Abstract
Purpose of review
To summarize the current status of blood screening to prevent transfusion-transmitted babesiosis.
Recent findings
Babesia microti has recently been determined to be the most common transfusion-transmitted pathogen in the United States. Patients who acquire transfusion transmitted babesiosis (TTB) often experience severe illness with an associated mortality rate of about 20%. Recent studies have demonstrated that laboratory screening using B. microti antibody and/or PCR assays can effectively identify infectious blood donors and that this approach may offer a cost effective means of intervention. Pathogen inactivation methods may offer an alternative solution. None of these methods has yet been licensed by FDA, however, and current efforts to prevent TTB rely on excluding blood donors who report having had babesiosis.
Summary
TTB imposes a significant health burden on the US population. Further research is needed to better inform decisions on optimal screening strategies and reentry criteria, but given the acute need and the currently available screening tools, initiation of blood donor screening to prevent TTB should be given high priority.
Keywords: Babesia microti, babesiosis, transfusion, screening
Introduction
Human babesiosis is a world-wide emerging protozoal disease caused by several species of intraerythrocytic protozoa that are transmitted primarily by hard-bodied (Ixodes) ticks but also through blood transfusion [1,2]. Transfusion-transmitted babesiosis (TTB) was first recognized in 1979, 10 years after the first tick transmitted case was described [3]. The causative agent was Babesia microti, the most common cause of human babesiosis and TTB [1,2]. B. microti infection is endemic in the Northeastern and northern Midwestern United States (Figure 1). A second species of babesia found in the far West (Babesia duncani) also has been shown to be transmitted through blood transfusion, but only a few cases of B. duncani infection have been described [4]. Babesiosis is difficult to identify in donors because about a quarter of adults experience asymptomatic B. microti infection and most patients have persistent asymptomatic infection for months following antibiotic therapy [5,6]. Thus, infected donors and transfusion personnel are unaware of the contaminated blood donation.
Figure 1. Human babesiosis and Lyme disease cases in the United States 2011–2013.
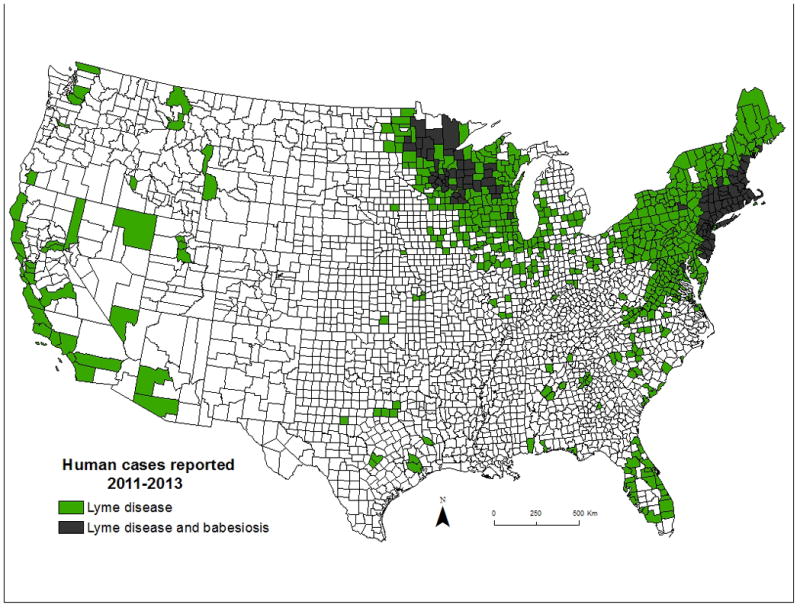
(from Diuk-Wasser M, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and Clinical consequences. Trends in Parasitol, 2016;32:30–42)
The problem of TTB
TTB Epidemiology
In 2008, the FDA announced that Babesia microti was the most frequent transfusion-transmitted microbial pathogen in the United States with more than 100 reported cases. The rate of TTB has continued to increase since that time [2]. Unlike tick-borne babesiosis that occurs primarily in endemic areas in the summer and early autumn, TTB occurs in both endemic and non-endemic areas year round. Babesia contaminated blood from endemic areas is occasionally transported to non-endemic areas. Furthermore, a resident of a non-endemic area can develop an asymptomatic B. microti infection while traveling to an endemic area and then donate blood upon returning home. The incubation period following transfusion is usually one to two months but may be as long as six months [2].
The risk of TTB varies widely because the distribution of babesiosis cases is not uniform, even within endemic regions [7–9]. The extent of blood donor exposure to B. microti infection has been estimated by serosurveys of donor populations. The first published report was by Popovsky et al. who found seropositivity rates of 3 – 5% in Massachusetts blood donors based on an immunofluorescence assay using a 1:16 cut-off [7]. Additional blood donor seroprevalence studies were subsequently carried out using the immunofluorescence assay with a positive cutoff titer of 1:64 [8,10–13] (Table 1). Seroprevalence values ranged from 0 to 2.5%, similar to the 2.5% seroprevalence result noted among healthy non-donor residents of Connecticut [8]. A recombinant antigen-based B. microti ELISA was used in a 2005 study to screen 3,490 Connecticut blood donors [9]. Rates of enzyme-linked immunosorbent assay (ELISA) positivity of 6.6% and 5.0% were observed in endemic and non-endemic areas, respectively, compared to 1.4% and 0.3%, respectively, on subsequent testing with an immunofluorescence assay. A subset of the immunofluorescence-positive samples were tested by a nested PCR assay and about half (53%) were found positive and presumably parasitemic. Overall, these studies indicate that a substantial fraction (1% to 5%) of the donor population in endemic areas has been infected with B. microti and is seropositive while a much smaller fraction is likely to be infectious.
