Abstract
Splicing of precursor messenger RNA is a critical step in regulating gene expression and major advances are being made in understanding the composition and structure of the enzymatic complex which performs splicing, termed the spliceosome. In parallel, there has been increased appreciation for diverse mechanisms by which alterations in splicing contribute to cancer pathogenesis. Key among these includes change-of-function mutations in genes encoding spliceosomal proteins. Such mutations are amongst the most common genetic alterations in myeloid and lymphoid leukemias, making efforts to therapeutically target cells bearing these mutations critical. To this end, recent studies have clarified that pharmacologic modulation of splicing may be preferentially lethal for cells bearing spliceosomal mutations and also may have role in the therapy of MYC-driven cancers. This has culminated in the initiation of a clinical trial of a novel oral spliceosome modulatory compound targeting the SF3B complex and several novel alternative approaches to target splicing are in development as reviewed here. There is therefore now a great need to understand the mechanistic basis of altered spliceosomal function in cancers and to study the effects of spliceosomal modulatory compounds in pre-clinical settings and in well-designed clinical trials.
Background
Basic Mechanisms of pre-mRNA splicing
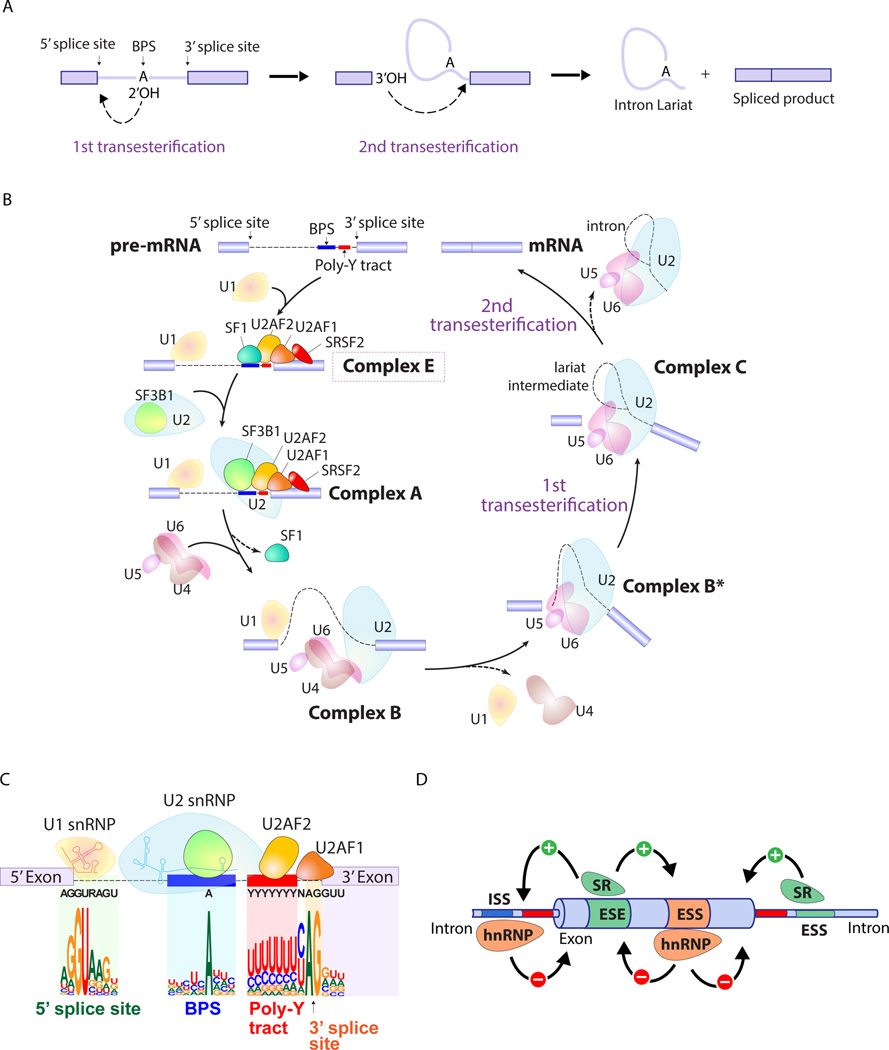
Aberrant regulation of gene expression is a well-known hallmark of cancer cells. As such, mRNA splicing, the process of removing introns from precursor messenger RNA (pre-mRNA) represents a critical step in the post-transcriptional regulation of gene expression. More than 95% of human genes are capable of generating multiple RNA species through alternative splicing and this process enables cells to generate a diversity of functionally distinct proteins from a single gene. mRNA splicing is carried out inside the nucleus by an enzymatic complex known as the spliceosome. The spliceosome is a metalloribozyme that consists of 5 small nuclear ribonucleoproteins (snRNPs; U1, U2, U4, U5, and U6 snRNPs), each of which contains its own small nuclear RNA (snRNA) complexed to a group of proteins, and more than 200 related proteins. Recent utilization of cryo-electron microscopy has enabled an unprecedented high-resolution view of each step in splicing (1–5). Although splicing is a complex multistep process (reviewed in refs (6–10) in detail), the crux of splicing catalysis consists of 2 sequential transesterification reactions (Figure 1A). Base-pairing of snRNAs to conserved sequences on pre-mRNA as well as interactions of numerous splicing accessory proteins and RNA-protein interactions are essential in guiding the massive spliceosomal complex to regions of pre-mRNA for splicing of the correct segments of RNA (Figures 1B-D). Here we present a simplified summary of the spliceosome assembly pathway and the factors required for exon definition (Figure 1B).
