Abstract
Recent studies suggest that correcting low serum bicarbonate levels may reduce the progression of kidney disease; however, few patients with chronic kidney disease have low serum bicarbonate. Therefore, we examined whether higher levels of serum bicarbonate within the normal range (20–30 mmol/l) were associated with better kidney outcomes in the African American Study of Kidney Disease and Hypertension (AASK) trial. At baseline and during follow-up of 1094 patients, the glomerular filtration rates (GFR) were measured by iothalamate clearances and events were adjudicated by the outcomes committee. Mean baseline serum bicarbonate, measured GFR, and proteinuria were 25.1 mmol/l, 46 ml/min per 1.73 m2, and 326 mg/g of creatinine, respectively. Each 1 mmol/l increase in serum bicarbonate within the normal range was associated with reduced risk of death, dialysis, or GFR event and with dialysis or GFR event (hazard ratios of 0.942 and 0.932, respectively) in separate multivariable Cox regression models that included errors-in-variables calibration. Cubic spline regression showed that the lowest risk of GFR event or dialysis was found at serum bicarbonate levels near 28–30 mmol/l. Thus, our study suggests that serum bicarbonate is an independent predictor of CKD progression. Whether increasing serum bicarbonate into the high-normal range will improve kidney outcomes during interventional studies will need to be considered.
Keywords: AASK (African American Study of Kidney Disease and Hypertension), acidosis, chronic kidney disease, survival
Long-term follow-up of participants in the African American Study of Kidney Disease and Hypertension (AASK) showed that >50% died or developed a doubling of serum creatinine or end-stage renal disease at 10 years of follow-up.1,2 This is particularly concerning as >80% of participants in the cohort phase of AASK were on renin–angiotensin blockers and blood pressure was maintained near recommended target values.1 Given that most patients with chronic kidney disease (CKD) progress over the long term, it is clear that other strategies are necessary to slow advancing CKD.
In addition to renin–angiotensin blockade, correcting metabolic acidosis might be a useful additional maneuver to prevent CKD progression. Metabolic acidosis resulting from impaired urinary acid excretion is a complication of CKD. There are some data suggesting a detrimental effect of low serum bicarbonate with outcomes in CKD. In a recent study, individuals with hypertensive nephropathy and serum bicarbonate <22 mmol/l treated with sodium citrate had improved surrogate markers of kidney disease, such as reduced urinary excretion of endothelin-1, transforming growth factor-β1, and albumin, compared with those who were not treated with alkali.3 In a randomized study of 134 CKD patients with serum bicarbonate levels in the range of 16–20 mmol/l, correcting low serum bicarbonate reduced the rate of kidney function decline when compared with placebo.4 Thus, those CKD patients with overt reduction in serum bicarbonate levels might benefit from correction of low serum bicarbonate levels. However, >85% of people with estimated glomerular filtration rate (eGFR) in the range of 30–49 ml/min per 1.73 m2 and >75% of those with eGFR in the range of 20–29 ml/min per 1.73 m2 do not have low serum bicarbonate (<22 mmol/l).5 Thus, it is important to examine whether serum bicarbonate in the upper limit of normal range is associated with better outcomes.
In a retrospective chart review of 5422 adults visiting a general medical clinic, compared with those with serum bicarbonate levels of 25–26 mmol/l, the low serum bicarbonate (<22 mmol/l) group had a higher hazard of kidney disease progression after adjusting for eGFR.6 In a study of 1106 veterans with mean eGFR of 37±17 ml/min per 1.73 m2, the lowest hazard of death was in those with baseline serum bicarbonate levels of 24–29 mmol/l, but the associations of serum bicarbonate with kidney outcomes were not specifically reported.7 These studies used GFR estimated from serum creatinine by the modification of diet in renal disease (MDRD) equation to adjust for confounding among the level of renal impairment, serum bicarbonate, and the outcomes. Even though these analyses controlled for eGFR, it is possible that lower serum bicarbonate levels might still reflect low true GFR,8 and therefore, these observational results could still be confounded by the level of kidney function. This report of the AASK cohort investigates the hypothesis that higher serum bicarbonate levels within the normal range are associated with improved renal outcomes, while rigorously controlling for GFR using iothalamate clearance, rather than eGFR, and other potential confounders.
RESULTS
Baseline characteristics
Characteristics of AASK study participants according to baseline serum bicarbonate (<20 mmol/l, 20–24.9 mmol/l, 25–29.9 mmol/l, and ≥30 mmol/l) are presented in Table 1. Only 4.3% of the AASK cohort (mean GFR 34±13 ml/min per 1.73 m2) had serum bicarbonate levels <20 mmol/l, whereas 35.5% had serum bicarbonate in the low-normal range (20–24.9 mmol/l). The lowest serum bicarbonate group had lower mean GFR and higher median baseline proteinuria. Body mass index was lower in the lowest bicarbonate group. Only 39% of the participants in the lowest bicarbonate group, but 83% of the participants in the highest bicarbonate group, were on diuretics at baseline.
