Abstract
Background
Conflicting results have been obtained regarding roles of Fc receptors and effector cells in models of active systemic anaphylaxis (ASA). In part, this might reflect the choice of adjuvant used during sensitization, as various adjuvants might differentially influence the production of particular antibody isotypes.
Objective
We developed an ‘adjuvant-free’ mouse model of ASA and assessed the contributions of components of the ‘classical’ and ‘alternative’ pathways in this model.
Methods
Mice were sensitized intra-peritoneally (i.p.) with ovalbumin at weekly intervals for 6 weeks and challenged i.p. with ovalbumin two weeks later.
Results
Wild-type animals developed immediate hypothermia and late-phase intra-peritoneal inflammation in this model. These features were reduced in mice lacking the IgE receptor FcεRI, the IgG receptor FcγRIII or the common γ-chain FcRγ. FcγRIV blockade resulted in a partial reduction of inflammation without any effect on hypothermia. Depletion of monocytes/macrophages with clodronate liposomes significantly reduced the hypothermia response. By contrast, depletion of neutrophils or basophils had no significant effects in this ASA model. Both the hypothermia and inflammation were dependent on platelet-activating factor (PAF) and histamine and were reduced in two types of mast cell (MC)-deficient mice. Finally, engraftment of MC-deficient mice with bone marrow-derived cultured MCs significantly exacerbated the hypothermia response, and restored inflammation to levels similar to those observed in wild-type mice.
Conclusion
Components of the classical and alternative pathways contribute to anaphylaxis in this adjuvant-free model, with key roles for mast cells and monocytes/macrophages.
Keywords: Rodents, Mouse model, Mast Cells/Basophils, Monocytes/Macrophages, Neutrophils, Antibodies, Fc Receptors, Allergy, Inflammation, Anaphylaxis
Capsule summary
FcεRI, FcγRIII, mast cells, histamine, and PAF are required for full development of hypothermia and numbers of infiltrating leukocytes in an adjuvant-free ASA model. Monocytes/macrophages also contribute to hypothermia in this model.
Introduction
Anaphylaxis is an acute, life-threatening systemic allergic reaction with a lifetime prevalence of 0.05% to 2.0% in developed countries1–3. In humans, it is largely accepted that anaphylaxis can be triggered by histamine and other mediators released in response to antigen cross-linking of IgE bound to its high-affinity receptor, FcεRI, on mast cells (MCs)1–3. However, IgG might also contribute to anaphylaxis in some patients, especially those treated with infused drugs such as therapeutic monoclonal antibodies4, 5.
Several mouse models of anaphylaxis have been developed and used to assess the contributions of IgE and IgG antibodies, and the roles of various effector cells and mediators. The analysis of passive local or systemic anaphylaxis (PSA) models has allowed identification of two major pathways of anaphylaxis in mice: a ‘classical’ pathway consisting of IgE, FcεRI, MCs and histamine6–8; and an ‘alternative’ pathway consisting of IgG, FcγRIII and/or FcγRIV5, 9–11, platelet-activating factor (PAF)12, 13 and, depending on the exact model used, either macrophages5, 14, basophils5, 13 and/or neutrophils5, 11, 15.
Active systemic anaphylaxis (ASA) models, which are arguably more reflective of the clinical situation, have generated more conflicting results. Some ASA models critically depend on IgE, FcεRI and MCs16–19, while some others can develop, at least with respect to the features analyzed, in IgE−/−, FcεRI−/− and/or MC-deficient mice9, 13, 20. Depending on the mouse strain and ASA model used, basophils have been shown either to contribute to the systemic response13, 19 or to play little to no significant role17, 21. Similarly, depletion of monocytes/macrophages or antibody-mediated neutrophil depletion reduces anaphylaxis in some but not all ASA models11, 17, 19, 21.
We hypothesize that such conflicting results in ASA models might reflect, at least in part, the choice of adjuvant used during sensitization, as various adjuvants might differentially influence the production of individual antibody isotypes. Moreover, in humans who develop anaphylactic reactivity, sensitization to antigen generally occurs in the absence of an artificial adjuvant. We therefore developed an ‘adjuvant-free’ mouse model of ASA and assessed the contributions of components of the ‘classical’ and ‘alternative’ pathways of anaphylaxis in this model.
Methods
Mice
C57BL/6J (WT) mice were purchased from Jackson Laboratories (Bar Harbor, Me) or Charles River (France). FcγRIII−/− mice (B6.129P2-Fcgr3tm1Sjv/J mice, backcrossed to C57BL/6 for 12 generations) were purchased from Jackson Laboratories. FcRγ−/− mice (B6.129P2 Fcer1gtm1Rav mice backcrossed to C57BL/6 for 12 generations) were from Taconic (New York, NY). C57BL/6-KitW-sh/W-sh mice were originally provided by Peter Besmer (Memorial Sloan-Kettering Cancer Center, NY, USA); we then backcrossed these mice to C57BL/6J mice for more than 11 generations22. FcεRI−/− mice7 (FcεRI alpha chain-deficient mice backcrossed to C57BL/6 for more than 8 generations and kindly provided by Jean-Pierre Kinet, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA), Mcpt8DTR mice (backcrossed to C57BL/6 for at least 10 generations)23, and Cpa3-Cre; Mcl-1fl/fl mice (backcrossed to C57BL/6 for at least 9 generations)24 were bred and maintained at the Stanford University Research Animal Facility. Aged-matched male mice were used in all experiments. Experiments in Fig 2 used C57BL/6J WT mice as controls. We used littermate controls in all other experiments. All animal care and experimentation was conducted in compliance with the guidelines of the National Institutes of Health and with the specific approval of the Institutional Animal Care and Use Committee of Stanford University and of the Animal Ethics committee CETEA (Institut Pasteur, Paris, France) registered under #C2EA-89.
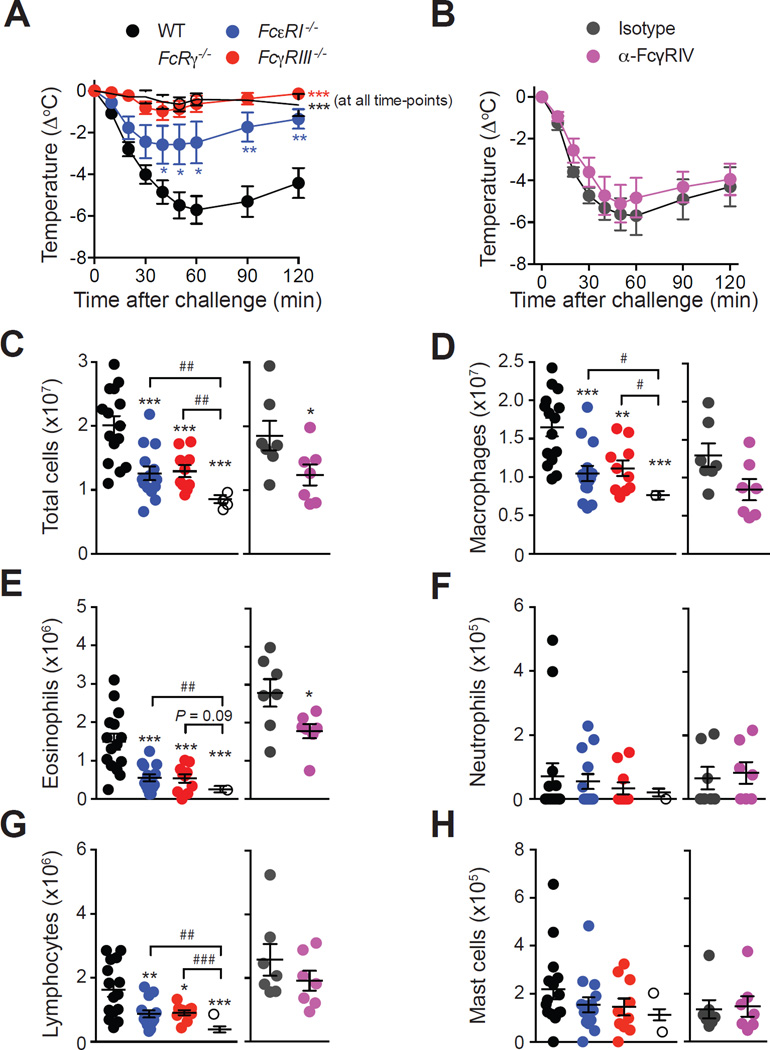
Figure 2. Roles of Fc receptors in OVA-induced ASA.
