Abstract
Purpose
The clinical utility of next generation sequencing (NGS) in breast cancer has not been demonstrated. We hypothesized we could perform NGS of a new biopsy from patients with metastatic triple negative breast cancer (TNBC) in a clinically actionable timeframe.
Experimental Design
We planned to enroll 40 patients onto a prospective study, Individualized Molecular Analyses Guide Efforts (IMAGE), to evaluate the feasibility of obtaining a new biopsy of a metastatic site, perform NGS (FoundationOne™), and convene a molecular tumor board to formulate treatment recommendations within 28 days. We collected blood at baseline and at time of restaging to assess cell-free circulating plasma tumor DNA (ptDNA).
Results
We enrolled 26 women with metastatic TNBC who had received ≥1 line of prior chemotherapy, and 20 (77%) underwent NGS of a metastatic site biopsy. Twelve (60%) evaluable patients received treatment recommendations within 28 days of consent. The study closed after 20 patients underwent NGS, based on protocol-specified interim futility analysis. Three patients went on to receive genomically directed therapies. Twenty-four of 26 patients had genetic alterations successfully detected in ptDNA. Among 5 patients, 4 mutations found in tumor tissues were not identified in blood and 4 mutations found in blood were not found in corresponding tumors. In nine patients, NGS of follow up blood samples showed 100% concordance with baseline blood samples.
Conclusions
This study demonstrates challenges of performing NGS on prospective tissue biopsies in patients with metastatic TNBC within 28 days, while also highlighting the potential use of blood as a more time efficient and less invasive method of mutational assessment.
INTRODUCTION
As patients and oncologists seek additional relevant data to help direct treatment in metastatic breast cancer, demand for genomic profiling of tumors is rapidly increasing. Therapeutic options for patients with metastatic disease for estrogen receptor-alpha (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) negative, or “triple negative” breast cancer (TNBC), are limited to chemotherapy. It is becoming increasingly clear that TNBC can be further divided into subtypes and that individual patients might benefit from targeted treatments (1, 2). Multiple genomic panels are now commercially available. However, in order for genomic testing to be clinically meaningful for patients with TNBC, who often have rapidly progressive disease, these tests must be performed in a timely fashion to allow for patients and physicians to implement therapies that might be rationally directed from patient-specific alterations.
Despite these challenges, there is strong interest in next generation sequencing (NGS) approaches to provide molecular profiles of tumors from individual patients for direction of treatment. One recent single-institution experience of 2,000 patients with advanced solid tumors demonstrated that comprehensive implementation of NGS tumor profiling for patients was feasible (3). However, the authors found that 23% of patients enrolled in the study were unable to undergo testing due to inadequate tissue or DNA. Notably, the majority of patients were enrolled on the study not to direct the immediate next course of therapy, but to direct future treatments that had no timeline. Presumably this is due to the time associated with testing and the fact that many patients with rapidly progressive cancer require therapy urgently. One concern with using NGS tumor profiling results to guide future treatment is that the mutational landscape of cancer evolves over time, so an isolated biopsy of metastatic cancer may not be representative of that cancer months or years in the future (4, 5). A single biopsy may also not be genetically representative of other sites of metastatic disease, although the oncogenic drivers of the primary tumor seem to be conserved in metastatic sites (6, 7).
There has been great interest in the use of “liquid biopsies” for cancer sequencing as an alternative to biopsies of metastatic tissues (8). It has been demonstrated that blood is an easily accessible source of circulating cell-free tumor DNA (9–16). Both normal and malignant cells shed DNA into the circulation, and NGS technologies can be used to detect circulating cell-free plasma tumor DNA (ptDNA), making blood a source for real-time genomic tumor profiling.
We hypothesized that we could efficiently create a molecular profile from tumors from patients with metastatic TNBC, and make treatment recommendations based on these results in a clinically actionable timeframe. We initiated a prospective clinical trial to investigate the feasibility of molecular profiling of patients’ tumors within 28 days from enrollment to treatment recommendations. Secondarily, we also investigated the use of ptDNA to genomically profile patients’ cancers, hypothesizing that blood offers an easily accessible source of tumor DNA.
PATIENTS AND METHODS
Participants
We initiated a prospective study designated Individualized Molecular Analyses Guide Efforts in Breast Cancer (IMAGE). Women with newly progressing metastatic TNBC with ECOG performance status of 0–2 who had received at least one line of prior chemotherapy were eligible. Patients were enrolled from clinics within the Johns Hopkins Medical Institutions. We obtained a new biopsy from a metastatic site for molecular profiling at study entry. Archived metastatic biopsy specimens were allowed only if patients had not commenced on a new systemic therapy. The protocol was approved by The Johns Hopkins Institutional Review Board. All patients provided written informed consent prior to enrollment onto the study.