Table 1.
Characteristics of AASK participants by baseline serum bicarbonate
| Serum (<20 mmol/l) | Serum (20–24.9 mmol/l) | Serum (25–29.9 mmol/l) | Serum (≥30 mmol/l) | P-value | |
|---|---|---|---|---|---|
| Number | 47 | 388 | 599 | 60 | |
| Age (years) | 54±11 | 54±11 | 55±10 | 55±10 | 0.167 |
| Male (%) | 51.1 | 62.4 | 61.3 | 60.0 | 0.514 |
| Randomized drug group (%) | |||||
| Ramipril | 34.0 | 42.3 | 39.2 | 35.0 | 0.514 |
| Metoprolol | 38.3 | 40.5 | 41.1 | 33.3 | 0.696 |
| Amlodipine | 27.7 | 17.3 | 19.7 | 31.7 | 0.034 |
| Randomized BP group (%) | |||||
| Strict control | 46.8 | 51.0 | 48.4 | 50.0 | 0.854 |
| Usual control | 53.2 | 49.0 | 51.6 | 50.0 | 0.854 |
| Atherosclerotic conditions (%) | 12.8 | 12.1 | 16.1 | 10.0 | 0.259 |
| Congestive heart failure (%) | 4.3 | 2.6 | 2.3 | 3.3 | 0.849 |
| Current or past smoking (%) | 63.8 | 62.4 | 54.9 | 53.3 | 0.088 |
| Body mass index (kg/m2) | 26.8±6.5 | 30.5±6.5 | 30.8±6.5 | 31.8±7.1 | <0.001 |
| Mean arterial pressure (mm Hg) | 116±16 | 113±15 | 114±16 | 117±19 | 0.286 |
| GFR (ml/min per 1.73 m2) | 34±13 | 43±14 | 49±13 | 49±12 | <0.001 |
| Urine protein/creatinine ratio (mg/g)a | 410 (50, 1110) | 130 (40, 580) | 60 (20, 230) | 70 (30, 260) | <0.001 |
| Serum albumin (g/dl) | 4.2±0.3 | 4.2±0.4 | 4.3±0.4 | 4.3±0.4 | 0.019 |
| Diuretic use (%) | 39.1 | 61.6 | 65.1 | 83.1 | <0.001 |
Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; ANOVA, analysis of variance; BP, blood pressure; GFR, glomerular filtration rate.
Median (interquartile range) presented.
P-values are calculated by ANOVA for continuous variables and χ2-values for dichotomous variables. Continuous measures are shown as mean±standard error.
Death, dialysis, or GFR event composite in entire cohort
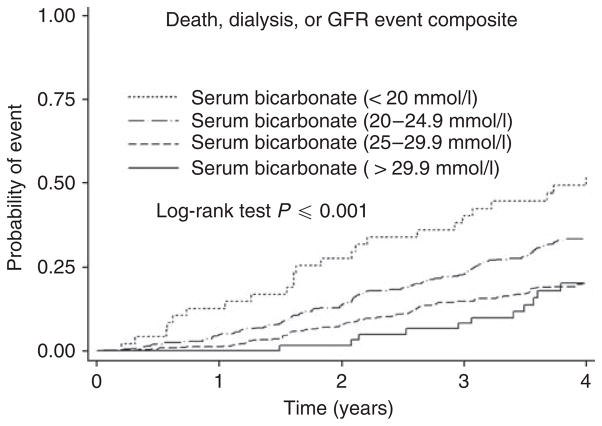
There were a total of 359 death, dialysis, or GFR composite events occurring over 4475 patient-years of follow-up (0.08 events per patient-year). The unadjusted cumulative incidence of this outcome by serum bicarbonate groups is shown in Figure 1. The event rates for those with baseline serum bicarbonate <20, 20–24.9, 25–29.9, and ≥30 mmol/l were 0.17, 0.10, 0.06, and 0.06 events per patient-year, respectively.
Figure 1. Probability of death or renal composite (dialysis or glomerular filtration rate (GFR) event) according to baseline serum bicarbonate levels.
Participants were categorized into four groups according to the baseline bicarbonate levels (<20 mmol/l, 20.0–24.9 mmol/l, 25.0–29.9 mmol/l, and ≥30 mmol/l). The unadjusted results are shown here.