Figure 1. Splicing catalysis, the spliceosome assembly pathway, and mechanisms of splice site selection.
(A) Diagram of the 2 sequential transesterification reactions that represent the crucial catalytic steps in intron removal during splicing. An adenine nucleotide (termed the "invariant adenine") of the branch point sequence (BPS) initiates the first transesterification and generates a free 5' exon and an intron-3' exon lariat. The 3' end hydroxyl of the free 5' exon then attacks the intron-3' exon junction, completing the splice and releasing a lariat RNA intron. (B) Pre-mRNA splicing is a dynamic process that involves several distinct spliceosomal complexes. The earliest complex (complex E) is established by binding of (i) U1 snRNP to the the 5’ splice site (SS), (ii) splicing factor 1 (SF1) to the BPS, (iii) U2AF2 (also known as U2AF65) to the polypyrimidine tract, (iv) U2AF1 (also known as U2AF35) to the 3’ SS. Formation of complex E in turn enhances the recruitment of U2 snRNP to the BPS and leads to the formation of complex A. SF3B1, a component of U2 snRNP, is involved in the binding to the BPS. The pre-assembled U4/U6.U5 tri-snRNP complex joins and the U1/U4 snRNPs are released to form the catalytically active complex B (complex B*), followed by the further conformational rearrangements that results in the formation of complex C. Complexes B and C catalyze the first and second esterification reactions, respectively, and mediate excision of the intron and ligation of the proximal and distal exon to synthesize mature mRNA. (C) A focus on complex E highlights consensus sequence elements recognized by U1 and U2 snRNPs as well as the U2AF complex. An intron is defined via (i) the 5’ SS, (ii) the 3’ SS, (iii) the branch point sequence (BPS), and (iv) the polypyrimidine (Poly-Y) tract. The definition of an intron depends on recognition of the 5’ SS and BPS by U1 and U2 snRNPs, respectively. The consensus sequences shown are those recognized by the major (U2-dependent spliceosome) which processes >95% of introns (as opposed to the minor U12-dependent spliceosome which recognizes different consensus sequences than those shown here). (D) In addition to sequences in mRNA recognized by the core spliceosome and the U2AF complex, accessory splicing regulatory proteins are essential in promoting or repressing splice site usage. Members of the serine/arginine (SR) family proteins control the pattern of alternative splicing by recognizing specific sequences in pre-mRNA named exonic and intronic splicing enhancers (ESE and ISE). SR proteins generally act as enhancers of splicing from nearby splice sites by interacting ESE and ISE, while heterozygous nuclear ribonucleoprotein particle (hnRNP) suppresses splicing by interacting with exonic and intronic splicing silencers (ESS and ISS).
An intron is defined by four consensus elements: (i) the 5’ splice site (5' SS; located at the 5’ end of the intron), (ii) the 3’ SS (located at the 3’ end of the intron), (iii) the branch point sequence (BPS) (located upstream of the 3’ SS), (iv) the polypyrimidine tract (located between the BPS and the 3’ SS) (Figure 1C). These sequences are critical in allowing the spliceosome to recognize nucleotide sequences as introns and to distinguish introns from exonic sequence. For the majority of introns, the 5' SS is characterized by a GU dinucleotide while the 3' SS contains an AG dinucleotide. These 2 sequences are not sufficient by themselves to define an intron in most cases and a variable stretch of pyrimidine nucleotides, called the polypyrimidine tract, further helps define the 3' SS. The polypyrimidine tract is situated between the 3' SS and the BPS, and also serves to recruit splicing factors to the 3' SS and BPS. The BPS, so-called as it consists of a nucleotide which initiates a nucleophilic attack on the 5′ SS to create a "branch" like structure, contains a conserved Adenosine nucleotide required for the first step of splicing (Figure 1A).
The early steps of spliceosome assembly are then achieved by binding of the 5’ SS and BPS by U1 and U2 snRNPs, respectively, through base-pairing interactions. U2 snRNP consists of SF3A, SF3B, and a 12S RNA subunit in which SF3B1 is involved in the binding to the BPS. The likelihood of splicing at a particular site is influenced by additional proteins outside of the core spliceosome. For example, members of the serine/arginine (SR) family proteins generally promote splicing by recognizing specific sequences in pre-mRNA named exonic and intronic splicing enhancers (ESE and ISE) (Figure 1D). SR proteins generally act as enhancers of splicing from nearby splice sites by interacting with these sequences and recruiting the U1 snRNP and U2AF to 5’ and 3’ SS, respectively. In contrast, heterozygous nuclear ribonucleoprotein particle proteins (hnRNPs) generally suppress splicing by interacting with exonic and intronic splicing silencers (ESS and ISS).
