple tissues. GagPol-specific CD4 and CD8 T-cells were quantified by ex vivo peptide pool stimulation followed by intracellular cytokine staining and polychromatic flow cytometric detection (Figure 2). AE delivery elicited robust bronchoalveolar lavage (BAL) responses to GagPol, ranging from 10–30% of CD4 T-cells and up to 25% of CD8 T-cells one month post-rAd5 (Figure 2B), consistent with previous studies [11]. IN delivery also induced substantial BAL responses, but these were significantly less than those elicited by AE 4 µm, ranging from 0.5–5% for most animals. GagPol responses following IM delivery were generally undetectable. This finding was inconsistent with 1–7% responding BAL CD8 T-cells observed previously following IM immunization [11, 12], and may be due to original antigenic sin interference from rAd5-Env co-administered with rAd5-GagPol (see below). Interpretation of the unprimed IM regimen in this experiment may thus be confounded. CD8 T-cell responses in a subset of animals from the 10µm AE and IN groups were also unexpectedly low. Taken together, along with our prior studies in which IN and IM delivery exhibit similar BAL T-cell priming with slightly greater responses elicited by IN [11, 12], we observed the following hierarchy for priming lung mucosal T cell responses: AE 4µm AE 10µm > IN IM. Following response peak, GagPol-specific T-cells contracted to similarly low levels ranging from 0–1.5% for all groups (week 29 and 39, data not shown).
Figure 2.
Airway mucosal T-cell response magnitude. (A) Experimental vaccination scheme. Sixteen rhesus macaques were immunized with three DNA primes encoding Env at one-month intervals. Eight weeks after the last DNA prime, a mixture of two rAd5 viruses encoding Env and GagPol immunogens was administered IN, IM, AE 4µm, or AE 10µm (n=4 per group). Antigen-specific T-cells were measured throughout the vaccination regimen in BAL by in vitro peptide stimulation and intracellular Th1 cytokine staining and flow cytometric quantitation. Unprimed GagPol-specific (B) and DNA-primed Env-specific (C) CD4+ (top) and CD8+ (bottom) T-cell responses are shown for each vaccination group at the indicated time point. Statistically significant (p<0.05, Wilcoxon rank-sum test) responses relative to the pre-immune measurement for each vaccine group are indicated. (D) Direct comparison of DNA-primed and unprimed BAL responses four weeks after rAd5 vaccination (week 20) is shown.
To test the boosting capability of each route, responses to a different immunogen, EnvSIV, were first primed with DNA intramuscularly three times at monthly intervals prior to boosting with rAd5 encoding Env via the various routes (Figure 2A). CD4 and CD8 responses to the DNA prime were modest (Figure 2C), as expected. Mucosal T-cell responses elicited by the rAd5 boost were similar in magnitude to unprimed responses by the same route (Figure 2B and [11, 12]). AE rAd5 boosting elicited the largest BAL response, again ranging from 10–30% of both CD4 and CD8 T-cell subsets. By contrast, responses to IN and IM delivery comprised 0–2% of CD4 T-cells and 0–5% of CD8 T-cells. Peak responses were typically detected four weeks post-rAd5 for the AE and IM groups, while IN responses were often delayed, consistent with previous experiments (data not shown). The boosting capabilities can thus be summarized as follows: AE 10µm AE 4µm > IN IM.
For comparison, peak primed and unprimed mucosal BAL responses were plotted together in Figure 2D. CD4 T-cell responses were similar in magnitude for Env and GagPol for all immunization routes, suggesting that systemic DNA did not prime CD4 BAL T-cells. The effect of DNA priming on CD8 T-cell responses was less clear due to the atypical unprimed GagPol response observed for most delivery conditions. Remarkably, CD8 responses to AE 4µm were robust for both Env and GagPol. Combined, AE rAd5 was more immunogenic than IN delivery in the respiratory mucosa, regardless of priming status.