To account for confounding between serum bicarbonate and other baseline factors, the association of serum bicarbonate with the composite outcome was next examined in Cox models (Table 2). After adjusting for age, gender, and randomization groups, each 1 mmol/l increase in serum bicarbonate was associated with an 11.1% reduction in the hazard of the clinical composite outcome of death, dialysis, or GFR events (hazard ratio (HR) 0.889, 95% confidence interval (CI) 0.859–0.921). Further adjustment for baseline-measured iothalamate GFR and proteinuria attenuated, but did not eliminate this association (HR 0.950, 95% CI 0.916–0.985). Even though GFR was measured directly based on iothalamate clearance in this cohort, recognizing that there could be errors in measurements of both proteinuria and GFR, we next built an errors-in-measurement Cox regression model adjusting for these covariables. The associations of serum bicarbonate with the above composite outcome remained significant in that model (Table 2). In sensitivity analyses, when further adjusted for baseline atherosclerotic conditions, congestive heart failure, mean arterial blood pressure, body mass index, smoking, serum albumin, and use of diuretics, each 1 mmol/l increase in serum bicarbonate was associated with a statistically significant 4% lower hazard of the composite death, dialysis, or GFR event (Table 2).
Table 2.
Association of each mmol/l increase in baseline serum bicarbonate with the hazard of death, dialysis, or GFR event in the entire cohort (N=1090) and those with baseline serum bicarbonate levels of 20–30 mmol/l (N=1017)
| Baseline | Death, dialysis, or GFR event
|
|||||
|---|---|---|---|---|---|---|
| Entire cohort
|
20–30 mmol/l
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Model 1a | 0.889 | 0.859–0.921 | <0.0001 | 0.869 | 0.829–0.910 | <0.0001 |
| Model 2b | 0.950 | 0.916–0.985 | 0.006 | 0.938 | 0.894–0.984 | 0.009 |
| Model 3c | 0.956 | 0.921–0.992 | 0.018 | 0.942 | 0.898–0.989 | 0.017 |
| Model 4d | 0.960 | 0.924–0.998 | 0.041 | 0.950 | 0.904–0.999 | 0.047 |
Abbreviations: CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio.
Model 1: adjusted for age, gender, and blood pressure and drug assignment.
Model 2: adjusted for Model 1 variables, GFR, and proteinuria at baseline.
Model 3: adjusted for Model 2 variables and accounting for measurement error in baseline GFR and proteinuria.
Model 4: adjusted for Model 3 variables, atherosclerotic conditions, congestive heart failure, mean arterial blood pressure, body mass index, smoking, serum albumin, and use of diuretics at baseline.
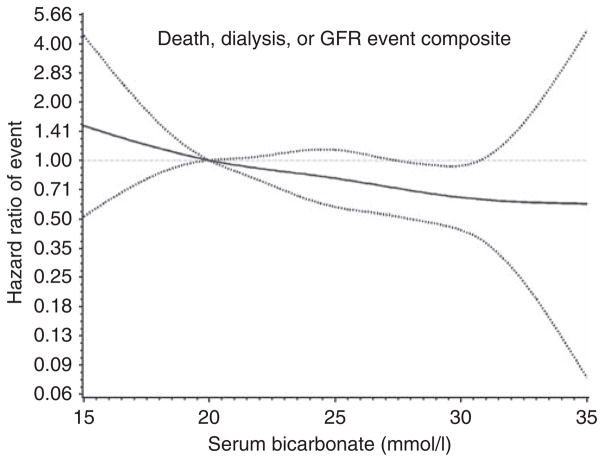
The results of a restricted cubic spline regression model adjusted for gender, age, trial assignment, GFR, and proteinuria are presented in Figure 2, which shows a linear inverse relationship between serum bicarbonate levels and the death, dialysis, or GFR event composite.
Figure 2. Restricted cubic spline regression model of the hazard of glomerular filtration rate (GFR) event, dialysis, or death among all participants by baseline serum bicarbonate levels after adjusting for gender, age, trial assignment, measured GFR, and proteinuria.
The estimated adjusted hazard ratio as a function of baseline serum bicarbonate, using a bicarbonate level of 20 mmol/l as the reference, with 95% pointwise confidence limits, is shown here.
