Abstract
Purpose
Multiparametric MRI (mpMRI) and fusion biopsy (FBx) detect more high-risk prostate cancer (PCa) and less low-risk PCa than systematic biopsy (SBx). However, there remains a small subset of patients where SBx captures higher grade disease than FBx. We aim to identify potential mechanisms for failure of FBx biopsy in detection of clinically significant (CS) PCa.
Methods
We reviewed a prospectively maintained database of patients undergoing mpMRI followed by FBx and SBx from 2007-2014. In patients disease upgraded to CS disease (Gleason ≥ 7) by SBx over FBx, independent re-review of MR imaging, archived biopsy imaging, and whole mount pathology, as well as needle coordinate mapping were conducted. Multivariate logistic regression analysis was performed to determine predictors for upgrading by SBx.
Results
Disease upgrading based on SBx over FBx occurred in 135/1003 (13.5%) patients, of which only 62 (6.2%) were to intermediate (Gleason=7) [N=51, 5.1%] or high risk PCa (Gleason≥8) [N=11, 1.1%]. On multivariate analysis, lower PSA (p <0.001), higher MRI prostate volume (p <0.001), and lower number of target cores (p=0.001) were predictors of upgrading by SBx. Main mechanisms for under-grading by FBx included mpMRI reader oversight, presence of MR invisible cancer, FBx technique error, and intra-lesion Gleason heterogeneity.
Conclusions
MRI and FBx rarely misses CS PCa, as only 62/1003 (6.2%) cases were upgraded to CS disease by SBx. Imaging and biopsy techniques are continually refined and further studies will help to clarify mechanisms of FBx failure and patient populations which benefit from SBx in addition to FBx.
Keywords: Prostatic neoplasms, Magnetic Resonance Imaging, Image-Guided Biopsy
Introduction
PSA-based screening paradigms and transrectal ultrasound (TRUS)-guided 12-core systematic biopsy (SBx) have resulted in overdiagnosis and overtreatment of low-risk prostate cancer (PCa). The addition of functional sequences in multiparametric magnetic resonance imaging (mpMRI) has improved PCa detection while MRI/ultrasound fusion-guided biopsy (FBx) has facilitated targeted biopsy of suspicious lesions 1. FBx now has a well-defined role in patients with prior negative TRUS biopsies and lesions in areas traditionally undersampled with SBx 2-5. Furthermore, FBx identifies more high-risk cases, while avoiding detection of clinically insignificant low-risk cancers 6-8. Nevertheless, there remains a small subset of patients where SBx captures higher grade disease than FBx. This is especially critical when clinically significant (CS) cancer is found on SBx and missed by FBx, with previous studies suggesting rates ranging from 3.5% - 13% 9-11. The reasons by which FBx misses disease identified by SBx are not clearly understood and have not been fully investigated.
The current study aims to identify predictors for disease upgrading by SBx over FBx and to define potential mechanisms by which FBx fails to capture CS disease.
Materials and Methods
Study Population
A retrospective review was performed of 1003 consecutive patients undergoing FBx for elevated PSA or abnormal DRE at the National Cancer Institute, National Institutes of Health from 2007-2014. All patients were enrolled in an institutional review board approved prospective trial of mpMRI and FBx (NCT00102544). Only initial biopsy sessions were included for analysis in patients with multiple biopsies during the study period. This cohort has been reported on previously; however, the current objective and analysis have not been published 9, 12.
Image acquisition and interpretation
Patients underwent a diagnostic 3.0 Tesla mpMRI (Achieva; Philips Healthcare, Andover, MA, USA) with a 16-channel surface coil positioned over the pelvis (SENSE; Philips Healthcare, Andover, MA, USA) and endorectal coil (BPX-30, Medrad Inc, Pittsburgh, PA, USA). MpMRI included triplanar T2-weighted, diffusion-weighted (DW) images with apparent diffusion coefficient mapping, high b value DW MRI (b = 2000s/mm2), and dynamic contrast-enhanced images. Lesion location, MRI suspicion score (MRI SS; 1-5 scale), and tumor diameter were recorded. All mpMRI studies were prospectively evaluated by two genitourinary radiologists (B.T. and P.L.C.) with 8 and 16 years of experience in prostate imaging, respectively.
