Abstract
Purpose
Alternative strategies to EGFR blockage by mAbs is necessary in order to improve the efficacy of therapy in patients with locally advanced or metastatic pancreatic cancer. One such strategy includes the use of NK cells to clear cetuximab-coated tumor cells, as need for novel therapeutic approaches to enhance the efficacy of cetuximab is evident. We show that IL-21 enhances NK cell-mediated effector functions against cetuximab-coated pancreatic tumor cells irrespective of KRAS mutation status.
Experimental Design
NK cells from normal donors or donors with pancreatic cancer were used to assess ADCC, IFN-γ release, and T cell chemotaxis towards human pancreatic cancer cell lines. The in vivo efficacy of IL-21 in combination with cetuximab was evaluated in a subcutaneous and intraperitoneal model of pancreatic cancer.
Results
NK cell lysis of cetuximab-coated wild-type and mutant KRAS pancreatic cancer cell lines was significantly higher following NK cell IL-21 treatment. In response to cetuximab-coated pancreatic tumor cells, IL-21 treated NK cells secreted significantly higher levels of IFN-γ and chemokines, increased chemotaxis of T cells, and enhanced NK cell signal transduction via activation of ERK and STAT1. Treatment of mice bearing subcutaneous or intraperitoneal EGFR-positive pancreatic tumor xenografts with mIL-21 and cetuximab led to significant inhibition of tumor growth, a result further enhanced by the addition of gemcitabine.
Conclusions
These results suggest that cetuximab treatment in combination with IL-21 adjuvant therapy in patients with EGFR-positive pancreatic cancer results in significant NK cell activation, irrespective of KRAS mutation status, and may be a potential therapeutic strategy.
Keywords: IL-21, pancreatic cancer, antibody-dependent cellular cytotoxicity, natural killer cells, cetuximab
INTRODUCTION
Overexpression of the epidermal growth factor receptor (EGFR) is observed in greater than 70% of pancreatic adenocarcinomas and is associated with disease progression and poor prognosis (1–4). Cetuximab (Erbitux™) is a monoclonal antibody that binds to the extracellular domain of the EGFR molecule and prevents ligand binding. Cetuximab-mediated inhibition of EGFR-mediated signal transduction in tumor cells leads to G1 arrest and the induction of apoptosis in vitro (5,6) and in murine xenograft models (7,8). Cetuximab has been approved by the FDA alone or in combination with the topoisomerase inhibitor irinotecan for the treatment of patients with irinotecan-refractory colorectal carcinoma. This regimen led to a significant increase in progression-free survival in colorectal cancer patients and led to complete or partial tumor shrinkage in over 20% of patients (9,10). However, cetuximab, like other EGFR-directed therapies, has produced objective clinical responses in only a minority of pancreatic cancer patients with EGFR-positive tumors (11). One explanation for this could be the presence of mutations in the KRAS oncogene, which results in constitutive activation of the MAPK pathway. This activating mutation stimulates the MAPK pathway downstream of EGFR, resulting in reduced cetuximab effectiveness.
NK cells are bone-marrow-derived, large granular lymphocytes that contain abundant cytolytic granules and express numerous cellular adhesion molecules (12,13). NK cells are unique in their constitutive expression of receptors for numerous cytokines (i.e. IL-12, -15, -18 and -21) and an activating receptor for the Fc region of IgG (FcγRIIIa) (14–16). In addition to their ability to mediate antibody-dependent cellular cytotoxicity (ADCC), FcR-activated NK cells also secrete factors such as IFN-γ, TNF-α and chemokines that inhibit tumor cell proliferation, enhance antigen presentation and stimulate the chemotaxis of T cells (17,18). NK cells constitutively express receptors for a number of cytokines including the IL-21 receptor. IL-21 promotes the maturation of murine NK cells and increases their expression of activating receptors (19,20). It was hypothesized that IL-21-mediated enhancement of NK cell FcR effector function would be a potential method of enhancing the effectiveness of cetuximab irrespective of the KRAS mutational status of the tumor cells.
In the present study, it was shown that NK cell ADCC and cytokine release in response to cetuximab-coated pancreatic cancer cells was significantly increased following IL-21 treatment. This effect was present for both wild-type and mutant KRAS pancreatic cancer cells, and the combination of IL-21 and cetuximab had robust in vivo anti-tumor efficacy. Notably, treatment of tumor bearing mice with gemcitabine and cetuximab in combination led to only a modest reduction in tumor burden, but this effect was markedly enhanced by the addition of IL-21. Further, pancreatic patient derived NK cells exhibited significantly higher ADCC against cetuximab-coated pancreatic tumor cells following IL-21 stimulation. These findings support a role for cytokine adjuvant therapy and cetuximab treatment in the setting of EGFR-positive pancreatic cancer patients.
MATERIALS AND METHODS
Cell lines, NK cells and reagents
The human pancreatic adenocarcinoma cell lines AsPc1, BxPc3, MiaPaCa2 and Panc-1 were a gift from Dr. Mark Bloomston (The Ohio State University). MDA-MB-453 (human breast adenocarcinoma, negative control) was obtained from the American Type Culture Collection (ATCC). The murine pancreatic cancer cell line Panc02 was a gift from Michael Hollingsworth (University of Nebraska Medical Center). Colorectal cancer cell lines HCT-116 MUT and HCT-116 WT were a gift from Dr. Terrence Williams (The Ohio State University). Cell lines were grown as previously described (21). Human natural killer (NK) cells were isolated from fresh peripheral blood leukopacks (American Red Cross, Columbus, OH) or pancreatic cancer patients (OSU IRB Protocol 2006C0046) by 30-min incubation with RosetteSep cocktail (Stem Cell Technologies) before Ficoll Hypaque (Sigma) density gradient centrifugation and cultured as previously described (22). Recombinant human interleukin-21 (rhu-IL-21) was supplied by ZymoGenetics, Inc (Seattle, WA).
Immunoblot analysis
The expression of EGFR was verified via immunoblot analysis. Lysates were prepared from human pancreatic cancer cell lines as previously described (23,24) and assayed for the expression of EGFR (Santa Cruz Biotechnology, Santa Cruz, CA) or β-Actin, as a loading control (Sigma-Aldrich, St. Louis, MO).
Flow cytometry of tumor cell lines
The expression of EGFR was evaluated by extracellular flow cytometry (21). Tumor cells were harvested by trypsanization and incubated on ice for 30 min in flow buffer (5% FBS in PBS) with EGFR-PE or isotype control antibodies (Santa Cruz Biotechnology). Cells were then washed and fixed in 1% formalin. Non-specific staining by an isotype control Ab was employed to determine the percent positive population.
Antibody-dependent cellular cytotoxicity assays
NK cells from normal donors or donors with pancreatic cancer were treated with IL-21 (10 ng/ml) overnight in RPMI 1640 media supplemented with 10% human AB serum media at 37°C. Eighteen hours later, cetuximab- or IgG-coated 51Cr-labeled tumor cells were incubated with NK cells at various effector:target (E:T) ratios. Following a 4 hr incubation, supernatants were harvested and percent lysis was calculated as previously described (25).
NK cell cytokine secretion
Tumor cells were treated with 100 μg/ml of cetuximab or IgG for 1 hr at 37°C. Purified NK cells were then added to wells at 2×105 cells/well in RPMI supplemented with 10% human AB serum media with or without IL-21 for varying lengths of time. Cell-free supernatants were harvested at the indicated time points and the cytokine and chemokine levels were measured using commercially available ELISA kits (R&D Systems) (26).
T-cell chemotaxis
Normal T cells were activated for 48 hrs with 1 μg/ml phytohemagglutinin and for 72 hrs with 500 pmol/L huIL-2. NK cell co-culture supernatants were placed in the lower chambers of a 24-well flat bottom plate. Medium supplemented with 1 μg/ml monokine induced by gamma interferon served as a positive control. Migration experiments were conducted by placing 2×105 purified activated T cells in 100 μl of 10% HAB medium in the upper chambers of 5 μm pore size Transwell inserts (Corning Inc, Corning, NY). The plates were incubated for 4 hrs at 37°C followed by a 10 min wsincubation at 4°C. The number of migrated T cells was determined by trypan blue exclusion.