Dialysis or GFR event composite in entire cohort
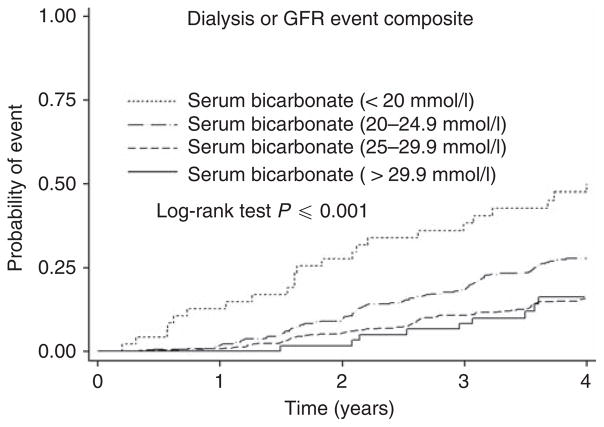
The unadjusted cumulative incidence of the dialysis or GFR event composite was also higher in the lowest bicarbonate group (Figure 3). There were a total of 278 events occurring over 4475 patient-years of follow-up (0.06 events per patient-year). The event rates for those with baseline bicarbonate <20, 20–24.9, 25–29.9, and ≥30 mmol/l were 0.15, 0.10, 0.05, and 0.05 events per patient-year, respectively.
Figure 3. Probability of the renal composite (dialysis or glomerular filtration rate (GFR) event) according to baseline serum bicarbonate levels.
Participants were categorized into four groups according to the baseline bicarbonate levels (<20 mmol/l, 20.0–24.9 mmol/l, 25.0–29.9 mmol/l, and ≥30 mmol/l). The unadjusted results are shown here.
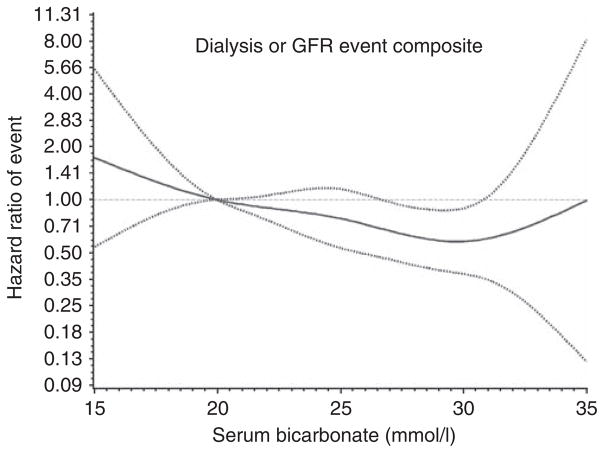
Each 1 mmol/l increase in serum bicarbonate was associated with a 6% lower hazard of the composite renal outcome of dialysis or GFR event (HR 0.940, 95% CI 0.902–0.980) after adjusting for age, gender, trial assignment, GFR, and proteinuria. This relationship persisted despite extensive adjustment for measurement error and extended covariables (Table 3). Results of a restricted cubic spline regression model adjusted for gender, age, trial assignment, GFR, and proteinuria suggest that the lowest risk of dialysis or GFR event is at serum bicarbonate levels in the range of 28–30 mmol/l (Figure 4).
Table 3.
Association of each mmol/l increase in baseline serum bicarbonate with the hazard of dialysis or GFR event in the entire cohort (N=1090) and those with baseline serum bicarbonate levels of 20–30 mmol/l (N=1017)
| Baseline | Dialysis or GFR event
|
|||||
|---|---|---|---|---|---|---|
| Entire cohort
|
20–30 mmol/l
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Model 1a | 0.872 | 0.838–0.907 | <0.0001 | 0.927 | 0.877–0.980 | < 0.0001 |
| Model 2b | 0.940 | 0.902–0.980 | 0.004 | 0.932 | 0.881–0.986 | 0.007 |
| Model 3c | 0.947 | 0.907–0.988 | 0.012 | 0.939 | 0.885–0.996 | 0.014 |
| Model 4d | 0.951 | 0.909–0.994 | 0.027 | 0.851 | 0.806–0.898 | 0.035 |
Abbreviations: CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio.
Model 1: adjusted for age, gender, and blood pressure and drug assignment
Model 2: adjusted for Model 1 variables, GFR, and proteinuria at baseline.
Model 3: adjusted for Model 2 variables and accounting for measurement error in baseline GFR and proteinuria.
Model 4: adjusted for Model 3 variables, atherosclerotic conditions, congestive heart failure, mean arterial blood pressure, body mass index, smoking, serum albumin, and use of diuretics at baseline.
Figure 4. Restricted cubic spline regression model of the hazard of glomerular filtration rate (GFR) event or dialysis among all participants by baseline serum bicarbonate levels after adjusting for gender, age, trial assignment, measured GFR, and proteinuria.
The estimated adjusted hazard ratio as a function of baseline serum bicarbonate, using a bicarbonate level of 20 mmol/l as the reference, with 95% pointwise confidence limits, is shown here.
Death in entire cohort
There were a total of 105 deaths (including deaths after end-stage renal disease) occurring over 4475 patient-years of follow-up (0.02 events per patient-year). There were no associations of serum bicarbonate with death (HR 0.987, 95% CI 0.923–1.056 for each 1 mmol/l increase in serum bicarbonate) in a Cox model adjusted for demographics, trial assignment, GFR, and proteinuria.