Tumor tissue Analysis
Surgical specimens were reviewed by the study pathologist and stained for ER, PR, HER2, and androgen receptor (AR) by immunohistochemistry in a CLIA-approved laboratory at Johns Hopkins. Specimens then underwent hybrid-capture based NGS (FoundationOne™, Foundation Medicine Inc., Cambridge, MA). Methods of the clinical cancer gene assay have been previously published and assay performance has been rigorously validated (17). In brief, DNA was extracted from formalin-fixed, paraffin-embedded tissue (≥ 1mm3) containing no less than 20% tumor nuclei by enzymatic digestion and subsequent purification. DNA was fragmented by sonication to 200 base pair segments. Indexed sequencing adapters were ligated to the DNA fragments and PCR amplified to yield >500 ng of sequencing library. Hybridization selection was performed using individually synthesized baits targeting 236 cancer-related genes and 47 introns of 19 genes frequently re-arranged in cancer. The Illumina HiSeq 2500 platform was used in 49 × 49 paired end sequencing. Sequence data was mapped to the human genome (hg19) using BWA aligner v0.5.9. Sequence data were analyzed through a computational analysis pipeline to call variants present in the sample, including substitutions, short insertions and deletions, rearrangements, and copy number variants.
Treatment Recommendations
Clinical data and genomic profiling reports were reviewed within a week of receipt by the Genomic Alterations In Tumors With Actionable Yields (GAITWAY) molecular profiling tumor board at Johns Hopkins, which interprets genetic alterations found in a patient’s tumor sample to identify potentially “actionable” genes and/or proteins and was previously described (18). A potentially actionable alteration was defined as a mutation that 1) has a US Food and Drug Administration (FDA)–approved therapy for the given tumor type, 2) has an FDA-approved therapy for a different tumor type, 3) may provide rationale for participation in a clinical trial, or 4) may lead to recommendations for genetic counseling and germline mutation testing. Recommendations were provided to the treating oncologist, and patients were followed for treatment decision and clinical outcomes.
Plasma DNA Analysis
Peripheral blood was obtained at study entry, and whenever possible, every 3–4 months and at time of progression. Blood samples and plasma DNA collection and preparation were performed as previously described (11). DNA was extracted from 1.5 to 3.0 mL plasma and used as input into sample preparation without sonication. Indexed sequencing adapters were ligated to the DNA fragments and PCR amplified to yield >2ug of sequencing library. Hybridization selection was performed using individually synthesized baits targeting full exons of 27 cancer-related genes (BRCA1, BRCA2, CCND1, CD274, CDH1, CDK4, CDK6, CDKN2A, CRKL, EGFR, ERBB2, ERRFI1, FGFR1, FGFR2, KRAS, MDM2, MET, MYC, NF1, PDCD1LG2, PTEN, PTPN11, SMO, TP53, VEGFA, FOXL2, MYCN) and partial exons of 34 cancer-related genes (ABL1, AKT1, ALK, ARAF, BRAF, BTK, CTNNB1, DDR2, ESR1, EZH2, FGFR3, FLT3, GNA11, GNAQ, GNAS, HRAS, IDH1, IDH2, JAK2, JAK3, KIT, MAP2K1, MAP2K2, MPL, MTOR, MYD88, NPM1, NRAS, PDGFRA, PDGFRB, PIK3CA, RAF1, RET, TERT) and introns of 6 genes frequently rearranged in cancer (ALK, EGFR, FGFR3, PDGFRA, RET, ROS1). The Illumina HiSeq 2500 platform was used in 176 × 176 paired end sequencing and >5000X unique coverage was generated for most samples. Sequence data were mapped to the human genome (hg19) using BWA MEM aligner v0.7.10. Sequence data were analyzed through a computational analysis pipeline which performs error corrections and calls variants present in the sample, including substitutions, short insertions and deletions and rearrangements. Mutational concordance for genes was then analyzed for patient samples where NGS of both tissue and blood were successfully performed and was qualitatively recorded as present in both or present in either tissue or blood. Only mutations (not amplifications) in these 27 genes were analyzed for concordance studies.
Statistical Methods
The primary objective was to assess feasibility of completing the process from consent to GAITWAY recommendations - including consent, biopsy, molecular profiling of the tumor sample, convening of a tumor board to discuss the results, and reporting of treatment suggestion based on molecular profiling - within 28 days. Secondary objectives included demonstrating the ability to make treatment suggestions based on the molecular profile of patients’ tumors, and to prospectively follow ptDNA in all patients who took part. The primary endpoint of this study was feasibility, defined as accomplishing the aforementioned steps within 28 days from consent, for at least 80% of patients. We planned to enroll 40 women, which would lead to a 90% confidence interval with maximum width of ±14%, which we judged would provide usefully precise estimates of the primary endpoints. If the primary objective of feasibility was met in 32 out of 40 patients (80%), the 90% confidence interval would be 67% to 90%. The protocol included an interim futility analysis of the first 20 patients who completed the process. The study would close for futility if the analysis predicted there was 25% or less probability of success at the end of the trial.
RESULTS
From September 2013 to April 2015, we enrolled 26 eligible women. The median age was 55 years (range: 25 to 78); 13 (50%) patients identified as white, and 11 (42.3%) as black; the median number of prior lines of chemotherapy in the metastatic setting was one (range: 0–4); and 65.4% of patients had visceral disease (Table 1). At the time of this analysis, the median duration of follow up for the 20 patients who underwent NGS was 7.5 months.