Biopsy Protocol
Patients with suspicious lesions on mpMRI underwent FBx using an office-based platform (UroNav, Philips/In Vivo Corp, Gainesville, FL, USA). T2-weighted images were segmented and co-registered via rigid registration with real-time TRUS images during biopsy. Suspicious lesions were displayed as centroid targets and sampled in axial and sagittal planes, generating two biopsy cores per target 13. This was directly followed by SBx in the same biopsy session. Specimens were reviewed by a single genitourinary pathologist (M.J.M) with 26 years of experience.
Study Design
Patients upgraded to higher risk disease based on SBx were identified by biopsy pathology. Low-risk disease was defined as Gleason 6 (3+3), intermediate-risk disease as Gleason 7 (3+4 and 4+3), and high-risk disease as Gleason ≥ 8 (4+4). CS PCa was defined as Gleason ≥7. In this subset of patients, independent non-blinded review of imaging was repeated to determine overlap of target(s) previously identified by mpMRI and sextant from SBx which revealed higher risk disease. Sextant overlap was defined as the presence of a suspicious lesion with subsequent FBx within the same sextant that revealed higher risk disease on SBx. In patients without overlap, review of imaging within the sextant that revealed higher risk disease on SBx was repeated to determine if a lesion was missed on initial review.
Biopsy mapping of tracked needle coordinates was performed on all sextant overlap patients who had their biopsy procedure after October 2011 (at which point biopsy mapping capability was integrated into the fusion-guided biopsy system) and were upgraded to CS disease by SBx over FBx (n = 25). The biopsy mapping process is previously described14. Briefly, the position of each biopsy specimen was annotated onto T2 weighted MRI by transposing the distal and proximal coordinates of the needle track from TRUS to MRI. Each biopsy core was modelled as a cylinder 4 mm in diameter and 16 mm in length, which represented the expected location of the biopsy core. Furthermore, in this subset of patients, review of archived imaging at time of needle deployment was conducted to determine the presence of registration and/or mechanical error. Archived imaging included the storage of cine recordings and still image captures throughout the entire procedure for each target. Registration error was defined as inaccurate superimposition of real-time TRUS imaging onto pre-acquired MRI, and mechanical error was defined as sampling outside the defined centroid “bullseye.”
In patients (13/25) who had radical prostatectomy, re-review of whole mount pathology was performed for correlation of biopsy pathology from SBx and FBx.
Statistical Analysis
Statistical analysis was performed utilizing IBM SPSS (version 21, Chicago, IL, USA). Univariate analyses were conducted using Mann-Whitney U test to compare distribution of continuous variables, and Fisher’s exact test and Pearson’s chi-square tests were used to compare proportions of categorical variables. To identify predictors of upgrading by SBx, univariate logistic regression was performed initially to obtain unadjusted odds ratios for several demographic and clinical variables. Subsequently, all the variables were put into a multivariate model to obtain adjusted odds ratios and control for confounding. Variables of interest for logistic regression were: age, race, PSA, abnormal DRE, prostate volume, history of prior prostate biopsy, and number of target cores. PSA, prostate volume, and number of target cores were evaluated for interaction via separate multivariable analyses. Statistical significance was predefined as two-sided p-value < 0.05.