Altered mRNA splicing in cancer
Growing evidence has revealed that mis-splicing of pre-mRNA can promote cancer initiation, maintenance, and/or progression. Genetic alterations in cancer that contribute to mis-splicing fall into 2 categories: (i) mutations falling within the mRNA sequence that is being spliced and thereby influencing splicing (so-called "cis-acting" mutations) and (ii) alterations in the level of expression or mutations in splicing factors which promote splicing of pre-mRNA (so-called "trans-acting" splicing factors).
Cis-acting mutations include those affecting the 5’ SS, 3’ SS, BPS, or splicing enhancer or silencer elements. Mutations with pathologic effects on splicing may therefore occur within introns or exons and include synonymous as well as non-synonymous mutations. Such mutations represent common mechanisms of inactivation of tumor suppressor genes (11). For example, recurrent synonymous mutations within TP53 occur adjacent to splice sites resulting in intron retention or activation of a cryptic splice site to produce a frameshifted mRNA subjected to nonsense mediated decay (12). Similarly, recurrent somatic mutations in APC resulting in exon skipping (13) or creation of a new splice site (14) in colon and lung cancer, respectively.
In 2011, recurrent somatic mutations affecting trans-acting spliceosome components were reported in hematopoietic malignancies (15, 16) and are currently among the most common class of mutations in patients with myelodysplastic syndromes (MDS) (15) and chronic lymphocytic leukemia (CLL) (17). These mutations occur predominantly in SF3B1 and U2AF1 (core spliceosomal components important in 3' SS recognition), SRSF2 (an SR protein), and ZRSR2 (which serves a function in the minor (U12-dependent) spliceosome in a role analogous to U2AF1) (recently reviewed (18, 19)). Mutations in these splicing factors have also been identified in solid tumors and include SF3B1 mutations in uveal melanoma (15–19%) (20–22), pancreatic ductal adenocarcinoma (4%) (23), and breast cancer (2–4%) (24, 25), as well as U2AF1 mutations in lung adenocarcinoma (3%) (26).
Mutations in SF3B1, U2AF1, or SRSF2 alter mRNA splicing preferences in a manner distinct from loss-of-function (27–31). Consistent with this change-of-function effect, mutations in SF3B1, U2AF1, and SRSF2 are invariably found as heterozygous point mutation at restricted amino acids and occur in a mutually exclusive manner with one another. We and others recently identified that cells bearing spliceosomal mutations depend on wildtype splicing function for survival (32–34), which appears to create a therapeutic window between spliceosomal-mutant cancer cells and normal cells for pharmacologic modulation of splicing.
In addition to mutations in splicing factors, mis-regulated expression of regulatory factors in the splicing machinery can also impact alternative splicing and promote cancer development. For example, SRSF1 is known to be upregulated in multiple cancers and transform cells by modulating alternative splicing of target genes, such as Ron (35) and S6K1 (36). Genetic alteration and/or mis-regulated expression of RBM5, RBM6, and RBM10 are also frequently observed and involved in the pathogenesis of cancers of the lung and other tissues (26, 37–39). These observations connect mis-regulation of RNA splicing to cancer pathogenesis.
Clinical-Translational Advances
As mentioned above, recent studies have suggested that spliceosomal-mutant malignancies are preferentially sensitive to pharmacologic or genetic modulation of splicing compared to spliceosomal-wildtype cancers or normal cells. To this end, natural products from several bacteria species and their analogs have been discovered that bind SF3B1 (and possibly other components of U2 snRNP) and block early spliceosome assembly. These compounds, which include E7107 (an analog of pladienolide B) (40), spliceostatin A (41), and the sudemycins (42), are thought to inhibit the exposure and binding of the branch point binding region of U2 snRNP to the BPS, thereby blocking the essential conformational change in U2 snRNP required for the transition from complex A to complex E (34, 43–45) (the activities and properties of these compounds have been reviewed recently in detail (46)). Although the downstream changes in the transcriptome and protein expression caused by these drugs are still largely unknown, results from preclinical evaluation of these compounds in genetic subsets of cancer are promising.
We recently demonstrated that in vivo treatment with E7107 increases retention of both constitutive and alternative introns as well as cassette exon skipping, consistent with E7107 inhibiting splicing catalysis. However, the magnitude of splicing inhibition following E7107 treatment was more severe in myeloid leukemias with Srsf2-mutant versus wildtype leukemias, resulting in decreased disease burden in both isogenic murine leukemia models and AML patient-derived xenograft (PDX) models with or without SRSF2 mutations (34). Similar preferential sensitivity was seen in Sf3b1K700E mutant hematopoietic cells after in vivo treatment with E7107 (47). An orally bioavailable analog of E7107, H3B-8800, has shown promising preclinical results in isogenic SRSF2 and SF3B1-mutant leukemias (48). These data have resulted in initiation of a phase I dose-escalation study of H3B-8800 for patients with spliceosomal-mutant MDS, AML, and CMML (clinicaltrials.gov identifier NCT02841540).
