Summary
Smoke inhalation injury is a serious medical problem that increases morbidity and mortality after severe burns. The National Repository of the American Burn Association houses data from 203,422 patients treated at 99 U.S. burn hospitals and centers between 2005 and 2014. These data show that the presence of smoke inhalation injury increases mortality (by nearly 24-fold) in burn patients under 60 years and in those with a total burned surface area between 0.1 and 19.9%.1 The incidence of smoke inhalation injury may increase exponentially in mass casualty, large-scale fires;2,3 14 (78%) of the 18 patients severely burned in the World Trade Center attack who were admitted to one burn center had inhalation injury.4 However, relatively little attention has been paid to this devastating condition, and the bulk of research is limited to preclinical basic science studies. This review discusses our current understanding of the pathophysiology of smoke inhalation injury, the best evidence-based treatments, and challenges and future directions in diagnostics and management.
Keywords: Smoke, inhalation injury, respiratory failure, clinical management, challenges in research
Pathophysiology of Smoke Inhalation Injury
Smoke inhalation injury is classified based upon the anatomical location of injury, specifically the upper airway (supraglottic), lower airway (infraglottic), or lung parenchyma. This section will focus on the pathophysiology of lower airway and parenchymal injuries, as these injuries contribute to clinical morbidity and mortality.
Airway Injury
The degree of airway injury depends on the duration of smoke exposure5 and the composition of the smoke. Although the upper airway (oropharynx) may have direct thermal burns from flame associated with overwhelming heat transfer, this type of injury is rare. The specific heat of air is quite low, and the upper airway has a relatively large surface area, high laminar flow, and highly efficient physiologic mechanisms for transferring heat to equilibrate temperature and minimize thermal injury.6,7 Therefore, most damage is attributable to chemical injury from noxious organic agents that are present in smoke, largely on the surface of the smoke particles, which are deposited in the lower airways and parenchyma according to their size.
Noxious smoke components stimulate the release of neuropeptides from peripheral endings of sensory neurons within the airways to induce neurogenic inflammation.8,9 The lung has an abundant network of vagal nerve sensory C-fibers, which contain proinflammatory peptides such as substance P, neurokinins, and calcitonin gene-related peptide.10,11 Plasma extravasation and edema then result as secondary responses.9,12–14 Neutral endopeptidase, the principal degradative enzyme targeting neuropeptides, has also been shown to play a pivotal role in smoke-induced airway changes.15
Although pathological changes in the airway secondary to smoke inhalation injury vary depending on numerous factors (e.g., chemical composition of smoke, duration and intensity of smoke exposure), direct injury along with neurogenic inflammation leads to major pathological changes that result in narrowing of airway lumina, ultimately restricting normal airflow to the alveoli. Airway luminal narrowing, which can lead to clinical problems, is attributable to 1) airway mucosal hyperemia, 2) formation of obstructive casts in the airway, and 3) bronchospasm.
Airway Pathophysiology
Mucosal hyperemia
The bronchial circulation provides arterial perfusion to the airway and related structures distal to the carina with approximately two-thirds of the venous return to the heart through the pulmonary veins. Anastomoses between the bronchial and pulmonary circulations, generally unimportant, become prominent after smoke inhalation injury. Three hours after smoke inhalation the bronchial circulation, normally approximately 1% of cardiac output, has increased by 10-fold to the trachea, 15-fold to the left main bronchus, and 20-fold to the right main bronchus.16 Blood flow is also increased in the distal airways, i.e., approximately 4- and 6-fold in the right and left lungs, respectively, with the increases being augmented in association with the increased cardiac output and hypermetabolic response elicited when inhalation injury is accompanied by a cutaneous burn.16,17
Increased airway blood flow is associated with airway mucosal edema, airway exudation of protein-rich fluid, increased pulmonary transvascular fluid flux, and flux of neutrophils and inflammatory mediators. Collectively, these responses lead to airway luminal narrowing with increased airway resistance, limitation of airflow to alveoli, fibrin clot and cast formation, fluid accumulation in the airways, parenchymal edema, and exacerbation of parenchymal inflammation and damage.
Studies using preclinical models have revealed the importance of those changes in the genesis of pulmonary dysfunction associated with smoke inhalation injury. For instance, ablation (ligation) of bronchial blood flow in conscious sheep decreases smoke-induced increases in pulmonary transvascular fluid flux (lung lymph flow).18–22 Ligation of the bronchial artery in dogs exposed to acrolein inhalation also delays pulmonary edema and lessens its magnitude.23 Additionally, neutrophil numbers and chemokine levels (e.g., IL-8) are significantly lower in animals with bronchial artery occlusion than in uninjured controls.20–22 These studies underscore the need for pharmacologic agents (perhaps aerosolized) that reduce airway edema.
Airway obstruction
Obstruction of the large and small airways is a life-threatening complication of smoke inhalation injury. Near total obstruction of a few proximal bronchi compromises ventilation of individual lung segments,24 whereas partial obstruction, which reduces ventilatory flow, can still produce hypoxia by inadequate oxygen saturation of blood passing through pulmonary capillaries in areas of ventilation-perfusion mismatching. Removal of obstructing airway casts immediately improves oxygenation and hemodynamics in patients with smoke inhalation injury.25 The resolution of obstructive casts in the treatment of smoke inhalation injury is critical, as the casts promote atelectasis; stasis of fluid and particulate matter, which contribute to pneumonia; and localized barotraumas.26
Obstructive airway casts are made up of debris including exfoliated airway epithelial cells, inflammatory cells, mucus, and protein-rich plasma exudates. In a preclinical model of severe smoke inhalation injury, nearly 100% of bronchial epithelial cells were exfoliated within 24 hours of exposure.27 Compromised airway integrity makes the airway and respiratory system vulnerable to infection and amplifies plasma leak and trans-endothelial migration of inflammatory cells into the airways. There is also growing evidence that neutrophils migrate into the airways from bronchial mucus glands.28,29 Because of impaired function of ciliary cells related to exfoliation, mucus clearance is reduced, allowing mucus to migrate distally to the lower airways and parenchyma.30
The protein-rich plasma that leaks into the airway following smoke inhalation contains procoagulant factors that promote fibrin formation in the airway, solidifying cast substrates and making them difficult to remove. The severity of airway obstruction is significantly attenuated by nebulization with anticoagulants31,32 or a tissue plasminogen activator that solubilizes clots.33 Bronchial artery ligation also significantly reduces formation of obstructive airway casts.21 Airway casts up to 5 cm long are common in patients with smoke inhalation injury. Extensive bronchial obstruction is seen in lung tissue of children who die after burns.27 When directly measured in preclinical models of burn and smoke inhalation injury, the mean cross-section diameter of the airway was reduced by about 29% in bronchi, 11% in bronchioles, and 1.2% in respiratory bronchioles.34 Bronchial obstruction peaked at 24 hours, whereas the bronchiolar obstruction score continued increasing over the ensuing 48 hours. In this study, approximately 10% of the bronchi scored showed airway obstruction between 90 and 100%.34
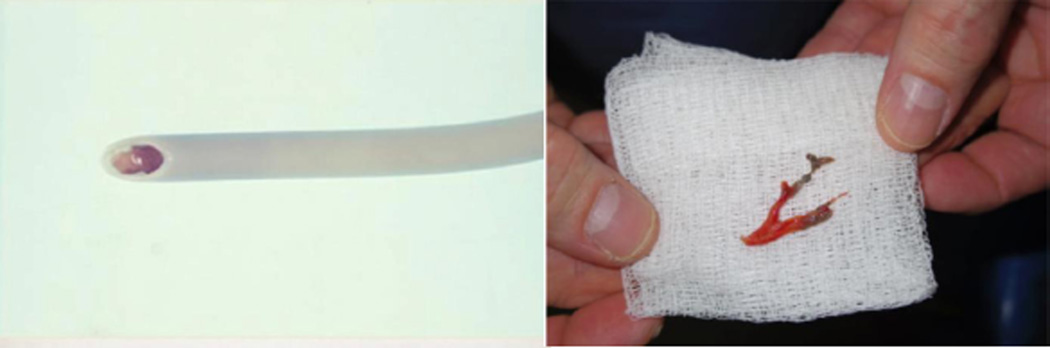
As shown in Figure 1, obstructive casts adhere to the airway wall and narrow the lumen. These may extend into the smaller airways through direct injury, gravity, and inadequate ciliary function, causing hypo- or non-ventilation of the alveoli. Blood vessels in these under-ventilated areas fail to constrict normally, causing a ventilation/perfusion mismatch. This transfer of blood flow from ventilated to non-ventilated areas results in poor oxygenation of arterial blood, which can lead to hypoxemic changes in organs. Obstruction of part of the bronchial tree results in hyperventilation and over-inflation of the non-occluded lungs, which increases airway pressure when volume-controlled mechanical ventilation is administered.35 Overstretching of the ventilated alveoli also induces synthesis and secretion of proinflammatory chemokines such as IL-8, which attracts neutrophils to the injured site to cause more tissue damage.36 Additionally, systemic hypoxia modulates various proinflammatory cytokines and inflammatory mediators.37–39 The use of anti-inflammatory agents along with standard treatments for smoke inhalation injury (i.e., anticoagulants, mucolytics, and bronchodilators) may be advisable.