Results
Risk category upgrading
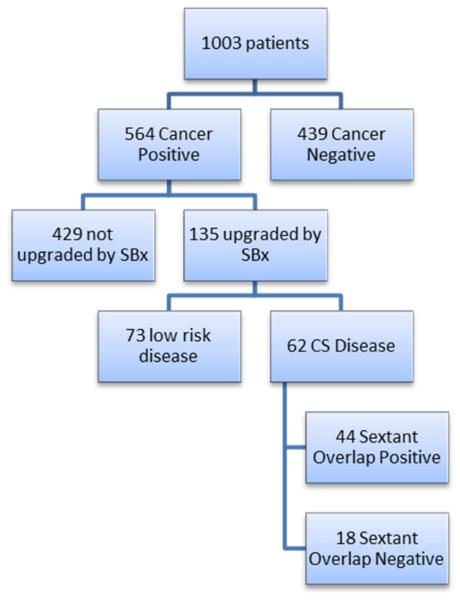
We identified 1003 patients who underwent mpMRI with FBx/SBx (Figure 1). A total of 564 patients (56.2 %) were found to harbor PCa. Disease risk category upgrading based on SBx results occurred in 135/564 (23.9%) patients. Gleason upgrading by biopsy type is presented in Table 1. The majority of upgrading (N=73, 54.1%) was due to FBx avoiding detection of Gleason 6 cancers. In the remaining 62/135 (45.9%), results from SBx revealed intermediate [N=51 (37.8%)] or high-risk disease [N=11 (8.1%)] while FBx showed benign or low-risk disease.
Figure 1.
Flow chart of risk category upgrading by systematic 12-core biopsy over MRI/TRUS fusion biopsy. CS= clinically significant; SBx = systematic 12-core biopsy.
Table 1.
Risk Category Upgrading by systematic-12 core biopsy over MRI/TRUS fusion biopsy
| Systematic Biopsy | ||||
|---|---|---|---|---|
| Fusion Biopsy | N=135 | Gleason 6 | Gleason 7 | Gleason ≥ 8 |
|
| ||||
| No Disease | 73 (54.1%) | 26 (19.3%) | 4 (3.0%) | |
|
| ||||
| Gleason 6 | - | 25 (18.6%) | 0 (0%) | |
|
| ||||
| Gleason 7 | - | - | 7 (5.2%) | |
Demographics are displayed in Table 2. On multivariate logistic regression analysis, lower PSA (0.92 [0.89-0.96], p<0.001), higher MRI prostate volume (1.13 [1.08-1.18], p<0.001), and lower number of target cores (0.86 [0.79-0.94], p=0.001) remained independent predictors for overall upgrading by SBx (Table 3). No significant interaction was observed among the significant variables (PSA, MRI prostate volume, number of target cores). No predictors for upgrading to CS disease by SBx were identified.
Table 2.
Demographics and clinical characteristics
| Upgraded by Systematic Biopsy |
Not upgraded by Systematic Biopsy |
P value | |
|---|---|---|---|
| N | 135 | 429 | - |
|
| |||
| Age, median (Range), years | 62.0 (40-79) | 64.0 (40-81) | .323 |
|
| |||
| PSA, median (Range), ng/mL | 5.6 (0.55-56.8) | 7.5 (0.33-231.60) | <.001 |
|
| |||
| Race | |||
| White, n (%) | 99 (74.4%) | 334 (78.6%) | |
| Black, n (%) | 26 (19.5%) | 68 (16.0%) | .592 |
| Other, n (%) | 8 (6.0%) | 23 (5.4%) | |
|
| |||
| Prior Prostate Biopsy, n (%) | 113 (83.75%) | 321 (74.8%) | .035 |
|
| |||
| Abnormal DRE, n (%) | 10 (7.4%) | 58 (13.5%) | .068 |
|
| |||
| Prostate Volume, median (Range), | 47.0 (20-220) | 41.0 (12-186) | <.001 |
| cm3 | |||
|
| |||
| # of target cores, median (Range) | 5.00 (2-14) | 6.00 (2-14) | <.001 |
Table 3.