Serum bicarbonate in the normal range and outcomes
Figures 2 and 4 suggest that higher serum bicarbonate levels within the range of 20–30 mmol/l were associated with improved survival and renal outcomes. We further examined these in analyses restricted to those with baseline serum bicarbonate levels of 20–30 mmol/l (n =1017). Cox regression models were carried out in the same fashion as described above and the results are summarized in Tables 2 and 3. These results suggest that even after extensive adjustment, each 1 mmol/l increase in baseline serum bicarbonate levels within the range of 20–30 mmol/l was associated with reduced risk of the composite of death, dialysis, or GFR event, as well as composite renal outcomes of dialysis or GFR event.
Sensitivity analyses
We conducted additional sensitivity analyses with adjusting for baseline eGFR estimated from the four-variable MDRD equation. These results were qualitatively similar to the models using iothalamate-measured GFR. For each 1 mmol/l increase in serum bicarbonate, the hazard of death, dialysis, or GFR event was lower (HR 0.958, 95% CI 0.924–0.993) in a multivariable Cox regression model adjusted for demographics, trial characteristics, baseline proteinuria, and eGFR. In additional sensitivity analyses of anion gap, the Pearson correlation coefficient between anion gap and serum bicarbonate was low at −0.14, even though it was statistically significant (P<0.0001). The R2 was 0.0196, indicating that <2% of the variability in serum bicarbonate was explained by anion gap. After adjustment for demographics and trial assignment, iothalamate-derived GFR, and proteinuria, anion gap was not associated with the death, dialysis, or GFR event composite (HR 0.991, 95% CI 0.949–1.036 for each unit increase in anion gap), or with dialysis or GFR event composite (0.989, 95% CI 0.940–1.040 for each unit increase in anion gap HR). Because protein intake might influence serum bicarbonate, a final sensitivity analysis was performed in which we adjusted for demographics, trial assignment, iothalamate-derived GFR, proteinuria, and calculated protein intake. Inclusion of calculated protein intake in the Cox models had no effect on the associations of serum bicarbonate with death, dialysis, or GFR event composite (for each mmol/l increase in serum bicarbonate: HR 0.950, 95% CI 0.916–0.986) and with the dialysis or GFR event composite (for each mmol/l increase in serum bicarbonate: HR 0.941, 95% CI 0.902–0.981) in the entire cohort (please see Model 2 in Tables 2 and 3 for comparison).
DISCUSSION
The Kidney Disease Outcome Quality Initiative practice guidelines recommend that serum bicarbonate be kept at a minimum of 22 mmol/l in those with CKD, a cutoff chosen based on the effect of metabolic acidosis on metabolic bone disease and nutritional parameters.9 However, the optimum serum bicarbonate in CKD might be different when considering renal and survival outcomes than for metabolic bone disease. In this investigation of the association of baseline serum bicarbonate on survival and renal outcomes in African Americans with hypertensive, non-dialysis-dependent CKD, higher serum bicarbonate is associated with reduced hazard of CKD progression and mortality. More importantly, in those within the range of serum bicarbonate levels of 20–30 mmol/l, there was an inverse linear relationship between serum bicarbonate levels and the hazard of death, dialysis, or GFR event composite, as well as dialysis or GFR event composite (Tables 2 and 3 and Figures 2 and 4), with the lowest hazard of kidney events observed as serum bicarbonate levels approach 30 mmol/l.
A strength of this study is that we have carefully considered confounding between baseline renal function and serum bicarbonate on the clinical end points. Prior studies investigating the association of baseline serum bicarbonate levels with CKD progression and mortality used the MDRD formula to estimate GFR.6,7 However, the accuracy of the MDRD equation was only 83%, even when accuracy was defined as predicting the measured GFR within a range of ±30%.10 Thus, eGFR may be substantially different from measured GFR in an individual. In a study in which the associations of serum bicarbonate with subsequent outcomes could largely reflect the associations of serum bicarbonate with baseline GFR, it is important that the baseline GFR be determined as accurately as possible. Furthermore, we recognize that there could be random measurement errors of GFR and proteinuria, which are important confounders of the hypothesis tested in this study. Therefore, we used additional errors-in-variables regression models to account for this. The results of this study suggest that after taking all of the above issues into account, the associations of baseline serum bicarbonate with clinical outcomes is not confounded by the level of baseline kidney function. These results support the results of prior studies that investigated the associations of baseline serum bicarbonate with CKD progression or mortality using the MDRD formula to estimate GFR.6,7
A major goal of this analysis is to describe the relationship between serum bicarbonate levels in the 20–30 mmol/l range with the outcomes in CKD. An earlier study suggested that correcting low serum bicarbonate (16–20 mmol/l) in CKD was associated with better renal outcomes.4 However, most patients with CKD do not have serum bicarbonate at such low levels, as was apparent in this study, in which <5% of this advanced CKD cohort had serum bicarbonate levels <20 mmol/l. This analysis supports the notion that baseline serum bicarbonate levels in the higher end of the normal range is associated with improved long-term renal function. These data suggest that individuals with CKD and serum bicarbonate in the low-normal range might also derive benefit from alkali therapy. Furthermore, maintaining serum bicarbonate in the high-normal range might improve long-term renal outcomes rather than maintaining it at a minimum of 22 mmol/l.