Table 1.
Babesia microti seroprevalence studies
| Study | Location | Years | No. subjects | B. microti seroprevalence | B. microti PCR positive |
|---|---|---|---|---|---|
| Popovsky et al.[7] | MA | 1988 | 779 | 3–5% | N/A |
| Leiby et al.[9] | CT | 1999 | 1,745 | 1.4% | 53% (10/19 seropositives) |
| Johnson et al.[10] | CT | 2000–2007 | 27,592 | 0.75–1.5% | N/A |
| Johnson et al.[11] | CT | 2009 | 1,002 | 2.5% | 0.3% (3/1002) |
| Tonnetti et al.[12] | MN | 2010–2011 | 2,150 | 2% | 2.4% (1/42 seropositives) |
| O’Brien et al.[13] | Canada | 2013 | 13,993 | 0% | 0% |
Geographic variation in case number helps to account for the large variation in TTB risk. The risk of acquiring babesiosis from transfused blood has been found to be as high as 1 case per 604 red blood cell (RBC) units in a small prospective surveillance study in Connecticut [14]. In contrast, the risk was only 1 case per 994,444 RBC units in a national American Red Cross Hemovigilance Program, which included areas where babesiosis is not endemic [15]. TTB risks that fall between these estimates include those noted in a Rhode Island Blood Center Program (1 case per 20,454 RBC units) and a Rhode Island/Brown University study (1 case per 8,532 RBC units) from 2005 to 2007 [16,17].
TTB disease severity
Patients who experience TTB are often seriously ill and the TTB mortality rate is about 20% [1,2]. Between 2005–2010, 11/307 (3.6%) transfusion related deaths reported to the FDA were due to TTB [18]. TTB patients are older than the general population (median age of 65 years; range <1 to 94 years) and most have at least some immune impairment [2]. Patients who most commonly acquire babesiosis through blood transfusion include neonates and the aged, those with hematologic or other cancers, sickle cell disease, thalassemia, cardiovascular or gastrointestinal surgery, organ transplantation, and trauma with posttraumatic splenectomy.
Prevention of TTB
It is clear that the current approach to prevention of TTB through exclusion of blood donors who report having had babesiosis is not sufficiently effective. Two successful approaches have been taken to prevent other transfusion-transmitted infections, pathogen inactivation of blood products and laboratory screening to identify and remove donations. Babesia inactivation has been shown to be feasible with methods that are in use for other pathogens but no licensed commercial products are yet available for this purpose. These methods include riboflavin, ultraviolet light, the Mirasol pathogen reduction system, photochemical inactivation with amotosalen, and long wavelength ultraviolet light [19–22]. A laboratory screening study to prevent TTB was first carried out in 2010–2011 [23]. This and subsequent studies suggest that B. microti antibody and/or PCR assays can be effective screening tools for preventing TTB.
Diagnostic and screening tests for TTB
Assays for B. microti infection
B. microti infects erythrocytes and its presence can be directly determined by microscopic examination of thin blood smears or by amplification of parasite DNA from whole blood or an erythrocyte fraction. A number of PCR assays for B. microti DNA have been developed, all relying on amplification of 18s rRNA sequences [24–28]. PCR is more sensitive than thin blood smear and is widely considered to be a confirmatory test [29]. A negative PCR result does not rule out babesiosis because parasite concentration may be below the limit of detection, [26,30]. Although parasitemia as low as 6 parasites in a milliliter of blood can be identified in principle [26], the limit of detection for these assays varies depending on such parameters as sample volume. Thus, while 6 parasites in a 1 milliliter volume of blood can be detected, 6 parasites circulating in an asymptomatically infected blood donor will not be detected unless the sample of blood analyzed contained those 6 parasites.
Assays that detect antibodies to B. microti have long been used to support a diagnosis of babesiosis in symptomatic patients, the majority of whom have detectable antibody. The most well-established serological test for clinical diagnosis has been the immunofluorescence assay has been the IFA using erythrocytes from parasitemic hamsters which contain B. microti whole cell antigens [31]. The timing of antibody appearance in the blood following infection has not been well established in humans, although limited studies indicate a lag time of approximately 7 to 14 days [32]. As with many other infections, immunoglobulin M is the earliest antibody isotype that is generated, followed by IgG over subsequent weeks [33]. Antibody concentration typically peaks at titers of ≥ 1:1,024 [6,33]. A small fraction of asymptomatically infected blood donors are infected just prior to donation. Thus, detection of IgM antibodies is relevant for blood screening.