Multivariate Logistic Regression for predicting disease risk category upgrading by systematic 12-core biopsy over MRI/TRUS Fusion Biopsy. OR, odds ratio; CI, 95% confidence interval.
| Variable | Univariate OR [CI] | P value | Multivariate OR [CI] |
P value |
|---|---|---|---|---|
| Age | 0.99 [.96 - 1.01] | 0.413 | 0.97 [.94 – 1.00] | 0.054 |
| PSA | 0.95 [.92 - .98] | 0.002 | 0.92 [.89 - .96] | <0.001 |
| Race | ||||
| White (reference) | 1 | - | 1 | - |
| Black | 1.29 [.78 – 2.14] | 0.323 | 1.09 [.61 – 1.92] | 0.777 |
| Other | 1.17 [.51 – 2.71] | 2.42 [.97 – 6.02] | 0.058 | |
| Prior Prostate Biopsy | 1.73 [1.04 – 2.87] | 0.034 | 1.60 [.94 – 2.75] | 0.086 |
| Abnormal DRE | 0.51 [.25 – 1.03] | 0.061 | 0.78 [.37 – 1.65] | 0.515 |
|
MRI Prostate Volume
(per 5 mL) |
1.07 [1.03 – 1.10] | <0.001 | 1.13 [1.08 – 1.18] | <0.001 |
| # of target cores | 0.84 [.77 - .91] | <0.001 | 0.86 [.79 - .94] | 0.001 |
MR Imaging Review
Of 62 patients upgraded to CS disease, 44 (71.0%) had a lesion identified on mpMRI with subsequent FBx in the same sextant that revealed intermediate or high-risk disease from SBx [MRI SS Low: 8, Moderate: 28, Moderate-High: 2, High: 5, Not Reported: 1; Tumor Diameter 0.5 – 1 cm: 23, 1.1 – 1.5 cm: 14, 1.6 – 2 cm: 7]. In the remaining 18/62 patients (29.0%), mpMRI failed to identify a targetable lesion within the sextant from SBx. On imaging re-review of these 18 patients by an expert genitourinary radiologist, 8 were found to have a lesion missed on initial review within the same sextant [MRI SS Low: 3, Low-Moderate: 1, Moderate: 4; Tumor Diameter 0.5 – 1 cm: 5, 1.1-1.5 cm: 3], whereas the remaining 10 patients had no visible lesion within the sextant (“MR Invisible”).
Mechanisms of SBx upgrading
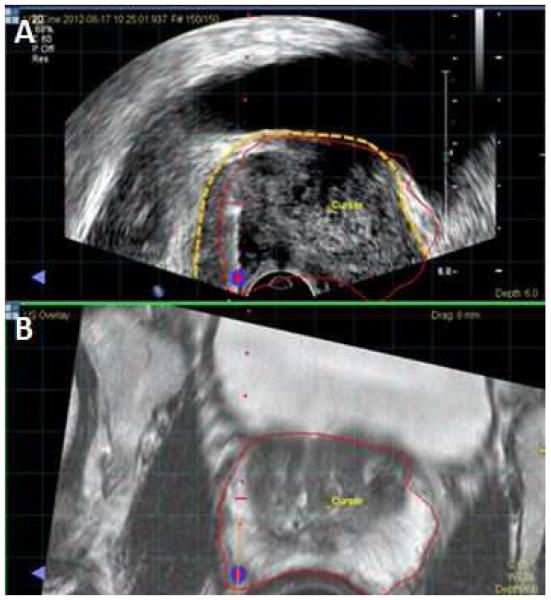
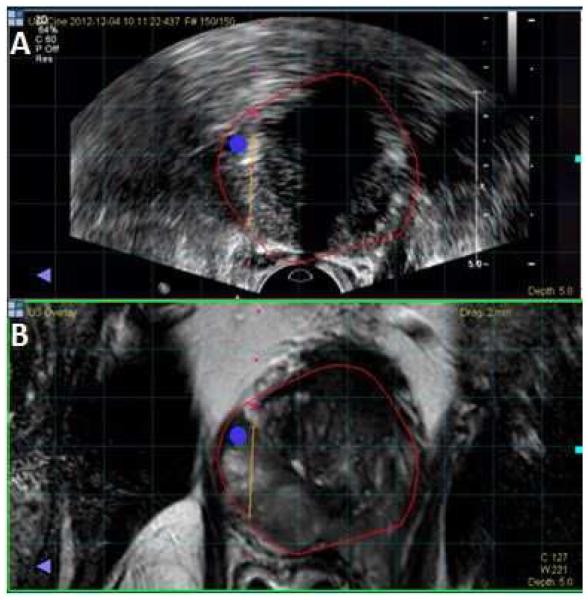
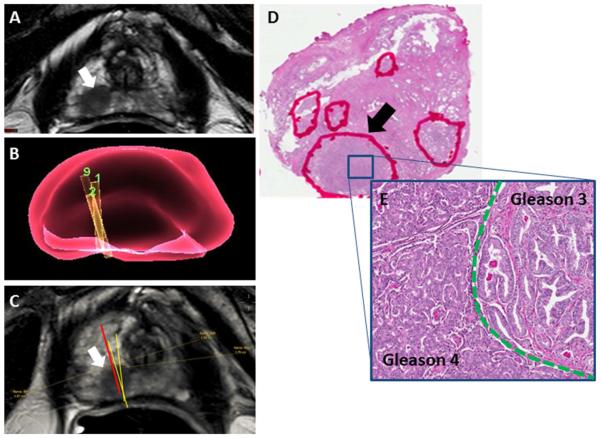
Biopsy mapping and archived imaging review was completed for 25/44 sextant overlap positive patients. In 25 patients, 5 (20%) cases of registration error (Figure 2) and 8 (32%) cases of mechanical error (Figure 3) were determined as potential sources of under-grading by FBx. Whole mount pathology review in 13/25 patients who had radical prostatectomy revealed intralesion Gleason heterogeneity as the mechanism for Gleason upgrading by SBx over FBx in 10 cases (Figure 4). In these 10 cases, predominant areas of both Gleason pattern 3 and pattern 4 could be identified within the same lesion, accounting for instances in which Gleason 6 on FBx could be upgraded to Gleason 7 found on SBx despite accurate targeting.