Peripheral blood T-cell responses
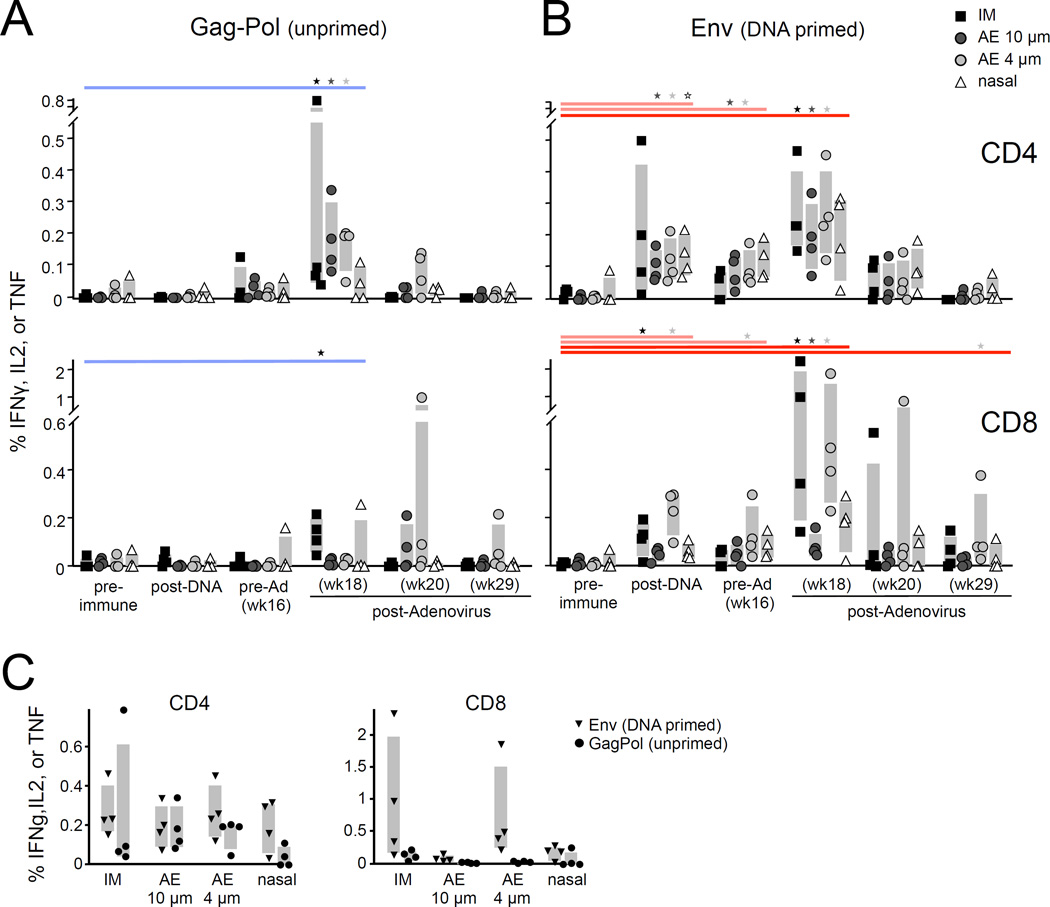
To study elicitation of systemic antigen-specific T-cells, we performed similar ICS analyses on PBMC samples. Low-magnitude, transient CD4 T-cell responses were observed to the unprimed Gag antigen for IM and both AE routes (Figure 3A), while only IM vaccination generated significant unprimed CD8 T-cell responses. These responses were generally below the limit of detection one month after rAd5. PBMC responses elicited by AE 4µm and 10µm immunization were not significantly different, whereas we previously observed higher magnitude responses by AE 4µm [11].
Figure 3.
Systemic T-cell response magnitude. Antigen-specific T-cell responses in PBMC were measured and plotted as in Figure 2. The unprimed GagPol-specific (A) and DNA-primed Env-specific (B) PBMC responses are shown. Statistically significant (p<0.05, Wilcoxon rank-sum test) responses relative to the pre-immune measurement for each vaccine group are indicated. (C) DNA-primed and unprimed PBMC responses are compared directly at peak (two weeks after rAd5, or week 18).
In the setting of systemic DNA-priming, all routes showed boosting CD4 responses relative to pre-Ad levels (IM, p=0.04; AE 10µm, p=0.15; AE 4µm, p=0.08; IN, p=0.33, wk 16 vs. 18; Figure 3B). By contrast, there was a striking difference among the delivery routes with respect to CD8 T-cell boosting. Only IM and AE 4µm boosted DNA-primed responses (IM, p=0.04, AE, p=0.09, wk 16 vs. 18), achieving 0.2–2% Env-specific CD8 T-cells (Figure 3B–C). Blood CD8 T-cell responses were not enhanced by either AE 10µm or IN rAd5 delivery. Thus AE 4µm is more similar to IM immunization with respect to boosting systemic CD8 T-cell responses, while AE 10µm and IN were less immunogenic.