Determining which diagnostic test or combination of tests is optimal for screening is complex. Use of antibody alone as a screening tool is problematic. Firstly, blood donors who have been very recently infected (window period) are by definition antibody negative. Secondly, people who have cleared a B. microti infection typically exhibit a measurable antibody titer lasting for months and sometimes years [6,30]. In endemic populations, the majority of seropositive individuals have been found to be PCR negative and thus are most likely to represent prior rather than active infections [34,35]. These individuals pose little or no risk to blood safety but would be subject to deferral based on detection of B. microti antibody. The use of PCR alone as a screening tool is also problematic. PCR is not a true gold standard for infection because a positive result may simply indicate the presence of “dead DNA” rather than active infection, although microbial DNA generally is rapidly cleared from the blood [6] [30]. Conversely, a small number of cases have been reported in which B. microti seropositive but PCR negative individuals were further tested for active infection by inoculation of their blood into hamsters from which B. microti was later recovered [30,36]. These cases demonstrate that some seropositive, PCR-negative donors harbor active B. microti infection. Although their numbers may be low, these individuals show that PCR testing by itself will not always detect active infection. Accordingly, donor screening models have been proposed incorporating both serology and PCR [36,37].
The performance of donor screening assays will be affected by the extent to which assay targets, whether protein or nucleic acid, are conserved across the repertoire of B. microti strains. The extent of genomic diversity within B. microti is not fully delineated due to the paucity of studies comparing genomes from a wide variety of isolates across endemic geographic regions. While available data point toward limited B. microti strain diversity [38–40] [Carpi G, Walter KS, Ben Mamoun C, et al, unpublished data], how adequately existing B. microti assays accommodate whatever diversity exists remains to be determined.
Blood screening policy
Given that B. microti causes more transfusion-transmitted infections than any other pathogen in the U.S. [22], development of approaches to mitigate TTB has been a high priority concern of the US Food and Drug Administration, which overseas blood safety. B. microti received initial FDA attention as one of several tick-borne agents posing a potential risk to the blood supply at a workshop convened in 1999 [41]. In 2008, the FDA held the first public workshop focused entirely on the risk of TTB and options to mitigate the problem [42]. Subsequently, TTB has been a topic addressed by the FDA’s Blood Products Advisory Committee in meetings in 2010 and 2015 [43,44]. A principal goal of these meetings has been to evaluate potential donor screening approaches and to explore how they might be implemented based on epidemiological risk factors for B. microti infection. While no assays for B. microti have yet been sanctioned by the FDA for diagnostic purposes or licensed for blood screening to date, serological and nucleic acid assays have been evaluated in several recent blood donor studies carried out under Investigational New Drug (IND) protocols, which is the first step towards licensure. At its May 2015 meeting, where preliminary results of these studies were presented, the Blood Products Advisory Committee voted in favor of nationwide serological screening for B. microti, supplemented by nucleic acid testing in high-risk areas. A nine state group comprising six New England states along with NY, MN and WI that was proposed as the high risk area was preferred over a 15 state group that included additional mid-Atlantic states.
Babesia blood donor screening studies on products seeking FDA licensure
The first study in which blood donors were screened for B. microti under an IND protocol was carried out by Young et al. in Rhode Island between 2010–2011 [23]. Donors were screened in parallel by both immunofluorescence and real-time PCR assays. Of 2,113 blood units tested, 26 (1.23%) were found seropositive at a 1:128 cut-off, while none were positive by PCR (Table 2). The rate of TTB decreased from 24 cases over the five years prior to the study to no cases in the year following commencement of the study. While the difference was not significant, this finding suggests that antibody-based screening, with or without PCR, can be effective in reducing TTB.
Table 2.
Summary of blood donor B. microti screening study results.
| Study | Regions screened | No. donors tested | Serologic assay | No. seropositive | No. PCR positive | No. PCR and seropositive | Specificity (95% CI)* | ||
|---|---|---|---|---|---|---|---|---|---|
| Endemic | Non-endemic | Endemic | Non-endemic | Endemic | Endemic | ||||
| Young, 2012 [23] | RI | N/A | 2,113 | IFA (AFIA) | 26/2,113 (1.23%) | N/A | 0 | 0/26 (0%) | N/A |
| Moritz, 2014 [34] | CT, MA | AZ, OK | 13,269 | IFA (AFIA) | 38/5,080 (0.75%) | 3/4,022 (0.075%) | 5/5,080 (0.1%) | 5/38 (13%) | 99.95% (99.82–99.99) |
| Levin, 2014 [45] | NY | AZ | 15,000 | ELISA | 46/5,000 (0.92%) | 8/5,000 (0.16%) | 1/5,000 (0.02%) | 1/46 (2.2%) | NA |
| Levin, 2016 [35] | NY | NM | 26,703 | ELISA | 38/13,757 (0.28%) | 11/8,363 (0.13%) | 8/13,757 (0.06%) | 8/38 (21%) | 99.93% (99.84–99.97) |
after exclusion of confirmed positives
Note: The sensitivity of the IFA (AFIA) assay for detection of PCR-positive patients with symptoms of babesiosis was reported as 81.6%, with non-detected cases presumed to be in the window period[34]. The sensitivity of the ELISA assay for PCR or blood smear positive patients with symptoms of babesiosis was 78.3%[35]. The two methods were statistically equivalent in sensitivity.