(A–B) OVA-induced hypothermia in OVA-sensitized WT, FcεRI−/−, FcγRIII−/− and FcRγ−/− mice (A), or WT mice treated with an anti-FcγRIV antibody or an isotype control (B). (C–H) Numbers of leukocytes in the PLF 3 days after challenge. Data are pooled from two (‘Isotype’ and ‘α-FcγRIV’; n=7/group) or three (all other groups; n=9–15/group) independent experiments. *, ** or *** = P < 0.05, 0.01 or 0.001.
OVA-induced adjuvant-free model of active anaphylaxis
Six to 8-week-old mice were sensitized intraperitoneally (i.p.) with 10 µg of endotoxin-free ovalbumin (Endograde OVA; BioVendor; < 0.01 EU endotoxin per injection) in 100 µL of PBS once a week for 6 weeks. Two weeks after the last i.p. sensitization, mice were challenged i.p. with 500 µg of OVA. Rectal measurements of body temperature were performed immediately before (time 0) and at different time points for up to 2 h after challenge. Mice were sacrificed at various time points after challenge (as indicated) for assessment of inflammatory cell numbers in the peritoneal cavity and histology.
Other methods
Please see this article’s Online Repository at www.jacionline.org for the methods for flow cytometry, peritoneal lavage and differential cell counts, depletion of basophils, monocytes/macrophages and neutrophils, blockade of FcγRIV, histologic analysis, measurement of serum OVA-specific IgG1 and IgG2c antibodies, IgE-mediated PSA, ASA with adjuvant, treatment with an H1 anti-histamine, a PAF receptor antagonist, quantification of histamine and PAF-AH, and generation and adoptive transfer of bone marrow-derived cultured mast cells (BMCMCs).
Statistical analyses
Results represent mean ± SEM or mean + SEM, with values for individual mice represented for quantifications of histamine, PAF-AH and leukocytes. We used an unpaired Student’s t test (body temperature) or an unpaired Mann-Whitney U test (all other data) to assess the significance of differences between two sets of data. P values < 0.05 are considered statistically significant.
Results
Development of an ‘adjuvant-free’ model of ASA in C57BL/6 mice
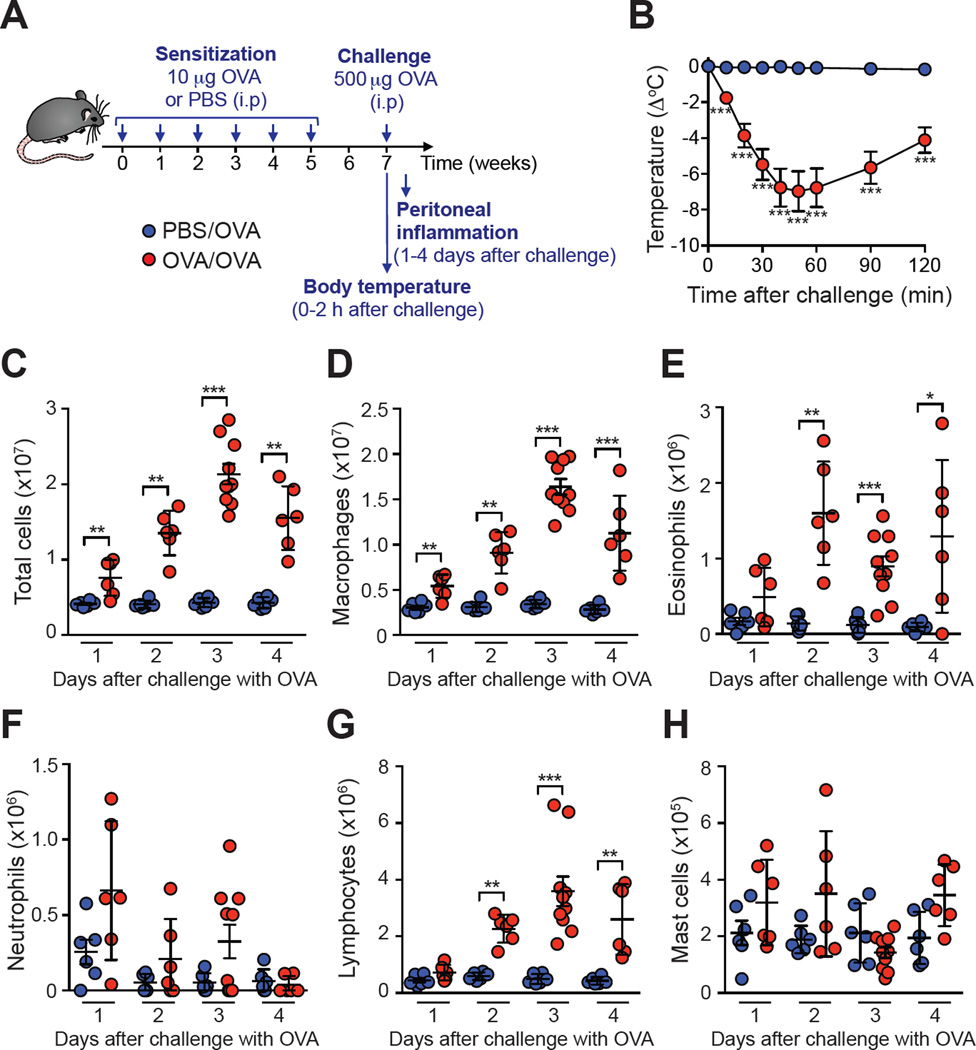
We developed an ‘adjuvant-free’ mouse model of ASA consisting of performing i.p. sensitizations with endotoxin-free OVA once a week for 6 weeks, and i.p. challenge with OVA two weeks after the last sensitization (Fig 1, A). OVA-sensitized C57BL/6 mice developed hypothermia following OVA challenge that was maximal at 30 min and decreased thereafter (Fig 1, B). Sensitized and challenged mice also developed a ‘late-phase’ inflammatory response with increased numbers of total cells, eosinophils, macrophages, and lymphocytes in the peritoneal cavity, with highest numbers of leukocytes on day 3 after challenge (Fig 1, C-H). Consistent with the increase in eosinophil numbers, we detected significant amounts of IL-5 in the plasma of 3 out of 5 OVA-sensitized mice 18 h (but not 72 h) after challenge, while levels of IL-4, IL-6, IL-10, TNF-α and IFN-γ were all below the detection limit of standard ELISAs at both time points (data not shown).
Figure 1. Development of hypothermia and intra-peritoneal inflammation in mice sensitized and challenged with OVA in the absence of adjuvant.
(A) Experimental outline. (B) Changes in body temperature following challenge with OVA in OVA-sensitized (‘OVA/OVA’) or PBS-treated (‘PBS/OVA’) mice. (C–H) Numbers of leukocytes in the peritoneal lavage fluid (PLF) at the indicated time points. Data are pooled from two or three independent experiments (n=6–10/group). *, ** or *** = P < 0.05, 0.01 or 0.001.
OVA-induced hypothermia and inflammation depend on the high-affinity IgE receptor FcεRI, the IgG receptor FcγRIII and their common activating subunit FcRγ
OVA sensitization induced significant elevation of OVA-specific IgG1 and IgG2c antibodies in C57BL/6 mice (see Fig E1, A & B in the Online Repository). We did not detect significant levels of OVA-specific IgE in sera by standard ELISA (data not shown). However, we observed degranulation of peritoneal cell-derived MCs (PCMCs) incubated in vitro with serum from OVA-sensitized mice followed by stimulation with OVA (see Fig E1, C & D in the Online Repository). Such degranulation was not observed with PCMCs incubated with serum from PBS-treated mice, or with serum from OVA-sensitized mice which had been pre-incubated with anti-IgE antibodies (see Fig E1, C & D in the Online Repository), demonstrating the presence of functional OVA-specific IgE in the serum of sensitized mice.