Quality of cellular responses
Since T-cell response quality correlates with disease protection in multiple animal models and human infections [26–28], we analyzed the combination of cytokines expressed by antigen-specific T-cells in each vaccine group. Three months after rAd5 primary immunization, the quality of the BAL memory CD4 T-cell (GagPol-specific) response differed between the AE groups and the IM group (p=0.05, AE 4µm; p=0.06, AE 10µm, Figure S1A). AE delivery shifted responses towards greater polyfunctionality as measured by simultaneous co-expression of IFNγ, IL-2, and TNF by a larger fraction of the antigen-specific cells. No significant differences were observed between vaccine delivery routes for CD8 T cells or at other time points.
We also assessed whether DNA priming influenced the quality of the response for each vaccine delivery route. At peak, BAL CD8 T-cell responses to IN immunization were more polyfunctional with DNA priming than without the systemic prime (Figure S1B). BAL responses were skewed towards IL-2 single positive and IL2+TNFα+ cells in the absence of DNA priming, while the primed response was comprised largely of IL2+TNFα-INFγ+ and IFNγsingle-positive cells. This is consistent with DNA priming driving the T cell response towards a more differentiated phenotype [29]. Response quality for other vaccination routes was not influenced by the systemic prime.
Humoral responses
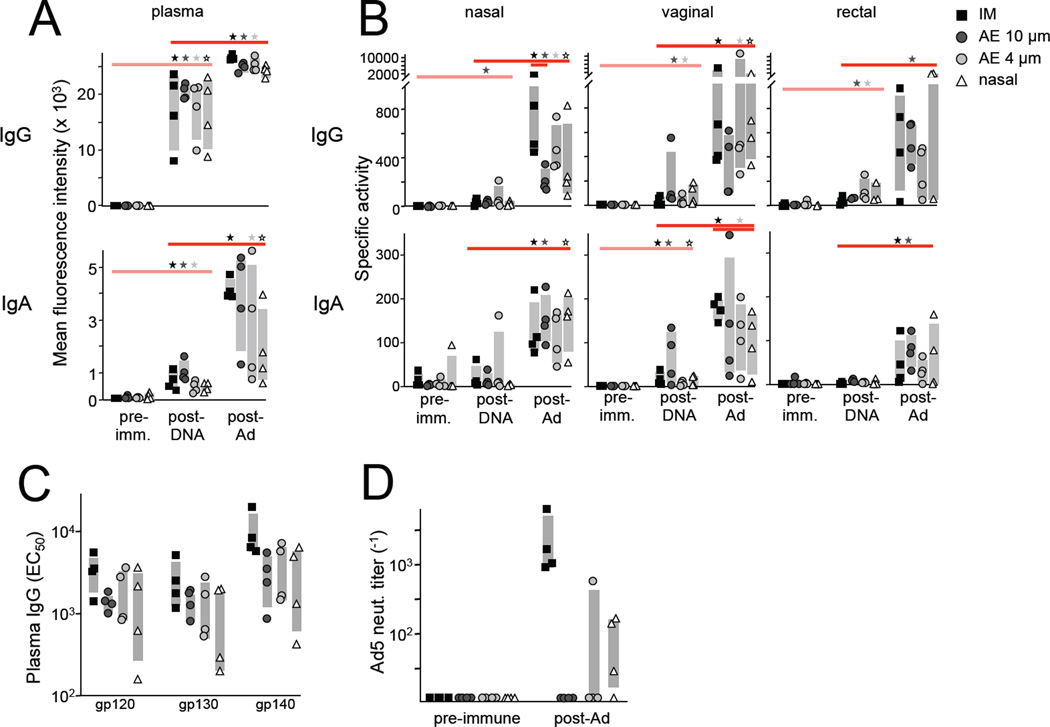
Env- and Gag-specific antibodies were measured in plasma and mucosal secretions following the DNA prime and rAd5 immunizations. DNA alone elicited significant plasma gp120-specific IgG and IgA responses (p<0.01 and p<0.03, respectively, Figure 4A), while nasal, vaginal, and rectal responses were low or undetectable (Figure 4B). Boosting with the viral vector significantly increased Env-specific plasma IgG or IgA for all delivery routes (p<0.05). Nasal, vaginal, and rectal mucosal responses were also boosted by rAd5 relative to post-DNA values, although statistical significance was not achieved for some delivery routes due to within group variability (Figure 4B). Similar trends were observed for antibodies specific for gp130 and gp140 Env antigens (data not shown). gp120-specific IgG or IgA mucosal responses generally were not significantly different between vaccination groups following rAd5 immunization, with the exception of greater nasal IgG following IM versus AE 10µm delivery and greater vaginal IgA following IM versus nasal delivery. Surprisingly, no significant antibody responses to the Gag immunogen were detected, likely due in part to greater inherent Env humoral immunogenicity.