Figure 1.
Obstructive airway casts taken form a 4 year old patient with burn and smoke inhalation injury. Typical ranges for pediatric airway size can be found in manuscript form Griscom, NT and Wohl, ME (1986) AJR146:233–237
Bronchospasm
The precise mechanisms by which smoke inhalation injury causes bronchospasm are poorly understood. Neuropeptides produced in the submucosa after airway injury are a potential cause of bronchoconstriction.40-43 Delivery of an aerosolized bronchodilator is standard at many burn centers and hospitals. In preclinical models, the non-specific adrenergic receptor agonist epinephrine has been shown to reduce (by β2 receptor agonism) the smoke-induced increase in airway pressure and improve pulmonary compliance in sheep with smoke exposure alone16 or combined with thermal burn.44 Aerosolized albuterol or epinephrine almost immediately reduces airway pressure (unpublished data). Taken together, the above observations suggest that smoke inhalation injury causes spasms of airway smooth muscle, further decreasing airway luminal cross-section area and impairing normal air flow.
Parenchymal injury
Pathophysiological changes of the lung parenchyma are accompanied by hypoxia as reflected by reduced PaO2/FiO2. This is associated with pulmonary edema, increased airway pressure, and decreased lung compliance. The severity of lung injury, which may be sufficient to induce acute respiratory distress syndrome (ARDS), depends on the presence of toxic agents and their concentration in the smoke, the size of the smoke particles, and the duration of smoke exposure. Pulmonary transvascular flux is increased approximately 5-fold. Factors responsible for fluid movement between compartments in the lung, pulmonary microvascular hydrostatic and oncotic pressures, and capillary permeability are related as expressed in the Starling-Landis equation.45 Jυ = Kf[(Pc-Pi)-a(πc-πi)], where Jυ is fluid movement between compartments, Pc is capillary hydrostatic pressure, Pi is interstitial hydrostatic pressure, πc is capillary oncotic pressure, π i is interstitial oncotic pressure, Kf is the filtration coefficient, and σ is the osmotic reflection coefficient.
The pulmonary microvascular endothelium is permeable not only to water, but also protein. Following smoke inhalation injury an increase in pulmonary microvascular pressure is accompanied by a decrease in plasma protein concentration, changes associated with a subsequent increase in lung lymph flow.46 The above observations are consistent with previous studies demonstrating that the pulmonary vascular filtration coefficient (index of permeability to small particles) is increased while the reflection coefficient (index of permeability to protein) is decreased,47,48 resulting in lung parenchymal edema with protein-rich exudates.
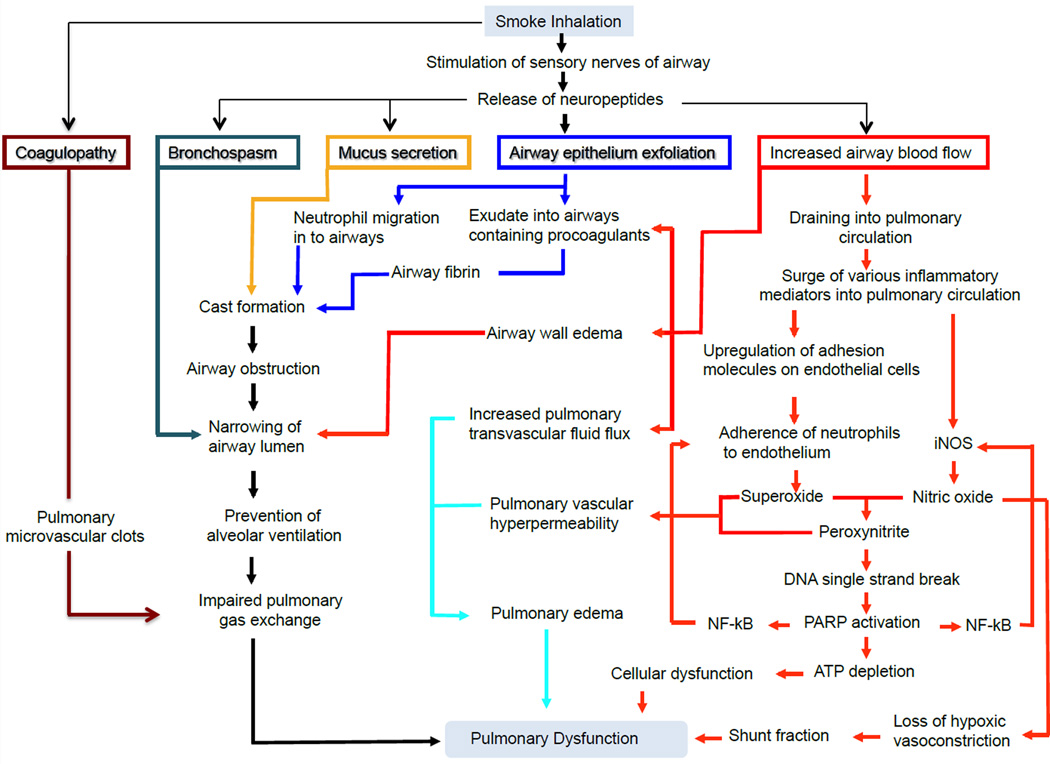
Recent studies have shown that the lung endothelium is also permeable to inflammatory cells such as neutrophils.46 Examination of neutrophil kinetics within the lung identified increased activation of circulating neutrophils (CD11b/syndecan-4 positive neutrophils), lung tissue myeloperoxidase activation, and greater numbers of neutrophils in lung lymph samples collected after smoke inhalation.46 Pulmonary parenchymal injury involves both the alveolar epithelium and the microvascular endothelium, suggesting that infusion of agents such as a neuronal nitric oxide synthase [nNOS] inhibitor, poly[ADP]ribose polymerase [PARP] inhibitor, or peroxynitrite decomposition catalyst into the bronchial circulation may provide therapeutic benefits by reducing pulmonary edema and improving gas exchange.49–51 Moreover, loss of epithelia integrity and compromised cellular function in the airway reduce bacterial clearance and increase the risk of infection of the airways and the lungs. Activation of proinflammatory mediators and elevated levels of reactive oxygen species contribute to tissue destruction and organ dysfunction following smoke inhalation.52–57 The many faceted pathophysiology of airway and parenchyma damage is summarized in Diagram 1.