Given the frequency and adverse prognosis of SF3B1 mutations in CLL (49), several studies have examined the zpotential efficacy of spliceosome inhibition in CLL. In vitro exposure of primary CLL cells to FD-895 (50), pladienolide B (50), or spliceostatin A (51) results in increased apoptosis of CLL cells compared with normal B-cells, regardless of SF3B1 mutational status. However attempts to study the efficacy of these compounds in vivo in the context of CLL have largely been limited by the lack of stable and robust PDX models of CLL (52) as well as genetically engineered CLL models with Sf3b1 mutations. One issue to consider in therapeutic targeting of spliceosomal-mutant CLL is that, distinct from myeloid malignancies where SF3B1 mutations are usually in the predominant clone, SF3B1 mutations in CLL are frequently subclonal (49, 53). Therefore, estimation of SF3B1 mutant allele frequencies may be needed to assess the impact of targeting the spliceosome in CLL.
To date there have been no studies testing the efficacy of SF3B1 binding agents based on the presence of spliceosomal gene mutations in epithelial cancers. However, several studies using unbiased approaches have revealed that a wide-range of MYC-dependent cancers are preferentially vulnerable to spliceosomal modulation. A genome-wide MYC-synthetic lethal screen in mammary epithelial cells identified several components of the spliceosome as preferentially required in cells with MYC overexpression (54). This observation motivated the authors to hypothesize that oncogenic MYC depends on normal spliceosomal functions for cell survival. Similarly, a genome-wide siRNA screen in patient-derived glioblastoma multiforme stem cells (GSCs) (55) identified PHF5A as differentially required for survival of GSCs over normal neuronal stem cells. PHF5A is known to form a bridge between the U2 snRNP and ATP-dependent RNA helicases and be involved in RNA splicing. In fact, knockdown of PHF5A resulted in GSC-specific intron retention and exon skipping events in hundreds of genes as well as preferential cell cycle arrest and loss of viability in GSCs, but not in untransformed neural stem cells. Intriguingly, these observations in GSCs were phenocopied by overexpression of MYC in untransformed neural stem cells. Taken together, therapeutic intervention with spliceosomal inhibition in MYC-driven cancers appears to be a promising approach to target a wide variety of solid and liquid tumors.
In addition to the use of spliceosome modulators, several recent clinical trials have highlighted potential therapeutic approaches for spliceosomal-mutant cancers by targeting biological processes not directly linked to splicing. For example, a pilot study of the telomerase inhibitor imetelstat for patients with the myeloproliferative neoplasm myelofibrosis demonstrated preferential effects of imetelstat in patients with SF3B1 or U2AF1 mutations versus patients without these mutations (complete response rate, 38% vs 4%, p=0.04) (56). However, testing of imetelstat in forms of MDS where >80% of patients harbor SF3B1 mutations, termed refractory anemia with ring sideroblasts (RARS) and RARS-t (a variant of RARS with thrombocytosis), revealed only modest effects in these patients. For these reasons and the need to define its therapeutic efficacy further, results from larger clinical trials of imetelstat in myeloid malignancy patients are clearly needed.
While spliceosomal gene mutations are a recently discovered feature of MDS, one of the oldest hallmarks of MDS is the presence of ineffective erythropoiesis associated with erythroid hyperplasia and apoptosis of red blood cell (RBC) precursors in the bone marrow. Recent data has identified excessive SMAD2/3 signaling as casually linked to pathologic erythropoiesis in MDS patients (57, 58). Consistent with this, lower-risk MDS patients treated with the SMAD2/3 inhibitor, luspatercept (ACE-536), achieved hematologic improvement and reduced RBC transfusion independence in a phase II, multicenter, open-label study (59, 60). Higher response rates were observed in patients with RARS MDS and SF3B1 mutations in this study. Luspatercept is a fusion protein containing a modified extracellular domain of the human activin receptor type IIB linked to a human IgG1 Fc domain, which sequesters TGF-β superfamily ligands to suppress SMAD2/3 activation (a so-called "ligand trap") (58). It is currently unclear if the improvements in anemia in low-risk MDS patients are due to an unrecognized link between SF3B1 mutations and TGF-β signaling or if the effects of luspatercept on erythropoiesis are unrelated to SF3B1 mutations. Several clinical trials of luspatercept are currently ongoing for MDS patients now (clinicaltrial.gov identifiers NCT02631070, NCT02268383, NCT01749514) and may clarify this association.