Figure 4.
Systemic and mucosal humoral responses. (A–B) Env- and Gag-specific antibodies were measured in plasma and mucosal (nasal, vaginal, rectal) secretions by Luminex. (A) gp120-specific plasma IgG (top) and IgA (bottom) are plotted for each animal as mean fluorescence intensity (MFI). Statistically significant (p<0.05) responses relative to the post-DNA measurement for each vaccine group are indicated along the top (Wilcoxon rank-sum test). “Post-DNA” and “post-Ad” refer to study week 14 (6 weeks post-DNA) and 20 (4 weeks post Ad), respectively. Gray bars reflect the interquartile range for each group. (B) Nasal, vaginal, and rectal IgG (top) and IgA (bottom) are plotted as specific activity (binding units / total Ig (µg/ml), where binding units = MFI*dilution). Between group comparisons within a time point are indicated by lines spanning group with significantly different responses. (C) Week 20 plasma IgG EC50 values for the indicated Env antigens were determined using a 4 parameter logistic curve fit model. (D) The plasma 90% adenovirus neutralizing titer was determined for each animal before and 4 weeks after the rAd5 immunization and plotted as the reciprocal dilution.
Circulating Env-specific humoral responses elicited by the rAd5 boost were further quantitated by titrating plasma to determine the half maximal effective concentration (EC50) for responses to three versions of the Env antigen. IM delivery typically induced the largest plasma IgG responses, with greater gp140-specific IgG than AE 10µm (p=0.05), AE 4µm (p=0.09), and IN (p=0.1) (Figure 4C). gp120- and gp130-specific responses followed a similar pattern. Thus IM delivery potentially offers a slight advantage with respect to boosting plasma IgG magnitude, but overall responses were similar among the groups.
We previously reported that little or no humoral response is generated to the viral vector when it is administered AE [11]. To compare the anti-vector response across the delivery routes used here, we measured serum Ad5 neutralizing antibodies four weeks after rAd5 vaccination. As expected, Ad5 titers were robust following IM delivery: 90% inhibitory plasma concentrations (IC90) values ranged from 1000–4000−1 in all animals (Figure 4D). IN delivery was less potent, but low-level responses were mounted in three of four animals. By contrast, AE was largely silent with respect to Ad5 immunogenicity with undetectable titers in seven of eight animals. Taken together with the humoral responses elicited against the Env insert, these data indicate that mucosal airway and parenteral vaccination achieve similar humoral responses to the immunogen, but the latter is much more immunogenic with respect to anti-vector responses. Between the mucosal routes, IN is more likely to generate vector-specific antibodies than AE.
Discussion
Respiratory mucosal vaccination has been well tolerated, immunogenic, and efficacious in humans as well as animal models of disease. The immunologic advantage gained by targeting vaccines to different regions of the respiratory tract, however, is not clear. Here we report distinct immune profiles following AE 4µm, AE 10µm, and IN delivery of rAd5 vaccine vector in rhesus macaques (summarized in Table 1). AE vaccination with either 4 or 10 µm diameter droplets elicits 1–2 log greater peak lung CD4 and CD8 T-cell responses than IN, which exhibited immunogenicity more similar to IM than AE. This was observed for both unprimed and systemic DNA-primed responses. BAL CD4 T-cell responses were generally not primed by DNA, regardless of vaccination route. A different immunogenicity hierarchy was observed for blood T-cell responses: IM and AE 4µm robustly boosted CD8 responses, while little boosting was observed by AE 10µm and IN. Tracking of AE droplets within respiratory mucosa further distinguished AE 4µm and 10µm immunization, with greater cellular uptake of material delivered by 4µm droplets. Mucosal and plasma humoral responses to the Env immunogen were similar regardless of the rAd5 delivery route. Upon longer follow up, systemically primed AE generates more durable rectal IgA titers than IM vaccination with the same prime [12]. Taken together, these data, combined with our previous T-cell priming observations [11, 12], suggest that while both 4µm and 10µm AE are far more immunogenic than IN, only 4µm AE elicits robust peripheral T-cells.