Diagram 1.
Diagnosis and Treatment
Although diagnostic and treatment strategies may differ between burn centers and hospitals, they should be guided by an understanding of the pathophysiology described above. The comorbid effect of inhalation injury per se and that of pneumonia, which is a frequent complication in burn patients with inhalation injury, mandates prompt diagnosis and initiation of treatment, including mechanical ventilation if needed. The comorbid effect of inhalation injury, which varies according to the extent of the thermal injury, is greatest (20% added mortality) in the region of the LA50 for all age groups. In those burn patients with inhalation injury who develop pneumonia (2/3 of patients by 7–8 days post burn), the comorbid effect is independent but additive, and mortality increases by a maximum of 60% when the extent of the burn is in the region of the LA50, with lesser effect on either side of the LA50.58
Fiberoptic bronchoscopy is commonly used to identify inhalation injury in the supra and infraglottic airway and does so with an accuracy of 86% and no false positive diagnoses.59 To supplement bronchoscopy, Oh and colleagues have developed a method of grading pulmonary changes on the admission chest CT to assess distal airway injury and the “degree and depth of damage to the airway mucosa.” On the basis of extent and severity of pulmonary opacification on the CT scan, smaller airway disease was identified, and when combined with fiberoptic bronchoscopy, this method was associated with a 12.7-fold increase in pneumonia, acute lung injury ALI/ARDS, and death.60 The grading process, which is lengthy and influenced by mechanical trauma and prior chest surgery, is currently being refined. In a swine model of inhalation injury, virtual bronchoscopy reliably identified airway narrowing, was comparable to fiberoptic bronchoscopy in grading inhalation injury, and correlated with PaO2-to-FiO2 ratios.61 The addition of 133Xenon ventilation-perfusion lung scan and pulmonary function tests can increase diagnostic accuracy to a small extent, but these are considered difficult to justify on the basis of cost for the modest increase in positive diagnoses.59
In an ovine model of inhalation injury, the six inert gas technique was used to characterize changes in lung airflow and blood flow induced by inhalation injury. The preponderant change occurred in the airway with a modest increase in true shunt and the appearance of a low airflow, high blood flow compartment in proportion to the dose of smoke administered.62 The importance of airway compromise has prompted a search for ventilation techniques that optimize airway patency, reduce ventilation-perfusion mismatching, and prevent the development of pneumonia. High frequency interrupted flow positive pressure ventilation has been reported to decrease the occurrence of pneumonia and is associated with increased survival of patients with inhalation injury.63 In a comparison of high frequency percussive ventilation, high frequency oscillatory ventilation, and high frequency jet ventilation, Allen et al. identified improvement in O2 and CO2 tensions; attenuation of lung inflammation and reduction in histologic evidence of lung injury; improvement in static lung compliance, ventilation, and oxygenation index; a decrease in ventilator-associated pneumonia; and improved survival with high frequency percussive ventilation as compared to the other modalities. 64 They concluded that high frequency percussive ventilation has the unique capacity to exploit both high and low frequency ventilation to favorably influence gas exchange while providing lung-protective, low tidal volume ventilatory support.
Clinicians in Japan have endorsed the use of fiberoptic bronchoscopy and repeated bronchoalveolar lavage for removal of endobronchial pseudomembranes and other debris as well as the use of high frequency percussive ventilation.65 Toon and colleagues in Australia utilize nebulized n2 agonists, heparin, and N-acetylcysteine (NAC) and have suggested early lung decontamination by lavage or nebulization of amphoteric, hypertonic chelating agents such as those used in treating chemically injured eyes.66
The importance of lung-protective ventilation has prompted evaluation of both ECMO and extracorporeal CO2 removal therapy. Each of these modalities has been effective in removing CO2 and reducing PaCO2, and EMCO has been effective as a salvage modality in individual patients with severe ALI and ARDS.67,68 Disappointingly, in a sheep model of inhalation injury, early use of the extracorporeal CO2 removal device had no effect on severity of lung injury or mortality.69
Independent of ventilatory modality, simple prone positioning has been reported to improve oxygenation in adult burn patients with severe ARDS in association with a prompt increase in PaO2/FiO2 ratio.70 In that study, survival at 48 hours was 78% but that decreased to 33% by the time of discharge. There were no unintended extubations, but facial pressure ulcers were observed in four of the treated patients. The authors concluded that prone positioning can be safely utilized in a burn ICU and achieves an increase in P/F ratio but with persistent high mortality. As an adjunctive consideration in the ventilatory management of patients with inhalation injury, White et al. have identified lower interbreath interval complexity as assessed by respiratory wave forms from spontaneous breathing trials as being associated with extubation failure in intubated patients.71 Greater interbreath irregularity was observed in those patients who were successfully extubated. The authors concluded that wave form analysis could be useful in predicting extubation success and reducing the need for reintubation.
Laboratory studies have identified nebulized tiotropium as an agent for possible use in the treatment of patients with inhalation injury.72 Additionally, the infusion of a tetracycline analog, as well as a platelet activating factor (PAF) inhibitor (CV 3988) and pentoxifylline, have each been reported to improve the acute respiratory distress induced by smoke inhalation in animal models.73–75 In an ovine model, CV3988 exerted a beneficial effect on PaO2, PaCO2, pulmonary dynamic resistance and static compliance, reduced airflow-perfusion mismatching, and decreased histologic evidence of pulmonary damage when administered post injury only.73 Clinical trials of each of those agents are anticipated. Antithrombin (AT), which has both anticoagulant and anti-inflammatory activity, has shown promise in limited clinical trials. Kowal-Vern and colleagues have reported that, in a group of nine burn patients treated with plasma-derived AT, airway resistance was decreased, oxygenation increased, and the incidence of pneumonia reduced compared to controls.76 In a subsequent study comparing patients with inhalation injury and minor burns with patients having inhalation injury and major burns, the authors detected AT levels only in the bronchoalveolar lavage fluid from patients with more extensive burns. In contrast, TNF-α and IL-6 levels were significantly increased in the bronchoalveolar lavage fluid at admission and days 3–6, as compared to plasma levels, which had decreased by days 3–6, suggesting a therapeutic role for aerosolized AT.76*
As noted above, a variety of therapeutic agents are used in clinical practice. These can be classified as 1) mucolytic agents, 2) anticoagulants, or 3) bronchodilators. These agents are included in the treatment protocol at Shriners Hospitals for Children®—Galveston, which is an example of best evidence-based care and is summarized Table 1.
Table 1.
Example of an evidence-based protocol for patients (0–18 years) with smoke inhalation injury.
|
Mucolytic Agents
N-acetylcysteine (NAC) is a powerful mucolytic agent commonly used in the treatment of smoke inhalation injury, and it is indicated for patients with abnormal inspissated mucus secretions.80 NAC is also an airway irritant and may directly induce bronchoconstriction. Thus, patients should be evaluated for signs of bronchospasm and a bronchodilator added if wheezing is present.