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
| Age, years | |
| Median (Range) | 55 (25–78) |
| Race | |
| White | 13 (50%) |
| Black | 11 (42.3%) |
| Asian | 1 (3.8%) |
| Other | 1 (3.8%) |
| ECOG | |
| 0 | 12 (46.2%) |
| 1 | 11 (42.3%) |
| 2 | 3 (11.5%) |
| BRCA status (germline) | |
| BRCA1/2 positive | 1 (3.8%) |
| BRCA negative | 15 (57.7%) |
| Unknown/Not Done | 10 (38.5%) |
| Prior (neo)adjuvant chemotherapy | |
| Yes | 24 |
| Anthracycline and taxane based | 16 |
| Anthracycline based | 2 |
| Taxane based | 5 |
| Other/unspecified | 2 |
| No | 2 |
| Prior (neo)adjuvant hormonal therapy* | 5 |
| Other | 1 |
| No. prior systemic therapy for metastatic disease | |
| All regimens - Median (Range) | 1.5 (0–4) |
| Chemotherapy | 1 (0–4) |
| Hormonal* | 1.5 (1–2) |
| Other/unknown | 1 clinical trial 1 PDL-1 trial |
| AR status (N=21) | |
| Positive | 5 (23.8%) |
| Negative | 16 (76.2%) |
Eligible patients could have previously had ER-positive and/or HER2-positive disease, but must have had confirmation of TNBC on their most recent tissue biopsy.
Twenty (77%) eligible patients underwent successful NGS of a metastatic site biopsy (Table 2). These included 12 new biopsies, and archival tissue from 8 recent biopsies. For patients with archival specimens, the median number of days from biopsy to registration was 13.5 (range 7 to 40), and these patients did not receive any new therapy during this period as specified by our protocol. Six patients did not undergo NGS due to either absence of a metastatic site amenable for biopsy (N=3) or inadequate tissue for NGS from archival (N=2) or new (N=1) biopsies. Of the 20 patients who underwent NGS, 12 (60%) patients received treatment recommendations within 28 days of consent (90% CI: 39%, 78%). The study met the predefined statistical endpoint for futility and closed after 20 patients underwent NGS. Failure to meet this time frame was due to difficulties in accessing archival tumor tissue (N=5) and need to retrieve additional tissue for molecular analysis (N=3).
Table 2.
Outcomes
| Signed Consent (N) | 28 |
| Registered | 26 |
| Screen Failures (N) | 6 |
| Tissue not available | 5 |
| Tissue not usable | 1 |
| Time from consent to tissue availability, days (range) | |
| All | 2.5 (0–21) |
| New specimen (N=12) | 0 (0–13) |
| Archival (N=8) | 4.5 (1–21) |
| Time from biopsy to specimen shipment, days (range) (N=12) | 4 (2–7) |
| Time from specimen shipment to report from FM, days (range) | 15.5 (12–30) |
| Time from report to tumor board, days (range) | 5 (3–10) |
| Recommendation within 28 days of biopsy | |
| Yes | 12 (60%) |
| No | 8 (40%) |
| Successful NGS of metastatic biopsy (N) | 20 |
| Potentially actionable mutation identified (N) | 15 |
| Tumor board recommended targeted therapy as possible next treatment (N) | 13 |
| Received targeted therapy as next treatment (4)* | 4 |
A patient with an AR+ tumor received bicalutamide on study, a patient with a BAP1 mutation received carboplatin/PARP inhibitor on study, a patient with a MAP2K1 amplification received trametinib off study, and a patient with an ERBB2 mutation received trastuzumab off study.
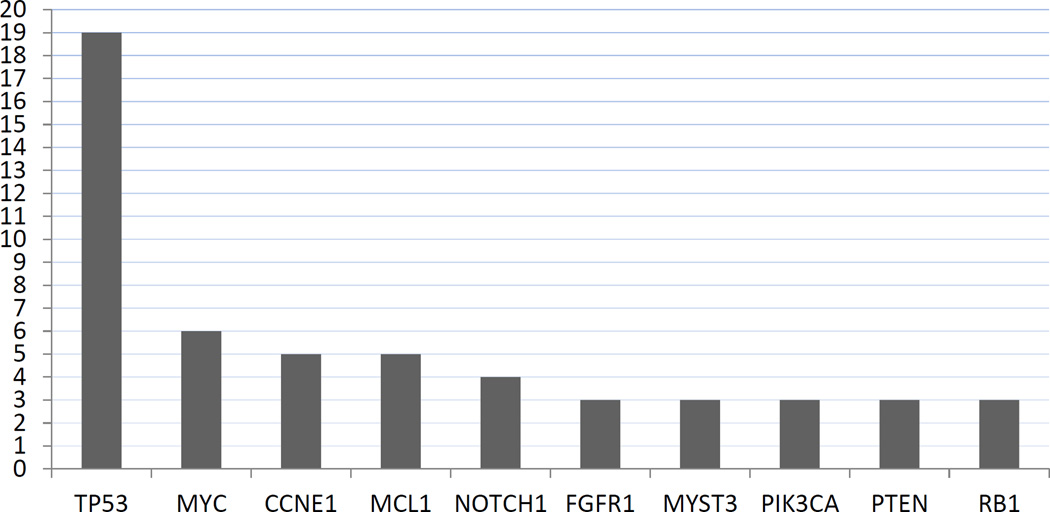
All 20 patients who underwent NGS of a metastatic site had at least one identifiable somatic alteration (Figure 1, Figure S1, Table S1). Members of the GAITWAY tumor board reviewed the clinical data, family history and genomic profile for each of the 20 participants and determined that 15 had at least one potentially actionable alteration. The board recommended targeted treatment as a possible next line of therapy in 13 patients. The recommendations were reported to the treating physician, who ultimately decided with the patient on the next administered therapy. Three patients went on to receive a NGS-identified targeted therapy as their next treatment (Table 2). One patient received AR-directed treatment as her next line of therapy (Table 2). The primary reasons nine patients did not receive targeted treatment were lack of geographically accessible or available clinical trials, deterioration of patient’s performance status precluding enrollment on recommended studies, and initiating another therapy prior to receiving the GAITWAY tumor board recommendations.