Table 1.
Summary of immune response magnitude by vaccine delivery route
| Immunization | Systemic* | BAL | Mucosal | Vector- specific neut. Ab |
|||||
|---|---|---|---|---|---|---|---|---|---|
| T cell | Antibody | T cell | Antibody | ||||||
| Primary | Boost | Primary** | Boost | Primary** | Boost | Primary | Boost | ||
| DNA (IM) | − | n.a. | ++ | n.a. | + | n.a. | − | n.a. | n.a. |
| Ad IM | +++ | ++ | ++ | +++ | ++ | ++ | n.a. | ++ | +++ |
| Ad IN | + | + | n.a. | ++ | ++ | ++ | n.a. | + | ++ |
| Ad AE 4µm | ++ | ++ | ++ | ++ | ++++ | ++++ | n.a. | +*** | + |
| Ad AE 10µm | ++ | + | + | ++ | +++ | ++++ | n.a. | + | − |
Immune responses were ranked by magnitude across all animals, followed by averaging the rank within each vaccine group.
Distal mucoasl IgA are more durable following rAd5 boost via AE 4um than IM [12].
n.a. = not assessed
Our tracking studies revealed greater cellular uptake of material delivered by AE 4µm than AE 10µm, particularly among cells residing in the lower respiratory tract. Our data indicate that more antigen-presenting cells (APC) within the respiratory mucosa are exposed to and engulf antigen when exposed to smaller aerosols. In turn, this likely results in a higher frequency of APC in draining lymph nodes and a larger quantity of antigen presented by each APC. Deeper penetration of the 4µm aerosol droplets may also target APC that drain to more distal or distinct lymph nodes that alter the trafficking of responding antigen-specific lymphocytes.
T-cell and antibody responses to the unprimed GagPol rAd5 insert were limited and lower than our previous observations reporting robust cellular and humoral responses elicited by the same rAd5-GagPol vaccination in both unprimed and DNA-primed settings [11, 12]. GagPol-specific responses were thus impaired in this study. Inhibition was possibly due to “original antigenic sin” from the prior exposure to Env DNA: i.e., the priming immunization resulted in Env-dominated responses when both GagPol and Env were co-delivered as a boost. Similar results have been previously noted for cellular responses [30–33].
rAd5 vector-specific antibodies were consistently elicited by IM and IN, but not AE. While vector-specific antibodies are not typically elicited by AE immunization [11], one animal immunized by AE 4µm in this study mounted Ad5-specific titers. This animal was among the strongest responders to the vaccination regimen within the AE 4µm group, with the largest CD4 T cell response to the DNA prime in both BAL and PBMC. These data suggest that robust CD4 helper T cells generated by the systemic prime specific for the insert encoded by a subsequent viral vector boost may facilitate development of vector-specific antibody responses upon AE boosting.
In summary, we show that small droplet aerosols generate robust mucosal T cell responses and, when primed systemically, boost both peripheral T cells and humoral responses. Similar magnitude lung T cell responses are generated by small droplet aerosols of rAd35 [11, 34] or Bacillus Calmette–Guérin (M. Roederer, unpublished), suggesting that these findings may extend to other vectors and vaccine platforms. Given good safety profiles of aerosol delivery in both humans and non-human primates, the historical success of aerosol vaccination with live-attenuated measles virus in children [3, 35, 36], and the promising results presented here, further consideration of aerosol immunization is warranted for elicitation of durable and robust mucosal immunity. These findings are pertinent for strategies to achieve mucosal immunity using heterologous systemic/mucosal vaccination.