Anticoagulants
Effects of aerosolized anticoagulants for treatment of smoke inhalation injury have been described in both preclinical and clinical studies. Aerosolized heparin (5,000 units in 3 ml) decreases airway cast formation, and combination treatment with NAC reduces ventilator days and mortality in pediatric patients.81–83 Heparin exerts a potent anticoagulant effect solely through binding to AT.84,85 Thus, its effect is limited when AT is deficient. In experimental animals with burn and smoke inhalation injury, the combination of aerosolized heparin and recombinant AT (aerosolized or intravenous) improves pulmonary function. These treatment approaches improve lung compliance, reduce pulmonary edema, and diminish airway obstruction better than control treatments.31,32 Systematic reviews confirm that inhaled anticoagulants improve survival and lessen morbidity in preclinical and clinical studies of smoke inhalation injury.83,86
Bronchodilators
Smoke inhalation injury to the lower airways results in a chemical tracheobronchitis, producing wheezing, mucosal sloughing, cast formation, and bronchospasms. Aerosolized bronchodilators are useful for several reasons. They induce bronchial muscle relaxation and stimulate mucociliary clearance. Additionally, bronchodilators decrease airflow resistance and improve dynamic compliance.80,86 Currently useful bronchodilators include albuterol, levalbuterol, and racemic epinephrine. These should be given when wheezing or bronchospasm occurs.80
The present treatment protocol used at Shriners Hospitals for Children (SHC) at Galveston, a best evidence-based protocol, is summarized in Table 1. Ventilator support management, which is often institution- or physician-specific and is discussed above, is not included in Table 1.
Challenges and future directions in smoke inhalation injury research
A major challenge in smoke inhalation injury research is the accurate diagnosis and grading of injury severity. Diagnosis of smoke inhalation injury can be complicated by preexisting morbidities, such as infection and by the presence of cutaneous burns and by preexisting infection. Cutaneous burns induce a massive generalized inflammatory response, as reflected by pathological changes in the lung.87 Accordingly, ALI may occur in patients with scald burns but no smoke exposure.88 Preexisting pulmonary infection can be mistakenly diagnosed as smoke inhalation injury or conversely mask the symptoms of inhalation injury. Additionally, lung injury and pneumonia arising from mechanical ventilation (typically if it is required for more than 48 hours) can alter the outcome of inhalation injury. Consequently, some of the commonly used diagnostic criteria89–94 have been of uncertain reliability that has compromised their use. Even those studies that have proposed specific criteria for diagnosis and grading of inhalation injury have not been universally accepted.59–62,89–101 The lack of diagnostic consensus supports the conduct of a large prospective multicenter randomized controlled trial to develop universal guidelines for diagnosing and grading the severity of smoke inhalation injury. Recent large animal translational studies (unpublished data) suggest that magnetic resonance imaging may be useful for assessing injury severity and extent of lung involvement. Further studies such as these should be conducted to aid development of new tools and methods for diagnosis and grading.
Successful treatment of smoke inhalation injury will depend on not only accurate diagnosis and grading of smoke inhalation injury, but also the development of therapies that target both airway and parenchymal injuries. Airway management should focus on 1) reducing airway hyperemia/edema, 2) ameliorating or preventing bronchospasm, 3) reducing mucus secretion, 4) preventing/lysing airway fibrin clots, and 5) repairing airway epithelium to improve mucociliary clearance. Lung parenchymal management should target 1) increased permeability of both the pulmonary microvascular and alveolar epithelium as well as 2) parenchymal inflammation.
Pilot studies of potential treatment strategies are underway including those focused on: 1) anti-inflammatory agents, 2) nitric oxide synthase (NOS) inhibitors, 3) reactive nitrogen species modulators, 4) antioxidants, 5) PARP inhibitors, 6) anti-mucus secretion agents, 7) anticoagulants, 8) fibrinolytic agents, 9) specific bronchodilators, 10) hydrogen sulfide donors, 11) neuropeptide modulators, and 12) cyclooxygenase inhibitors. Additionally, cellular therapy currently in the preclinical stage offers the possibility of accelerating airway healing and favorably altering the pathophysiologic changes. We have recently found that administration of adipose tissue-derived mesenchymal stem cells either intravenously102 or through nebulization (unpublished data) may hold potential for treating smoke inhalation damage.
Other strategies should be developed to counter endothelial hyperpermeability, for which there is currently no FDA-approved drug. An exciting area of research in this regard is the exploration of the role of potent permeability factors, adhesion molecules, intercellular tight junction molecules, and endothelial glycocalyx integrity disruption. Studies investigating changes in fluid movement across the alveolar epithelium may focus on mechanisms involving Na/K and Na/K-ATPase pumps. Electron or confocal microscopic studies should reveal actual damage to both the endothelium and alveolar epithelium.
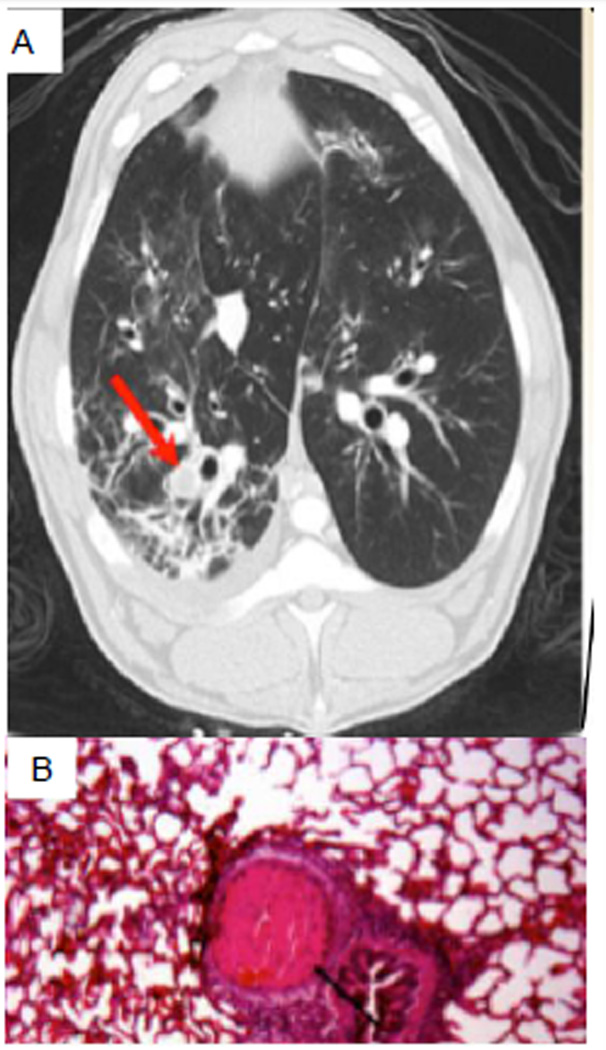
Future studies should also focus on approaches to attenuating airway and parenchymal coagulopathy. To this end, identification of coagulopathic changes within lung tissue is a particularly important goal. Burn patients experience a hypercoagulable state 24 hours after injury, as seen by high levels of the activated factors VII, thrombin/AT complexes, and plasminogen activator inhibitor type-1.103 In both patients and experimental animals with burn and smoke inhalation, hypercoagulation is associated with a severe fall in plasma concentrations of AT, the most potent endogenous anticoagulant. AT deficiency following cutaneous burn is correlated with total surface area burn, presence of smoke inhalation, length of ICU and hospital stay, morbidity severity, and mortality.104–107 However, the role of coagulopathies within the pulmonary parenchyma is understudied. Whether isolated “pure” smoke inhalation causes coagulopathy in the lung tissue itself is unknown. We recently conducted CT scans of the lungs of sheep exposed to smoke and noted blood clots in pulmonary arteries of different sizes (Fig. 3), a finding that was confirmed by both postmortem macroscopic and microscopic examination of the lung tissue. Clinical studies are needed to confirm these results, supporting the routine use of chest CT scans in burn patients with suspected smoke inhalation injury, and clarify the coagulopathic and pathophysiologic mechanisms involved.
Another research area deserving attention is the acute and long-term effects of smoke inhalation on extrapulmonary organ dysfunction, particularly changes in the central nervous system. Because these acute symptoms can be masked by analgesics and sedatives in burn patients, careful neurological examination should be considered in future studies. Long-term follow up studies on the neurological status of burn patients with smoke inhalation are of particular interest, as a recent preclinical study has shown that neuronal and astrocyte dysfunction/death occurs after smoke inhalation (unpublished).