A subsequent study by Moritz et al. under IND consisted of parallel testing by immunofluorescence and PCR assays of 13,269 paired plasma and whole blood samples from donors in endemic (MN, WI, MA, CT) and non-endemic (AZ, OK) regions [34]. The immunofluorescence assay, an automated version termed AFIA, yielded a 0.75% seropositivity rate in an endemic region comprising MA and CT. Five of 38 (13%) seropositive samples in this group were also positive by PCR and hence potentially infectious. In the non-endemic region, three out of 4,022 donors were positive by AFIA at a 1:64 cut-off, of which two were found negative by B. microti Western blot and assumed to be false positive, while the third was assumed to be a true positive based on a positive Western blot. Antibody test specificity was 99.95%, which increased to 99.98% at a 1:128 cut-off.
Levin et al. developed a microplate ELISA utilizing a combination of B. microti peptide antigens derived from proteins of the BMN1 family [45]. Using an epitope mapping approach, unique, immunodominant peptides from BMN1-9 (also known as BmSA1) and BMN1-17 were identified based on reactivity with sera from patients with symptomatic B. microti infections. In an initial study, 15,000 blood donors from endemic (NY) and non-endemic (AZ) regions were screened by ELISA [45]. Repeat reactive rates of 1.08% and 0.40% were observed in endemic and non-endemic regions, respectively, which were reduced to 0.92% and 0.16% upon application of a revised cut-off. A second study was carried out in 2013 in which approximately 27,000 donor samples were screened by ELISA, with repeat reactive samples reflexed to PCR, immunofluorescence and immunoblot assays[35]. In this study, with application of an adjusted cut-off, the rate of seropositive donors in a region of NY that combined areas of greater and lesser endemicity was 0.28%, while the rate in a non-endemic region (NM) was 0.13%. Nine out of 45 (20%) seropositive donors from endemic regions were found positive by PCR; none were found in the non-endemic region. Using immunoblot to identify and exclude true positives in the non-endemic region, the specificity of the ELISA was 99.93%.
A follow-on study by Bloch et al. showed that 75% of seropositive donors from the preceding study remained seropositive, and 2 out of 5 donors that were initially positive by PCR remained PCR positive, one year later [46]. Consistent with other reports [6,30], this study suggested that both seropositivity and parasite DNA may persist in some donors for relatively long periods of time following infection. These findings may be of particular significance with respect to consideration of criteria for re-entry of donors who are deferred based on B. microti screening results.
Detection of window period infections
As window period infections cannot be detected serologically by definition, direct methods for parasite detection must be used. In the study by Moritz et al., all donors were tested by PCR in parallel with the immunofluorescence (AFIA) serological method. Results showed no PCR-positive donors among the AFIA-negative group [34]. A subsequent report from the same group described results of ongoing screening in which a total of 84,209 donors in endemic regions of CT, MA, MN and WI had been tested by AFIA and PCR. This yielded 331 (0.4%) that were reactive by either AFIA or PCR. Eight blood donors were AFIA negative but PCR positive (0.01%), all of whom were tested between June and August, which are peak tick transmission months for B. microti [36]. The study by Levin et al. relied on serology as a first step, so that ELISA negative/PCR positive donors were not identifiable; however, a subset of samples in that study were screened by PCR in parallel with ELISA. None of 2,522 samples tested were found positive by PCR and negative by ELISA. In summary, window period infections have been demonstrated at low frequency, on the order of 2.4% of all donors found positive for B. microti antibodies or DNA based on the results of one study [36]. The cost-effectiveness of detecting window period infections by screening with PCR or other similarly sensitive direct methods is an issue under current debate (see below).
ELISA vs immunofluorescence assays for use in screening
Overall, the specificities of the AFIA method and the peptide ELISA for B. microti were statistically equivalent, as were the percentage of seropositive samples that were also positive by PCR and thus potentially infectious (Table 2). Both assays have been positioned for FDA licensure for blood screening and according to the manufacturers have been adapted for high volume throughput [23,35].
The trade-off between overdetection and underdetection of B. microti-infected or exposed donors is a function of assay methodology and stringency, in addition to geography and timing of screening. Cut-offs for both immunofluorescence and ELISA methods have been subject to modification based on study results in order to balance the number of donors that would be detected with active infection against those with previous exposure but not actively infected, who would be unnecessarily deferred. Combining serological assays with PCR either serially, in a reflex algorithm, or in parallel, presents potential options to improve overall accuracy in identification of infectious donors. Serial algorithms result in higher specificity which is important to offset the lower positive predictive value that is associated with screening in low-prevalence populations. Ultimately, these questions will be subject to analyses of cost-effectiveness and effect on decreasing the donor pool (see below).
Cost effectiveness of screening
A cost-effective screening program to prevent TTB would ideally not exceed the target threshold cost that is used for interventions mitigating other infectious risks of transfusion.