Figure 1. Most Frequent Somatic Genomic Alterations in Metastatic Biopsies of 20 Triple Negative Breast Cancers.
Shown are the frequencies of genetic alterations identified by NGS in the 20 metastatic lesions from triple negative breast cancer patients. Alterations include point mutations and copy number alterations.
The GAITWAY tumor board regarded AR-expression as a potential therapeutic target, based on previously published work showing the AR antagonist bicalutamide has clinical activity in AR-expressing breast cancer (19). Five out of twenty-one (23.8%) evaluable patients had AR-expressing tumors, and AR-directed treatment was recommended as a possible next line of therapy in four patients. All four of these patients had additional targetable alterations identified by NGS (Table 2).
Four patients in the study received treatment consistent with GAITWAY tumor board recommendations. Patient 1–005 had AR+ TNBC, and was treated with single-agent bicalutamide as an off-label use. Bicalutamide is a non-steroidal anti-androgen with evidence of activity in ER-/PR-/AR+ breast cancer (19). After two weeks of treatment she had progression of disease in the skin and the drug was stopped. Patient 1–017 had a BAP1 E498fs*38 mutation and BAP1 loss in her tumor. Bap1 is a BRCA1-associated tumor suppressor protein. A clinical trial of a PARP-inhibitor was recommended as a possible next step in treatment, as PARP-inhibitors have demonstrated efficacy in patients with BRCA mutations (20). She was treated with a PARP-inhibitor in combination with carboplatin. After 2 cycles of treatment she had stable disease, with eventual disease progression after 4 cycles. Patient 1–021 had a MAP2K1 amplification in her tumor and was recommended a clinical trial with a MEK inhibitor as a possible next therapeutic choice. Trametinib is one such oral tyrosine kinase inhibitor approved to treat BRAF V600E or V600K mutated melanoma in combination with dabrafenib. Her treating oncologist then started off-label, single-agent trametinib and documented clinical response in the breast and clavicle. After several weeks of treatment she had disease progression. Finally, patient 1–023 had two known ERBB2 kinase domain mutations in her tumor (D769H and V777L) that in pre-clinical models confer sensitivity to HER2-directed treatment (21). Off-label HER2-directed treatment was recommended as a potential next treatment for her. She received trastuzumab alone for 10 months and then had progression of disease. Capecitabine was then added to trastuzumab, with stable disease for 9 months.
Members of the GAITWAY tumor board also considered whether particular genetic alterations were suggestive of potential germline mutations, for example, BRCA1, BRCA2 or TP53 mutations. In eight (40%) patients genetic counseling was recommended to further investigate family history and potential need for formal germline genetic testing.