Supplementary Material
Supplemental Figure S1. T-cell response quality. The combination of cytokines expressed by antigen-specific cells in response to cognate peptide stimulation was measured by flow cytometry as in Figure 2. The proportion of the total antigen-specific T-cell population expressing each possible combination of cytokines is shown as a pie chart for each vaccination group; colored wedges correspond to cytokine profile indicated at right. (A) Unprimed BAL CD4+ T-cell quality at memory (13 weeks post-rAd5) for the Gag/Pol-specific response is shown. (B) BAL CD8+ T-cell quality at peak (4 weeks post-rAd5) for the unprimed (Gag) and DNA primed (Env) responses for each rAd5 vaccination route. Significantly different pie charts are indicated with p-values.
Highlights.
Intranasal and lung immunization induce distinct immune responses
Small aerosol drops provide the optimal lung immunization modality
Unlike parenteral and intranasal, lung immunization induces no anti-vector immunity
Acknowledgments
We are grateful to Dr. Bob Bailer of the Vaccine Research Center (VRC) Immunology Core Laboratory for adenovirus serology; JP Todd of the VRC Laboratory of Animal Medicine for animal protocol preparation, animal procedure scheduling, and administering immunizations; the VRC Flow Cytometry Core for maintenance of LSR II flow cytometric instrumentation; members of the VRC ImmunoTechnology Section for helpful scientific discussions; Judith T. Lucas, Yong Lin, Vicki C. Ashley, and R. Glenn Overman for expert technical assistance; and BIOQUAL, Inc. for animal housing, handling, and tissue collection.
This work was supported by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH and by a cooperative agreement (W81XWH-07-2- 0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. The authors declare no conflict of interest.
References
- 1.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 2.Johansson EL, Wassen L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infection and immunity. 2001;69:7481–7486. doi: 10.1128/IAI.69.12.7481-7486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23:3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. Journal of virology. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revaz S, Dudler J. [Clinical manifestations of gout] Revue medicale suisse. 2007;3:728–730. [PubMed] [Google Scholar]
- 6.de Swart RL, Kuiken T, Fernandez-de Castro J, Papania MJ, Bennett JV, Valdespino JL, et al. Aerosol measles vaccination in macaques: preclinical studies of immune responses and safety. Vaccine. 2006;24:6424–6436. doi: 10.1016/j.vaccine.2006.05.125. [DOI] [PubMed] [Google Scholar]
- 7.de Swart RL, LiCalsi C, Quirk AV, van Amerongen G, Nodelman V, Alcock R, et al. Measles vaccination of macaques by dry powder inhalation. Vaccine. 2007;25:1183–1190. doi: 10.1016/j.vaccine.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Bergquist C, Lagergard T, Holmgren J. Anticarrier immunity suppresses the antibody response to polysaccharide antigens after intranasal immunization with the polysaccharide-protein conjugate. Infection and immunity. 1997;65:1579–1583. doi: 10.1128/iai.65.5.1579-1583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills KH, Cosgrove C, McNeela EA, Sexton A, Giemza R, Jabbal-Gill I, et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infection and immunity. 2003;71:726–732. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert review of vaccines. 2004;3:643–654. doi: 10.1586/14760584.3.6.643. [DOI] [PubMed] [Google Scholar]
- 11.Song K, Bolton DL, Wei CJ, Wilson RL, Camp JV, Bao S, et al. Genetic immunization in the lung induces potent local and systemic immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22213–22218. doi: 10.1073/pnas.1015536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton DL, Song K, Wilson RL, Kozlowski PA, Tomaras GD, Keele BF, et al. Comparison of systemic and mucosal vaccination: impact on intravenous and rectal SIV challenge. Mucosal immunology. 2012;5:41–52. doi: 10.1038/mi.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 14.Cranage MP, Fraser CA, Cope A, McKay PF, Seaman MS, Cole T, et al. Antibody responses after intravaginal immunisation with trimeric HIV-1 CN54 clade C gp140 in Carbopol gel are augmented by systemic priming or boosting with an adjuvanted formulation. Vaccine. 2011;29:1421–1430. doi: 10.1016/j.vaccine.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. Journal of virology. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gall JG, Lizonova A, EttyReddy D, McVey D, Zuber M, Kovesdi I, et al. Rescue and production of vaccine and therapeutic adenovirus vectors expressing inhibitory transgenes. Molecular biotechnology. 2007;35:263–273. doi: 10.1007/BF02686012. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen H, Rasmussen C, Lempicki M, Durham R, Brough D, King CR, et al. TNFerade Biologic: preclinical toxicology of a novel adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene. Cancer gene therapy. 2002;9:951–957. doi: 10.1038/sj.cgt.7700518. [DOI] [PubMed] [Google Scholar]