This cost threshold has traditionally been 1 million dollars per quality-of-life-years (QALY) [47]. QALY is a generic measure of disease burden that includes both quality and quantity of life lived. One year of life in perfect health is equivalent to a QUALY of 1 while death is equivalent to a QALY of 0. Several cost effectiveness studies have been carried out to estimate the costs of different B. microti blood screening programs. Each has arrived at different conclusions regarding the cost effectiveness of TTB screening for similar programs. Two studies found that effective programs could be devised that would cost less than the 1 million dollars per QALY threshold [37,48]. One of these found that universal donor antibody screening for B. microti in endemic areas of the US is the most promising approach while the other found that universal PCR in four endemic states would be most cost effective. In contrast, a third study found that no program that significantly decreased TTB would fall within the 1 million dollars per QALY threshold [47]. The reason for the variability in estimates is the lack of robust data for underlying assumptions. Some of the critical assumptions used in these models that varied were TTB transmission probability (how many units of blood will result in a single infection), the risk and severity of TTB complications, the impact of babesiosis on quality of life, the ability of screening to avert TTB (test performance), and the costs of screening. In general, the most cost effective strategies involved a single laboratory screening test used in a limited number of endemic States. The consensus of these studies is that additional research is needed to more accurately quantify these variables.
Deferral and reentry strategies
The endpoint for all babesia screening programs is to discard blood donations from anyone who is found to have babesia infection and to defer future donations until there is assurance that their blood is no longer infectious. Once non-infectious, reentry into the blood donor pool is possible. In order to achieve these goals, screening programs must distinguish infectious from non-infectious donors and determine when a person becomes non-infectious. Unfortunately, our current knowledge of the basic biology of human babesial infection is limited, including the minimum infectious dose, the best determination of infectiousness, and the duration of human infectiousness. It is likely that the minimum infectious dose from a blood transfusion is only a few (~5) babesia, based on evidence from transfusion transmission of Plasmodia. If so, this would exceed the ability of current direct detection techniques such as smear, PCR, and antigen capture to identify all B. microti-infected donor blood. Previous studies have shown that babesia infection may persist asymptomatically for as long as two years in untreated immunocompromised hosts and as long as a year in immune intact persons who have been given standard antibiotic therapy [6]. Given these complexities, a completely effective blood screening approach may be very difficult to develop, with the more realistic goal being a significant reduction in TTB incidence rather than total eradication. Although several approaches for TTB prevention have been studied, including exclusion based on history of babesiosis, antibody screening, and antibody screening with PCR, a blood screening method has yet to be licensed by the FDA. A similar challenge exists in regard to reentry strategies. The current standard is to bar anyone with a previous history of babesiosis or laboratory evidence of previous infection from ever donating again. This presupposes that babesial infection can never be eradicated. Contrary to this conservative approach, current data suggest that babesial infection does not exceed two years in immune intact persons. At a time when meeting demands for blood is a challenge, it will be important to devise approaches that allow donors to reenter the donor pool without putting recipients at significantly increased risk of TTB. One possible approach is to rescreen donors a year after they have tested positive and allow them to donate if repeat antibody and PCR screening results are negative.
Conclusions
TTB imposes a significant health burden on the US population. The number of cases is increasing, often with serious or fatal consequences. Several screening methods have been developed and validated based on antibody and PCR assays, but none has yet been licensed by the FDA. Pathogen inactivation methods likewise have not been licensed for use on babesia. Further research is needed to gain a better understanding of such fundamental issues as the minimal infectious dose and the duration of infectiousness, in order to inform decisions on optimal screening strategies and reentry criteria. Given the acute need and the currently available screening tools, however, initiation of blood donor screening to prevent TTB should be given high priority.
Key points.
Babesia microti has recently been determined to be the most common transfusion-transmitted pathogen in the United States.
Patients who acquire transfusion transmitted babesiosis (TTB) often experience severe illness with an associated mortality rate of about 20%.
Recent studies have demonstrated that laboratory screening using B. microti antibody and/or PCR assays can effectively identify potentially infectious donors.
A cost-effective screening program to prevent TTB would ideally not exceed the target threshold cost that is used for interventions mitigating other infectious risks of transfusion.
Although further research is needed to better inform decisions on optimal screening strategies and reentry criteria, given the acute need and the currently available screening tools, initiation of blood donor screening to prevent TTB should be given high priority.
Acknowledgments
Funding source: The authors acknowledge support by the Gordon and Llura Gund Foundation (to P.J.K.).
Footnotes
Conflicts of interest: Dr. Levin is President and Chief Scientific Officer of Immunetics, Inc. which has developed a B. microti ELISA intended for use in blood donor screening. He is the PI on NIH grant # R44HL127698 for development of a B. microti screening assay. Dr. Krause is a coinvestigator on this grant.