Intracellular flow cytometry
Intracellular levels of phospho-ERK and phospho-STAT1 within NK cells were detected using a pERK-FITC mAb or a pSTAT1-FITC mAb in combination with the NK cell marker CD56 (BD Biosciences) (27). The percentage of positively staining cells was calculated for the specified cell population.
In vitro generation of human myeloid-derived suppressor cells
Peripheral blood mononuclear cells (PBMC) were isolated directly from fresh peripheral blood leukopacks (American Red Cross) as previously described (22). Monocytes were isolated from PBMC by positive selection using CD14 MicroBeads (Miltenyi Biotec) and cultured in RPMI 1640 media supplemented with 10% human AB serum and 10 ng/ml IL-6 and GM-CSF (Peprotech, Rocky Hill, NJ) to generate myeloid-derived suppressor cells (MDSCs). Cytokines were replenished every 2–3 days.
Transfection of Panc02 cells with human EGFR, luciferase, and GFP
Human EGFR vectors were a gift from Dr. Anil Rustgi (University of Pennsylvania, Philadelphia, PA). The empty pFb-neo vector was obtained from Stratagene (La Jolla, CA). After plasmids were expanded in bacterial cells, they were transiently transfected into the Phoenix-Ampho viral packaging cell line using the calcium phosphate chloroquine method (22). Luciferase/GFP plasmid DNA vectors (ACT PBase and PB Transposon) were obtained from Dr. Tian Xu (Yale University, New Haven, CT). The presence of the EGFR protein was confirmed by immunoblot analysis and extracellular flow cytometry. The presence of the luciferase+GFP vector was confirmed by fluorescent microscopy.
Murine Tumor Model
The ability or cetuximab to bind to tumor-expressed EGFR in murine models in vivo has been previously demonstrated (28–31). Age-matched female 01B74 nude athymic nude mice (NCI-Frederick, MD) were injected s.c. in the right flank with 1×106 Panc02EGFR, Panc02neo, or Panc02 cells in 100 μl PBS or were injected i.p. with 5×105 Panc02EGFR/luc+GFP cells in 100 μl PBS. Tumors became palpable approximately 2–3 days later. All treatments were administered i.p. thrice weekly in 200 μl of PBS. Subcutaneous tumor volumes were calculated 3x/week as follows: tumor volume = 0.5 × ((large diameter) × (small diameter)2). Quantification of intraperitoneal tumor burden was performed using Xenogen IVIS software to quantify nonsaturated bioluminescence in regions of interest. Treatments continued until tumors were greater than 2 cm in maximum dimension (s.c. model) or until average radiance exceeded 1.5×109 p/s/cm2/sr via IVIS Bioluminescence imaging (i.p. model), at which time mice were sacrificed. Mice received an i.p. injection of an anti-asialo GM1 Ab (for depletion of NK cells) or normal rabbit serum (control) on days −3, −1, +1, and +3 with respect to tumor inoculation and every 4 days thereafter or an i.p. injection of a clodronate-containing liposome at 1.0 mg/kg (for depletion of monocytes) or PBS-containing liposomes (control) on day 0 with respect to tumor inoculation, and given a 0.5 mg/kg dose every 4 days thereafter. The efficiency of depletion was >98% as confirmed by flow cytometric analysis of tumor cells and splenocytes. All animal protocols were approved by the Ohio State University Animal Care and Use Committee and mice were treated in accordance with institutional guidelines for animal care.
Statistics
Tumor cell lysis as measured by ADCC, IFN-γ and chemokine release as measured by ELISA, and T cell chemotaxis as measured by a transwell assay system was analyzed by analysis of variance (ANOVA). Tumor growth data was analyzed by mixed effect models, incorporating repeated measures for each tumor.
RESULTS
Human pancreatic cell lines express EGFR
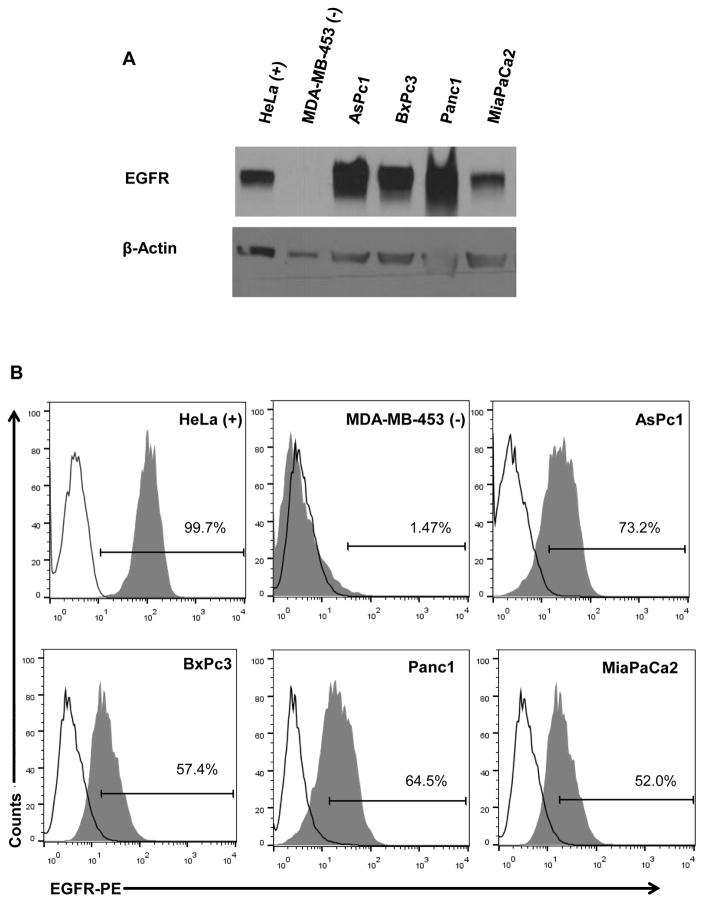
The expression of EGFR protein was validated in four human pancreatic cancer cell lines via immunoblot analysis and flow cytometry. There was expression of the EGFR protein in all cell lines tested irrespective of KRAS mutational status (Fig. 1A). In general, the expression of EGFR as determined by immunoblot analysis was consistent with surface expression of EGFR as measured by flow cytometric analysis (Fig. 1B).
Figure 1. Expression of EGFR in human pancreatic cancer cell lines.
(A) Lysates were prepared from human pancreatic cancer cell lines and subjected to immunoblot analysis with antibodies directed against EGFR. Nitrocellulose membranes were probed for β-actin to control for loading. HeLa and MDA-MB-453 served as positive and negative controls, respectively. (B) The percentage of cells expressing EGFR was assessed by flow cytometry using an anti-EGFR-PE Ab. Experiments are representative of two separate determinations.