The potential mechanism explaining the finding that the lowest hazard of CKD progression is in the upper range of normal is speculative, but may involve renal ammonia production. Nath et al.11 showed that metabolic acidosis increases renal ammonia production, activates the alternative complement pathway, and contributes to tubulointerstitial injury in a remnant kidney model. However, increased renal ammonia production has been observed in animals with normal serum bicarbonate levels after renal ablation–infarction, presumably as a means to maintain normal systemic acid–base balance.12 Thus, if serum bicarbonate levels are in the range of 28–30 mmol/l, renal ammonia production and tubulointerstitial fibrosis might be maximally attenuated. Therefore, administration of alkali in early stages of CKD, when serum bicarbonate is in the normal range, might reduce ammonia generation and provide a means to slow tubulointerstitial fibrosis.
Other effects of chronic metabolic acidosis include metabolic bone disease, skeletal muscle wasting, hypoalbuminemia, insulin resistance, abnormal thyroid function, and elevated C-reactive protein.13–17 In non-dialysis-dependent CKD, treating metabolic acidosis attenuates elevations in parathyroid hormone,18 reduces protein degradation,19 reduces blood urea nitrogen,20,21 and raises 1,25-dihydroxy-vitamin D levels.22
Although this study and a prior study7 suggest that the optimal serum bicarbonate range is about 28–30 mmol/l in the CKD population, the optimal range of serum bicarbonate in dialysis patients might be lower. In two hemodialysis studies, the lowest hazard of death was associated with serum bicarbonate levels of 20–22 mmol/l.23,24 However, in another study of hemodialysis patients, those with serum bicarbonate > 22 mmol/l had lower hazard of death.25
The AASK trial was designed to examine the effects of blood pressure control and blood pressure agents on the slope of GFR decline. Hence, a limitation of our study is that it is a secondary analysis of an existing database. It is also possible that the serum bicarbonate level could have been falsely low in some participants at baseline owing to delays in centrifugation of the blood sample and the measurement of total CO2,26 as samples were analyzed at a central laboratory. In addition, higher serum bicarbonate levels might reflect compensation for respiratory acidosis. However, if indeed this is the case, there are no biological rationale for how respiratory acidosis is associated with improved survival and reduced kidney disease progression in CKD.
In summary, the results of this investigation suggest that higher serum bicarbonate levels within the normal range are associated with reduced hazard of mortality and CKD progression. Furthermore, serum bicarbonate level is an independent predictor of CKD progression. Interventional trials are warranted to determine the renoprotective effects of maintaining serum bicarbonate levels between 28 and 30 mmol/l in individuals with CKD.
METHODS
Study participants
The details of the AASK trial have been published earlier.1,2,27 African Americans, aged 18 to 70 years, with hypertensive CKD (defined by a GFR between 20 and 65 ml/min per 1.73 m2 by renal clearance of iodine I125 iothalamate clearance and diastolic blood pressure >95 mm Hg) were eligible for the study. Patients were excluded if they had elevated fasting or random blood glucose, treatment for diabetes, urinary protein to creatinine ratio of >2.5, accelerated or malignant hypertension, serious systemic disease, congestive heart failure, or a specific indication for or contra-indication to a study drug.
AASK trial
The AASK study was a 3 × 2 factorial design. Participants were randomized to ramipril, metoprolol, or amlodipine, and to one of two blood pressure goals (a usual mean arterial pressure of 102–107 mm Hg or a low mean arterial pressure goal of ≤92 mm Hg). Recruitment into the full-scale trial began in February 1995, with planned follow-up through to September 2001. Using standardized forms, trained personnel obtained data on baseline demographic, clinical, and laboratory data. At a seated position, 10–20 ml of blood was collected in serum separator tubes, allowed to clot at room temperature for at least 30 min, and then centrifuged. Serum samples were mailed overnight in a frozen pack to the Central Biochemistry Laboratory at the Cleveland Clinic for standardized measurements. Serum bicarbonate was measured using either the kinetic ultraviolet method (Roche Hitachi 747 autoanalyzer, Roche, Indianapolis, IN) or a CO2 electrode (Beckman CX3 Delta autoanalyzer, Beckman, Brea, CA). Urinary protein excretion was expressed as the urinary protein to creatinine ratio from a 24-h urine collection. The primary outcome was the rate of change in iothalamate GFR, which was measured twice during baseline, and at months 3, 6, and every 6 months thereafter for a 3.5-to 6.5-year follow-up period. A main secondary outcome was a composite of death, end-stage renal disease (dialysis or transplantation), or GFR event (defined as a GFR reduction by 50% or by 25 ml/min per 1.73 m2 from the mean of two GFR measurements at baseline). The outcomes committee adjudicated the above events.