References
- **1.Vannier E, Krause PJ. Human babesiosis. N Engl J Med [Internet] 2012 Jun 21;366(25):2397–407. doi: 10.1056/NEJMra1202018. [cited 2014 Jul 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22716978 This manuscript is a general review of human babesiosis. [DOI] [PubMed] [Google Scholar]
- **2.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med [Internet] 2011 Oct 18;155(8):509–19. doi: 10.7326/0003-4819-155-8-201110180-00362. [cited 2013 Nov 30] Available from: http://annals.org/article.aspx?articleid=474991 This manuscript is the most comprehensive case series of TTB that has been published. [DOI] [PubMed] [Google Scholar]
- 3.Jacoby GA, Hunt JV, Kosinski KS, Demirjian ZN, Huggins C, Etkind P, et al. Treatment of Transfusion-Transmitted Babesiosis by Exchange Transfusion. 2009 doi: 10.1056/NEJM198011063031906. http://dx.doi.org/101056/NEJM198011063031906. [DOI] [PubMed]
- 4.Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, et al. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36(7):779–89. doi: 10.1016/j.ijpara.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Krause PJ, McKay K, Gadbaw J, Christianson D, Closter L, Lepore T, et al. Increasing Health Burden of Human Babesiosis in Endemic Sites. Am J Trop Med Hyg [Internet] 2003 Apr 1;68(4):431–6. [cited 2014 Nov 24] Available from: http://www.ajtmh.org/content/68/4/431.long. [PubMed] [Google Scholar]
- 6.Krause PJ, Spielman A, Telford SR, Sikand VK, McKay K, Christianson D, et al. Persistent Parasitemia after Acute Babesiosis. N Engl J Med [Internet] 1998;339(3):160–5. doi: 10.1056/NEJM199807163390304. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9664092. [DOI] [PubMed] [Google Scholar]
- 7.Popovsky MA, Lindberg LE, Syrek AL, Page PL. Prevalence of Babesia antibody in a selected blood donor population. Transfusion [Internet] Jan;28(1):59–61. doi: 10.1046/j.1537-2995.1988.28188127955.x. [cited 2013 Nov 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/3341068. [DOI] [PubMed] [Google Scholar]
- 8.Krause PJ, Telford SR, Ryan R, Hurta AB, Kwasnik I, Luger S, et al. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol [Internet] 1991 Jan;29(1):1–4. doi: 10.1128/jcm.29.1.1-4.1991. [cited 2013 Nov 30] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=269691&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leiby DA, Chung APS, Gill JE, Houghton RL, Persing DH, Badon S, et al. Demonstrable parasitemia among Connecticut blood donors with antibodies to Babesia microti. Transfusion [Internet] 2005 Nov;45(11):1804–10. doi: 10.1111/j.1537-2995.2005.00609.x. [cited 2013 Nov 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16271108. [DOI] [PubMed] [Google Scholar]
- *10.Johnson ST, Cable RG, Tonnetti L, Spencer B, Rios J, Leiby Da. Seroprevalence of Babesia microti in blood donors from Babesia-endemic areas of the northeastern United States: 2000 through 2007. Transfusion [Internet] 2009 Dec;49(12):2574–82. doi: 10.1111/j.1537-2995.2009.02430.x. [cited 2014 Nov 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19821951 This manuscript reports on a large B. microti serosurvey study of 27,000 blood donations over 7 years. [DOI] [PubMed] [Google Scholar]
- 11.Johnson ST, Van Tassell ER, Tonnetti L, Cable RG, Berardi VP, Leiby Da. Babesia microti real-time polymerase chain reaction testing of Connecticut blood donors: potential implications for screening algorithms. Transfusion [Internet] 2013 Feb 27;:1–6. doi: 10.1111/trf.12125. [cited 2013 Nov 21] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23445322. [DOI] [PubMed]
- 12.Tonnetti L, Thorp AM, Deisting B, Bachowski G, Johnson ST, Wey AR, et al. Babesia microti seroprevalence in Minnesota blood donors. Transfusion [Internet] 2013 Aug;53(8):1698–705. doi: 10.1111/j.1537-2995.2012.03948.x. [cited 2013 Nov 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23145838. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien SF, Delage G, Scalia V, Lindsay R, Bernier F, Dubuc S, et al. Seroprevalence of Babesia microti infection in Canadian blood donors. Transfusion. 2016;56(1):237–43. doi: 10.1111/trf.13339. [DOI] [PubMed] [Google Scholar]
- 14.Gerber MA, Shapiro ED, Krause PJ, Cable RG, Badon SJ, Ryan RW. The risk of acquiring Lyme disease or babesiosis from a blood transfusion. J Infect Dis [Internet] 1994 Jul;170(1):231–4. doi: 10.1093/infdis/170.1.231. [cited 2016 Jul 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/8014507. [DOI] [PubMed] [Google Scholar]
- 15.Tonnetti L, Eder AF, Dy B, Kennedy J, Pisciotto P, Benjamin RJ, et al. Transfusion-transmitted Babesia microti identified through hemovigilance. Transfusion [Internet] 2009 Dec;49(12):2557–63. doi: 10.1111/j.1537-2995.2009.02317.x. [cited 2016 Jul 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19624607. [DOI] [PubMed] [Google Scholar]
- 16.Asad S, Sweeney J, Mermel LA. Transfusion-transmitted babesiosis in Rhode Island. Transfusion [Internet] 2009 Dec;49(12):2564–73. doi: 10.1111/j.1537-2995.2009.02380.x. [cited 2013 Nov 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19761547. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Gubernot DM, Nakhasi HL, Mied PA, Asher DM, Epstein JS. Transfusion-transmitted babesiosis in the United States: Summary of a workshop. Transfusion. 2009;49(12):2759–71. doi: 10.1111/j.1537-2995.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 18.Cushing M, Shaz B. Transfusion-transmitted babesiosis: achieving successful mitigation while balancing cost and donor loss _3746 1404..1407. Transfusion. 2012;52:1404–7. doi: 10.1111/j.1537-2995.2012.03746.x. [DOI] [PubMed] [Google Scholar]