IL-21 enhances NK cell lytic activity against cetuximab-coated EGFR-positive pancreatic cancer cells
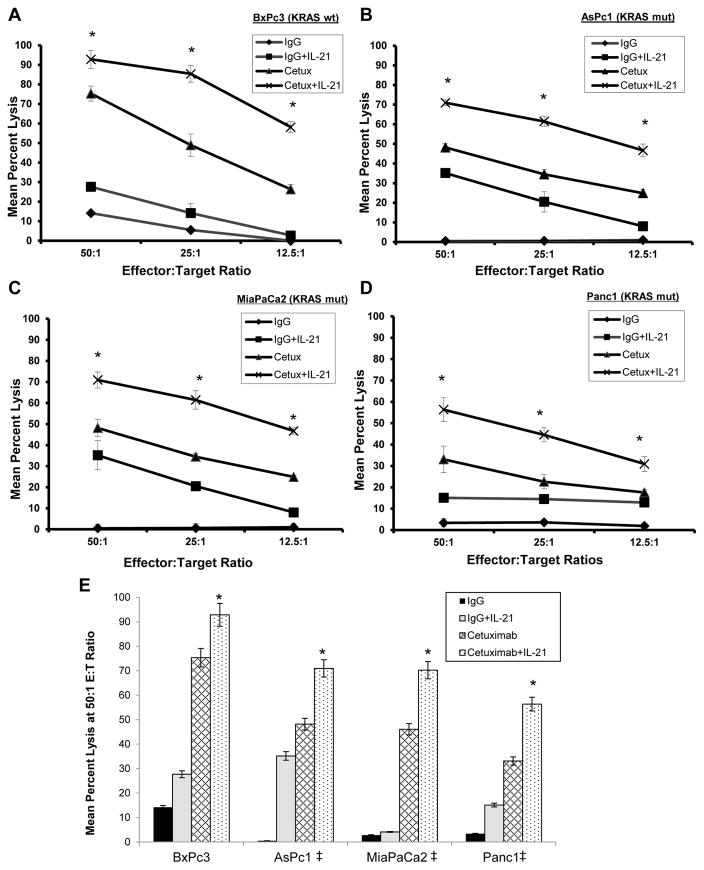
It was hypothesized that the lytic activity of normal human NK cells against cetuximab-coated pancreatic cancer cell lines would be enhanced in vitro following the pre-treatment of NK cells with IL-21, irrespective of KRAS mutation status. ADCC assays were performed using normal donor NK cells and various EGFR-positive pancreatic cancer tumor targets. There was a statistically significant enhancement of NK cell-mediated ADCC against cetuximab-coated targets following IL-21 activation as compared to control conditions (p<0.001). The lysis of wild-type KRAS (wtKRAS) BxPc3 tumor cells (Fig. 2A) was on the same order as that of mutant KRAS (mutKRAS) AsPc1 (Fig. 2B), MiaPaCa2 (Fig. 2C), and Panc1 (Fig. 2D) pancreatic tumor cells (p<0.001). In each instance, lysis of the tumor cells by untreated NK cells was low even at the 50:1 E:T ratio. Stimulation of NK cells with IL-21 or treatment of tumor cells with cetuximab led to modest gains in tumor cell killing. However, the combination of IL-21 and cetuximab treatment led to marked levels of ADCC as compared to controls (p<0.001). A graphical representation of this cytotoxicity data at the 50:1 E:T ratio is presented in Fig. 2E.
Figure 2. IL-21 enhances antibody-dependent cellular cytotoxicity of NK cells against pancreatic cancer cells.
Purified human NK cells were incubated overnight in medium alone or in medium supplemented with 10 ng/ml IL-21. The lytic activity of IL-21-activated NK cells was then assessed in a standard 4 hr chromium release assay using cetuximab-coated KRAS wild-type (A) or KRAS mutant (B–D) pancreatic cancer cells as targets. (E) Graphical summary of cytotoxicity data of four pancreatic cancer cell lines at the 50:1 E:T ratio. ‡Denotes presence of a KRAS mutation. The percentage of lysis was calculated as previously described. Each graph depicts the results from one representative donor ± SD. Three normal donors were tested per cell line. The asterisk (*) denotes p<0.001 versus all conditions shown.
IL-21 enhances the NK cell response to KRAS mutant and KRAS wild-type colorectal cancer cell lines
To further determine the efficacy of cetuximab and IL-21 in the treatment of KRAS mutant cancers, NK cells were obtained from healthy donors and used in an ADCC assay as described above. EGFR expression was validated in colorectal cancer cell lines HCT-116 MUT (mutKRAS) and HCT-116 WT (wtKRAS) via flow cytometry (Fig. S1A). In experiments similar to those in Figure 2, it was determined that there was a marked increase in ADCC when NK cells were treated with IL-21 and cetuximab in combination, as compared to either treatment alone, irrespective of KRAS mutation status (Fig. S1B–C, p<0.001). Notably, the overall level of cytotoxicity in the combination group was similar for both cell lines. This result suggests that ADCC by IL-21-activated NK cells is largely unaffected by the KRAS mutational status of these cancer cells.
NK cells from pancreatic cancer patients exhibit enhanced ADCC in the presence of IL-21
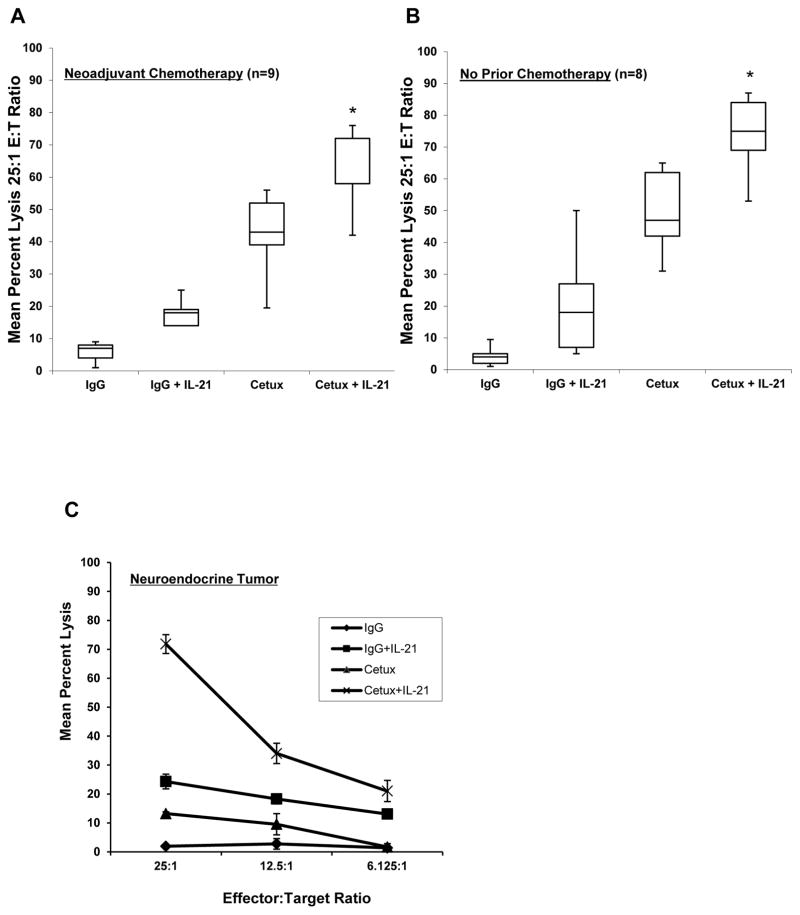
To further determine the efficacy of cetuximab and IL-21 in the setting of pancreatic cancer, NK cells were obtained from pancreatic cancer patients prior to surgical resection and utilized in ADCC assays as described above. Of the 17 patients included in this analysis, 9 had received neoadjuvant chemotherapy and 8 had no chemotherapy prior to surgical resection (Fig. 3A–B). In all cases, treatment of patient NK cells with IL-21 resulted in greater mean percent lysis of cetuximab-coated AsPc1 tumor (mutKRAS) compared to controls (p<0.001). Patients that underwent neuroendocrine tumor resection exhibited significant ADCC activity and served as a control (Fig. 3C).
Figure 3. IL-21 enhances antibody-dependent cellular cytotoxicity of pancreatic patient NK cells against KRAS mutant pancreatic cancer cells.
Purified NK cells from patients with pancreatic adenocarcinoma were incubated overnight with medium alone or in medium supplemented with 10 ng/ml IL-21. The lytic activity of IL-21-activated NK cells was then assessed in a standard 4 hr chromium release assay using cetuximab-coated KRAS mutant AsPc1 tumor cells. The mean percent lysis at 25:1 E:T ratio for 9 patients who received neoadjuvant chemotherapy (A) or the 8 patients who had not received chemotherapy prior to surgical resection (B) is shown in box plots. The box plots represent the median and interquartile range, with I bars showing the range for each group. (C) NK cell lysis of a patient that underwent neuroendocrine tumor resection ± SD. Experiments are representative of two separate determinations. The asterisk (*) denotes p<0.001 versus all conditions shown.