Figure 2.
Screen Capture from fusion biopsy platform. A. Fused image with MR prostate segmentation (red line) overlaid on top of ultrasound. The ultrasound prostate border is highlighted by the dotted yellow line. The MR segmentation does not perfectly align with the ultrasound prostate border, illustrating the presence of registration error. B. T2 weighted MRI with prostate border segmented in red.
Figure 3.
Screen capture from fusion biopsy platform. A. Fused image with MR prostate segmentation (red line) overlaid on top of ultrasound. Blue circle represents a bullseye that marks the lesion for targeting. Yellow line represents a virtual depiction of biopsy needle course at time of biopsy. Biopsy needle missed the blue circle and coursed to the right, illustrating the presence of mechanical error. B. T2 weighted MRI with prostate border segmented in red.
Figure 4.
A. T2-weighted MR image demonstrating a lesion in the right apical peripheral zone (white arrow) B. Virtual 3D representation of biopsy needle cores from biopsy session in right apical sextant C. Mapping of needle coordinates onto MRI. Red line = systematic core in right apical sextant (Gleason 3+4); Yellow lines = targeted fusion biopsy cores of lesion within the sextant (Gleason 3+3) D. Whole mount H&E section of prostate tissue from radical prostatectomy with multiple foci of tumor (marked by red circles); Lesion of interest in right apical peripheral zone (black arrow). At higher magnification within the lesion (unmagnified) (E), the area is predominantly composed by infiltrating, well-formed glands with branched and more open lumen, and focal intraluminal eosinophilic crystalloids, corresponding to Gleason pattern 3 (right side of the image). The second component shows poorly-formed glands, some of them fused and more complex with glomeruloid pattern and cribriform cellular proliferation, corresponding to Gleason pattern 4 (left side of the picture). (5X)
Discussion
Current PCa detection strategies have limitations and exploration of novel biomarkers, genomic analysis, and imaging has been employed to bridge the deficiency15-17. Though mpMRI and FBx are significant improvements over the status quo, in a small number of patients it reports a ‘false negative’ that is captured on SBx. The current study represents a comprehensive investigation in identifying potential mechanisms by which FBx under-grades CS disease.