We then assessed responses of C57BL/6 mice lacking the IgG receptor FcγRIII, the high-affinity IgE receptor FcεRI, or their common activating subunit FcRγ. OVA-induced hypothermia was abolished in FcRγ−/− mice and FcγRIII−/− mice, and partially reduced in FcεRI−/− mice, as compared to WT mice (Fig 2, A). Antibody-mediated blockade of FcγRIV had no effect on immediate hypothermia (Fig 2, B). WT, FcεRI−/− and FcγRIII−/− mice had similar levels of intra-peritoneal leukocytes at baseline (except for a small increase in macrophages and lymphocytes in FcγRIII−/− mice) (see Fig E2 in the Online Repository). However, we observed decreased numbers of total cells, macrophages, eosinophils, and lymphocytes in the peritoneal cavity of FcεRI−/− and FcγRIII−/− mice, as compared to WT mice 3 days after challenge (Fig 2, C-G). These numbers were further decreased in mice lacking the common FcRγ (Fig 2, C-G). Antibody-mediated blockade of FcγRIV induced only a moderate decrease of peritoneal leukocyte numbers at day 3, with reduced numbers of total cells and eosinophils as compared with mice treated with an isotype control antibody (Fig 2, C-G).
Monocyte/Macrophage depletion with clodronate liposomes decreases immediate hypothermia and enhances late-phase intra-peritoneal inflammation
We injected OVA-sensitized mice with clodronate liposomes (or PBS liposomes, as a control) 24 h before challenge with OVA to deplete monocytes/macrophages and assess their potential contribution to anaphylaxis via the alternative pathway. Clodronate liposome-treated mice exhibited depletion of circulating CD11bhigh monocytes at the time of OVA challenge, with no effect on blood Ly6G+ neutrophils (see Fig E3, A, C & D in the Online Repository). OVA challenge led to a decrease in CD11b expression in blood monocytes in PBS liposome-treated mice at day 3 (see Fig E3, A in the Online Repository). Consistent with a previous report25, we found similar percentages of blood monocytes in both PBS liposome- and clodronate liposome-treated mice at day 3 (i.e. 4 days after the injection of liposomes) (see Fig E3, A, D & E in the Online Repository). However, F4/80+ macrophages were depleted in the spleen of clodronate liposome-treated mice at this time point, with no effect on neutrophils (see Fig E3, B, F & G in the Online Repository). Confirming the efficiency of depletion, we also found that levels of peritoneal macrophages were reduced by 73% in clodronate liposome-treated mice as compared to control mice at day 3 (Fig 3, E).
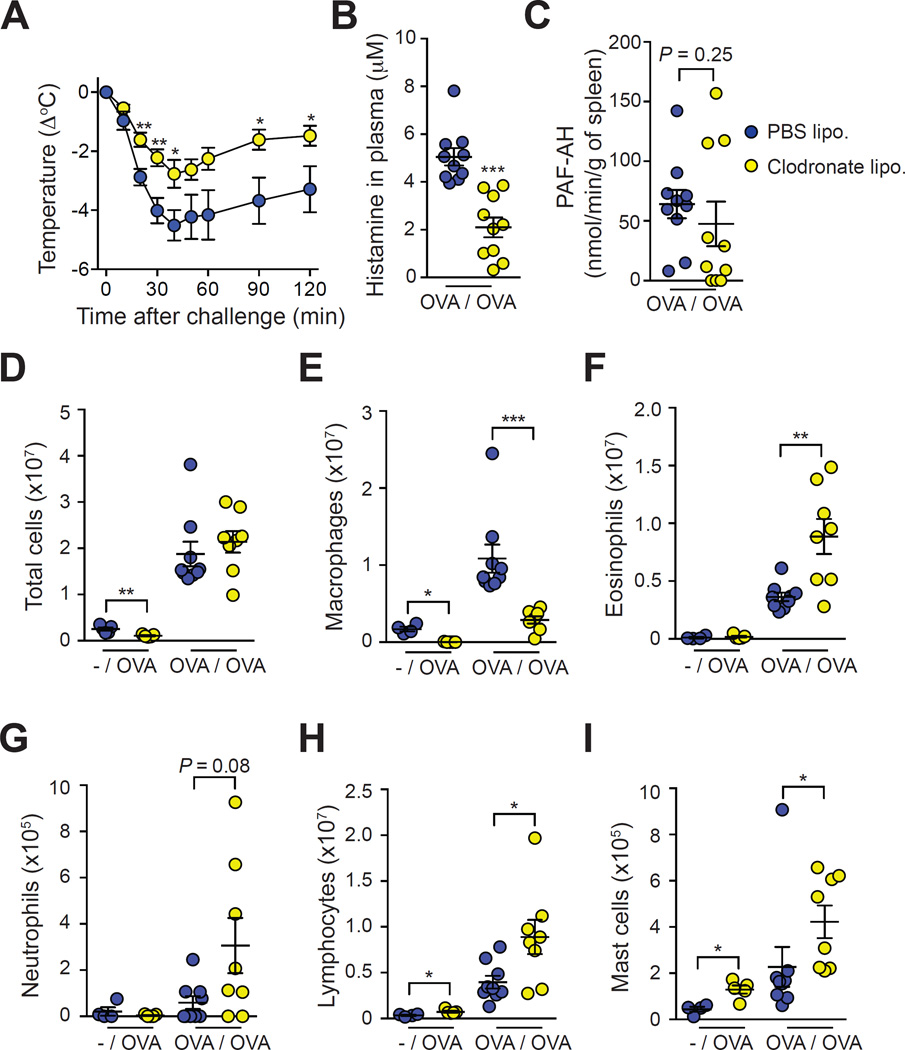
Figure 3. Assessment of the effect of monocyte/macrophage depletion on OVA-induced ASA.
Mice were treated with clodronate liposomes (‘Clodronate lipo.’) or PBS liposomes (‘PBS lipo.’) 24 h before challenge. (A) OVA-induced hypothermia. (B) Levels of histamine in the plasma 20 min after challenge. (C) PAF-AH activity in the spleen 20 min after challenge. (D–I) Numbers of leukocytes in the PLF 3 days after challenge in non-sensitized mice (‘-/OVA’) or OVA-sensitized mice (‘OVA/OVA’). Data are pooled from three independent experiments for all OVA-sensitized groups (total n=8–9/group) and from one experiment for non-sensitized controls (n=4–5/group). *, ** or *** = P < 0.05, 0.01 or 0.001.
In line with previous reports17, 26, treatment with clodronate liposomes reduced features of anaphylaxis in an ASA model using sensitization with OVA together with the adjuvant alum and Bordetella pertussis toxin (see Fig E4, B & C in the Online Repository). Treatment with clodronate liposomes also significantly diminished the hypothermia response in OVA-sensitized and challenged mice in the adjuvant-free ASA model (Fig 3, A). Clodronate liposome-treated mice had lower levels of histamine in the plasma 20 min after challenge, as compared to PBS liposomes-treated mice (Fig 3, B). Since such a reduction in histamine levels could reflect a toxic effect of the liposomes on MCs, we also assessed responses of mice treated with clodronate or PBS liposomes in a MC-dependent IgE-mediated PSA model. Hypothermia was slightly but not significantly reduced (except at 10 min after challenge) in clodronate liposomes-treated mice, which suggests that the clodronate liposomes have only modest effects on MC activity in this model (see Fig E3, H in the Online Repository). The activity of PAF acetylhydrolase (PAF-AH, an enzyme which degrades PAF) has been shown to inversely correlate with the severity of anaphylaxis in human27. However, we found similar PAF-AH activity in the spleen of mice treated with clodronate or PBS liposomes 20 min after challenge (Fig 3, C). By contrast, clodronate liposome-treated mice displayed significantly higher levels of eosinophils, lymphocytes and MCs than PBS liposome-treated mice in the peritoneal cavity 3 days after challenge (Fig. 3, F-I). Levels of blood neutrophils were also higher in such clodronate liposome-treated, macrophage-depleted mice as compared to control mice at day 3 (see Fig E3, A & C in the Online Repository). However, we also found a small but significant increase in lymphocyte and MC numbers 3 days after OVA challenge in naïve mice treated with clodronate liposomes, which could reflect some pro-inflammatory effects of the clodronate liposomes (Fig 3, D-I).
Neutrophils are not required for OVA-induced hypothermia and inflammation
We injected OVA-sensitized mice with an anti-Gr-1 antibody (or an isotype control antibody) 40 h before and 24 h after challenge with OVA to deplete neutrophils. Such treatment lead to complete ablation of circulating neutrophils at the time of challenge (see Fig E5, A & C in the Online Repository), and neutrophils remained absent in the blood and spleen of anti-Gr-1-treated mice 3 days after challenge (see Fig E5, A-C & F in the Online Repository). By contrast, treatment with anti-Gr-1 antibodies did not deplete circulating monocytes or spleen macrophages (and the percentages of these cells were even slightly higher than those in mice treated with the isotype control antibody) (see Fig E5, A, B, D, E & G in the Online Repository).