Clinical trials are currently underway to test new smoke inhalation injury treatments. One pilot clinical trial (at SHC Galveston) is investigating the use of nebulized epinephrine (non-specific adrenergic agent) in patients with severe burns and smoke inhalation. The rationale for the use of nebulized epinephrine is that it may exert beneficial effects by acting on all adrenergic receptors (not just the β receptors), ultimately inducing bronchodilation, reducing airway hyperemia and edema, and limiting both fluid and inflammatory mediator fluxes to the lung parenchyma (bronchial artery ablation effect). Another approach currently being investigated is oral administration of high doses of the antioxidant vitamin E. This multicenter study (SHC Galveston, SHC Houston, and Southwestern Medical Center in Dallas) has 24 enrolled subjects and in whom direct assessments of pulmonary function will be made.
In the event of pulmonary failure, lung transplantation bears consideration; however, this approach is limited by the presence of large burns, which preclude use of immunosuppressive therapy because of high infection risk. A solution might lie in the development of bioengineered, non-immunogenic lung constructs that could be populated by endogenous cells, with the patient maintained by a period of extracorporeal lung support.
Further considerations in smoke inhalation injury research include questions regarding particular populations such as children and the role of underlying pathology such as chronic obstructive pulmonary disease. Perhaps differing strategies are more apt to produce benefit, such as the use of adrenergic agonists in children, who have more active receptors than adults. Furthermore, glucocorticoids or certain types of anti-inflammatory agents may be more effective in the face of chronic lung disease; this should be addressed in specific trials. Finally, attention should be given to prevention and treatment of long-term sequelae such as bronchiectasis and recurrent lung infections.
Conclusion
Despite recent advances in critical care and the management of burn patients, smoke inhalation injury continues to increase significantly the morbidity and mortality in burn patients. This is related, it least in part, to the difficulty in reliably grading the severity of inhalation injury and the paucity of evidence-based, generally accepted therapeutic interventions. Clinical trials should be undertaken to address these issues. Basic and preclinical translational studies should focus on determining molecular and cellular mechanisms that underlie both airway and lung parenchymal injury as well as the development of novel treatment approaches including the application of regenerative medicine and bioengineering. The authors recommend that an expert consensus conference be organized in the near future to establish a comprehensive protocol for a large prospective multicenter, ideally multinational, clinical trial. In that trial the noted gaps in clinical care and pathophysiologic understanding could be definitively addressed to reduce the morbidity and increase the salvage of patients with inhalation injury.
Figure 2.
Smoke inhalation causes pulmonary vascular clot: A) Chest CT scan in sheep with smoke inhalation injury (24hrs post-injury); and B) Postmortem (24hrs) lung tissue histology. Arrow indicates clot. Pictures represent data from 6 sheep.
Table 2.
Pediatric vs. adult burn patients: differences in pathpphysiologic variables
| Variables | Pediatric patients | Adult patients |
|---|---|---|
| Airway compliance | Higher1 | Lower2 |
| Fluid creep risk | Higher3 | Lesser4 |
| Pulmonary edema | Frequent5 | Less frequent6 |
| Tidal Volume | 9–10mL/kg7 | 6–8mL/kg8 |
| Airway pressure | Lower9 | Higher10 |
| Tracheostomy Complication: lumen narrowing scar |
Frequent11 | Less frequent12 |
| Acute right heart failure | Frequent13 | Less frequent14 |
Sarnaik AP, Pediatr Crit Care Med. 2004.
Endorf FW, J Burn Care Res. 2007.
Oscier C, Anaesthesia. 2014.
Goh CT, Paediatr Respir Rev. 2015.
Ruddy RM, pediatric Clinics of North America, 1994.
Ashbaugh DG, Lancet. 1967.
Sousse LE, J Am Coll Surg. 2015.
ARDS Net Study, N Engl J Med. 2000.
Thompson BT, Crit Care Clin. 2011.
Villar J, Crit Care Med. 2006.
Sen S, Burns. 2015.
Qing Y, Zhonghua Shao Shang Za Zhi. 2011.
Gonzales JN, Austin J Vasc Med. 2015.
Agarwal N, J Trauma. 1983.
Acknowledgments
This study was supported by the Department of Defense (W81XWH-1220086), National Institutes of Health, (P50GM060338, UL1TR001439, T32GM008256, R01GM056687, and R01HD049471), and Shriners Hospitals for Children (84050 and 84080).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Further details on preclinical management and specific therapy for systemic toxicity of specific smoke compounds can be found in textbooks “Fisherman’s Pulmonary Diseases and Disorders”77 and “Total Burn Care”78 and a review article by R.H. Demling.79
Contributors
PE, BAP, OS, SW, RM, HS, and DH planned this review and were responsible for the design, coordination, drafting, and finalization of the manuscript. PE, OS, and DH obtained funding.
Conflicts of interest
The authors have no conflicts of interest to declare.
Search Strategy and Selection Criteria
We searched PubMed (all dates) using “inhalation,” “airway,” “lung,” “pulmonary,” or “ventilation” combined with “thermal,” “burn,” or “smoke” as search terms. The resulting articles were also searched for relevant citations.
References
- 1.National Burn Repository, 2015 report: American burn Association. http://www.ameriburn.org/2015NBRAnnualReport.pdf.
- 2.Pittman HS, Schatzki R. Pulmonary effects of the Cocoanut Grove fire; a 5 year follow up study. N Engl J Med. 1949;241(25):1008. doi: 10.1056/NEJM194912222412503. [DOI] [PubMed] [Google Scholar]
- 3.Saffle JR. The 1942 fire at Boston’s Cocoanut Grove nightclub. Am J Surg. 1993;166(6):581–591. doi: 10.1016/s0002-9610(05)80661-0. [DOI] [PubMed] [Google Scholar]
- 4.Yurt RW, Bessey PQ, Bauer GJ, et al. A regional burn center’s response to a disaster: September 11, 2001, and the days beyond. J Burn Care Rehabil. 2005;26(2):117–124. doi: 10.1097/01.bcr.0000155543.46107.e6. [DOI] [PubMed] [Google Scholar]
- 5.Kimura R, Traber LD, Herndon DN, Linares HA, Lubbesmeyer HJ, Traber DL. Increasing duration of smoke exposure induces more severe lung injury in sheep. J Appl Physiol (1985) 1988;64(3):1107–1113. doi: 10.1152/jappl.1988.64.3.1107. [DOI] [PubMed] [Google Scholar]
- 6.Moritz AR, Henriques FC, McLean R. The Effects of Inhaled Heat on the Air Passages and Lungs: An Experimental Investigation. Am J Pathol. 1945;21(2):311–331. [PMC free article] [PubMed] [Google Scholar]
- 7.Rong YH, Liu W, Wang C, Ning FG, Zhang GA. Temperature distribution in the upper airway after inhalation injury. Burns. 2011;37(7):1187–1191. doi: 10.1016/j.burns.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Role of neural mechanisms in airway defense. In: Chretien J, Dusser D, editors. Environmental Impact in the Airways. New York: Marcel Dekker; 1996. pp. 93–121. [Google Scholar]