Open questions
Systematic analyses of mutations in cancer have shown that >50% of human tumors possess one or more mutational hotspots (61). These data underscore the importance of developing therapeutic strategies to target cancer cells bearing such gain-of-function mutations. Of these hotspots, 81% arise in two or more tumor types, suggesting that many hotspot mutations confer a selective advantage across diverse lineages. SF3B1 and U2AF1 mutations are included among such newly defined hotspots and further efforts to define the functionally relevant downstream mis-spliced events present in spliceosomal-mutant cancers will be essential in furthering our understanding of these mutations and developing therapies targeting cells bearing these mutations. Although much has been learned about how mutations in SRSF2 and U2AF1 alter RNA recognition and splicing, more effort to define the allele-specific effects of different SF3B1 mutational hotspots on splicing and gene expression will be critical. Moreover, understanding the effects of spliceosomal gene mutations in the context of mutations commonly co-occurring with them, such as commonly co-existing mutations in SRSF2/IDH2 and U2AF1/ASXL1 as well as enrichment of SF3B1 mutations in patients with inv(3) MDS/AML and del(13q) CLL may reveal novel contributions of splicing mutations to cancer (62–64).
Given the preferential sensitivity of spliceosomal-mutant cells to SF3B1 binding agents, further effort to decipher the mechanistic effects of these compounds on gene expression and splicing are now needed. In addition, ongoing efforts may soon determine the potential efficacy of candidate compounds with effects on splicing beyond SF3B1 binding agents. Increasing evidence supports a role for protein arginine methyltansferase (PRMT) family proteins as splicing regulators. PRMT5 has been shown to play an essential role in regulating splicing (65) as deletion of Prmt5 in several cell types results in reduced methylation of Sm proteins, suboptimal maturation of snRNP complexes, as well as aberrant constitutive and alternative splicing of mRNAs (66). Importantly, PRMT5 suppression in MYC-driven lymphomas results in exon skipping and intron retention coincident with loss of tumor maintenance (67). These findings strongly suggest that targeting PRMTs may have importance for spliceosomal-mutant malignancies as well as MYC-driven tumors. In addition, inhibition of phosphorylation of SR proteins may represent another method to perturb splicing pharmacologically. SR proteins have conserved arginine- and serine-rich domains, which are subject to phosphorylation by multiple kinases, including the SR protein kinases and the CDC2-like kinases. Although the role of phosphorylation of these domains remains to be clarified, modulation of SR protein phosphorylation clearly impacts splicing (68, 69). Characterization of the effects of these new classes of compounds on splicing and potential effects on spliceosomal-mutant malignancies may represent novel therapeutic approaches for conquering malignancies with aberrant spliceosomal catalysis.
Acknowledgments
Grant Support
AY is supported by the Aplastic Anemia and MDS Research Foundation. OAW is supported by grants from the Edward P. Evans Foundation, the Dept. of Defense Bone Marrow Failure Research Program (BM150092 and W81XWH-16-1-0059), NIH/NHLBI (R01 HL128239), an NIH K08 Clinical Investigator Award (1K08CA160647-01), the Josie Robertson Investigator Program, a Damon Runyon Clinical Investigator Award, an award from the Starr Foundation (I8-A8-075), the Leukemia and Lymphoma Society, and the Pershing Square Sohn Cancer Research Alliance.
Footnotes
Potential Conflicts of Interest
O. Abdel-Wahab is a consultant/advisory board member for and reports receiving commercial research grants from H3 Biomedicine Inc. No potential conflicts of interest were disclosed by the other author.
Authors' Contributions
Conception and Design: A. Yoshimi, O. Abdel-Wahab
Writing, review, and/or revision of the manuscript: A. Yoshimi, O. Abdel-Wahab
References
- 1.Nguyen TH, Galej WP, Bai XC, Savva CG, Newman AJ, Scheres SH, et al. The architecture of the spliceosomal U4/U6.U5 tri-snRNP. Nature. 2015;523:47–52. doi: 10.1038/nature14548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan R, Yan C, Bai R, Wang L, Huang M, Wong CC, et al. The 3.8 A structure of the U4/U6.U5 tri-snRNP: Insights into spliceosome assembly and catalysis. Science. 2016;351:466–475. doi: 10.1126/science.aad6466. [DOI] [PubMed] [Google Scholar]
- 3.Yan C, Hang J, Wan R, Huang M, Wong CC, Shi Y. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science. 2015;349:1182–1191. doi: 10.1126/science.aac7629. [DOI] [PubMed] [Google Scholar]