IL-21 enhances production of IFN-γ and T cell attracting chemokines by NK cells co-cultured with cetuximab-coated EGFR-positive pancreatic cancer cells
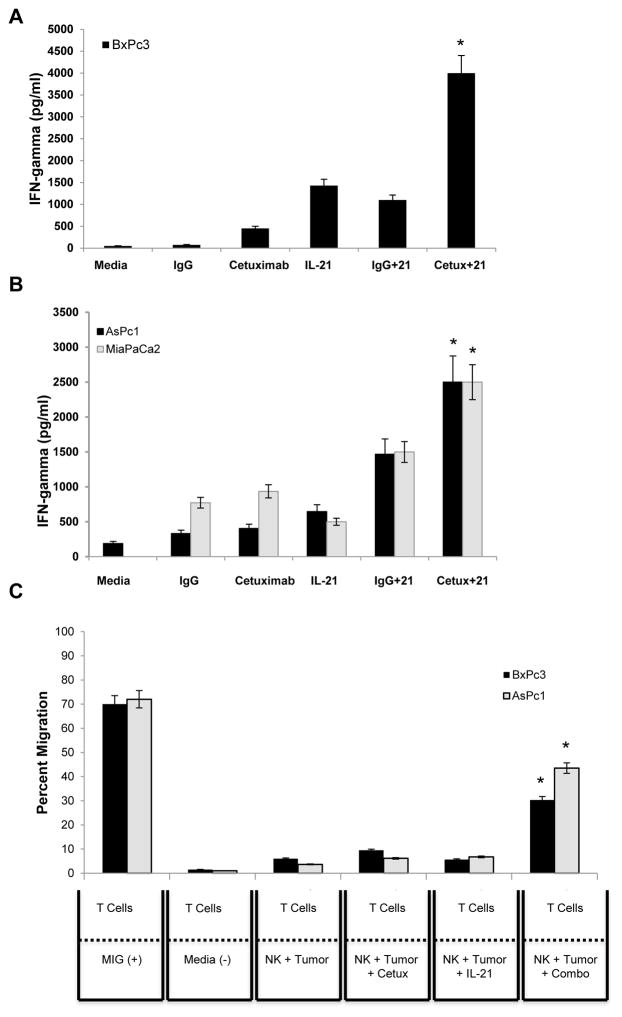
Although the cytolytic potential of NK cells is widely appreciated, the capability of NK cells to initiate and shape the adaptive immune response by providing an early source of IFN-γ has become evident (32). It was hypothesized that there would be elevated production of IFN-γ by NK cells in response to co-stimulation of the NK cell IL-21R and FcR. As predicted, exposure of IL-21-activated NK cells to cetuximab-coated wtKRAS BxPc3 (Fig. 4A) and mutKRAS AsPc1 and MiaPaca2 cells (Fig. 4B) resulted in increased production of IFN-γ (p<0.001) as compared to control conditions. Dual stimulation of NK cells resulted in robust NK cell IFN-γ production for up to 72 hrs (Fig. S2A–B, p<0.001). Cetuximab-coated mutKRAS AsPc1 tumor cells were also a potent co-stimulus for NK cell production of the chemokines IL-8, MIP-1α, MIP-1β, and RANTES (Fig. S3A–D, p<0.001).
Figure 4. Human NK cells secrete high levels of IFN-γ and NK culture supernatants stimulate the migration of T cells.
The KRAS wild-type BxPc3 (A) and KRAS mutant AsPc1 and MiaPaCa2 (B) cell lines were cultured with human NK cells in an in vitro tumor co-culture assay. Control conditions consisted of tumor cells and NK cells cultured with medium alone, IgG alone, cetuximab alone, IL-21 alone or the combination of IgG and IL-21. Culture supernatants were harvested at 48 hrs and analyzed for IFN-γ by ELISA. (C) Chemotaxis of activated T cells in response to culture supernatants derived from NK cells costimulated with cetuximab-coated KRAS wild-type BxPc3 or KRAS mutant AsPc1 tumor cells and IL-21. Culture medium supplemented with monokine induced by gamma-interfereon (MIG) was included as a positive control. Each graph depicts the results from one representative donor ± SD. Three normal donors were tested per cell line. The asterisk (*) denotes p<0.001 versus all conditions shown.
NK cell supernatants from co-stimulated NK cells induce chemotaxis of activated T cells
To confirm the functional activity of NK cell-derived chemokines, supernatants from co-cultures of tumor cells with NK cells were tested for their ability to stimulate T cell migration. A greater percentage of T cells were recruited to supernatant factors released from the culture of IL-21-activated NK cells with cetuximab-coated tumor cells as compared to control conditions (Fig. 4C, p<0.001). Importantly, chemotaxis was not greatly affected by the mutational status of KRAS.
Myeloid-derived suppressor cells inhibit NK cell ADCC and production of IFN-γ
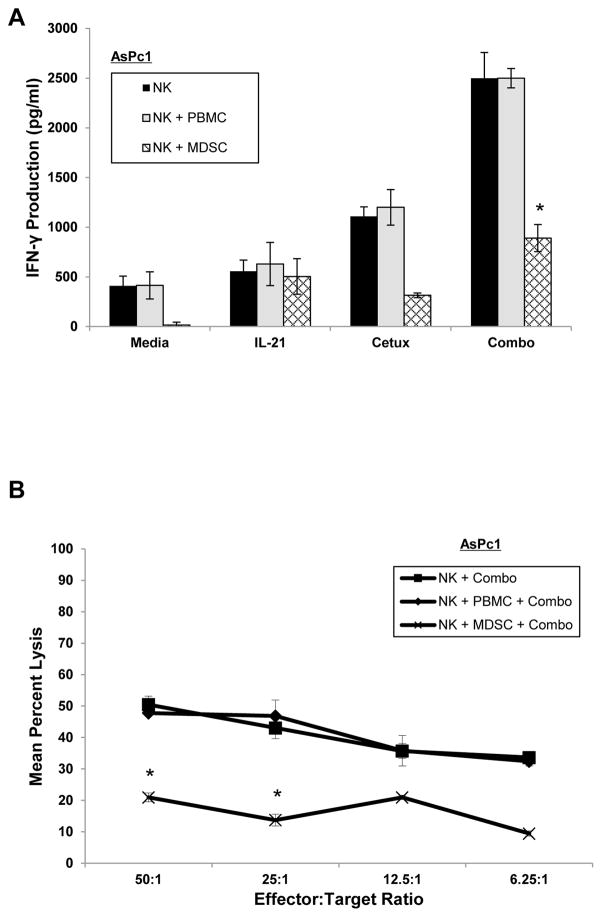
Recently, our group has shown that MDSCs induced by tumor-derived factors are elevated in patients with gastrointestinal malignancies, inhibit T cell proliferation and effector functions, and correlate with progressive disease (33). Therefore, autologous PBMC-derived MDSCs, or control PBMCs, and healthy donor NK cells were cultured at a 0.5:1 ratio and added to co-culture with AsPc1 (mutKRAS) tumor cells. Co-culture conditions included IL-21-activated NK cells, cetuximab-coated tumor cells or the combination. When MDSCs were added to the co-culture, IFN-γ production by NK cells cultured with mutKRAS AsPc1 tumor cells was markedly inhibited as compared to control-treated NK cells (Fig. 5A). Furthermore, MDSCs impaired IL-21-activated NK cell ADCC activity against cetuximab-coated mutKRAS AsPc1 tumor cells (Fig. 5B).
Figure 5. MDSCs inhibit NK cell IFN-γ production and ADCC.
Autologous PBMC-derived MDSCs or control PBMCs were co-cultured with healthy donor NK cells at a 0.5:1 ratio. In vitro generated MDSCs were found to be suppressive to T cell proliferation by CFSE assay (data not shown). (A) Purified IL-21-activated NK cells were co-cultured with cetuximab-coated KRAS mutant AsPc1 cancer cells alone or in the presence of autologous PBMCs or MDSCs. Control conditions consisted of medium alone, IL-21 alone, or cetuximab alone. Culture supernatants were harvested at 48 hrs and analyzed for IFN-γ by ELISA. (B) Purified human NK cells were incubated overnight in medium alone in medium containing 10 ng/ml of IL-21. The lytic activity was tested against cetuximab-coated KRAS mutant AsPc1 cancer cells in the presence of autologous PBMCs or MDSCs in a standard 4 hr chromium release assay. Each graph depicts the results from one representative donor ± SD. Three normal donors were tested per cell line. The asterisk (*) denotes p<0.001 versus all conditions shown.