Statistical analysis
Baseline characteristics were summarized and compared between the four serum bicarbonate groups (defined a priori using clinical criteria) using independent two-sample t-tests or Wilcoxon rank-sum tests as appropriate for continuous variables and Fisher’s exact test for categorical variables.
Examination of death, dialysis, or GFR event composite
A series of Cox regression models were fit to relate the clinical composite outcome of GFR event, dialysis, and death to serum bicarbonate as a continuous variable using different levels of covariate adjustment, with stratification of the baseline hazard by clinical center. Follow-up time was censored at the administrative end date of the study and permanent loss-to-follow-up. The initial model included only the AASK intervention groups and the basic demographic factors of age and gender as covariates. Next, as the most important biological confounder of the associations of serum bicarbonate with outcomes is baseline level of kidney function, this model was adjusted for the mean of the two baseline measurements of iothalamate GFR and logarithm of the baseline 24-h urinary protein to creatinine ratio. A two-slope spline model was used for iothalamate GFR, with separate slope below and above 40 ml/min per 1.73 m2, because the association of GFR with the clinical composite was found to be stronger at lower than at higher GFR levels.
In the third step, an errors-in-variables regression calibration approach28 was applied to the Cox regression from step 2 to adjust for measurement error in the iothalamate GFR and log-transformed urine protein to creatinine measurements. This step was necessary because both these variables have nontrivial random variation, so that conventional Cox regression analysis without an errors-invariables adjustment may not fully control for confounding between bicarbonate and baseline renal function. Based on the observed variation in measured GFR between the two baseline assessments, and the average variation between log-transformed urine protein to creatinine ratio over 6-month interval in the follow-up period of the trial, the regression calibration model assumed measurement error variances of 0.0146 for log-transformed GFR and of 0.324 for log-transformed urine protein/creatinine ratio. The variance estimates correspond roughly to assumed coefficients of variation of 15 and 58% for GFR and urine protein/creatinine ratio, respectively, and reflect both assay measurement error and short-term biological fluctuations. In the last step, atherosclerotic conditions, congestive heart failure, mean arterial pressure, smoking, body mass index, serum albumin, and use of diuretics at baseline were added to the above model. The results of the final model were interpreted as exploratory, as we recognized that some of these additional factors could be on the causal pathway between effects of bicarbonate and renal outcome (thus leading to a risk of overadjustment).
To examine whether higher serum bicarbonate levels in the normal range (20–30 mmol/l) were associated with better outcomes, two approaches were adopted. In the first approach, the above Cox regression models were examined in the sub-group of patients with serum bicarbonate levels in the 20–30 mmol/l range. In the second approach, we modified the above Cox regression analyses by using a four-degree of freedom restricted cubic regression spline basis matrix to graphically model the relationship between serum bicarbonate levels and the clinical composite outcome.29 Because the cubic splines provided smooth functions over the range of serum bicarbonate in the data set, the results are relatively insensitive to the selection of the knot points. This analysis was run with adjustment for age, gender, trial assignment, iothalamate-measured GFR, and logarithm of urinary protein to creatinine ratio, using serum bicarbonate of 20 mmol/l as the reference.
Examination of dialysis or GFR event composite
The above analyses were repeated with dialysis or GFR event composite as the outcome of interest.
Examination of death
The above analyses were repeated with death as the outcome of interest. Deaths included those that occurred after the onset of end-stage renal disease.
Sensitivity analyses
Baseline GFR estimated from the four-variable MDRD equation was used instead of the iothalamate GFR to examine the associations of serum bicarbonate with the composite outcomes in Cox models.
Correlation between anion gap (calculated as the difference between sodium and the sum of chloride and bicarbonate) and bicarbonate was tested using a Pearson correlation coefficient. In an additional set of analyses, anion gap was used instead of serum bicarbonate as the predictor variable in Cox models of time to death, dialysis, or GFR event composite or dialysis or GFR event composite.