- 4.Wan R, Yan C, Bai R, Huang G, Shi Y. Structure of a yeast catalytic step I spliceosome at 3.4 A resolution. Science. 2016;353:895–904. doi: 10.1126/science.aag2235. [DOI] [PubMed] [Google Scholar]
- 5.Yan C, Wan R, Bai R, Huang G, Shi Y. Structure of a yeast activated spliceosome at 3.5 A resolution. Science. 2016;353:904–911. doi: 10.1126/science.aag0291. [DOI] [PubMed] [Google Scholar]
- 6.Daguenet E, Dujardin G, Valcarcel J. The pathogenicity of splicing defects: mechanistic insights into pre-mRNA processing inform novel therapeutic approaches. EMBO Rep. 2015;16:1640–1655. doi: 10.15252/embr.201541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y, Rio DC. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu Rev Biochem. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TH, Galej WP, Fica SM, Lin PC, Newman AJ, Nagai K. CryoEM structures of two spliceosomal complexes: starter and dessert at the spliceosome feast. Curr Opin Struct Biol. 2016;36:48–57. doi: 10.1016/j.sbi.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papasaikas P, Valcarcel J. The Spliceosome: The Ultimate RNA Chaperone and Sculptor. Trends Biochem Sci. 2016;41:33–45. doi: 10.1016/j.tibs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Sahebi M, Hanafi MM, van Wijnen AJ, Azizi P, Abiri R, Ashkani S, et al. Towards understanding pre-mRNA splicing mechanisms and the role of SR proteins. Gene. 2016;587:107–119. doi: 10.1016/j.gene.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 11.Jung H, Lee D, Lee J, Park D, Kim YJ, Park WY, et al. Intron retention is a widespread mechanism of tumor-suppressor inactivation. Nat Genet. 2015;47:1242–1248. doi: 10.1038/ng.3414. [DOI] [PubMed] [Google Scholar]
- 12.Supek F, Minana B, Valcarcel J, Gabaldon T, Lehner B. Synonymous mutations frequently act as driver mutations in human cancers. Cell. 2014;156:1324–1335. doi: 10.1016/j.cell.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Montera M, Piaggio F, Marchese C, Gismondi V, Stella A, Resta N, et al. A silent mutation in exon 14 of the APC gene is associated with exon skipping in a FAP family. J Med Genet. 2001;38:863–867. doi: 10.1136/jmg.38.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecina-Slaus N, Majic Z, Musani V, Zeljko M, Cupic H. Report on mutation in exon 15 of the APC gene in a case of brain metastasis. J Neurooncol. 2010;97:143–148. doi: 10.1007/s11060-009-0001-7. [DOI] [PubMed] [Google Scholar]
- 15.Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365:1384–1395. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 17.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 18.Dvinge H, Kim E, Abdel-Wahab O, Bradley RK. RNA splicing factors as oncoproteins and tumour suppressors. Nat Rev Cancer. 2016;16:413–430. doi: 10.1038/nrc.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott LM, Rebel VI. Acquired mutations that affect pre-mRNA splicing in hematologic malignancies and solid tumors. J Natl Cancer Inst. 2013;105:1540–1549. doi: 10.1093/jnci/djt257. [DOI] [PubMed] [Google Scholar]
- 20.Furney SJ, Pedersen M, Gentien D, Dumont AG, Rapinat A, Desjardins L, et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;3:1122–1129. doi: 10.1158/2159-8290.CD-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbour JW, Roberson ED, Anbunathan H, Onken MD, Worley LA, Bowcock AM. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet. 2013;45:133–135. doi: 10.1038/ng.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin M, Masshofer L, Temming P, Rahmann S, Metz C, Bornfeld N, et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet. 2013;45:933–936. doi: 10.1038/ng.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–360. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsafadi S, Houy A, Battistella A, Popova T, Wassef M, Henry E, et al. Cancer-associated SF3B1 mutations affect alternative splicing by promoting alternative branchpoint usage. Nat Commun. 2016;7:10615. doi: 10.1038/ncomms10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darman RB, Seiler M, Agrawal AA, Lim KH, Peng S, Aird D, et al. Cancer-Associated SF3B1 Hotspot Mutations Induce Cryptic 3' Splice Site Selection through Use of a Different Branch Point. Cell Rep. 2015;13:1033–1045. doi: 10.1016/j.celrep.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 29.DeBoever C, Ghia EM, Shepard PJ, Rassenti L, Barrett CL, Jepsen K, et al. Transcriptome sequencing reveals potential mechanism of cryptic 3' splice site selection in SF3B1-mutated cancers. PLoS computational biology. 2015;11:e1004105. doi: 10.1371/journal.pcbi.1004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilagan JO, Ramakrishnan A, Hayes B, Murphy ME, Zebari AS, Bradley P, et al. U2AF1 mutations alter splice site recognition in hematological malignancies. Genome Res. 2015;25:14–26. doi: 10.1101/gr.181016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E, Ilagan JO, Liang Y, Daubner GM, Lee SC, Ramakrishnan A, et al. SRSF2 Mutations Contribute to Myelodysplasia by Mutant-Specific Effects on Exon Recognition. Cancer Cell. 2015;27:617–630. doi: 10.1016/j.ccell.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis Liang Fei HM, Chatrikhi Rakesh, Prasad Sameer, Yu Jovian, Gao Shaojian, Kielkopf Clara, Bradley Robert K, Varmus Harold. Wild-type Splicing Factor U2AF1 inhibits splicing associated with a recurrent U2AF1 mutant in human lung cancers and is required for cell survival. BioArxive [Google Scholar]