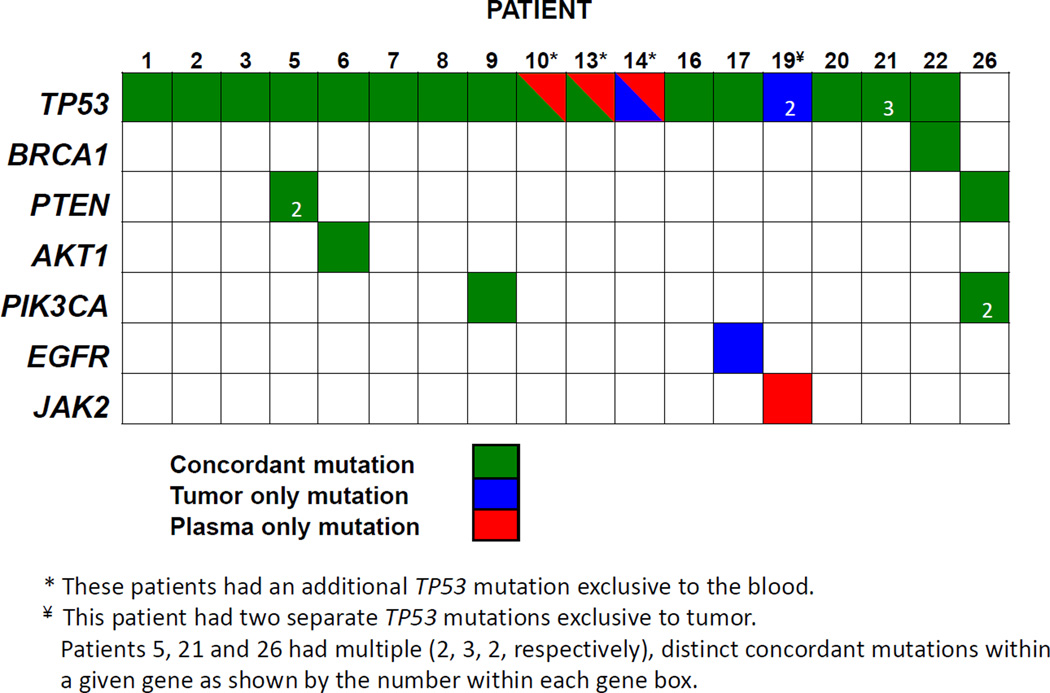
We successfully collected baseline blood samples from all 26 patients. Two of 26 baseline patient samples had insufficient cell-free DNA (cfDNA) for NGS, yielding inadequate coverage for accurate mutation identification. NGS of baseline ptDNA samples was successfully performed and detected at least one mutation in all remaining 24 patients, including six patients who did not have successful NGS of tumor tissue (Table 3). For patients who had NGS analysis of both tumor and blood (N=18) (Figure 2), 23 of 33 mutations (70%) were concordant. A total of 5 patients had discordant results between tumors and tissues. In 3 patients (patients 14, 17 and 19), 4 mutations found in tumor tissues were not identified in the blood including two separate TP53 mutations found only in the metastatic biopsy for patient 19. Conversely, 4 mutations found in blood were not found in corresponding tumor tissues in 4 patients (patients 10, 13, 14 and 19), though none were deemed targets for therapy. Interestingly patients 14 and 19 did not have any mutations found in both tissue and blood.
Table 3.
Sequencing results of ptDNA
| Patient ID | Time Point | Gene | Alteration (AA) |
ptDNA- Allelic Frequency (%) |
|---|---|---|---|---|
| 1 | 0m | TP53 | R342* | 20.1 |
| 1 | fu1 | TP53 | R342* | 48.0 |
| 2 | 0m | TP53 | Y163C | 31.66 |
| 2 | fu1 | TP53 | Y163C | 7.32 |
| 2 | fu2 | TP53 | Y163C | 41.95 |
| 3 | 0m | TP53 | M66fs*57 | 33.93 |
| 3 | fu1 | TP53 | M66fs*57 | 0.15 |
| 3 | fu2 | TP53 | M66fs*57 | 7.50 |
| 4 | 0m | TP53 | L206* | 1.64 |
| 4 | 0m | BRCA1 | E1849* | 1.21 |
| 5 | 0m | TP53 | R209fs*6 | 0.63 |
| 5 | 0m | PTEN | Y188fs*2 | 0.08 |
| 5 | 0m | PTEN | T319fs*6 | 0.10 |
| 6 | 0m | TP53 | R175H | 16.96 |
| 6 | 0m | AKT1 | E17K | 8.27 |
| 7 | 0m | TP53 | R248Q | 1.27 |
| 8 | 0m | TP53 | Y234C | 47.05 |
| 9 | 0m | TP53 | P219fs*2 | 0.076 |
| 9 | 0m | PIK3CA | V344G | 20.12 |
| 10 | 0m | TP53 | E294fs*51 | 0.29 |
| 10 | fu1 | TP53 | E294fs*51 | 0.39 |
| 10 | fu2 | TP53 | E294fs*51 | 0.20 |
| 10 | 0m | TP53 | R249W | 3.24 |
| 10 | fu1 | TP53 | R249W | 0.71 |
| 10 | fu2 | TP53 | R249W | 1.08 |
| 11 | 0m | TP53 | R110P | 2.02 |
| 12 | 0m | TP53 | I195T | 59.28 |
| 13 | 0m | TP53 | R273L | 2.44 |
| 13 | fu1 | TP53 | R273L | 1.86 |
| 13 | 0m | TP53 | Y163C | 0.6 |
| 13 | fu1 | TP53 | Y163C | 10.22 |
| 14 | 0m | TP53 | R213fs*34 | 0.21 |
| 15 | 0m | TP53 | R248Q | 27.98 |
| 15 | 0m | TP53 | splice site 376-1G>A |
0.65 |
| 16 | 0m | TP53 | Y234del | 34.06 |
| 17 | 0m | TP53 | A276G | 10.51 |
| 17 | fu | TP53 | A276G | 24.48 |
| 17 | fu | BRCA2 | S2001C | 0.79 |
| 18 | 0m | TP53 | R213* | 15.38 |
| 19 | 0m | JAK2 | V617F | 1.31 |
| 20 | 0m | TP53 | E221fs*4 | 0.1077 |
| 21 | 0m | TP53 | T253N | 25.17 |
| 21 | 0m | TP53 | N247K | 25.15 |
| 21 | 0m | TP53 | R175H | 0.73 |
| 22 | 0m | TP53 | P151R | 1.65 |
| 22 | 0m | BRCA1 | E23fs*17 | 30.00 |
| 24 | 0m | NF1 | Q83* | 2.97 |
| 24 | fu | NF1 | Q83* | 23.97 |
| 24 | 0m | CDH1 | S111fs*6 | 18.95 |
| 24 | fu | CDH1 | S111fs*6 | 27.21 |
| 24 | 0m | PIK3CA | H1047R | 18.12 |
| 24 | fu | PIK3CA | H1047R | 25.58 |
| 26 | 0m | PTEN | C296fs*12 | 0.101 |
| 26 | 0m | PIK3CA | M1043I | 0.081 |
| 26 | 0m | PIK3CA | N1044K | 0.082 |
Allelic frequency is the percentage of mutant DNA relative to total DNA as assessed by NGS
Figure 2. Plasma and tumor mutation concordance.