As expected, lower PSA, higher MRI prostate volume, and lower number of target cores were predictors for upgrading to higher risk disease based on SBx over FBx. Shakir et al. demonstrated that the diagnostic utility of FBx is greatest in patients with higher PSA, and at lower PSA thresholds, SBx resulted in greater detection of low-risk disease 18. Therefore, the lower PSA average in the cohort upgraded by SBx likely reflects the finding that most instances of upgrading by SBx were due to detection of Gleason 6 cancers. Walton Diaz et al. identified increased yield of PCa detection with FBx compared to SBx in patients with larger prostate volumes 19, yet also illustrated a consistent downward trend of cancer detection rate as prostate volume increased. It is conceivable that FBx accuracy is impacted with enlarged prostates due to increased operator-dependent deformation during the biopsy procedure, resulting in registration error. Alternatively, the heterogeneous nature of the transition zone on mpMRI presents a diagnostic challenge, and transition zone cancers may be mis-characterized.
Despite representing a small proportion overall, it is important to delineate mechanisms by which FBx missed CS disease. Cash et al. 20 examined 61 patients in which SBx detected cancer where FBx did not, and proposed failure of FBx technique and falsely high initial PI-RADS score as mechanisms for a negative FBx. However, the lack of needle tracking and biopsy mapping to further classify failure of FBx technique were limitations. The current study reviewed biopsy core mapping of both SBx and FBx cores in 25/44 patients that were upgraded to CS disease in addition to assessment of whole mount histopathologic specimens in patients undergoing radical prostatectomy. Biopsy mapping allows for accurate documentation of biopsy locations, re-biopsy of specific sites, and focal therapy planning 21-23.
We propose 4 potential mechanisms for failure of FBx in identifying CS disease: mpMRI reader oversight (retrospective identification of lesion missed on initial review), mpMRI invisible cancers, inaccurate sampling of lesion during FBx due to registration and/or mechanical error, and intra-lesion Gleason heterogeneity. The mechanism(s) involved in under-grading by FBx are not mutually exclusive and presence of multiple errors can compound to yield inaccurate results.
In the 62 patients upgraded to CS PCa by SBx, mpMRI identified a lesion within the sextant that showed CS disease in the majority (n = 44, 71%). However, mpMRI failed to identify a lesion within the sextant in 10 cases. MpMRI is not without its limitations, with negative predictive values ranging from 63% to 98% for CS disease 24, 25.
The second mechanism of FBx under-grading entailed inaccurate sampling of lesions during FBx. FBx is a challenging technique comprised of numerous integrated steps for reliable and correct results. Accuracy may be affected by multiple factors including precise registration and superimposition of real-time TRUS with previously acquired MRI, (mechanical) hand-eye coordination and steadiness during navigation and needle deployment, and meticulous real-time adjustments for prostate motion/deformation during the procedure 26. Biopsy mapping and archived imaging review in 25/44 patients with sextant overlap revealed 5 cases of registration error and 8 cases of mechanical error. However, these represent a small proportion of the entire 1000 patient biopsy cohort.
Lastly, review of whole mount pathology in the 13/25 patients who had radical prostatectomy revealed intra-lesion Gleason heterogeneity as the mechanism for Gleason upgrading by SBx over FBx in 10 cases. Histological grade heterogeneity in PCa is a well-established concept in the setting of multifocal adenocarcinoma and intra-tumor heterogeneity 27-29. Current practice at our institution is to obtain 2 biopsy cores (axial, sagittal) per lesion; however, there is potential consideration in saturating lesions (especially larger lesions) with additional cores during FBx to gain the most accurate representation of Gleason distribution within a lesion, which is an area currently under investigation. Sampling of larger lesions with additional cores may be achieved with division into sub-regions of interest or systematic sampling at arbitrary intervals. This however would be balanced with the morbidity of obtaining additional cores.