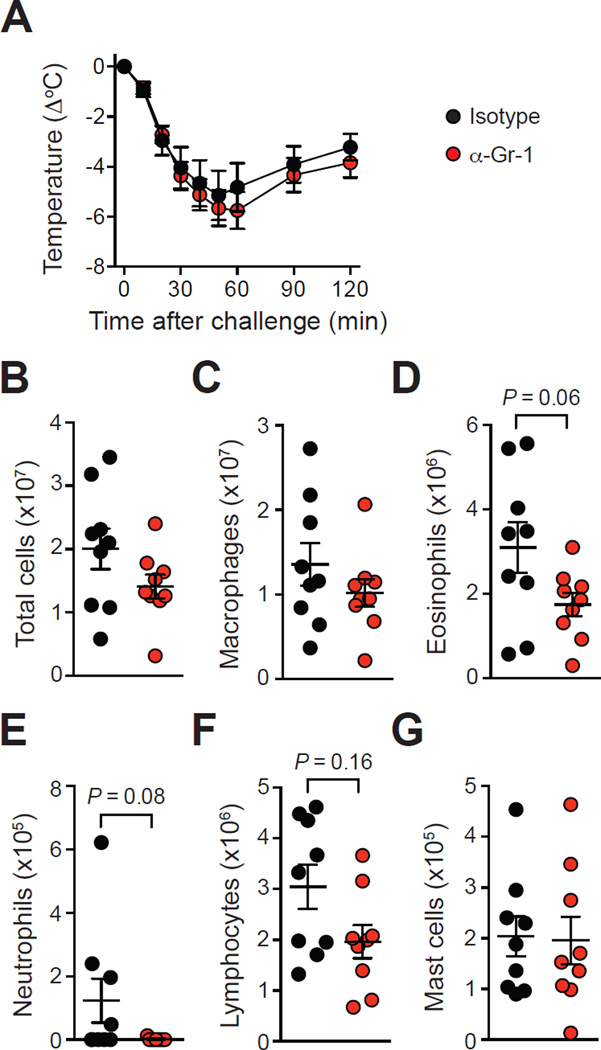
In agreement with findings obtained in IgG-mediated PSA models or ASA models using adjuvants for the sensitization5, 11, we found that anti-Gr-1-treated mice developed markedly reduced features of anaphylaxis as compared to isotype control-treated mice in an ASA model using sensitization with OVA together with the adjuvants alum and Bordetella pertussis toxin (see Fig E4, D & E in the Online Repository). By contrast, isotype control-treated mice and anti-Gr-1-treated mice developed similar levels of immediate hypothermia following challenge with OVA (Fig 4, A). Both groups also had similar levels of leukocytes in the peritoneal cavity 3 days after challenge (although eosinophil and lymphocyte numbers were slightly decreased in the anti-Gr-1-treated group as compared to isotype control-treated mice, this difference did not reach statistical significance) (Fig 4, B-G).
Figure 4. Assessment of antibody-mediated depletion of neutrophils on OVA-induced ASA.
OVA-sensitized mice were treated with an anti-Gr-1 neutrophil-depleting antibody (‘α-Gr-1’) or an isotype control (‘Isotype’) 40 h before and 24 h after challenge with OVA. (A) OVA-induced hypothermia (B–G) Numbers of leukocytes in the PLF 3 days after challenge. Data are pooled from three independent experiments (total n=9/group).
Cpa3-Cre+; Mcl-1fl/fl mice develop less OVA-induced hypothermia and inflammation
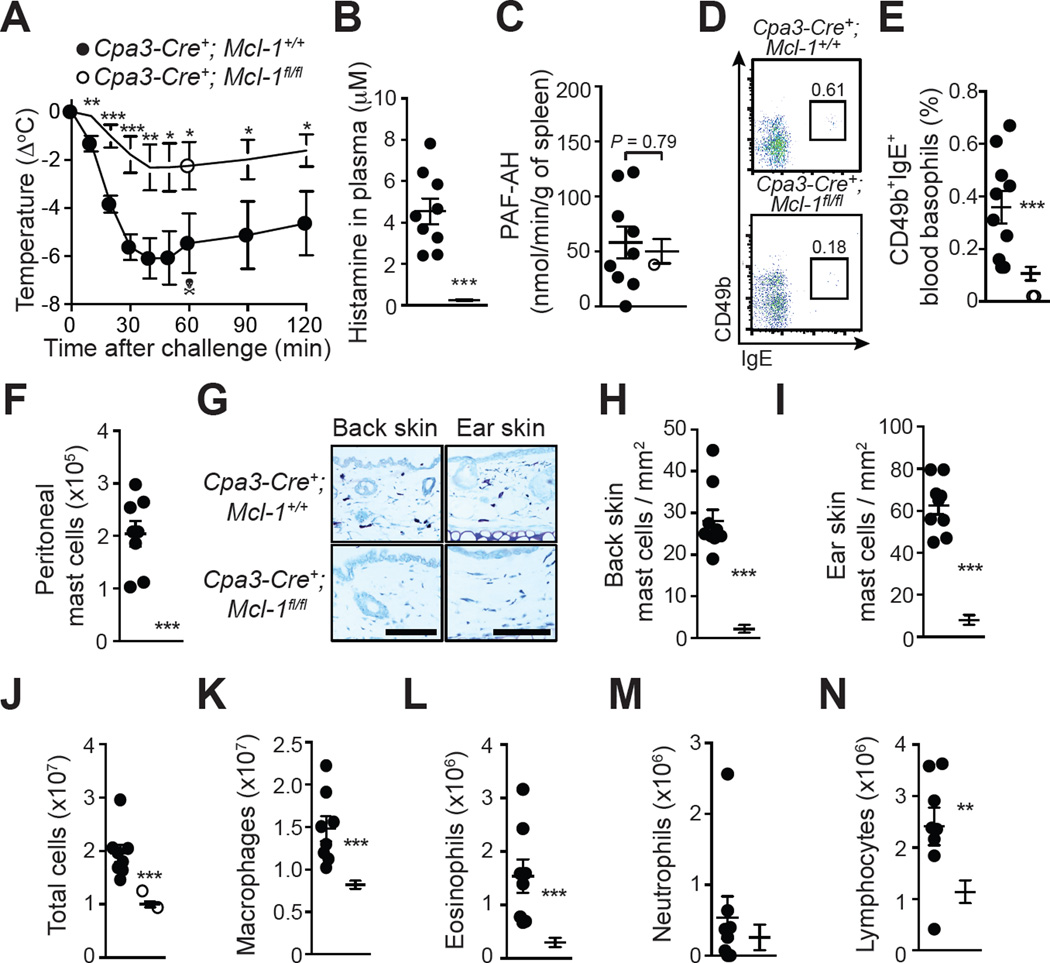
We next assessed responses of MC-deficient, basophil-depleted Cpa3-Cre+; Mcl-1fl/fl mice24 in this ‘adjuvant-free’ ASA model. Cpa3-Cre+; Mcl-1fl/fl mice remained highly deficient in MCs and had reduced numbers of basophils even after repeated sensitizations and challenge with OVA (Fig 5, D-I). These mice developed similar levels of OVA-specific IgG1 and IgG2c antibodies as compared to Cpa3-Cre+; Mcl-1+/+ littermate control mice (see Fig E6, A & B in the Online Repository), and displayed similar amounts of antigen specific-IgE activity in the serum (see Fig E6 C & D in the Online Repository), suggesting that MCs and basophils do not contribute substantially to the sensitization phase in this model. However, sensitized Cpa3-Cre+; Mcl-1fl/fl mice developed significantly reduced levels of hypothermia following challenge with OVA, suggesting an important role for MCs and/or basophils in this feature of the model (Fig 5, A). Cpa3-Cre+; Mcl-1fl/fl mice had markedly reduced levels of histamine in the plasma but similar PAF-AH activity in the spleen 20 min after challenge (Fig 5, B & C). Cpa3-Cre+; Mcl-1fl/fl mice also had diminished intra-peritoneal inflammation, with decreased numbers of total leukocytes, macrophages, eosinophils, and lymphocytes, as compared to Cpa3-Cre+; Mcl-1+/+ mice, indicating that MCs and/or basophils can also importantly contribute to the late phase leukocyte numbers in this model (Fig 5, J-N).