Shown are concordance between mutations found in tumor tissue and mutations found in a paired blood samples. Copy number alterations were not performed on blood samples and were therefore not analyzed. Concordance between tissue and blood for a given mutation is represented in green, while mutations found exclusively in tissue or blood are represented as blue and red, respectively. Patients 5, 21 and 26 had multiple distinct mutations within a given gene with the number of mutations shown for each gene (2 for PTEN, 3 for TP53, 2 for PIK3CA, respectively). Three patients had additional, separate TP53 mutations found only in blood (*), while one patient had two separate TP53 mutations found only in tumor (¥).
In addition to baseline blood samples, we obtained 12 follow up blood samples from nine patients every 3–4 months for analysis. Two of nine initial follow up samples (FU1) were unevaluable due to insufficient input cfDNA. In seven of seven (100%) evaluable FU1 samples and in three of three (100%) subsequent follow up samples (FU2), NGS identified all mutations found in baseline samples. In the patient unable to undergo NGS tissue analysis with follow up blood collected (patient 24), NGS identified three of three mutations seen at a baseline blood sample. In one of seven patients, NGS identified a new mutation at FU1 not seen at baseline or in the tumor sample (patient 17).
In general, patients with follow up blood samples displayed changing allelic fractions of ptDNA for any given mutation (Table 3), reflective of disease progression and in some cases response to therapy. Notably for patient 3, who had brain and other organ metastases, an initial baseline TP53 mutation was detected in both tumor and plasma, with an allelic frequency in blood of 33%. In fact, this percentage was high enough that we tested her germline DNA and confirmed that this was indeed a somatic mutation. The ptDNA sample at baseline was taken prior to removal of a metastatic brain lesion, and the brain lesion itself was used for NGS on this study. A subsequent follow up blood sample demonstrated a remarkably lower allelic fraction (0.15%) of mutant TP53. Ultimately the patient progressed systemically and succumbed to her disease, and blood samples obtained at the time of progression demonstrated an increase in mutant TP53 ptDNA (7.5%).
DISCUSSION
In this prospective study of patients with advanced TNBC, we obtained a new biopsy of metastatic disease, performed genomic analysis and clinical interpretation, and returned information to the referring physician and patient. Our goal was to complete these steps within a clinically relevant time frame of 28 days, but the study was terminated after meeting pre-specified criteria for futility. NGS in tissue could not be performed in 8 of 20 (40%) patients within 28 days due to sampling issues, including difficulty accessing archival tumor tissue, and need for repeat submission of tumor tissue due to inadequate initial sample. In contrast to NGS of tissue samples, we successfully obtained and performed NGS on plasma samples in 24 of 26 patients, and were able to identify mutations in all 24 patients’ ptDNA. Because levels of plasma DNA are variable, we found that there were inadequate quantities of cfDNA for NGS in two of 26 patients, using ~1.5 ml of plasma. Although we now obtain additional blood tubes to mitigate this issue, in the current study plasma exhaustion accounted for the inability to sequence these 2 patient samples. Our results demonstrate the clinical challenges of obtaining prospective tissue biopsies for sequencing in patients with metastatic triple negative breast cancers within 28 days, while also highlighting the potential use of blood as a more time efficient method of cancer gene sequencing.
Our study emphasizes several pragmatic limitations regarding the ability to use NGS of tumor tissue for clinical management, including obtaining a recent or new biopsy for analysis. Although other studies have used archival specimens for NGS, it is now well established that tumor heterogeneity and clonal evolution after therapy can alter the genomic landscape of metastatic lesions. For example, recent findings suggest that ESR1 (estrogen receptor-alpha) ligand binding domain mutations occur predominantly, if not exclusively, in metastatic breast cancer patients after endocrine therapies (22–24), and that ptDNA can detect additional mutations not found in tissue biopsies (13, 25, 26). Thus, for molecular tumor profiling, a new biopsy prior to initiating a new therapy would in theory yield the most relevant information to guide patient care. But obtaining tissue sufficient for NGS in patients with metastatic TNBC in a timely way was difficult. Six patients out of 26 could not undergo NGS tumor testing due to either inaccessible tissue (N=5) or insufficient tissue for analysis (N=1). It is important to note that these six patients had already consented to this study, indicating that their treating physicians believed they had accessible tumors amenable to biopsy. Biopsy also added significant delay, as it requires scheduling with a specialized team. We purposefully chose to test our feasibility hypothesis in patients with TNBC since their rapidly progressive disease and limited standard of care treatment options represent an especially challenging group of patients for benchmarking timed molecular analyses. Moreover, a 28-day timeframe was chosen, as this is often a required “washout” period in clinical trials of new therapies. However, our results demonstrate the real world challenges in performing such studies, including technical difficulties of obtaining tissue, DNA requirements for NGS, and the need for patients to start therapy if results are not delivered in a relevant clinical timeframe.