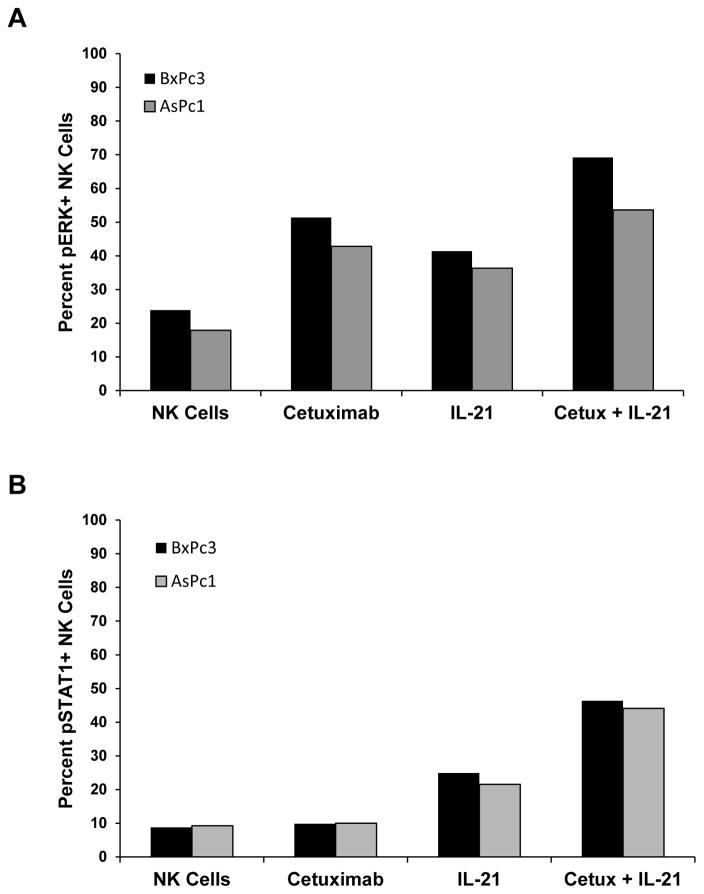
Co-stimulation of NK cells with Ab-coated pancreatic cancer cells and IL-21 results in enhanced activation of ERK and STAT1
It was hypothesized that the interaction of the human NK cell FcR with cetuximab-coated pancreatic tumor cells and the IL-21 receptor in the presence of IL-21 would activate the MAPK and JAK/STAT signaling pathways and result in enhanced phosphorylation of ERK and STAT1, respectively, within NK cells. Human NK cells were co-cultured with cetuximab-coated pancreatic cancer cells in the presence or absence of IL-21. After a 30 min incubation period, culture supernatants were collected and cells were dual stained for CD56 and activated (phosphorylated) ERK (pERK) or activated (phosphorylated) STAT1 (pSTAT1). As predicted, NK cells stimulated with antibody-coated mutKRAS AsPc1 tumor cells and IL-21 demonstrated the highest level of ERK activation with 53.5% of cells staining positively for pERK (Fig. 6A, Fig. S4A). NK cells co-cultured with cetuximab-coated mutKRAS AsPc1 pancreatic cancer cells or stimulated with IL-21 alone exhibited 42.8% and 36.3% staining, respectively, while only 17.9% of resting NK cells demonstrated activation of ERK (Fig. 6A, Fig. S4A). Thus, cetuximab-coated tumor cells are a strong stimulus that results in significant induction of the MAPK signaling pathway. Similarly, NK cells stimulated with antibody-coated tumor cells and IL-21 demonstrated the highest level of STAT1 activation with 44.1% of cells staining positively for pSTAT1 (Fig. 6B, Fig. S4C). NK cells co-cultured with cetuximab-coated mutKRAS AsPc1 pancreatic cancer cells or stimulated with IL-21 alone exhibited 9.98% and 21.5% staining, respectively, while only 9.26% of resting NK cells demonstrated activation of STAT1 (Fig. 6B, Fig. S4C). Thus, IL-21 is a strong stimulus that results in significant induction of the JAK/STAT signaling pathway. The percent increase in NK cells for both pERK and pSTAT1 was greater with the combination of IL-21 and cetuximab versus either condition alone (Fig. 6, Fig. S4). Similar results were obtained for the wtKRAS cell line BxPc3 (Fig. 4, Fig. S4B, Fig. S4D). Thus, the combination treatment led to concurrent activation of NK cells via the MAPK and JAK/STAT pathways that was not present in the control conditions.
Figure 6. NK cell signal transduction is enhanced by IL-21 receptor and Fc receptor engagement by cetuximab-coated mutant KRAS AsPc1 tumor cells.
Purified human NK cells were stimulated with 10 ng/ml of IL-21 and co-cultured with cetuximab-coated KRAS mutant AsPc1 pancreatic cancer cells for 30 mins. Control conditions consisted of media alone, IL-21-stimulated NK cells alone, or cetuximab-coated tumor cells alone. Following incubation, cells were collected and underwent dual flow cytometry staining using anti-CD56 and (A) anti-phospho-ERK1/2 or (B) anti-phospho-STAT1 antibodies. Percentages reported are of dual positive populations (Q2). Each plot depicts the results from one representative donor. Three normal donors were tested per cell line.
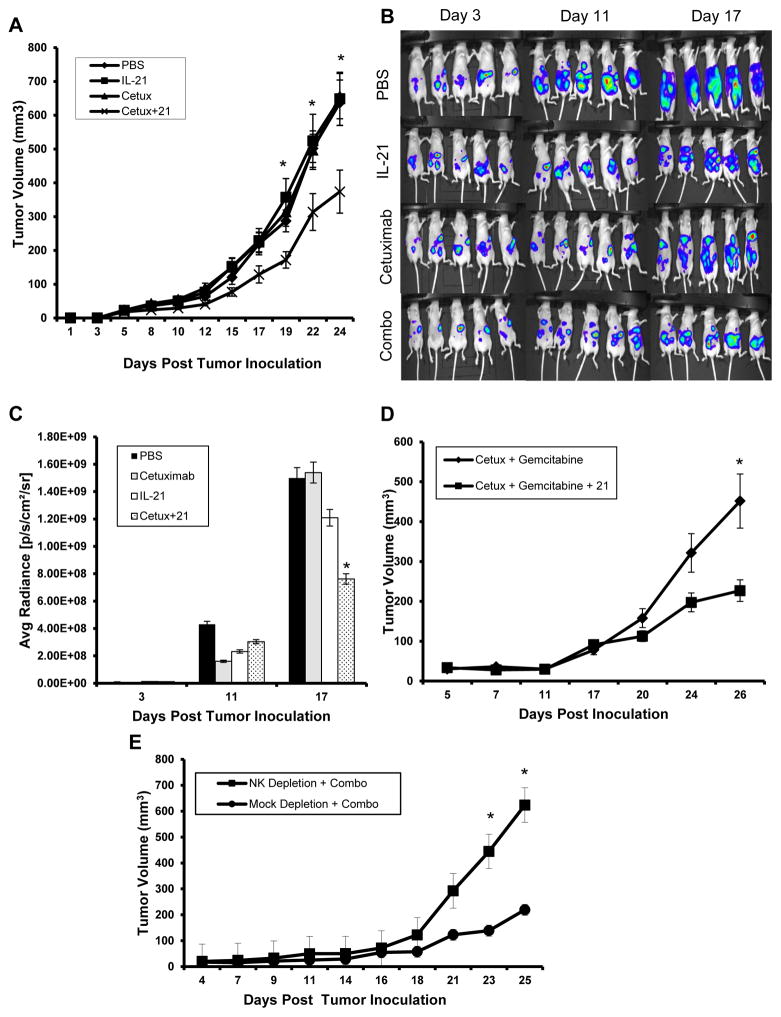
IL-21 enhances the therapeutic efficacy of cetuximab in a murine model of pancreatic cancer
A murine subcutaneous tumor model of EGFR-positive pancreatic cancer was employed in order to determine the therapeutic efficacy of IL-21 and cetuximab in vivo. The murine pancreatic tumor cell line Panc02 was engineered to express the human EGFR protein (PANC02EGFR). Tumors were generated in athymic nu/nu mice by subcutaneous injection of 1x106 PANC02EGFR tumor cells. There was no significant difference in tumor growth or tumor histology at the microscopic level as compared to non-transformed tumors or those expressing the empty vector (Fig. S5). Mice bearing PANC02EGFR tumors were then treated with PBS, IL-21 (5 μg), cetuximab (0.5 mg/kg) or IL-21 plus cetuximab i.p. three times per week. There was no significant difference in tumor volumes at baseline. However, by day 24 post tumor inoculation, there was a significant reduction in tumor volume in mice receiving the combination regimen as compared to the other treatment groups (Fig. 7A, p<0.01). To further evaluate the therapeutic efficacy of IL-21 and cetuximab in vivo, a murine intraperitoneal tumor model of EGFR-positive pancreatic cancer was employed. Here, plasmid vectors encoding luciferase and GFP were transfected into the murine pancreatic tumor cell line that was modified to express human EGFR (Panc02EGFR/luc+GFP). Tumor burden was quantified by comparing average radiance in mice treated with PBS, IL-21 or cetuximab alone, to the combination of IL-21 and cetuximab. By day 11, there was a significant reduction in tumor burden of mice treated with IL-21 and cetuximab versus mice treated with PBS or either agent alone (Fig. 7B–C, p<0.001). Expression of EGFR was necessary for NK cell-mediated ADCC (Fig. S6A) and the efficacy of the combination of IL-21 and cetuximab was dependent on the expression of human EGFR in the Panc02 cell line (Fig. S6B).