Figure 5. Assessment of OVA-induced ASA in genetically MC-deficient and basophil-depleted Cpa3-Cre; Mcl-1fl/fl mice.
(A) OVA-induced hypothermia in OVA-sensitized Cpa3-Cre+; Mcl-1+/+ and Cpa3-Cre+; Mcl-1fl/fl mice. (B) Levels of histamine in the plasma 20 min after challenge. (C) PAF-AH activity in the spleen 20 min after challenge. (D–E) Representative FACS profile (D) and percentage (E) of blood basophils (CD49b+; IgE+) 24 h before challenge. (F) Numbers of MCs in the PLF 3 days after challenge. (G–I) Toluidine blue staining for MCs (G) and MC numbers (H, I) in sections of back skin and ear pinna. (J-N) Numbers of leukocytes in the PLF 3 days after challenge. Data are pooled from three independent experiments (total n=9–14/group). *, ** or *** = P < 0.05, 0.01 or 0.001. The crossbones symbol indicates death of one mouse. Scale bars: 100 µm.
Basophils are not required for OVA-induced hypothermia and inflammation
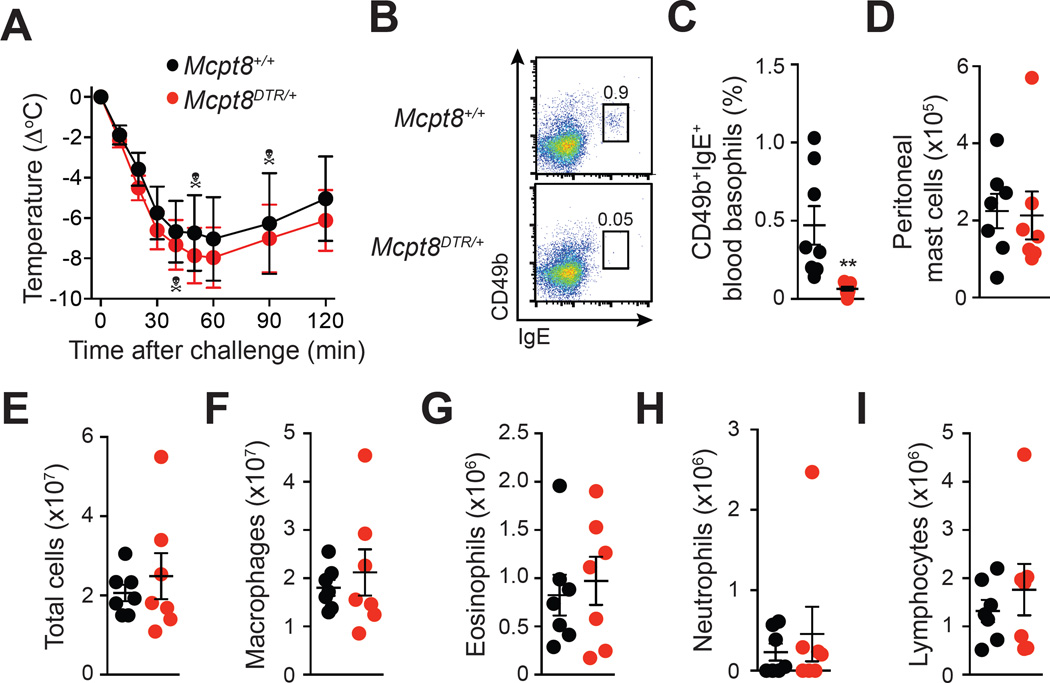
We assessed the potential role of basophils using Mcpt8DTR mice, which express the diphtheria toxin (DT) receptor in basophils only, and in which basophils can be selectively ablated by injection of DT23. We first confirmed that treatment with DT induces ablation of basophils, but not MCs, in OVA-sensitized Mcpt8DTR mice (Fig 6, B-D). We ruled out a significant role for basophils in this model by showing that DT-mediated depletion of basophils in OVA-sensitized Mcpt8DTR mice does not affect OVA-induced hypothermia (Fig 6, A) or late phase intra-peritoneal leukocyte numbers (Fig 6, E-I).
Figure 6. Assessment of the effect of diphtheria toxin-mediated basophil depletion on OVA-induced ASA in Mcpt8DTR mice.
OVA-sensitized Mcpt8+/+ and Mcpt8DTR mice were treated with diphtheria toxin 48 h before and 24 h after challenge with OVA. (A) OVA-induced hypothermia. (B-C) Representative FACS profile (B) and percentage (C) of blood basophils (CD49b+; IgE+) 2 h before challenge. (D–I) Numbers of leukocytes in the PLF 3 days after challenge. Data are pooled from two independent experiments (total n=7–9/group). ** = P < 0.01. Each crossbones symbol indicates death of one mouse.
MCs exacerbate OVA-induced hypothermia and inflammation
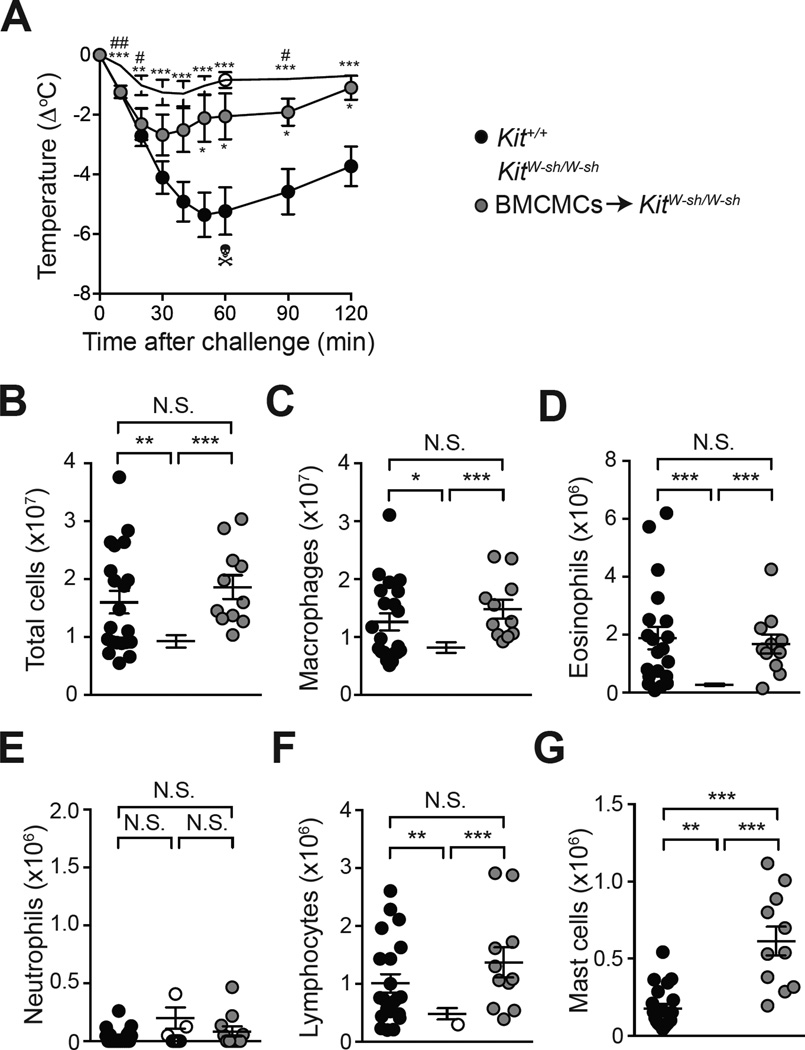
We used Kit mutant MC-deficient KitW-sh/W-sh mice to assess further the contributions of MCs in this ‘adjuvant-free’ ASA model. Since KitW-sh-W-sh mice have many KIT related phenotypic abnormalities beside their MC deficiency 28–33 we also included a group of KitW-sh/W-sh mice that had been engrafted with bone marrow-derived cultured MCs (BMCMCs➔KitW-sh/W-sh mice) both i.p. and i.v. 12 weeks before the first sensitization with OVA.