Estimated protein intake was calculated from the baseline 24-h urine urea nitrogen excretion by the formula 6.25(urine urea nitrogen in g/day +(0.031 g nitrogen/kilogram/day)(weight in kg)).30
In all of the above models, key assumptions of the Cox regression models, including linear effects of baseline factors and proportional hazards over time, were evaluated by preliminary diagnostic analyses. First, quadratic terms were tested for each continuous covariate to test for the presence of nonlinear effects of continuous covariates. Only GFR and albumin had nonlinear associations with the outcomes. Therefore, both linear and quadratic terms were included for these variables in each of the Cox regressions described above. Second, interactions of each predictor with follow-up time were tested to evaluate the assumption of proportional hazards. No significant deviation from proportional hazards was detected.
Acknowledgments
The AASK study was conducted by the AASK investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with investigators of the AASK study and does not necessarily reflect the opinions of the AASK study or the NIDDK. This work is supported by NIDDK Grants RO1-DK077298 and RO1-DK078112 awarded to SB.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Appel LJ, Wright JT, Jr, Greene T, et al. Long-term effects of reninangiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic ki kidney disease in African Americans. Arch Intern Med. 2008;168:832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 3.Phisitkul S, Khanna A, Simoni J, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77:617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 4.de Brito-Ashurst I, Varagunam M, Raftery MJ, et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SN, Abramowitz M, Hostetter TH, et al. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009;54:270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009;24:1232–1237. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertow GM, Moe SM. Calcification or classification? J Am Soc Nephrol. 2005;16:293–295. doi: 10.1681/ASN.2004121115. [DOI] [PubMed] [Google Scholar]
- 9.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35:S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 11.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76:667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HY, Baylis C, Verlander JW, et al. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol. 2007;293:F1238–F1247. doi: 10.1152/ajprenal.00151.2007. [DOI] [PubMed] [Google Scholar]
- 13.Brungger M, Hulter HN, Krapf R. Effect of chronic metabolic acidosis on thyroid hormone homeostasis in humans. Am J Physiol. 1997;272:F648–F653. doi: 10.1152/ajprenal.1997.272.5.F648. [DOI] [PubMed] [Google Scholar]
- 14.Clase CM, Kiberd BA, Garg AX. Relationship between glomerular filtration rate and the prevalence of metabolic abnormalities: results from the Third National Health and Nutrition Examination Survey (NHANES III) Nephron Clin Pract. 2007;105:c178–c184. doi: 10.1159/000100489. [DOI] [PubMed] [Google Scholar]
- 15.Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med. 2008;25:798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Lofberg E, Gutierrez A, Anderstam B, et al. Effect of bicarbonate on muscle protein in patients receiving hemodialysis. Am J Kidney Dis. 2006;48:419–429. doi: 10.1053/j.ajkd.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Mathur RP, Dash SC, Gupta N, et al. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006;28:1–5. doi: 10.1080/08860220500461187. [DOI] [PubMed] [Google Scholar]
- 19.Reaich D, Channon SM, Scrimgeour CM, et al. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am J Physiol. 1993;265:E230–E235. doi: 10.1152/ajpendo.1993.265.2.E230. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins D, Burton PR, Bennett SE, et al. The metabolic consequences of the correction of acidosis in uraemia. Nephrol Dial Transplant. 1989;4:92–95. [PubMed] [Google Scholar]
- 21.Papadoyannakis NJ, Stefanidis CJ, McGeown M. The effect of the correction of metabolic acidosis on nitrogen and potassium balance of patients with chronic renal failure. Am J Clin Nutr. 1984;40:623–627. doi: 10.1093/ajcn/40.3.623. [DOI] [PubMed] [Google Scholar]
- 22.Lu KC, Lin SH, Yu FC, et al. Influence of metabolic acidosis on serum 1,25(OH)2D3 levels in chronic renal failure. Miner Electrolyte Metab. 1995;21:398–402. [PubMed] [Google Scholar]
- 23.Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:661–671. [PubMed] [Google Scholar]
- 24.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation, of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 25.Wu DY, Shinaberger CS, Regidor DL, et al. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1:70–78. doi: 10.2215/CJN.00010505. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum B. Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis. 2000;35:1068–1071. doi: 10.1016/s0272-6386(00)70041-2. [DOI] [PubMed] [Google Scholar]
- 27.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14:S154–S165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll R, Ruppert D, Stefanski LA, et al. Measurement Error in Nonlinear Models: A Modern Perspective, Second Edition. 2. Chapman & Hall; Florence, KY: 2006. [Google Scholar]
- 29.Heinzl H, Kaider A, Zlabinger G. Assessing interactions of binary time-dependent covariates with time in Cox proportional hazards regression models using cubic spline functions. Stat Med. 1996;15:2589–2601. doi: 10.1002/(SICI)1097-0258(19961215)15:23<2589::AID-SIM373>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]