Figure 7. The combination of IL-21 and cetuximab enhances tumor regression in a mouse xenograft model.
(A) Nude mice were inoculated s.c. with 1×106 Panc02EGFR tumor cells. Once tumors reached 50–100 mm3 in size, treatment began thrice weekly with i.p. injections of PBS, 5 μg IL-21, 0.5 mg/kg cetuximab, or the combination. Tumor growth was measured by calipers thrice weekly and tumor volumes were calculated as described in the Methods section. Results shown are average tumor volumes of n=7 mice per group ± SD. The asterisk (*) denotes p<0.01 versus all conditions shown. (B) Nude mice were inoculated i.p. with 5×105 Panc02EGFR/Luc+GFP tumor cells. Treatment began on day 3 and was administered as described above. Mice were anesthetized on days 3, 11, and 17 using isoflurane. Approximately 10 min post luciferin injection, mice were imaged at an exposure time of 1 sec. (C) Xenogen IVIS software was used to quantify nonsaturated bioluminescence in regions of interest. Radiance greater than 5.5×106 p/s/cm2/sr was assumed to be indicative of viable luciferase-labeled tumor cells while emissions below this range were considered as background. Results shown are average radiance of n=5 mice per group ± SD. The asterisk (*) denotes p<0.001 versus all conditions shown. (D) Nude mice bearing Panc02EGFR tumors were treated i.p. with low dose gemcitabine (15 mg/kg) and cetuximab (0.5 mg/kg) with or without mIL-21 (5 μg) thrice weekly. Tumor volume was calculated as described above. Results shown are average tumor volumes of n=7 mice per group ± SD. The asterisk (*) denotes p<0.007 versus control condition shown. (E) Nude mice bearing Panc02EGFR tumors were depleted of NK cells by an i.p. injection of anti-asialo GM1 or mock depleted by an i.p. injection of normal rabbit serum and treated with PBS or the combination of IL-21 and cetuximab. Results shown are average tumor volumes of n=5 mice per group ± SD. The asterisk (*) denotes p<0.002 versus control condition shown.
IL-21 further enhances the therapeutic efficacy of a gemcitabine and cetuximab regimen in a murine model of pancreatic cancer
Given that gemcitabine is widely used in the treatment of pancreatic cancer, it was determined whether the addition of IL-21 could enhance the anti-tumor effects of cetuximab and gemcitabine combination therapy against EGFR-positive pancreatic tumor cells. Therapy with gemcitabine plus cetuximab resulted in modest tumor reduction by day 26, however, the addition of IL-21 to this regimen led to a further and significant reduction in tumor volume (Fig. 7D, p<0.007).
The anti-tumor effects of IL-21 and cetuximab are dependent on NK cells
To determine whether the therapeutic efficacy of IL-21 and cetuximab was dependent on the presence of NK cells or monocytes, mice bearing Panc02EGFR tumors were depleted of either NK cells (via administration of anti-asialo GM1) or monocytes (via administration of clodronate-containing liposomes) and then treated with PBS or with IL-21 and cetuximab (Fig. 7E, Fig. S7). The effectiveness of combination therapy was noticeably reduced following NK cell depletion as compared to mock-depleted mice (Fig. 7E, p<0.002). In contrast, the efficacy of combination therapy did not appear to be greatly dependent on the presence of the monocyte compartment (Fig. S7)
DISCUSSION
In the current study it was demonstrated that IL-21 can enhance NK cell-mediated effector functions against cetuximab-coated pancreatic tumor cells. Stimulation of NK cells with IL-21 elicited greater NK cell-mediated ADCC against Ab-coated pancreatic cancer cells compared to control conditions in both healthy donors and pancreatic cancer patients. In addition, costimulation of NK cells in this fashion induced the secretion of IFN-γ and chemokines with the ability to direct the chemotaxis of activated T cells. An increase in levels of phosphorylated STAT1 were found in NK cells following IL-21:IL-21R interactions, and heightened levels of phosphorylated ERK were identified following stimulation of NK cell FcR with cetuximab-coated tumor cells. Thus, the mechanism for the observed synergistic actions of the combination therapy likely lies in the induction of non-overlapping pathways of cellular activation within NK cells. Co-administration of IL-21 and cetuximab to tumor-bearing mice resulted in reduced tumor burden in both a subcutaneous and intraperitoneal model of pancreatic adenocarcinoma. Notably, the addition of IL-21 to a regimen of cetuximab and gemcitabine further reduced tumor burden in vivo, suggesting that the addition of cytokines to standard chemotherapy-antibody regimens could have beneficial effects. The effectiveness of combination therapy was dependent on NK cells, and minimally affected by the depletion of monocytes. The enhancement of the NK cell response to EGFR-expressing pancreatic tumor targets by IL-21 occurred irrespective of KRAS mutational status. Indeed, IL-21 enhanced NK cell lytic activity against a cetuximab-coated colorectal cancer cell line expressing wtKRAS, as well as the identical cell line transfected with mutKRAS. These data demonstrate the ability of IL-21 to enhance NK cell activation in response to cetuximab-coated targets, independently of the intracellular signaling pathways that are operational within the cancer cell.
Alternative strategies are necessary in order to improve the efficacy of mAb therapy in patients with locally advanced or metastatic EGFR-positive pancreatic cancer. One such strategy would be to make use of the immune system to clear cetuximab-coated tumor cells. Given the ability of the innate immune system to recognize targets coated in IgG, our group has suggested that the efficacy of mAb therapy could be enhanced via the administration of immune stimulatory cytokines with the capacity to activate FcR-bearing NK cells. IL-21 is a pleiotropic cytokine produced by activated CD4+ T cells that affects the differentiation, maturation, and function of a number of lymphoid and myeloid cells. It has also been shown to enhance the proliferation and cytotoxicity of NK cells and CD8+ T cells and increase their secretion of IFN-γ (34). Systemic expression of IL-21 in vivo by plasmid-mediated delivery revealed that IL-21 could inhibit the growth of melanoma and fibrosarcoma tumors, and this inhibition was found to be mediated through enhanced cytolytic activity of NK cells (35). A study comparing the activity of intraperitoneally delivered IL-2, IL-15, and IL-21 against syngeneic tumors in mice showed that IL-21 had the most potent antitumor activity and resulted in substantially enhanced long-term survival (36). IL-21 was found to enhance rituximab activity in a cynomolgus monkey model of B cell depletion and in mouse B cell lymphoma models where IL-21-activated innate immune effectors, increased the ADCC of rituximab-coated targets, mobilized B cells into peripheral blood and worked in vivo in synergy with rituximab to yield significant survival benefits over either agent alone (37). These prior results support the use of IL-21 to enhance cetuximab therapy of pancreatic cancers.