- 19.Tonnetti L, Thorp AM, Reddy HL, Keil SD, Goodrich RP, Leiby DA. Riboflavin and ultraviolet light reduce the infectivity of Babesia microti in whole blood. Transfusion [Internet] 2013 Apr;53(4):860–7. doi: 10.1111/j.1537-2995.2012.03791.x. [cited 2016 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22803831. [DOI] [PubMed] [Google Scholar]
- 20.Tonnetti L, Proctor MC, Reddy HL, Goodrich RP, Leiby DA. Evaluation of the Mirasol pathogen [corrected] reduction technology system against Babesia microti in apheresis platelets and plasma. Transfusion [Internet] 2010 May;50(5):1019–27. doi: 10.1111/j.1537-2995.2009.02538.x. [cited 2016 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20030791. [DOI] [PubMed] [Google Scholar]
- 21.Grellier P, Benach J, Labaied M, Charneau S, Gil H, Monsalve G, et al. Photochemical inactivation with amotosalen and long-wavelength ultraviolet light of Plasmodium and Babesia in platelet and plasma components. Transfusion [Internet] 2008 Aug;48(8):1676–84. doi: 10.1111/j.1537-2995.2007.01762.x. [cited 2016 Jul 4] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18503613. [DOI] [PubMed] [Google Scholar]
- 22.Lobo CA, Cursino-Santos JR, Alhassan A, Rodrigues M. Babesia: an emerging infectious threat in transfusion medicine. Knoll LJ, editor. PLoS Pathog [Internet] 2013 Jan;9(7):e1003387. doi: 10.1371/journal.ppat.1003387. [cited 2014 Nov 25] Available from: http://dx.plos.org/10.1371/journal.ppat.1003387. [DOI] [PMC free article] [PubMed]
- *23.Young C, Chawla A, Berardi V, Padbury J, Skowron G, Krause PJ. Preventing transfusion-transmitted babesiosis: Preliminary experience of the first laboratory-based blood donor screening program. Transfusion. 2012;52(7):1523–9. doi: 10.1111/j.1537-2995.2012.03612.x. This manuscript reports on the first laboratory based donor screening program and highlights benefits and problems associated with B. microti donor screening. [DOI] [PubMed] [Google Scholar]
- 24.Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman a, Thomford JW, et al. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol [Internet] 1992 Aug;30(8):2097–103. doi: 10.1128/jcm.30.8.2097-2103.1992. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=265450&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol [Internet] 2012 Mar;50(3):903–8. doi: 10.1128/JCM.05848-11. [cited 2014 Dec 2] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3295123&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloch EM, Lee T-H, Krause PJ, Telford SR, Montalvo L, Chafets D, et al. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion [Internet] 2013 Oct;53(10):2299–306. doi: 10.1111/trf.12098. [cited 2013 Nov 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23362840. [DOI] [PubMed] [Google Scholar]
- 27.Rollend L, Bent SJ, Krause PJ, Usmani-Brown S, Steeves TK, States SL, et al. Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector Borne Zoonotic Dis [Internet] 2013 Nov;13(11):784–90. doi: 10.1089/vbz.2011.0935. [cited 2015 Nov 28] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3822370&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G, Wormser GP, Zhuge J, Villafuerte P, Ip D, Zeren C, et al. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis [Internet] 2015:1–7. doi: 10.1016/j.ttbdis.2015.03.001. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1877959X15000382. [DOI] [PubMed]
- 29.Krause PJ, Telford S, Spielman a, Ryan R, Magera J, Rajan TV, et al. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol [Internet] 1996 Nov;34(11):2791–4. doi: 10.1128/jcm.34.11.2791-2794.1996. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=229405&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Leiby Da, Johnson ST, Won KY, Nace EK, Slemenda SB, Pieniazek NJ, et al. A longitudinal study of Babesia microti infection in seropositive blood donors. Transfusion [Internet] 2014 Sep;54(9):2217–25. doi: 10.1111/trf.12622. [cited 2014 Nov 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24673297 This manuscript demonstrates the persistence of parasitemia and babesia antibody among asymptomatic blood donors infected with B. microti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chisholm ES, Ruebush TK, Sulzer AJ, Healy GR. Babesia microti infection in man: evaluation of an indirect immunofluorescent antibody test. Am J Trop Med Hyg [Internet] 1978 Jan 1;27(1 Pt 1):14–9. doi: 10.4269/ajtmh.1978.27.14. [cited 2013 Nov 25] Available from: http://europepmc.org/abstract/MED/343608/reload=0. [DOI] [PubMed] [Google Scholar]
- *32.Gumber S, Nascimento FS, Rogers KA, Bishop HS, Rivera HN, Xayavong MV, et al. Experimental transfusion-induced Babesia microti infection: dynamics of parasitemia and immune responses in a rhesus macaque model. Transfusion [Internet] 2016;56(June) doi: 10.1111/trf.13521. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26892459 This manuscript provides the first insights into the timing of parasitemia and immunological responses to B. microti infection using a non-human primate model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruebush TK, Chisholm ES, Sulzer AJ, Healy GR. Development and persistence of antibody in persons infected with Babesia microti. Am J Trop Med Hyg [Internet] 1981 Jan;30(1):291–2. doi: 10.4269/ajtmh.1981.30.291. [cited 2016 Jan 6] Available from: http://www.ncbi.nlm.nih.gov/pubmed/7011069. [DOI] [PubMed] [Google Scholar]