- 9.Nadel JA. Neutral endopeptidase modulates neurogenic inflammation. Eur Respir J. 1991;4(6):745–754. [PubMed] [Google Scholar]
- 10.Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;(147 Suppl 1):S202–S211. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dakhama A, Larsen GL, Gelfand EW. Calcitonin gene-related peptide: role in airway homeostasis. Curr Opin Pharmacol. 2004;4(3):215–220. doi: 10.1016/j.coph.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Lange M, Enkhbaatar P, Traber DL, et al. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol (1985) 2009;107(1):176–184. doi: 10.1152/japplphysiol.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302(3):839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 14.Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol (1985) 1991;71(6):2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- 15.Jacob S, Deyo DJ, Cox RA, Traber DL, Herndon DN, Hawkins HK. Mechanisms of toxic smoke inhalation and burn injury: role of neutral endopeptidase and vascular leakage in mice. Toxicol Mech Methods. 2009;19(3):191–196. doi: 10.1080/15376510902725649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange M, Hamahata A, Traber DL, et al. Preclinical evaluation of epinephrine nebulization to reduce airway hyperemia and improve oxygenation after smoke inhalation injury. Crit Care Med. 2011;39(4):718–724. doi: 10.1097/CCM.0b013e318207ec52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enkhbaatar P, Murakami K, Shimoda K, et al. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003;167(7):1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 18.Abdi S, Herndon DN, Traber LD, et al. Lung edema formation following inhalation injury: role of the bronchial blood flow. J Appl Physiol (1985) 1991;71(2):727–734. doi: 10.1152/jappl.1991.71.2.727. [DOI] [PubMed] [Google Scholar]
- 19.Efimova O, Volokhov AB, Iliaifar S, Hales CA. Ligation of the bronchial artery in sheep attenuates early pulmonary changes following exposure to smoke. J Appl Physiol (1985) 2000;88(3):888–893. doi: 10.1152/jappl.2000.88.3.888. [DOI] [PubMed] [Google Scholar]
- 20.Hamahata A, Enkhbaatar P, Sakurai H, Nozaki M, Traber DL. Effect of ablated bronchial blood flow on survival rate and pulmonary function after burn and smoke inhalation in sheep. Burns. 2009;35(6):802–810. doi: 10.1016/j.burns.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita N, Enkhbaatar P, Maybauer DM, et al. Impact of bronchial circulation on bronchial exudates following combined burn and smoke inhalation injury in sheep. Burns. 2011;37(3):465–473. doi: 10.1016/j.burns.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai H, Johnigan R, Kikuchi Y, Harada M, Traber LD, Traber DL. Effect of reduced bronchial circulation on lung fluid flux after smoke inhalation in sheep. J Appl Physiol (1985) 1998;84(3):980–986. doi: 10.1152/jappl.1998.84.3.980. [DOI] [PubMed] [Google Scholar]
- 23.Hales CA, Barkin P, Jung W, Quinn D, Lamborghini D, Burke J. Bronchial artery ligation modifies pulmonary edema after exposure to smoke with acrolein. J Appl Physiol (1985) 1989;67(3):1001–1006. doi: 10.1152/jappl.1989.67.3.1001. [DOI] [PubMed] [Google Scholar]
- 24.Thomas HM, 3rd, Garrett RC. Strength of hypoxic vasoconstriction determines shunt fraction in dogs with atelectasis. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(1):44–51. doi: 10.1152/jappl.1982.53.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Nakae H, Tanaka H, Inaba H. Failure to clear casts and secretions following inhalation injury can be dangerous: report of a case. Burns. 2001;27(2):189–191. doi: 10.1016/s0305-4179(00)00100-5. [DOI] [PubMed] [Google Scholar]
- 26.Pruitt BA, Jr, Cioffi WG. Diagnosis and treatment of smoke inhalation. J Intensive Care Med. 1995;10(3):117–127. doi: 10.1177/088506669501000303. [DOI] [PubMed] [Google Scholar]
- 27.Cox RA, Jacob S, Zhu Y, et al. Airway obstruction and bacterial invasion in autopsy tissue of pediatric burn victims. J Burn Care Res. 2014;35(2):148–153. doi: 10.1097/BCR.0b013e31828e62b8. [DOI] [PubMed] [Google Scholar]
- 28.Cox RA, Burke AS, Jacob S, et al. Activated nuclear factor kappa B and airway inflammation after smoke inhalation and burn injury in sheep. J Burn Care Res. 2009;30(3):489–498. doi: 10.1097/BCR.0b013e3181a28e13. [DOI] [PubMed] [Google Scholar]
- 29.Cox RA, Burke AS, Oliveras G, et al. Acute bronchial obstruction in sheep: histopathology and gland cytokine expression. Exp Lung Res. 2005;31(9–10):819–837. doi: 10.1080/01902140600574967. [DOI] [PubMed] [Google Scholar]
- 30.Cox RA, Mlcak RP, Chinkes DL, et al. Upper airway mucus deposition in lung tissue of burn trauma victims. Shock. 2008;29(3):356–361. doi: 10.1097/shk.0b013e31814541dd. [DOI] [PubMed] [Google Scholar]
- 31.Enkhbaatar P, Cox RA, Traber LD, et al. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med. 2007;35(12):2805–2810. doi: 10.1097/01.ccm.0000291647.18329.83. [DOI] [PubMed] [Google Scholar]
- 32.Enkhbaatar P, Esechie A, Wang J, et al. Combined anticoagulants ameliorate acute lung injury in sheep after burn and smoke inhalation. Clin Sci (Lond) 2008;114(4):321–329. doi: 10.1042/CS20070254. [DOI] [PubMed] [Google Scholar]
- 33.Enkhbaatar P, Murakami K, Cox R, et al. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22(1):70–75. doi: 10.1097/01.shk.0000129201.38588.85. [DOI] [PubMed] [Google Scholar]
- 34.Cox RA, Burke AS, Soejima K, et al. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 35.Dreyfuss D, Martin-Lefevre L, Saumon G. Hyperinflation-induced lung injury during alveolar flooding in rats: effect of perfluorocarbon instillation. Am J Respir Crit Care Med. 1999;159(6):1752–1757. doi: 10.1164/ajrccm.159.6.9805018. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H, Teramoto H, Uetani K, Igawa K, Shimizu E. Cyclic stretch upregulates interleukin-8 and transforming growth factor-beta1 production through a protein kinase C-dependent pathway in alveolar epithelial cells. Respirology. 2002;7(2):103–109. doi: 10.1046/j.1440-1843.2002.00377.x. [DOI] [PubMed] [Google Scholar]
- 37.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276(4 Pt 1):C812–C820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 38.Madjdpour C, Jewell UR, Kneller S, et al. Decreased alveolar oxygen induces lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L360–L367. doi: 10.1152/ajplung.00158.2002. [DOI] [PubMed] [Google Scholar]
- 39.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia increases leukocyte emigration and vascular permeability in conscious rats. J Appl Physiol (1985) 2000;89(4):1561–1568. doi: 10.1152/jappl.2000.89.4.1561. [DOI] [PubMed] [Google Scholar]
- 40.Jones J, McMullen MJ, Dougherty J. Toxic smoke inhalation: cyanide poisoning in fire victims. Am J Emerg Med. 1987;5(4):317–321. doi: 10.1016/0735-6757(87)90360-3. [DOI] [PubMed] [Google Scholar]
- 41.Lundquist P, Rammer L, Sorbo B. The role of hydrogen cyanide and carbon monoxide in fire casualties: a prospective study. Forensic Sci Int. 1989;43(1):9–14. doi: 10.1016/0379-0738(89)90116-3. [DOI] [PubMed] [Google Scholar]