Currently, tumor NGS is used most often to identify genomic alterations that may provide rationale for targeted therapies. This feature depends on the frequency of actionable mutations, which varies widely depending on tumor type, and changes over time as new drugs come under investigation and become commercially available. For example, despite the TP53 gene being altered in 95% of patients in this study, our GAITWAY tumor board did not consider these mutations targetable. If future therapies that effectively target mutant p53 are successfully developed, this would greatly impact the use of NGS for TNBC patients. Of the 20 patients with successful NGS, only four (20%) went on to receive a targeted treatment guided by NGS, although 15 had GAITWAY-determined targetable aberrations. For the majority of those patients with targetable alterations, the only directed treatments available were via clinical studies. In most cases these studies were not available locally. This issue of study availability is consistent with findings at other academic centers, and will require additional resources and careful study designs to accommodate numerous, low frequency mutations (3, 27, 28).
At the same time, we were able to successfully obtain and perform NGS on ptDNA from the majority of participants (24 of 26). Beyond the ease of obtaining blood versus tissue, liquid biopsies may have other advantages over biopsy of a single metastatic site. For example, inter- and intra-tumor heterogeneity is an important consideration when using tissue biopsy specimens as the source of genomic testing (29). Emerging evidence suggests that ptDNA represents mutational burden from disparate clonal populations in patients with metastatic disease (13, 30) due to blood acting as a reservoir for all metastatic sites shedding ptDNA into the circulation. In accord with this notion, our analysis demonstrates that additional mutations were detected in ptDNA that were not found in the tumor biopsy. For example, three patients had a second TP53 mutation found exclusively in blood, possibly reflecting mutations from other metastatic sites. Interestingly, in one patient (patient 19) with two TP53 mutations found only in the tumor, a JAK2 V617F mutation was seen only in the blood at relatively low allelic frequency. Since JAK2 V617F mutations are almost exclusively found in hematologic diseases (e.g. polycythemia vera), there is a possibility that that this ptDNA mutation may reflect an undiagnosed hematologic disorder. Importantly, ruxolitinib is an approved JAK inhibitor for the treatment of JAK2 V617F hematologic diseases. Further verification of the origin of this patient’s JAK2 mutation is ongoing.
For the majority of patients, the same mutations were identified in tumor and blood (23/33, 70%) if both were successfully sequenced. Although we previously reported a higher degree of mutational concordance when tissue and blood were obtained concurrently (9), there were important differences in the current study, notably, the use of NGS for ptDNA detection, rather than digital PCR. It is known that allelic frequencies in ptDNA are often extremely low, and the current sensitivity of NGS may not allow for detection of mutations in plasma that are derived from low-level subclonal variants present in a metastatic site. Newer technologies and bioinformatics pipelines may enable improved sensitivity for future NGS studies. Another limitation of our ptDNA analysis is the inability to assess copy number changes in blood. During this study period, copy number analysis of ptDNA was still in development and therefore could not be reported. Finally, NGS generally requires a higher amount of input cfDNA compared to digital PCR. We had inadequate DNA quantity to obtain enough NGS coverage to confidently assess mutations in ptDNA for two of 26 patients. Future studies can avoid this limitation by obtaining additional tubes for each blood draw.
The ease of blood sampling also enables tracking mutational evolution of metastatic disease over time along with response to therapies. We collected serial measurements in 9 patients and our data do indeed demonstrate that mutations can be monitored qualitatively and quantitatively in a serial fashion, supporting results by others that serial NGS of ptDNA is feasible (9–16). Small studies in breast and other cancers have shown that a change in ptDNA can predict for change in disease status in advance of clinical or imaging-based evidence (11, 16, 31, 32). Our study also suggests ptDNA can be used to track response to therapies, including neurosurgical removal of brain metastases as describe above. It will be important to confirm whether ptDNA can be used as a way to monitor disease burden and response to therapies, and determine if changing therapies in patients who do not have a decrease in ptDNA after starting treatment improves clinical outcomes.
In summary, we have shown the clinical challenges of using new metastatic biopsies for molecular profiling of actionable mutations within a 28-day timeframe. This study highlights the need for easier, quicker mutational profiling methods in patients with metastatic TNBC, and suggests NGS of blood as a potential alternative to tissue. We envision that NGS of ptDNA could be used to identify more patients than tissue biopsy for enrollment onto clinical trials. Larger studies investigating the utility of NGS of ptDNA in tracking metastatic disease and predicting for response to treatment are needed.
Supplementary Material
Statement of Translational Relevance.