There are no tests without shortcomings and it is prudent to evaluate them with a critical eye. MpMRI and FBx miss a number of CS prostate cancers (62/564), only 2% of which represents high-risk disease. The study findings reveal that the number of cases under-graded on FBx can be further reduced with meticulous imaging review to avoid missed lesions and careful attention to biopsy technique. Factors associated with under-grading, though statistically significant, are unlikely to be clinically meaningful as they are not actionable items in patient selection for additional SBx due to the number of patients who would require a combined FBx/SBx biopsy to capture such a limited amount of cases.
Strengths of this study include a large patient cohort, well-established standardized imaging/biopsy protocol, and biopsy mapping with archived biopsy imaging review to infer potential mechanisms of FBx under-grading. However, a number of limitations are apparent. Firstly, it was assumed in sextant overlap patients that the systematic core that revealed higher risk disease sampled the lesion pre-identified in the sextant; however, it is possible that the systematic core sampled a region in the sextant but outside the lesion, in which case multifocality within the sextant and/or presence of an “MR Invisible” lesion could be theoretical reasons for SBx upgrading. Biopsy mapping and archived biopsy imaging review was only performed in a limited subset of patients upgraded by SBx to CS disease as the technology was not introduced until 2011. Furthermore, biopsy mapping of coordinates onto MRI assumes accurate registration in order to transpose TRUS coordinates onto MRI. However, several factors such as prostate motion and/or deformation of the prostate during the procedure can affect the registration leading to error. Phantom studies with the Uronav platform have demonstrated that the accuracy of the system is approximately 2.4 ± 1.2 mm 30. Therefore, there is a small degree of error present in mapping of needle coordinates based on accuracy of the registration. Lastly, as there is currently no method for objective measurement of registration error integrated into any platform, presence of registration error and mechanical error was determined by examining archived biopsy imaging at the time of biopsy of the lesion of interest.
Conclusion
Disease risk category upgrading to CS disease by SBx over FBx occurred in a small proportion of patients; thus, FBx is highly accurate and identifies the vast majority CS disease.
Mechanisms for disease risk category upgrading by SBx over FBx include: mpMRI reader oversight, mpMRI invisible cancers, registration error and/or mechanical error, and intra-lesion Gleason heterogeneity. Additional studies will help identify patients that benefit from SBx in addition to FBx.
Acknowledgements
Financial Funding: This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute, Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field.
This research was also made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program
Standard Abbreviations
- mpMRI
multiparametric magnetic resonance imaging
- FBx
MRI/TRUS fusion-guided biopsy
- SBx
systematic 12-core biopsy
- PCa
prostate cancer
- CS
clinically significant
- TRUS
transrectal ultrasound
- MRI SS
MRI Suspicion Score
- PSA
prostate specific antigen
- DRE
digital rectal exam
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.George AK, Turkbey B, Valayil SG, et al. A urologist's perspective on prostate cancer imaging: past, present, and future. Abdom Radiol (NY) 2016 doi: 10.1007/s00261-016-0751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nix JW, Turkbey B, Hoang A, et al. Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU Int. 2012;110:E694. doi: 10.1111/j.1464-410X.2012.11503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sankineni S, George AK, Brown AM, et al. Posterior subcapsular prostate cancer: identification with mpMRI and MRI/TRUS fusion-guided biopsy. Abdom Imaging. 2015;40:2557. doi: 10.1007/s00261-015-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound Fusion-targeted Prostate Biopsy in Men With Previous Negative Biopsies: Impact on Repeat Biopsy Strategies. Urology. 2015;86:1192. doi: 10.1016/j.urology.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radtke JP, Boxler S, Kuru TH, et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate Cancer Prostatic Dis. 2015;18:288. doi: 10.1038/pcan.2015.29. [DOI] [PubMed] [Google Scholar]