- 42.Terrill JB, Montgomery RR, Reinhardt CF. Toxic gases from fires. Science. 1978;200(4348):1343–1347. doi: 10.1126/science.208143. [DOI] [PubMed] [Google Scholar]
- 43.Vogel SN, Sultan TR, Ten Eyck RP. Cyanide poisoning. Clin Toxicol. 1981;18(3):367–383. doi: 10.3109/15563658108990043. [DOI] [PubMed] [Google Scholar]
- 44.Lopez E, Fujiwara O, Lima-Lopez F, et al. Nebulized Epinephrine Limits Pulmonary Vascular Hyperpermeability to Water and Protein in Ovine With Burn and Smoke Inhalation Injury. Crit Care Med. 2016;44(2):e89–e96. doi: 10.1097/CCM.0000000000001349. [DOI] [PubMed] [Google Scholar]
- 45.Starling EH. On the absorption of fluid from the connective tissue spaces. Journal of Physiology. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehberg S, Yamamoto Y, Sousse LE, et al. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med. 2013;41(12):e439–e446. doi: 10.1097/CCM.0b013e318298ad3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enkhbaatar P, Kikuchi Y, Traber LD, et al. Effect of inhaled nitric oxide on pulmonary vascular hyperpermeability in sheep following smoke inhalation. Burns. 2005;31(8):1013–1019. doi: 10.1016/j.burns.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Isago T, Traber LD, Herndon DN, Abdi S, Fujioka K, Traber DL. Determination of pulmonary microvascular reflection coefficient in sheep by venous occlusion. J Appl Physiol (1985) 1990;69(6):2311–2316. doi: 10.1152/jappl.1990.69.6.2311. [DOI] [PubMed] [Google Scholar]
- 49.Hamahata A, Enkhbaatar P, Lange M, et al. Direct delivery of low-dose 7-nitroindazole into the bronchial artery attenuates pulmonary pathophysiology after smoke inhalation and burn injury in an ovine model. Shock. 2011;36(6):575–579. doi: 10.1097/SHK.0b013e3182360f2e. [DOI] [PubMed] [Google Scholar]
- 50.Hamahata A, Enkhbaatar P, Lange M, et al. Administration of a peroxynitrite decomposition catalyst into the bronchial artery attenuates pulmonary dysfunction after smoke inhalation and burn injury in sheep. Shock. 2012;38(5):543–548. doi: 10.1097/SHK.0b013e31826e9c54. [DOI] [PubMed] [Google Scholar]
- 51.Hamahata A, Enkhbaatar P, Lange M, et al. Administration of poly(ADP-ribose) polymerase inhibitor into bronchial artery attenuates pulmonary pathophysiology after smoke inhalation and burn in an ovine model. Burns. 2012;38(8):1210–1215. doi: 10.1016/j.burns.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Basadre JO, Sugi K, Traber DL, Traber LD, Niehaus GD, Herndon DN. The effect of leukocyte depletion on smoke inhalation injury in sheep. Surgery. 1988;104(2):208–215. [PubMed] [Google Scholar]
- 53.Morita N, Shimoda K, Traber MG, et al. Vitamin E attenuates acute lung injury in sheep with burn and smoke inhalation injury. Redox Rep. 2006;11(2):61–70. doi: 10.1179/135100006X101020. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen TT, Cox CS, Jr, Herndon DN, et al. Effects of manganese superoxide dismutase on lung fluid balance after smoke inhalation. J Appl Physiol (1985) 1995;78(6):2161–2168. doi: 10.1152/jappl.1995.78.6.2161. [DOI] [PubMed] [Google Scholar]
- 55.Niehaus GD, Kimura R, Traber LD, Herndon DN, Flynn JT, Traber DL. Administration of a synthetic antiprotease reduces smoke-induced lung injury. J Appl Physiol (1985) 1990;69(2):694–699. doi: 10.1152/jappl.1990.69.2.694. [DOI] [PubMed] [Google Scholar]
- 56.Virag L. Poly(ADP-ribosyl)ation in asthma and other lung diseases. Pharmacol Res. 2005;52(1):83–92. doi: 10.1016/j.phrs.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto Y, Enkhbaatar P, Sousse LE, et al. Nebulization with gamma-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model. Shock. 2012;37(4):408–414. doi: 10.1097/SHK.0b013e3182459482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunt JL, Agee RN, Pruitt BA., Jr Fiberoptic bronchoscopy in acute inhalation injury. J Trauma. 1975;15(8):641–549. doi: 10.1097/00005373-197508000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Oh JS, Chung KK, Allen A, et al. Admission chest CT complements fiberoptic bronchoscopy in prediction of adverse outcomes in thermally injured patients. J Burn Care Res. 2012;33(4):532–538. doi: 10.1097/BCR.0b013e318237455f. [DOI] [PubMed] [Google Scholar]
- 61.Kwon HP, Zanders TB, Regn DD, et al. Comparison of virtual bronchoscopy to fiberoptic bronchoscopy for assessment of inhalation injury severity. Burns. 2014;40(7):1308–1315. doi: 10.1016/j.burns.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Pruitt BA, Jr, Cioffi WG, Shimazu T, Ikeuchi H, Mason AD., Jr Evaluation and management of patients with inhalation injury. J Trauma. 1990;30(12 Suppl):S63–S68. doi: 10.1097/00005373-199012001-00015. [DOI] [PubMed] [Google Scholar]
- 63.Cioffi WG, Graves TA, McManus WF, Pruitt BA., Jr High-frequency percussive ventilation in patients with inhalation injury. J Trauma. 1989;29(3):350–354. doi: 10.1097/00005373-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Allan PF, Osborn EC, Chung KK, Wanek SM. High-frequency percussive ventilation revisited. J Burn Care Res. 2010;31(4):510–520. doi: 10.1097/BCR.0b013e3181e4d605. [DOI] [PubMed] [Google Scholar]
- 65.Ogura H, Sumi Y, Matsushima A, et al. [Smoke inhalation injury: diagnosis and respiratory management] Nihon Geka Gakkai Zasshi. 2005;106(12):740–744. [PubMed] [Google Scholar]
- 66.Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Crit Care Resusc. 2010;12(1):53–61. [PubMed] [Google Scholar]
- 67.Askegard-Giesmann JR, Besner GE, Fabia R, Caniano DA, Preston T, Kenney BD. Extracorporeal membrane oxygenation as a lifesaving modality in the treatment of pediatric patients with burns and respiratory failure. J Pediatr Surg. 2010;45(6):1330–1335. doi: 10.1016/j.jpedsurg.2010.02.106. [DOI] [PubMed] [Google Scholar]
- 68.Lynch JE, Hayes D, Jr, Zwischenberger JB. Extracorporeal CO(2) removal in ARDS. Crit Care Clin. 2011;27(3):609–625. doi: 10.1016/j.ccc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Kreyer S, Scaravilli V, Linden K, et al. Early Utilization of Extracorporeal CO2 Removal for Treatment of Acute Respiratory Distress Syndrome Due to Smoke Inhalation and Burns in Sheep. Shock. 2016;45(1):65–72. doi: 10.1097/SHK.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 70.Hale DF, Cannon JW, Batchinsky AI, et al. Prone positioning improves oxygenation in adult burn patients with severe acute respiratory distress syndrome. J Trauma Acute Care Surg. 2012;72(6):1634–1639. doi: 10.1097/TA.0b013e318247cd4f. [DOI] [PubMed] [Google Scholar]
- 71.White CE, Batchinsky AI, Necsoiu C, et al. Lower interbreath interval complexity is associated with extubation failure in mechanically ventilated patients during spontaneous breathing trials. J Trauma. 2010;68(6):1310–1316. doi: 10.1097/TA.0b013e3181da90db. [DOI] [PubMed] [Google Scholar]
- 72.Jonkam C, Zhu Y, Jacob S, et al. Assessment of combined muscarinic antagonist and fibrinolytic therapy for inhalation injury. J Burn Care Res. 2012;33(4):524–531. doi: 10.1097/BCR.0b013e31823dc7da. [DOI] [PubMed] [Google Scholar]
- 73.Ikeuchi H, Sakano T, Sanchez J, Mason AD, Jr, Pruitt BA., Jr The effects of platelet-activating factor (PAF) and a PAF antagonist (CV-3988) on smoke inhalation injury in an ovine model. J Trauma. 1992;32(3):344–349. discussion 9–50. [PubMed] [Google Scholar]