- 33.Zhou Q, Derti A, Ruddy D, Rakiec D, Kao I, Lira M, et al. A chemical genetics approach for the functional assessment of novel cancer genes. Cancer Res. 2015;75:1949–1958. doi: 10.1158/0008-5472.CAN-14-2930. [DOI] [PubMed] [Google Scholar]
- 34.Lee SC, Dvinge H, Kim E, Cho H, Micol JB, Chung YR, et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nature medicine. 2016;22:672–678. doi: 10.1038/nm.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, et al. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol Cell. 2005;20:881–890. doi: 10.1016/j.molcel.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bechara EG, Sebestyen E, Bernardis I, Eyras E, Valcarcel J. RBM5, 6, and 10 differentially regulate NUMB alternative splicing to control cancer cell proliferation. Mol Cell. 2013;52:720–733. doi: 10.1016/j.molcel.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Oh JJ, Razfar A, Delgado I, Reed RA, Malkina A, Boctor B, et al. 3p21.3 tumor suppressor gene H37/Luca15/RBM5 inhibits growth of human lung cancer cells through cell cycle arrest and apoptosis. Cancer Res. 2006;66:3419–3427. doi: 10.1158/0008-5472.CAN-05-1667. [DOI] [PubMed] [Google Scholar]
- 39.Rintala-Maki ND, Goard CA, Langdon CE, Wall VE, Traulsen KE, Morin CD, et al. Expression of RBM5-related factors in primary breast tissue. J Cell Biochem. 2007;100:1440–1458. doi: 10.1002/jcb.21134. [DOI] [PubMed] [Google Scholar]
- 40.Kotake Y, Sagane K, Owa T, Mimori-Kiyosue Y, Shimizu H, Uesugi M, et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol. 2007;3:570–575. doi: 10.1038/nchembio.2007.16. [DOI] [PubMed] [Google Scholar]
- 41.Kaida D, Motoyoshi H, Tashiro E, Nojima T, Hagiwara M, Ishigami K, et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat Chem Biol. 2007;3:576–583. doi: 10.1038/nchembio.2007.18. [DOI] [PubMed] [Google Scholar]
- 42.Fan L, Lagisetti C, Edwards CC, Webb TR, Potter PM. Sudemycins, novel small molecule analogues of FR901464, induce alternative gene splicing. ACS chemical biology. 2011;6:582–589. doi: 10.1021/cb100356k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnal S, Vigevani L, Valcarcel J. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov. 2012;11:847–859. doi: 10.1038/nrd3823. [DOI] [PubMed] [Google Scholar]
- 44.Effenberger KA, Urabe VK, Jurica MS. Modulating splicing with small molecular inhibitors of the spliceosome. Wiley Interdiscip Rev RNA. 2016 doi: 10.1002/wrna.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folco EG, Coil KE, Reed R. The anti-tumor drug E7107 reveals an essential role for SF3b in remodeling U2 snRNP to expose the branch point-binding region. Genes Dev. 2011;25:440–444. doi: 10.1101/gad.2009411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SC, Abdel-Wahab O. Therapeutic targeting of splicing in cancer. Nature medicine. 2016;22:976–986. doi: 10.1038/nm.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obeng RJC Esther A, Seiler Michael, Chen Michelle C, Campagna PJS Dean R, Schneider Rebekka K, Lord Allegra M, Wang Lili, Gambe MEM Rutendo G, Ali Abdullah M, Raza Azra, Yu Lihua, Buonamici Silvia, Smith Peter G, Mullally Ann, Wu Catherine J, Fleming Mark D, Ebert Benjamin L. Physiologic expression of Sf3b1K700E causes impaired erythropoiesis, aberrant splicing, and sensitivity to pharmacologic spliceosome modulation. Cancer Cell. doi: 10.1016/j.ccell.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buonamici AY Silvia, Thomas Michael, Seiler Michael, Chan Betty, Caleb Benjamin, Darman Rachel, Fekkes Peter, Karr Craig, Keaney Gregg, Klimek Virginia, Kunii Kaiko, Lee Linda, Lee Stanley Chun-Wei, Liu Xiang, Meeske Carol, Mizui Yoshiharu, Padron Eric, Park Eunice, Pazolli Ermira, Prajapati Sudeep, Taylor Justin, Wang John, Warmuth Markus, Yu Lihua, Zhu Ping, Abdel-Wahab Omar, Smith Peter. H3B-8800, an orally bioavailable modulator of the SF3b complex, shows efficacy in spliceosome-mutant myeloid malignancies. 58th ASH Annual Meeting & Exposition abstract. [Google Scholar]