IL-21 has now entered phase I and II clinical testing and promising efficacy has been reported. In a phase I trial of subcutaneous recombinant human IL-21 in patients with metastatic melanoma or renal cell carcinoma, 69% of patients achieved an overall response of stable disease or better, which correlated with significant enhancement of granzyme B expression in NK cells following administration of IL-21, with similar trends being observed for IFN-γ and perforin mRNA (38). An examination of patient blood samples from two phase I trials of intravenous rhIL-21 in metastatic melanoma and renal cell carcinoma confirmed increases in perforin and granzyme B mRNA in CD8+ T cells and CD56+ NK cells, suggesting enhanced cytotoxic potential by these cell types (39). An expanded phase IIA trial of IL-21 in 10 additional patients with stage IV malignant melanoma resulted in one complete response and one partial response, with correlative studies showing increases in serum soluble CD25, mRNA for IFN-γ, perforin, and granzyme B in CD8+ and NK cells, and increased frequencies of CD25+ NK and CD8+ T cells (40). A multianalyte profiling of serum proteins in patients with advanced metastatic melanoma or renal cell carcinoma showed increased levels of biomarkers indicative of lymphocyte activation, acute phase response, myeloid activation and leukocyte chemotaxis/trafficking following treatment with IL-21 (41). Thus, IL-21 appears to have profound effects on both innate and specific immune effector cells that translates into clinical efficacy.
The use of interleukins in combination with monoclonal antibody therapy to boost immune function against cancer cells has been the topic of a few clinical studies. An analysis of NK cell functionality in patients with CLL in response to IL-21 stimulation showed an increase in CD25 expression, IFN-γ production, natural cytotoxicity and ADCC against rituximab-coated targets (42). The ability of IL-21 to enhance the activity of gemcitabine/cetuximab therapy in vivo is notable and suggests that therapeutic combinations or mAbs with chemotherapy and immune activating agents can mediate both direct cytotoxicity and indirect immunologic tumor cell destruction. In support of this concept our group conducted a phase I trial of paclitaxel and trastuzumab in combination with IL-12 in patients with HER2/neu-expressing malignancies and showed that there was increased activation of extracellular signal-regulated kinases in peripheral blood mononuclear cells and increased levels of IFN-γ and several chemokines in patients with clinical benefit (43). Correlative studies in a phase I trial of rhIL-21 in combination with cetuximab in patients with metastatic colorectal cancer, where 9 of 15 patients achieved stable disease, revealed that patient NK cells exhibited increased cytotoxicity against K562 target cells at the one week time point as well as a significant increase in soluble CD25 (44). Overall, these clinical trials of cytokines in combination with a mAb show that IL-21 has potential as an immune-stimulating therapy and has the ability to enhance cytolytic activity of NK cells in combination with mAb therapy. The ineffectiveness of standard chemotherapy and even newly developed checkpoint inhibitors provides significant impetus to develop strategies for optimizing antibody-based therapies in the setting of pancreatic cancer.
Despite promising preclinical work, phase II and phase III trials have consistently failed to show efficacy of cetuximab treatment in advanced pancreatic cancer either alone or in combination with cytotoxic agents (45). One of the defining features of pancreatic adenocarcinomas is a high rate of activating mutations in the KRAS oncogene (>90%). When constitutively activated, KRAS can activate MAPK signal transduction independent of EGFR, thus rendering EGFR inhibitors (e.g., erlotinib or gefitinib) ineffective. Genetic expression of mutant KRASG12D or KRASG12V specifically in the murine pancreas proved to be sufficient to initiate acinar-ductal metaplastic lesions and pancreatic intraepithelial neoplastic lesions, which progressed with long latency to invasive metastatic pancreatic adenocarcinoma (46). With the ability of oncogenic KRAS to drive/promote pancreatic cancer cell survival, there have been considerable efforts to develop direct inhibitors of this pathway. However, clinical attempts to directly interrupt KRAS activity have failed, and KRAS is considered by some researchers to be undruggable (47). While strides have been made to target molecules downstream of RAS through RAF inhibitors or MEK inhibitors, it has become clear that activation of RAS results in the recruitment of multiple branching signaling pathways that will be difficult to abrogate. Despite failure in targeting molecules downstream of RAS, a recent report suggested that priming of activated T cells with a bispecific anti-EGFR and anti-CD3 antibody could suppress MDSC differentiation and attenuation of their suppressive activity in the tumor microenvironment, even when the tumors express a mutant form of KRAS (48). This finding suggests that immunotherapy could be beneficial in pancreatic cancer treatments and that immune-based therapies targeting the EGFR may be a way to overcome KRAS mutations.
Although mutations in the KRAS oncogene can bypass the direct downstream effects of anti-EGFR mAbs like cetuximab, the present study shows that the immune response to cetuximab therapy can be significantly enhanced by the addition of IL-21 in the setting of oncogenic KRAS. IL-21 markedly enhances NK cell lysis of cetuximab-coated targets in vitro and improves the elimination of cetuximab-coated mutKRAS cells. IL-21 is also able to augment antigen-specific T cell responses and, at the same time, promote NK cell survival, functioning as a link between innate immune responses and adaptive immune responses (49). The ability of IL-21 activated NK cells to lyse cetuximab-coated pancreatic and colorectal tumor cells irrespective of KRAS mutation status suggests that the oncogenic pathways driving pancreatic cancer growth do not greatly affect, in a positive or negative fashion, the ability of NK cells to lyse those cells. In addition, the secretion of IFN-γ and T cell chemotactic factors such as IL-8, MIP-1α, MIP-1β and RANTES by NK cells that come into contact with cetuximab-coated mutKRAS pancreatic cell lines was markedly enhanced in the presence of IL-21. This would indicate that the combination therapy has the ability to attract T cells to the mutKRAS tumor microenvironment. Considering that IL-21 activates NK cells against mAb-coated tumor cells, an examination of negative regulators of NK cell function is also instructive. Tumor cells induce immune suppressor cells, like MDSCs, that can produce reactive oxygen species to influence the tumor microenvironment. We show that MDSCs significantly inhibit NK cell ADCC and IFN-γ production in the current model. Therefore, inhibition or depletion of MDSC function could enhance antibody therapy. These in vitro results of IL-21 and cetuximab against mutKRAS cancer cells were confirmed in two models of pancreatic cancer and results indicate that IL-21 can activate NK cells to lyse cetuximab-coated EGFR-positive tumor cells. Yang et al. (2013) created a novel EGFR-positive murine tumor cell line and demonstrated that cetuximab-induced tumor regression depends on both innate and adaptive immunity components, including CD8+ T cells, MyD88, and FcγR (50). Figure 7E shows that the efficacy of the combination therapy was almost fully dependent on NK cells, as a depletion of NK cells via the administration of anti-asialo GM1 Ab abrogated the effects of IL-21 and cetuximab. In addition, Figure S6 shows that monocytes had little impact on tumor growth. Due to the limitations of the present model, we did not evaluate the role played by T cells. However, given the ability of IL-21R/FcR dual-activated NK cells to produce chemokines with the ability to stimulate T cell chemotaxis, we believe that T cells would likely contribute to the anti-tumor effects of IL-21 and cetuximab in an immunocompetent host. Continued development of methods to enhance NK cell activity in the setting of cetuximab therapy of pancreatic cancer are warranted.