- 6.Valerio M, Donaldson I, Emberton M, et al. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review. Eur Urol. 2015;68:8. doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014;191:1749. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastinehad AR, Abboud SF, George AK, et al. Reproducibility of Multiparametric MRI and Fusion-Guided Prostate Biopsy: Multi-Institutional External Validation by a Propensity Score Matched Cohort. J Urol. 2016 doi: 10.1016/j.juro.2015.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313:390. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salami SS, Ben-Levi E, Yaskiv O, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int. 2015;115:562. doi: 10.1111/bju.12938. [DOI] [PubMed] [Google Scholar]
- 11.Radtke JP, Kuru TH, Boxler S, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. 2015;193:87. doi: 10.1016/j.juro.2014.07.098. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui MM, George AK, Rubin R, et al. Efficiency of Prostate Cancer Diagnosis by MR/Ultrasound Fusion-Guided Biopsy vs Standard Extended-Sextant Biopsy for MR-Visible Lesions. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong CW, Rais-Bahrami S, Walton-Diaz A, et al. Comparison of magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int. 2015;115:772. doi: 10.1111/bju.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkbey B, Xu S, Kruecker J, et al. Documenting the location of prostate biopsies with image fusion. BJU Int. 2011;107:53. doi: 10.1111/j.1464-410X.2010.09483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George AK, Pinto PA, Rais-Bahrami S. Multiparametric MRI in the PSA screening era. Biomed Res Int. 2014;2014:465816. doi: 10.1155/2014/465816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frye TP, Pinto PA, George AK. Optimizing Patient Population for MP-MRI and Fusion Biopsy for Prostate Cancer Detection. Curr Urol Rep. 2015;16:50. doi: 10.1007/s11934-015-0521-y. [DOI] [PubMed] [Google Scholar]
- 17.Eggener SE, Badani K, Barocas DA, et al. Gleason 6 Prostate Cancer: Translating Biology into Population Health. J Urol. 2015;194:626. doi: 10.1016/j.juro.2015.01.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakir NA, George AK, Siddiqui MM, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol. 2014;192:1642. doi: 10.1016/j.juro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walton Diaz A, Hoang AN, Turkbey B, et al. Can magnetic resonance-ultrasound fusion biopsy improve cancer detection in enlarged prostates? J Urol. 2013;190:2020. doi: 10.1016/j.juro.2013.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cash H, Gunzel K, Maxeiner A, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure. BJU Int. 2015 doi: 10.1111/bju.13327. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chelluri R, Kilchevsky A, George AK, et al. Prostate Cancer Diagnosis on repeat MRI-TRUS Fusion Biopsy of Benign Lesions: Recommendations for Repeat Sampling. J Urol. 2016 doi: 10.1016/j.juro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raskolnikov D, Rais-Bahrami S, George AK, et al. The role of image guided biopsy targeting in patients with atypical small acinar proliferation. J Urol. 2015;193:473. doi: 10.1016/j.juro.2014.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felker ER, Margolis DJ, Nassiri N, et al. Prostate cancer risk stratification with magnetic resonance imaging. Urol Oncol. 2016 doi: 10.1016/j.urolonc.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wysock JS, Mendhiratta N, Zattoni F, et al. Predictive Value of Negative 3T Multiparametric Prostate MRI on 12 Core Biopsy Results. BJU Int. 2016 doi: 10.1111/bju.13427. [DOI] [PubMed] [Google Scholar]
- 26.Tay KJ, Gupta RT, Rastinehad AR, et al. Navigating MRI-TRUS fusion biopsy: optimizing the process and avoiding technical pitfalls. Expert Rev Anticancer Ther. 2016;16:303. doi: 10.1586/14737140.2016.1131155. [DOI] [PubMed] [Google Scholar]
- 27.Aihara M, Wheeler TM, Ohori M, et al. Heterogeneity of prostate cancer in radical prostatectomy specimens. Urology. 1994;43:60. doi: 10.1016/s0090-4295(94)80264-5. [DOI] [PubMed] [Google Scholar]
- 28.Ruijter ET, van de Kaa CA, Schalken JA, et al. Histological grade heterogeneity in multifocal prostate cancer. Biological and clinical implications. J Pathol. 1996;180:295. doi: 10.1002/(SICI)1096-9896(199611)180:3<295::AID-PATH663>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.Arora R, Koch MO, Eble JN, et al. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer. 2004;100:2362. doi: 10.1002/cncr.20243. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]