We found no significant difference in the severity of anaphylaxis between Kit+/+ mice and MC-deficient Kit W-sh/W-sh mice in an ASA model using sensitization with OVA together with the adjuvants alum and Bordetella pertussis toxin (see Fig E4, F & G in the Online Repository), confirming previous findings obtained using MC-deficient KitW/W-v mice9. By contrast, MC-deficient KitW-sh/W-sh mice developed significantly less hypothermia following challenge with OVA as compared to Kit+/+ mice (Fig 7, A). BMCMCs➔KitW-sh/W-sh mice developed significantly more hypothermia than did KitW-sh/W-sh mice following challenge with OVA, although levels of hypothermia in these mice did not reach those of Kit+/+ mice (Fig 7, A). The intermediate body temperature response in MC-engrafted KitW-sh/W-sh mice compared with WT or MC-deficient KitW-sh/W-sh mice is consistent with our previously reported data in a peanut-induced ASA model19. We think it very likely that the technical limitations of such systemic MC engraftment experiments contributed to the intermediate temperature response seen in MC-engrafted KitW-sh/W-sh mice. Specifically, compared to the corresponding WT mice, MC-engrafted KitW-sh/W-sh mice had similar levels of MCs in the peritoneal cavity and mesenteric windows, and even higher numbers of MCs in the spleen. However, these mice had no detectable MCs in the skin, thus eliminating the numerically large population of skin MCs as a potential source of mediators in such mice (Fig 7, and see Fig E7 in the Online Repository).
Figure 7. Responses of WT mice, MC-deficient KitW-sh/W-sh mice, and KitW-sh/W-sh mice engrafted with bone marrow-derived cultured MCs in OVA-induced ASA.
(A) OVA-induced hypothermia in Kit+/+ mice, MC-deficient KitW-sh/W-sh mice and KitW-sh/W-sh mice engrafted with WT BMCMCs. (B-G) Numbers of leukocytes in the PLF 3 days after challenge. Data are pooled from three to five independent experiments (total n=12–25/group). *, ** or *** = P < 0.05, 0.01 or 0.001 vs. Kit+/+ group (A) or indicated group (B-G).# or = ## = P < 0.05 or 0.01 vs. BMCMCs➔KitW-sh/W-sh group (A). The crossbones symbol indicates death of one mouse.
Finally, MC-deficient KitW-sh/W-sh mice developed significantly diminished numbers of leukocytes in the late phase intra-peritoneal inflammation, as compared to Kit+/+ mice (Fig 7, B-F). Confirming the role of MCs in this feature of our ASA model, engraftment of KitW-sh/W-sh mice with BMCMCs restored levels of total peritoneal leukocytes, neutrophils, eosinophils, macrophages and lymphocytes quantified 3 days after challenge to those observed in Kit+/+ mice (Fig 7, B-F). Altogether, these results show that MCs can amplify both the immediate hypothermia and the late phase inflammatory reaction in KitW-sh/W-sh mice in this ‘adjuvant-free’ ASA model.
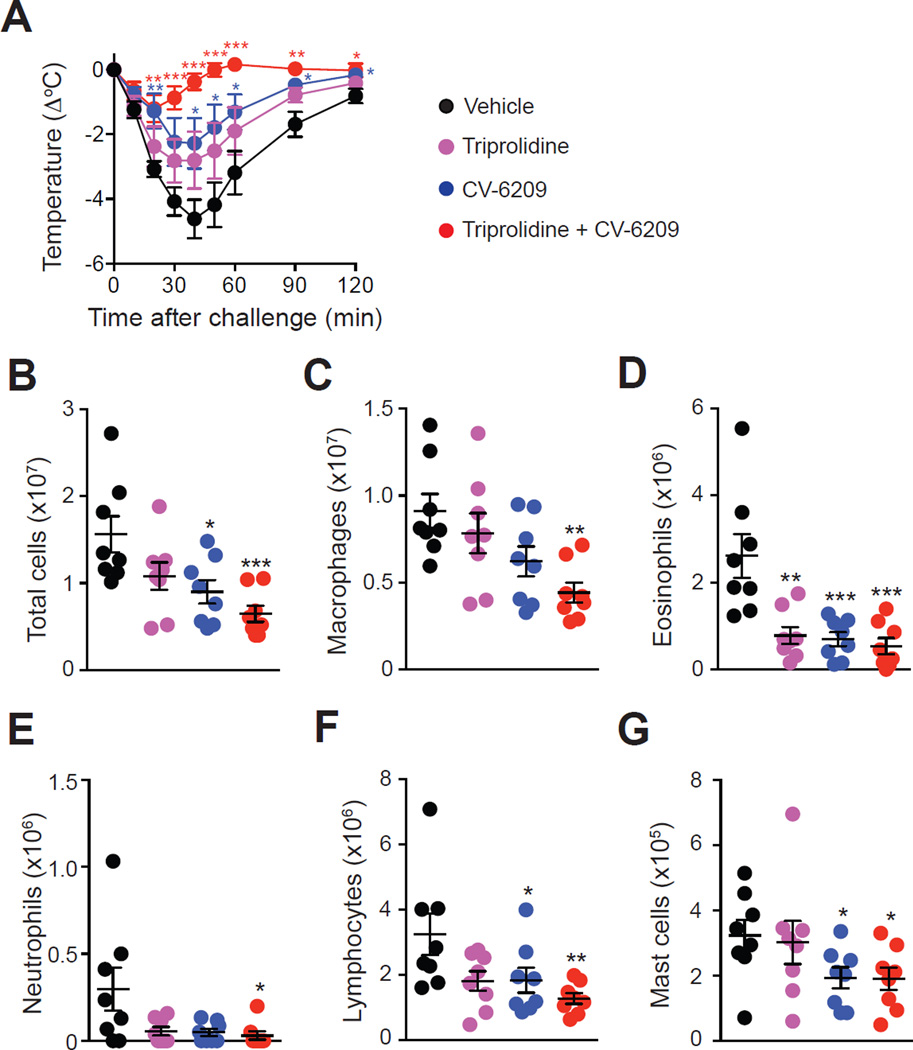
Histamine and PAF contribute to OVA-induced hypothermia and inflammation
Pre-treatment of mice with the platelet-activating factor (PAF) receptor antagonist, CV-6209, significantly decreased both the immediate hypothermia and the numbers of leukocytes in late phase inflammatory response (Fig 8). Pre-treatment with the H1 anti-histamine, triprolidine, slightly reduced the immediate hypothermia and numbers of leukocytes in the late phase response (Fig 8), although differences did not reach statistical significance except for eosinophil numbers (Fig 8, D). However, combined treatment with the anti-histamine and PAF receptor antagonist almost entirely blocked both the hypothermia and the increased numbers of leukocytes observed in the inflammatory responses (Fig 8). By contrast, treatment with triprolidine markedly reduced both the hypothermia and numbers of intra-peritoneal leukocytes in mice pre-treated with clodronate liposomes, while CV-6209 had no significant effects on these features (see Fig E8 in the Online Repository). Collectively, these data demonstrate the involvement of both histamine and PAF in this ASA model in WT mice, and suggest that monocytes/macrophages represent the main source of PAF in this model.
Figure 8. Roles of histamine and PAF in OVA-induced ASA.
OVA-sensitized mice were treated with the H1 anti-histamine triprolidine or with the PAF receptor antagonist CV-6209 alone or in combination 30 min before and 1 day after challenge with OVA. Control mice were injected with vehicle (saline) only. (A) OVA-induced hypothermia. (B-G) Numbers of leukocytes in the PLF 3 days after OVA challenge. Data are pooled from two independent experiments (total n=8/group). *, ** or *** = P < 0.05, 0.01 or 0.001 vs. vehicle group.
Discussion
Most ASA models employ adjuvants during the sensitization phase, and such methods typically prime the animals to exhibit allergic reactions that require little or no contributions from MCs and IgE. We hypothesized that the use of adjuvants might boost the production of certain IgG isotypes, favoring the activation of ‘alternative’ pathways of anaphylaxis, which might mask contributions from the ‘classical’ IgE- and MC-dependent pathway. We therefore designed a new ‘adjuvant-free’ model of ASA, and assessed the potential roles of various components of the ‘classical’ and ‘alternative’ pathways in that model.