- 74.Ogura H, Cioffi WG, Okerberg CV, et al. The effects of pentoxifylline on pulmonary function following smoke inhalation. J Surg Res. 1994;56(3):242–250. doi: 10.1006/jsre.1994.1038. [DOI] [PubMed] [Google Scholar]
- 75.Zhou X, Wang D, Ballard-Croft CK, Simon SR, Lee HM, Zwischenberger JB. A tetracycline analog improves acute respiratory distress syndrome survival in an ovine model. Ann Thorac Surg. 2010;90(2):419–426. doi: 10.1016/j.athoracsur.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 76.Kowal-Vern A, Orkin BA. Antithrombin in the treatment of burn trauma. World J Crit Care Med. 2016;5(1):17–26. doi: 10.5492/wjccm.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Enkhbaatar P. Fishman’s Pulmonary Diseases and Disorders. 5th. McGraw-Hill Education; 2015. Thermal lung injury and acute smoke inhalation. [Google Scholar]
- 78.Traber DL, Herndon DN, Enkhbaatar P, Maybauer MO, Maybauer DM. Pathophysiology of inhalation injury. In: Herndon DN, editor. Total Burn Care. 4th 2013. [Google Scholar]
- 79.Demling RH. Smoke inhalation lung injury: an update. Eplasty. 2008;8:e27. [PMC free article] [PubMed] [Google Scholar]
- 80.Sadowska AM. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2012;6(3):127–135. doi: 10.1177/1753465812437563. [DOI] [PubMed] [Google Scholar]
- 81.Brown M, Desai M, Traber LD, Herndon DN, Traber DL. Dimethylsulfoxide with heparin in the treatment of smoke inhalation injury. J Burn Care Rehabil. 1988;9(1):22–25. doi: 10.1097/00004630-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998;19(3):210–212. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Miller AC, Rivero A, Ziad S, Smith DJ, Elamin EM. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30(2):249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 84.Olson ST, Bjork I. Predominant contribution of surface approximation to the mechanism of heparin acceleration of the antithrombin-thrombin reaction. Elucidation from salt concentration effects. J Biol Chem. 1991;266(10):6353–6564. [PubMed] [Google Scholar]
- 85.Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992;267(18):12528–1238. [PubMed] [Google Scholar]
- 86.Walker PF, Buehner MF, Wood LA, et al. Diagnosis and management of inhalation injury: an updated review. Crit Care. 2015;19:351. doi: 10.1186/s13054-015-1077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns. 2008;34(4):441–451. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Zak AL, Harrington DT, Barillo DJ, Lawlor DF, Shirani KZ, Goodwin CW. Acute respiratory failure that complicates the resuscitation of pediatric patients with scald injuries. J Burn Care Rehabil. 1999;20(5):391–399. doi: 10.1097/00004630-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 89.Agee RN, Long JM, 3rd, Hunt JL, et al. Use of 133xenon in early diagnosis of inhalation injury. J Trauma. 1976;16(3):218–224. doi: 10.1097/00005373-197603000-00007. [DOI] [PubMed] [Google Scholar]
- 90.Lin WY, Kao CH, Wang SJ. Detection of acute inhalation injury in fire victims by means of technetium-99m DTPA radioaerosol inhalation lung scintigraphy. Eur J Nucl Med. 1997;24(2):125–129. doi: 10.1007/BF02439543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peitzman AB, Shires GT, 3rd, Teixidor HS, Curreri PW, Shires GT. Smoke inhalation injury: evaluation of radiographic manifestations and pulmonary dysfunction. J Trauma. 1989;29(9):1232–1238. doi: 10.1097/00005373-198909000-00008. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 92.Reske A, Bak Z, Samuelsson A, Morales O, Seiwerts M, Sjoberg F. Computed tomography--a possible aid in the diagnosis of smoke inhalation injury? Acta Anaesthesiol Scand. 2005;49(2):257–260. doi: 10.1111/j.1399-6576.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 93.Schall GL, McDonald HD, Carr LB, Capozzi A. Xenon ventilation-perfusion lung scans. The early diagnosis of inhalation injury. JAMA. 1978;240(22):2441–2445. [PubMed] [Google Scholar]
- 94.Teixidor HS, Rubin E, Novick GS, Alonso DR. Smoke inhalation: radiologic manifestations. Radiology. 1983;149(2):383–387. doi: 10.1148/radiology.149.2.6622680. [DOI] [PubMed] [Google Scholar]
- 95.Brown DL, Archer SB, Greenhalgh DG, Washam MA, James LE, Warden GD. Inhalation injury severity scoring system: a quantitative method. J Burn Care Rehabil. 1996;17(6 Pt 1):552–557. doi: 10.1097/00004630-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 96.Hollingsed TC, Saffle JR, Barton RG, Craft WB, Morris SE. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166(6):592–596. doi: 10.1016/s0002-9610(05)80662-2. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 97.Khoo AK, Lee ST, Poh WT. Tracheobronchial cytology in inhalation injury. J Trauma. 1997;42(1):81–85. doi: 10.1097/00005373-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 98.Madnani DD, Steele NP, de Vries E. Factors that predict the need for intubation in patients with smoke inhalation injury. Ear Nose Throat J. 2006;85(4):278–280. [PubMed] [Google Scholar]
- 99.Masanes MJ, Legendre C, Lioret N, Saizy R, Lebeau B. Using bronchoscopy biopsy to diagnose early inhalation injury. Macroscopic and histologic findings. Chest. 1995;107(5):1365–1369. doi: 10.1378/chest.107.5.1365. [DOI] [PubMed] [Google Scholar]
- 100.Petroff PA, Hander EW, Clayton WH, Pruitt BA. Pulmonary function studies after smoke inhalation. Am J Surg. 1976;132(3):346–351. doi: 10.1016/0002-9610(76)90391-3. [DOI] [PubMed] [Google Scholar]
- 101.Ryan CM, Schoenfeld DA, Thorpe WP, Sheridan RL, Cassem EH, Tompkins RG. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338(6):362–366. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 102.Ihara K, Fujuda S, Enkhtaivan B, et al. Abstract 19670: Adipose-derived Mesenchymal Stem Cells Attenuate Pulmonary Microvascular Hyperpermeability After Smoke Inhalation. Circulation. 2015;132(Suppl 3):A19670. doi: 10.1371/journal.pone.0185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Avello A, Lorente JA, Cesar-Perez J, et al. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb Res. 1998;89(2):59–64. doi: 10.1016/s0049-3848(97)00291-0. [DOI] [PubMed] [Google Scholar]
- 104.Kowal-Vern A, McGill V, Walenga JM, Gamelli RL. Antithrombin III concentrate in the acute phase of thermal injury. Burns. 2000;26(1):97–101. doi: 10.1016/s0305-4179(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 105.Kowal-Vern A, Walenga JM, McGill V, Gamelli RL. The impact of antithrombin (H) concentrate infusions on pulmonary function in the acute phase of thermal injury. Burns. 2001;27(1):52–60. doi: 10.1016/s0305-4179(00)00057-7. [DOI] [PubMed] [Google Scholar]
- 106.Lavrentieva A, Kontakiotis T, Bitzani M, et al. The efficacy of antithrombin administration in the acute phase of burn injury. Thromb Haemost. 2008;100(2):286–290. [PubMed] [Google Scholar]
- 107.Niedermayr M, Schramm W, Kamolz L, et al. Antithrombin deficiency and its relationship to severe burns. Burns. 2007;33(2):173–178. doi: 10.1016/j.burns.2006.06.011. [DOI] [PubMed] [Google Scholar]