Molecular profiling of tumors engenders hope that specific molecular alterations can be matched with targeted therapies. Due to tumor heterogeneity, one would prefer to obtain the mutational status of a new tissue biopsy for informing decisions regarding next line therapy. We prospectively enrolled patients with metastatic triple negative breast cancer (TNBC) and obtained a recent or new biopsy, and subjected this to next generation sequencing (NGS) with FoundationOne™. Our goal was to perform molecular profiling on a recent biopsy and convey recommendations back to the referring physician in 28 days or less. We met our interim analysis endpoint for futility, and the study was stopped after 26 patients were enrolled. However, we also showed that NGS of plasma DNA had high concordance with mutations found in tissue biopsies, and allowed for subsequent follow up/monitoring using blood. These results demonstrate the potential of “liquid biopsies” for mutational profiling and serial monitoring.
Acknowledgments
Conflict of Interest Disclosures: Dr. Park is a paid member of the scientific advisory boards of Horizon Discovery, LTD and Loxo Oncology and has ownership interest in Loxo Oncology. Under separate licensing agreements between Horizon Discovery, LTD and The Johns Hopkins University, Dr. Park and Dr. Lauring are entitled to a share of royalties received by the University on sales of products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Stearns received research funding from Abbvie, Celgene, Medimmune, Merck, Novartis, Pfizer, and PUMA. Dr. Connolly received research funding from Novartis, Genentech, Merrimack, Clovis, and PUMA. Dr. Wolff received research funding from Myriad. Drs. Ali, Clark, Kennedy, Lipson, Miller, Otto, Ross, and Stephens and Ms. Young are employees of Foundation Medicine.
Funding/Support: This work was supported by The Conquer Cancer Foundation’s Young Investigator Award, The Pearl M. Stetler Research Fund, NIH (CA088843, GM007309, CA168180, CA167939), The Avon Foundation, Commonwealth Foundation, the Santa Fe Foundation, The Canney Foundation, Cigarette Restitution Fund, The Cindy Rosencrans Fund for Triple Negative Breast Cancer Research, and the Marcie and Ellen Foundation. Foundation Medicine analyzed samples free of charge.
Role of the Funder/Sponsor: None of the funding sources influenced the design, interpretation or submission of this manuscript.
Footnotes
Author Contributions: Drs. Parsons and Park had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conception and design: HAP, JAB, GLR, JLauring, BHP, VS
Development of methodology: HAP, JAB, TC, DL, MK, DJZ, JLee, SC, DC, RLC
Acquisition of data: HAP, JAB, ACM, JZ, SS, TC, DL, SMA, MK, SJ, DAV, CG, JA, JLee, AW, RC, BHP, VS
Analysis and interpretation of data: All authors
Writing, review, and/or revision of the manuscript: All authors
References
- 1.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meric-Bernstam F, Brusco L, Shaw K, Horombe C, Kopetz S, Davies MA, et al. Feasibility of Large-Scale Genomic Testing to Facilitate Enrollment Onto Genomically Matched Clinical Trials. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.60.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtaza M, Dawson SJ, Pogrebniak K, Rueda OM, Provenzano E, Grant J, et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat Commun. 2015;6:8760. doi: 10.1038/ncomms9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao ZM, Zhao B, Bai Y, Iamarino A, Gaffney SG, Schlessinger J, et al. Early and multiple origins of metastatic lineages within primary tumors. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:2140–2145. doi: 10.1073/pnas.1525677113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nature medicine. 2016 doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz LA, Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins MJ, Jelovac D, Barnathan E, Blair B, Slater S, Powers P, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3462–3469. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaver JA, Jelovac D, Balukrishna S, Cochran RL, Croessmann S, Zabransky DJ, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2643–2650. doi: 10.1158/1078-0432.CCR-13-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. The New England journal of medicine. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 13.Chu D, Paoletti C, Gersch C, VanDenBerg D, Zabransky D, Cochran R, et al. ESR1 mutations in circulating plasma tumor DNA from metastatic breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature medicine. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Science translational medicine. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stearns V, Park BH. Gene Mutation Profiling of Breast Cancers for Clinical Decision Making: Drivers and Passengers in the Cart Before the Horse. JAMA Oncol. 2015;1:569–570. doi: 10.1001/jamaoncol.2015.0761. [DOI] [PubMed] [Google Scholar]
- 19.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 21.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer discovery. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 26.Spoerke JM, Gendreau S, Walter K, Qiu J, Wilson TR, Savage H, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nature communications. 2016;7:11579. doi: 10.1038/ncomms11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartnik NJ, Roberts J, Scott, Gornick MC, Le LQ, Chinnaiyan AM. Are oncologists using genome sequencing results to inform patient care? Journal of Clinical Oncology. 2015;(suppl):e12525. abstr. [Google Scholar]
- 28.André F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER) The Lancet Oncology. 2014;15:267–274. doi: 10.1016/S1470-2045(13)70611-9. [DOI] [PubMed] [Google Scholar]
- 29.Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Research. 2014;16:210. doi: 10.1186/bcr3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer discovery. 2015 doi: 10.1158/2159-8290.CD-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Science translational medicine. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 32.Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26:1715–1722. doi: 10.1093/annonc/mdv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.