In the present manuscript it has been demonstrated that the administration of IL-21 is able to activate FcR-bearing NK cells, and enhance their ability to recognize and eliminate cetuximab-coated tumor cells via the induction of ADCC and the release of cytokines with anti-tumor activity. In addition, there is evidence that NK cell elimination of Ab-coated targets proceeds largely independent of target cell KRAS mutational status. It was effectively demonstrated in vitro, with healthy donor and pancreatic cancer patient NK cells, and in vivo that this interplay between antibody therapy and the innate immune system can lead to improved anti-tumor effects.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Although pancreatic cancers over-express the epidermal growth factor receptor (EGFR), 95% of patients have mutations in the KRAS oncogene, rendering the use of monoclonal antibodies (mAb) against EGFR ineffective. Given the interactions between the innate immune system and antibody therapy, our group has suggested here that the efficacy of mAb therapy can be enhanced via the administration of immune stimulatory cytokines, mainly interleukin-21 (IL-21), with the capacity to activate NK cells. The present study shows that the activation of natural killer (NK) cells by IL-21 enhances NK cell mediated effector functions against cetuximab-coated pancreatic tumor cells irrespective of KRAS mutation status. Notably, the addition of IL-21 to a regimen of cetuximab and gemcitabine further reduced tumor burden in vivo, suggesting that the addition of cytokines to standard chemotherapy-antibody regimens could have beneficial effects for patients with pancreatic cancer that have limited therapeutic options.
Acknowledgments
This work was supported by NIH Grants P01 CA095426 (M. Caligiuri), P30 CA016058 (M. Caligiuri), CA84402, K24 CA93670 (W.E. Carson, III), T32 GM068412 (ACJ-R) and T32 CA009338.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6. [PubMed] [Google Scholar]
- 2.Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23(1–2):74–9. doi: 10.1159/000093497. [DOI] [PubMed] [Google Scholar]
- 3.Hemming AW, Davis NL, Kluftinger A, Robinson B, Quenville NF, Liseman B, et al. Prognostic markers of colorectal cancer: an evaluation of DNA content, epidermal growth factor receptor, and Ki-67. J Surg Oncol. 1992;51(3):147–52. doi: 10.1002/jso.2930510304. [DOI] [PubMed] [Google Scholar]
- 4.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Fan Z, Shang BY, Lu Y, Chou JL, Mendelsohn J. Reciprocal changes in p27(Kip1) and p21(Cip1) in growth inhibition mediated by blockade or overstimulation of epidermal growth factor receptors. Clin Cancer Res. 1997;3(11):1943–8. [PubMed] [Google Scholar]
- 6.Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95(4):1897–905. doi: 10.1172/JCI117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, et al. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6(5):1936–48. [PubMed] [Google Scholar]
- 8.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6(6):2166–74. [PubMed] [Google Scholar]
- 9.Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Faloppi L, Andrikou K, Cascinu S. Cetuximab: still an option in the treatment of pancreatic cancer? Expert Opin Biol Ther. 2013;13(5):791–801. doi: 10.1517/14712598.2013.786697. [DOI] [PubMed] [Google Scholar]
- 12.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76(12):2421–38. [PubMed] [Google Scholar]
- 13.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 14.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180(4):1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson WE, Lindemann MJ, Baiocchi R, Linett M, Tan JC, Chou CC, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85(12):3577–85. [PubMed] [Google Scholar]
- 16.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162(8):4511–20. [PubMed] [Google Scholar]
- 17.Bluman EM, Bartynski KJ, Avalos BR, Caligiuri MA. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J Clin Invest. 1996;97(12):2722–7. doi: 10.1172/JCI118726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somersalo K, Carpen O, Saksela E. Stimulated natural killer cells secrete factors with chemotactic activity, including NAP-1/IL-8, which supports VLA-4- and VLA-5-mediated migration of T lymphocytes. Eur J Immunol. 1994;24(12):2957–65. doi: 10.1002/eji.1830241206. [DOI] [PubMed] [Google Scholar]
- 19.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172(4):2048–58. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 20.Burgess SJ, Marusina AI, Pathmanathan I, Borrego F, Coligan JE. IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J Immunol. 2006;176(3):1490–7. doi: 10.4049/jimmunol.176.3.1490. [DOI] [PubMed] [Google Scholar]
- 21.Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, et al. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13(21):6419–28. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 22.Kondadasula SV, Roda JM, Parihar R, Yu J, Lehman A, Caligiuri MA, et al. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood. 2008;111(8):4173–83. doi: 10.1182/blood-2007-01-068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesinski GB, Badgwell B, Zimmerer J, Crespin T, Hu Y, Abood G, et al. IL-12 pretreatments enhance IFN-alpha-induced Janus kinase-STAT signaling and potentiate the antitumor effects of IFN-alpha in a murine model of malignant melanoma. J Immunol. 2004;172(12):7368–76. doi: 10.4049/jimmunol.172.12.7368. [DOI] [PubMed] [Google Scholar]
- 24.Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker M, et al. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J Natl Cancer Inst. 2004;96(17):1331–42. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 25.Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, et al. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31(10):3016–25. doi: 10.1002/1521-4141(2001010)31:10<3016::aid-immu3016>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE., 3rd Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177(1):120–9. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 27.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-cated tumor cells. J Clin Invest. 2002;110(7):983–92. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prewett M, Rockwell P, Rockwell RF, Giorgio NA, Mendelsohn J, Scher HI, et al. The biologic effects of C225, a chimeric monoclonal antibody to the EGFR, on human prostate carcinoma. J Immunother Emphasis Tumor Immunol. 1996;19(6):419–27. doi: 10.1097/00002371-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Prewett M, Rothman M, Waksal H, Feldman M, Bander NH, Hicklin DJ. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinoma xenografts in nude mice. Clin Cancer Res. 1998;4(12):2957–66. [PubMed] [Google Scholar]
- 30.Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5(4):909–16. [PubMed] [Google Scholar]
- 31.Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89(1):74–82. [PubMed] [Google Scholar]
- 32.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5(10):996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, et al. Patients with pancreatic adenocarcinoma exhibit elvated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64(2):149–59. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63(24):9016–22. [PubMed] [Google Scholar]
- 36.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173(2):900–9. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 37.Krejsa CM, Holly RD, Heipel M, Bannink KM, Johnson R, Roque R, et al. Interleukin-21 enhances rituximab activity in a cynomolgus monkey model of B cell depletion and in mouse B cell lymphoma models. PLoS One. 2013;8(6):e67256. doi: 10.1371/journal.pone.0067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt H, Brown J, Mouritzen U, Selby P, Fode K, Svane IM, et al. Safety and clinical effect of subcutaneous human interleukin-21 in patients with metastatic melanoma or renal cell carcinoma: a phase I trial. Clin Cancer Res. 2010;16(21):5312–9. doi: 10.1158/1078-0432.CCR-10-1809. [DOI] [PubMed] [Google Scholar]
- 39.Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13(12):3630–6. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 40.Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15(6):2123–9. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 41.Dodds MG, Frederiksen KS, Skak K, Hansen LT, Lundsgaard D, Thompson JA, et al. Immune activation in advanced cancer patients treated with recombinant IL-21: multianalyte profiling of serum proteins. Cancer Immunol Immunother. 2009;58(6):843–54. doi: 10.1007/s00262-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eskelund CW, Nederby L, Thysen AH, Skovbo A, Roug AS, Hokland ME. Interleukin-21 and rituximab enhance NK cell functionality in patients with B-cell chronic lymphocytic leukaemia. Leuk Res. 2011;35(7):914–20. doi: 10.1016/j.leukres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Bekaii-Saab TS, Roda JM, Guenterberg KD, Ramaswamy B, Young DC, Ferketich AK, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009;8(11):2983–91. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steele N, Anthony A, Saunders M, Esmarck B, Ehrnrooth E, Kristjansen PE, et al. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. Br J Cancer. 2012;106(5):793–8. doi: 10.1038/bjc.2011.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luedke E, Jaime-Ramirez AC, Bhave N, Carson WE. Monoclonal antibody therapy of pancreatic cancer with cetuximab: potential for immune modulation. J Immunother. 2012;35(5):367–73. doi: 10.1097/CJI.0b013e3182562d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 47.Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11(11):775–91. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakur A, Schalk D, Tomaszewski E, Kondadasula SV, Yano H, Sarkar FH, et al. Microenvironment generated during EGFR targeted killing of pancreatic tumor cells by ATC inhibits myeloid-derived suppressor cells through COX2 and PGE2 dependent pathway. J Transl Med. 2013;11:35. doi: 10.1186/1479-5876-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16(4):559–69. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21(1):91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.