- **34.Moritz ED, Winton CS, Johnson ST, Krysztof DE, Townsend RL, Foster Ga, et al. Investigational screening for Babesia microti in a large repository of blood donor samples from nonendemic and endemic areas of the United States. Transfusion [Internet] 2014 Sep;54(9):2226–36. doi: 10.1111/trf.12693. [cited 2014 Nov 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24865803 This manuscript describes one of the largest and most comprehensive studies carried out using B. microti immunofluorescence and PCR blood screening assays. [DOI] [PubMed] [Google Scholar]
- **35.Levin AE, Williamson PC, Bloch EM, Clifford J, Cyrus S, Shaz BH, et al. Serologic screening of United States blood donors for Babesia microti using an investigational enzyme immunoassay. Transfusion [Internet] 2016 doi: 10.1111/trf.13618. 00. Available from: http://doi.wiley.com/10.1111/trf.13618 This manuscript reports a major study demonstrating application of an ELISA screening assay followed by PCR for B. microti screening of blood donors. [DOI] [PMC free article] [PubMed]
- 36.Moritz ED, Stramer SL. Blood donation screening for Babesia microti: feasibility and results. ISBT Sci Ser [Internet] 2015;10(S1):169–72. Available from: http://doi.wiley.com/10.1111/voxs.12169. [Google Scholar]
- 37.Bish EK, Moritz ED, El-Amine H, Bish DR, Stramer SL. Cost-effectiveness of Babesia microti antibody and nucleic acid blood donation screening using results from prospective investigational studies. Transfusion [Internet] 2015 doi: 10.1111/trf.13136. 00:n/a – n/a. Available from: http://doi.wiley.com/10.1111/trf.13136. [DOI] [PubMed]
- 38.Benach JL, White DJ, McGovern JP, Jacovina MM. Immunological relationships of Long Island isolates of Babesia microti. Am J Trop Med Hyg [Internet] 1979 Jul;28(4):643–8. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/88904. [PubMed] [Google Scholar]
- 39.Cornillot E, Dassouli A, Garg A, Pachikara N, Randazzo S, Depoix D, et al. Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. PLoS One [Internet] 2013;8(9):e72657. doi: 10.1371/journal.pone.0072657. [cited 2016 Jul 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24023759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemieux JE, Tran AD, Freimark L, Schaffner SF, Goethert H, Andersen KG, et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol [Internet] 2016;1(June):16079. doi: 10.1038/nmicrobiol.2016.79. Available from: http://www.nature.com/articles/nmicrobiol201679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burckhardt John C. Federal Register /Workshop on the Potential for Transfusion-Transmission of Tickborne Agents. Fed Regist [Internet] 1998;63(228):65598. Available from: https://www.federalregister.gov/articles/1998/11/27/98-31606/workshop-on-the-potential-for-transfusion-transmission-of-tickborne-agents. [Google Scholar]
- 42.Shuren J. Approaches to Reduce Risk of Transfusion-Transmitted Babesiosis in the United States; Public Workshop. Fed Regist. 2008;73(E8-15799):39972. [Google Scholar]
- 43.Food and Drug Administration. Blood products advisory committee 104th Meeting. Topic I: Risk of Babesia infection by blood transfusion and the status of laboratory tests. 2012:1–24. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm205013.htm.
- 44.BPAC. Blood Products Advisory Committee Meeting Issue Summary - Strategies for Implementation of Antibody and Nucleic Acid-Based Testing for Babesia microti in Blood Donors [Internet] 2015 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM446274.pdf.
- 45.Levin AE, Williamson PC, Erwin JL, Cyrus S, Bloch EM, Shaz BH, et al. Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay. Transfusion [Internet] 2014 Sep;54(9):2237–44. doi: 10.1111/trf.12763. [cited 2014 Nov 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24995863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloch EM, Levin AE, Williamson PC, Cyrus S, Shaz BH, Kessler D, et al. A prospective evaluation of chronic Babesia microti infection in seroreactive blood donors. Transfusion [Internet] 2016;00:1–8. doi: 10.1111/trf.13617. Available from: http://doi.wiley.com/10.1111/trf.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodell AJ, Bloch EM, Krause PJ, Custer B. Costs, consequences, and cost-effectiveness of strategies for Babesia microti donor screening of the US blood supply. Transfusion [Internet] 2014 Sep;54(9):2245–57. doi: 10.1111/trf.12805. [cited 2014 Nov 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25109338. [DOI] [PubMed] [Google Scholar]
- 48.Simon MS, Leff JA, Pandya A, Cushing M, Shaz BH, Calfee DP, et al. Cost-effectiveness of blood donor screening for Babesia microti in endemic regions of the United States. Transfusion [Internet] 2014 Mar;54(3 Pt 2):889–99. doi: 10.1111/trf.12492. [cited 2014 Jul 21] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4039174&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diuk-Wasser M, Vannier E, Pandya A, Krause PJ. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and Clinical consequences. Trends in Parasitol. 2016;32:30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