Our sensitization protocol resulted in production of OVA-specific IgG1 and IgG2c antibodies (see Fig E1 & E4 in the Online Repository), two isotypes that can induce anaphylaxis through FcγRIII and/or FcγRIV5, 10, 11. Indeed, we found that FcγRIII−/− mice developed markedly reduced hypothermia and reduced numbers of peritoneal leukocytes as compared to WT mice in this model (Fig 2). We used an anti-FcγRIV blocking antibody to assess the contribution of this receptor, and found no role for FcγRIV in the hypothermia response, and a relatively minor contribution to numbers of intra-peritoneal leukocytes (Fig 2). Altogether, our results confirm the important involvement of FcγRIII in allergic shock10, 11, 17, 26, 34 and reveal that this receptor also has an important role in the development of antigen-induced allergic inflammation.
Although we did not detect significant levels of OVA-specific IgE in the sera of most mice by classical ELISA (data not shown), we demonstrated the presence of functionally active specific IgE in serum from OVA-sensitized mice using an ex vivo MC activation test (see Fig E1 & E6 in the Online Repository). These levels of antigen-specific IgE were sufficient to contribute to anaphylaxis and allergic inflammation, since mice lacking the high-affinity IgE receptor FcεRI displayed significantly diminished hypothermia and numbers of intra-peritoneal leukocytes as compared to WT mice (Fig 2). These results are in agreement with previous findings showing less hypothermia in FcεRI−/− or IgE−/− mice in models of peanut-induced ASA16, 17, 35. However, our results are in sharp contrast with reports showing no evidence for involvement of IgE or FcεRI in models of OVA-induced ASA which employ alum as an adjuvant during the sensitization phase9, 10, 20. In line with the strong contributions of both FcγRIII and FcεRI in our model, we found that FcRγ−/− mice, which lack the common activating subunit of Fcε and Fcγ receptors36, were completely protected from hypothermia, and developed even lower numbers of intra-peritoneal leukocytes than FcεRI−/− or FcγRIII−/− mice (Fig 2).
In mice, FcγRIII is expressed on all myeloid cells37 while FcεRI is mainly expressed on MCs and basophils38. Depending on the model used, MCs, basophils, monocytes/macrophages and/or neutrophils have been reported to contribute to ASA11, 13, 14, 16–19, 21, 26, 35. Several studies using passive or active models of anaphylaxis have reported an important role for monocytes/macrophages in the immediate hypothermia response following antigen challenge5, 14, 17, 21, 26, 39–41. We confirmed these findings in an ASA model using the adjuvants alum and Bordetella pertussis toxin for the sensitization (see Fig E4 in the Online Repository). Our depletion experiments also demonstrated that monocytes/macrophages contributed to the hypothermia response in our adjuvant-free ASA model (Fig 2). By contrast, little is known about the function of monocytes/macrophages in the late phase allergic inflammation following anaphylaxis. We found that mice treated with clodronate liposomes to deplete monocytes/macrophages exhibited significantly increased numbers of intra-peritoneal leukocytes (Fig 2). This raises the possibility that monocytes/macrophages might contribute to the resolution phase in this ASA model. However, care should be taken in the interpretation of these findings, since such increased leukocyte numbers might also reflect, at least in part, pro-inflammatory effects of the clodronate liposomes. Indeed, we also found slightly increased numbers of intra-peritoneal MCs and lymphocytes in non-sensitized mice treated with clodronate liposomes.
The importance of neutrophils and basophils in anaphylaxis is debated15. Some reports indicate that antibody-mediated depletion of neutrophils can reduce IgG2-mediated passive systemic anaphylaxis (PSA)5, 11 and ASA11 in mice, while others found no role for neutrophils in the hypothermia reaction following antigen challenge in IgG-mediated PSA13, 41 or ASA19, 21 models. In the present study, we used anti-Gr-1 antibodies to deplete neutrophils. We found that such treatment markedly reduced anaphylaxis in an ASA model using the adjuvants alum and Bordetella pertussis toxin for the sensitization (see Fig E4 in the Online Repository). By contrast, we found no significant contribution for neutrophils in either the immediate hypothermia reaction or the late phase accumulation of intra-peritoneal leukocytes in the adjuvant-free ASA model (Fig 4). Similarly, some reports indicate a contribution of basophils to IgG-mediated PSA5, 13, 34 or ASA11, 17, 19, while others found no significant role for basophils in anaphylaxis models21, 41, 42. Here, we used conditional basophil-deficient Mcpt8DTR mice23, and found no roles for basophils in either the immediate hypothermia reaction or the late phase allergic inflammation in our adjuvant-free ASA model (Fig 6).
It is largely agreed that MCs play important roles in food allergy and anaphylaxis14, 43–45. While most of the literature on the roles of MCs in experimental anaphylaxis is based on data obtained using Kit mutant genetically MC-deficient mice, several new models have been developed in which the MC deficiency is not dependent on mutations affecting c-kit structure or expression24, 30–32, 46–49. While discordant findings have been reported in some disease models in newer versus older MC-deficient strains30, 46, 47, 50, 51, the importance of MCs to both IgE-mediated PSA24, 47, 52 and peanut-induced ASA19 has been confirmed using multiple MC-deficient mouse strains. In agreement with these findings, we demonstrate here that both KitW-sh/W-sh mice and Kit-independent Cpa3-Cre; Mcl-1fl/fl MC-deficient mice developed reduced immediate hypothermia reactions and diminished numbers of late phase intra-peritoneal leukocytes in our adjuvant-free ASA model (Fig 5 & 7). We obtained additional evidence for an important contribution of MCs in this model by showing that engraftment of genetically MC-deficient KitW-sh/W-sh mice with bone marrow–derived cultured MCs partially restored the immediate hypothermia and completely restored the ‘late phase’ allergic inflammation induced by antigen challenge (Fig 7).
These results stand in marked contrast with previous reports9, 13 and our own data (see Fig E4 in the Online Repository) showing that anaphylaxis can fully develop following challenge with OVA in MC-deficient mice sensitized with OVA together with the adjuvant alum. One potential explanation for such results is that MCs are particularly potent at promoting allergic reactions at low levels of antibodies, and/or that increased levels of antibodies (promoted by the use of adjuvants during the sensitization phase) lead to a greater contribution of alternative pathways that mask or render redundant the role of MCs. Indeed, previous reports show that the contribution of MCs to some ASA models is greater when using low doses of adjuvant and/or antigen for the sensitization and low doses of antigen for the challenge17, 19. Our results are also in line with previous reports demonstrating that genetically MC-deficient mice exhibit significantly diminished OVA-induced allergic airway inflammation when sensitized without adjuvant53–56, but can fully develop airway inflammation when alum is employed as an adjuvant during the sensitization phase53, 57.
Finally, consistent with previous reports, we found that both histamine and PAF contributed to the immediate hypothermia reaction in WT mice14, 20, 31, 58–60. These two mediators also contributed to the late phase inflammatory reaction, with a more pronounced effect of PAF (Fig 8). By contrast, treatment with a PAF receptor antagonist had no effect in clodronate liposome-treated mice, suggesting that monocytes/macrophages represent the major source of PAF in this adjuvant-free ASA model. As expected, we found that plasma histamine levels were markedly reduced in MC- and basophil-deficient Cpa3-Cre+; Mcl-1fl/fl mice. More surprisingly however, we also found reduced plasma histamine levels in clodronate liposome-treated mice (although the reduction was not as substantial as that found in Cpa3-Cre+; Mcl-1fl/fl mice), suggesting that monocytes/macrophages might also directly or indirectly contribute to histamine release in this model.
In conclusion, we demonstrate here that FcεRI- and FcγRIII-dependent signaling, histamine, and PAF are required for the full development of hypothermia and intra-peritoneal leukocyte accumulation in an ‘adjuvant-free’ model of ASA. In this model, both MCs and monocytes/macrophages are critically involved in the immediate hypothermia reaction. In addition, MCs are also required for the full development of intra-peritoneal inflammation, as assessed as numbers of intra-peritoneal leukocytes. Our data thus strongly support the hypothesis that MCs and monocytes/macrophages are the main effector cells of anaphylaxis in this setting.
Supplementary Material
Key messages.
The IgE receptor FcεRI, the IgG receptor FcγRIII, histamine, and PAF contribute to hypothermia and leukocyte numbers in an adjuvant-free active systemic anaphylaxis (ASA) model.
Mast cells are required for full development of hypothermia and leukocyte numbers in this ASA model, with no significant role for basophils or neutrophils.