- 49.Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526:525–530. doi: 10.1038/nature15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kashyap MK, Kumar D, Villa R, La Clair JJ, Benner C, Sasik R, et al. Targeting the spliceosome in chronic lymphocytic leukemia with the macrolides FD-895 and pladienolide-B. Haematologica. 2015;100:945–954. doi: 10.3324/haematol.2014.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larrayoz M, Blakemore SJ, Dobson RC, Blunt MD, Rose-Zerilli MJ, Walewska R, et al. The SF3B1 inhibitor spliceostatin A (SSA) elicits apoptosis in chronic lymphocytic leukaemia cells through downregulation of Mcl-1. Leukemia. 2016;30:351–360. doi: 10.1038/leu.2015.286. [DOI] [PubMed] [Google Scholar]
- 52.Xargay-Torrent S, Lopez-Guerra M, Rosich L, Montraveta A, Roldan J, Rodriguez V, et al. The splicing modulator sudemycin induces a specific antitumor response and cooperates with ibrutinib in chronic lymphocytic leukemia. Oncotarget. 2015;6:22734–22749. doi: 10.18632/oncotarget.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu TY, Simon LM, Neill NJ, Marcotte R, Sayad A, Bland CS, et al. The spliceosome is a therapeutic vulnerability in MYC-driven cancer. Nature. 2015;525:384–388. doi: 10.1038/nature14985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubert CG, Bradley RK, Ding Y, Toledo CM, Herman J, Skutt-Kakaria K, et al. Genome-wide RNAi screens in human brain tumor isolates reveal a novel viability requirement for PHF5A. Genes Dev. 2013;27:1032–1045. doi: 10.1101/gad.212548.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM, et al. A Pilot Study of the Telomerase Inhibitor Imetelstat for Myelofibrosis. N Engl J Med. 2015;373:908–919. doi: 10.1056/NEJMoa1310523. [DOI] [PubMed] [Google Scholar]
- 57.Zhou L, Nguyen AN, Sohal D, Ying Ma J, Pahanish P, Gundabolu K, et al. Inhibition of the TGF-beta receptor I kinase promotes hematopoiesis in MDS. Blood. 2008;112:3434–3443. doi: 10.1182/blood-2008-02-139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suragani RN, Cadena SM, Cawley SM, Sako D, Mitchell D, Li R, et al. Transforming growth factor-beta superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nature medicine. 2014;20:408–414. doi: 10.1038/nm.3512. [DOI] [PubMed] [Google Scholar]
- 59.Giagounidis UP Aristoteles, Germing Ulrich, Götze PK Katharina, Mayer Karin, Ottmann Oliver, Radsak TW Markus, Haase Detlef, Hankin Monty, Wilson XZ Dawn, Laadem Adberrahmane, Sherman Matthew L, aKM Attie. Luspatercept Treatment Leads to Long Term Increases in Hemoglobin and Reductions in Transfusion Burden in Patients with Low or Intermediate-1 Risk Myelodysplastic Syndromes (MDS): Preliminary Results from the Phase 2 PACE-MDS Extension Study. 57th ASH Annual Meeting & Exposition abstract.2015. [Google Scholar]
- 60.Platzbecker UG Uwe, Giagounidis Aristoteles, Götze Katharina, Kiewe Philipp, Mayer Karin, Ottmann Oliver, Radsak Markus, Wolff Thomas, Haase Detlef, Hankin Monty, Wilson Dawn, Zhang Xiaosha, Laadem Abderrahmane, Sherman Matthew L, Attie Kenneth M. Biomarkers of Ineffective Erythropoiesis Predict Response to Luspatercept in Patients with Low or Intermediate-1 Risk Myelodysplastic Syndromes (MDS): Final Results from the Phase 2 PACE-MDS Study. 57th ASH Annual Meeting & Exposition abstract.2015. [Google Scholar]
- 61.Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016;34:155–163. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–698. doi: 10.1182/blood-2016-01-693879. [DOI] [PubMed] [Google Scholar]
- 64.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. quiz 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez SE, Petrillo E, Beckwith EJ, Zhang X, Rugnone ML, Hernando CE, et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature. 2010;468:112–116. doi: 10.1038/nature09470. [DOI] [PubMed] [Google Scholar]
- 66.Bezzi M, Teo SX, Muller J, Mok WC, Sahu SK, Vardy LA, et al. Regulation of constitutive and alternative splicing by PRMT5 reveals a role for Mdm4 pre-mRNA in sensing defects in the spliceosomal machinery. Genes Dev. 2013;27:1903–1916. doi: 10.1101/gad.219899.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koh CM, Bezzi M, Low DH, Ang WX, Teo SX, Gay FP, et al. MYC regulates the core pre-mRNA splicing machinery as an essential step in lymphomagenesis. Nature. 2015;523:96–100. doi: 10.1038/nature14351. [DOI] [PubMed] [Google Scholar]
- 68.Araki S, Dairiki R, Nakayama Y, Murai A, Miyashita R, Iwatani M, et al. Inhibitors of CLK protein kinases suppress cell growth and induce apoptosis by modulating pre-mRNA splicing. PloS one. 2015;10:e0116929. doi: 10.1371/journal.pone.0116929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakuma M, Iida K, Hagiwara M. Deciphering targeting rules of splicing modulator compounds: case of TG003. BMC Mol Biol. 2015;16:16. doi: 10.1186/s12867-015-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]