- 18.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. Journal of virology. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates NL, Stacey AR, Nolen TL, Vandergrift NA, Moody MA, Montefiori DC, et al. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol. 2013;6:692–703. doi: 10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24:297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 21.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day WC, Berendt RF. Experimental tularemia in Macaca mulatta: relationship of aerosol particle size to the infectivity of airborne Pasteurella tularensis. Infection and immunity. 1972;5:77–82. doi: 10.1128/iai.5.1.77-82.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Druett HA, Henderson DW, Packman L, Peacock S. Studies on respiratory infection. I. The influence of particle size on respiratory infection with anthrax spores. The Journal of hygiene. 1953;51:359–371. doi: 10.1017/s0022172400015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ICRP. Human respiratory tract model for radiological protection. Elsevier Health Sciences; 1994. [Google Scholar]
- 25.Cheng YS, Irshad H, Kuehl P, Holmes TD, Sherwood R, Hobbs CH. Lung deposition of droplet aerosols in monkeys. Inhalation toxicology. 2008;20:1029–1036. doi: 10.1080/08958370802105413. [DOI] [PubMed] [Google Scholar]
- 26.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 27.Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–972. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 28.Lin L, Finak G, Ushey K, Seshadri C, Hawn TR, Frahm N, et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol. 2015;33:610–616. doi: 10.1038/nbt.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews Immunology. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 30.Da Silva DM, Pastrana DV, Schiller JT, Kast WM. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimeric human papillomavirus virus-like particle vaccines. Virology. 2001;290:350–360. doi: 10.1006/viro.2001.1179. [DOI] [PubMed] [Google Scholar]
- 31.Liu XS, Dyer J, Leggatt GR, Fernando GJ, Zhong J, Thomas R, et al. Overcoming original antigenic sin to generate new CD8 T cell IFN-gamma responses in an antigen-experienced host. Journal of immunology. 2006;177:2873–2879. doi: 10.4049/jimmunol.177.5.2873. [DOI] [PubMed] [Google Scholar]
- 32.Zompi S, Harris E. Original antigenic sin in dengue revisited. Proc Natl Acad Sci U S A. 2013;110:8761–8762. doi: 10.1073/pnas.1306333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Good MF, Zevering Y, Currier J, Bilsborough J. 'Original antigenic sin', T cell memory, and malaria sporozoite immunity: an hypothesis for immune evasion. Parasite Immunol. 1993;15:187–193. doi: 10.1111/j.1365-3024.1993.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 34.Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, et al. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J Immunol. 2014;193:1799–1811. doi: 10.4049/jimmunol.1400676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dilraj A, Cutts FT, de Castro JF, Wheeler JG, Brown D, Roth C, et al. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet. 2000;355:798–803. doi: 10.1016/s0140-6736(99)95140-1. [DOI] [PubMed] [Google Scholar]
- 36.Hokey DA, Wachholder R, Darrah PA, Bolton DL, Barouch DH, Hill K, et al. A nonhuman primate toxicology and immunogenicity study evaluating aerosol delivery of AERAS-402/Ad35 vaccine: Evidence for transient t cell responses in peripheral blood and robust sustained responses in the lungs. Hum Vaccin Immunother. 2014;10:2199–2210. doi: 10.4161/hv.29108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. T-cell response quality. The combination of cytokines expressed by antigen-specific cells in response to cognate peptide stimulation was measured by flow cytometry as in Figure 2. The proportion of the total antigen-specific T-cell population expressing each possible combination of cytokines is shown as a pie chart for each vaccination group; colored wedges correspond to cytokine profile indicated at right. (A) Unprimed BAL CD4+ T-cell quality at memory (13 weeks post-rAd5) for the Gag/Pol-specific response is shown. (B) BAL CD8+ T-cell quality at peak (4 weeks post-rAd5) for the unprimed (Gag) and DNA primed (Env) responses for each rAd5 vaccination route. Significantly different pie charts are indicated with p-values.