Monocytes/macrophages contribute to hypothermia in this ASA model.
Acknowledgments
We thank Mariola Liebersbach for her help with mouse breeding, Chen Liu for processing slides for histological analysis, and all members of the Galli lab and Bruhns lab for discussions.
Declaration of funding sources:
B.B. was supported by a stipend from the Pasteur - Paris University (PPU) International Ph.D. Program. R.S. was supported by the Lucile Packard Foundation for Children’s Health and the Stanford NIH/NCRR CTSA award number UL1 RR025744; P.S. was supported by a Max Kade Fellowship of the Max Kade Foundation and the Austrian Academy of Sciences and a Schroedinger Fellowship of the Austrian Science Fund (FWF): J3399-B21 and is supported by a European Commission Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2014 655153); T.M. was supported by Marie Curie International outgoing Fellowship for Career Development (299954) and is supported by a “Charge de recherches” fellowship of the Belgian National Fund for Scientific Research (F.R.S-FNRS); N.G. was the recipient of a fellowship from the French “Fondation pour la Recherche Médicale FRM”; P.B acknowledges support from the European Research Council (ERC)–Seventh Frame-work Program (ERC-2013-CoG 616050), the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Institut Pasteur; L.L.R. acknowledges support from the French “Fondation pour la Recherche Médicale FRM”, the Stanford Pediatric Research Fund of the Lucile Packard Foundation for Children’s Health and the Stanford CTSA (National Institutes of Health grant UL1 RR025744), the National Institutes of Health grant K99AI110645, the European Commission Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2014 656086) and the French ‘Institut National de la Santé et de la Recherche Médicale’ (INSERM); S.J.G. acknowledges support from National Institutes of Health grants AI023990, CA072074, AI070813, AR067145, U19 AI104209, NS 080062, the Tobacco-Related Disease Research Program at University of California, and the Department of Pathology, Stanford University School of Medicine.
Abbreviations used
- ASA
Active systemic anaphylaxis
- BMCMC
Bone marrow-derived cultured mast cell
- Cpa3
Carboxypeptidase A3
- DT
Diphtheria toxin
- DTR
Diphtheria toxin receptor
- MC
Mast cell
- Mcpt
Mast cell protease
- OVA
Ovalbumin
- PAF
Platelet-activating factor
- PAF-AH
Platelet-activating factor acetylhydrolase
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors state that they have no conflicts of interest.
References
- 1.Lieberman P, Camargo CA, Jr, Bohlke K, Jick H, Miller RL, Sheikh A, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 3.Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125:S161–S181. doi: 10.1016/j.jaci.2009.12.981. [DOI] [PubMed] [Google Scholar]
- 4.Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A. 2011;108:12413–12418. doi: 10.1073/pnas.1105695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Clay CD, et al. Rapid desensitization of mice with anti-FcγRIIb/FcγRIII mAb safely prevents IgG-mediated anaphylaxis. J Allergy Clin Immunol. 2013;132:1375–1387. doi: 10.1016/j.jaci.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wershil BK, Mekori YA, Murakami T, Galli SJ. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987;139:2605–2614. [PubMed] [Google Scholar]
- 7.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 8.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 9.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and FcγRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of Fc epsilonRI alpha chain results in upregulation of FcγRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between FcεRI and FcγRIII for limiting amounts of FcR beta and gamma chains. J Clin Invest. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Million M, Fioramonti J, Zajac JM, Bueno L. Effects of neuropeptide FF on intestinal motility and temperature changes induced by endotoxin and platelet-activating factor. Eur J Pharmacol. 1997;334:67–73. doi: 10.1016/s0014-2999(97)01142-4. [DOI] [PubMed] [Google Scholar]
- 13.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 16-7. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson F, Mancardi DA, Albanesi M, Bruhns P. Neutrophils in local and systemic antibody-dependent inflammatory and anaphylactic reactions. J Leukoc Biol. 2013;94:643–656. doi: 10.1189/jlb.1212623. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179:6696–6703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 17.Arias K, Chu DK, Flader K, Botelho F, Walker T, Arias N, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol. 2011;127:1552–1561. doi: 10.1016/j.jaci.2011.03.044. e1. [DOI] [PubMed] [Google Scholar]
- 18.Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, et al. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am J Pathol. 2012;180:1535–1546. doi: 10.1016/j.ajpath.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:881–888. doi: 10.1016/j.jaci.2013.06.008. e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 21.Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011;6:e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6- KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 26.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 27.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 28.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127. doi: 10.1016/bs.ai.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaudenzio N, Sibilano R, Starkl P, Tsai M, Galli SJ, Reber LL. Analyzing the Functions of Mast Cells In Vivo Using ‘Mast Cell Knock-in’ Mice. J Vis Exp. 2015 doi: 10.3791/52753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutier H, Gillis CM, Iannascoli B, Godon O, England P, Sibilano R, et al. IgG subclasses determine pathways of anaphylaxis in mice. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2016.03.028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 37.Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 38.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 39.Lazar G, Jr, Lazar G, Kaszaki J, Olah J, Kiss I, Husztik E. Inhibition of anaphylactic shock by gadolinium chloride-induced Kupffer cell blockade. Agents Actions. 1994;41(Spec No):C97–C98. doi: 10.1007/BF02007784. [DOI] [PubMed] [Google Scholar]
- 40.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–457. doi: 10.1016/j.jaci.2004.12.1125. quiz 58. [DOI] [PubMed] [Google Scholar]
- 41.Jiao D, Liu Y, Lu X, Liu B, Pan Q, Liu Y, et al. Macrophages are the dominant effector cells responsible for IgG-mediated passive systemic anaphylaxis challenged by natural protein antigen in BALB/c and C57BL/6 mice. Cell Immunol. 2014;289:97–105. doi: 10.1016/j.cellimm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 47-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons FE. Anaphylaxis: Recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124:625–636. doi: 10.1016/j.jaci.2009.08.025. quiz 37-8. [DOI] [PubMed] [Google Scholar]
- 45.Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015;8:444–463. doi: 10.1038/mi.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Otsuka A, Kubo M, Honda T, Egawa G, Nakajima S, Tanizaki H, et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS One. 2011;6:e25538. doi: 10.1371/journal.pone.0025538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol. 2012;188:1809–1818. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR. Hematopoietic Kit Deficiency, rather than Lack of Mast Cells, Protects Mice from Obesity and Insulin Resistance. Cell Metab. 2015;21:678–691. doi: 10.1016/j.cmet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Kasprick A, Yu X, Scholten J, Hartmann K, Pas HH, Zillikens D, et al. Conditional depletion of mast cells has no impact on the severity of experimental epidermolysis bullosa acquisita. Eur J Immunol. 2015;45:1462–1470. doi: 10.1002/eji.201444769. [DOI] [PubMed] [Google Scholar]
- 52.Forster A, Blissenbach B, Machova A, Leja S, Rabenhorst A, Wilmschen S, et al. Dicer is indispensable for the development of murine mast cells. J Allergy Clin Immunol. 2015;135:1077–1080. doi: 10.1016/j.jaci.2014.10.005. e4. [DOI] [PubMed] [Google Scholar]
- 53.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 56.Yu M, Eckart MR, Morgan AA, Mukai K, Butte AJ, Tsai M, et al. Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma. J Clin Invest. 2011;121:3133–3143. doi: 10.1172/JCI43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibamoto T, Liu W, Cui S, Zhang W, Takano H, Kurata Y. PAF, rather than histamine, participates in mouse anaphylactic hypotension. Pharmacology. 2008;82:114–120. doi: 10.1159/000141516. [DOI] [PubMed] [Google Scholar]
- 59.Arias K, Baig M, Colangelo M, Chu D, Walker T, Goncharova S, et al. Concurrent blockade of platelet-activating factor and histamine prevents life-threatening peanut-induced anaphylactic reactions. J Allergy Clin Immunol. 2009;124:307–314. doi: 10.1016/j.jaci.2009.03.012. 14 e1-2. [DOI] [PubMed] [Google Scholar]
- 60.Wang M, Shibamoto T, Tanida M, Kuda Y, Kurata Y. Mouse anaphylactic shock is caused by reduced cardiac output, but not by systemic vasodilatation or pulmonary vasoconstriction, via PAF and histamine. Life Sci. 2014;116:98–105. doi: 10.1016/j.lfs.2014.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.