Abstract
As the most frequent plasma protein, albumin constitutes more than 50% of the serum proteins in healthy individuals. It has a key role in oncotic pressure maintenance and it is known as a versatile protein carrier for transportation of various endogenous and exogenous ligands. Reduced amounts of albumin in the body will lead to different kinds of diseases such as hypovolemia and hypoproteinemia. It also has various indications in shocks, burns, cardiopulmonary bypass, acute liver failure and etc. Further applications in research consist of cell culture supplement, drug delivery carrier and protein/drug stabilizer. So, the demand for albumin increased annually worldwide. Due to different applications of albumin, many efforts have been accomplished to achieve albumin during a long period of time. In this review, an overview of serum albumin and different purification methods are summarized.
Keywords: Human serum albumin, Purification, Chromatography, Immunoaffinity
Introduction
The name of albumin protein is taken from Albumen (etymologically goes back to Albus).1 There exist different types of albumin, including ovalbumin, human serum albumin (HSA), and bovine serum albumin (BSA) which are described briefly in Table 1. Albumin is the most predominant circulating protein in healthy adults (normal physiological concentration is 0.6 Mm). It is synthesized in the liver and it causes 80% of plasma colloid osmotic pressure (COP). Since last century, due to critical physiological and biopharmaceutical function of albumin, efforts have been made to achieve high pure and qualified albumin in order to be utilized in therapeutic and research approaches. Human albumin has the highest demand among other biopharmaceutical solutions. Currently, the annual request of albumin is guessed approximately 500 metric tons in the world.2 Till now various researchers and teams have tried to innovate new albumin production methods. Traditional techniques such as fractionation have been developed and currently diverse chromatographic techniques are used to attain albumin with high yield and purity. So, in the following review, different methods of albumin purification, production and cons and pros of each of them will be discussed.
Table 1. Types of albumin.
| Albumin type | definition | M.W (Da) | pI | aa No. | applications | Cause of use |
| OVA | A highly functional food protein | 47000 | 4.8 | 385 | • Carrier for drug delivery in food matrix design. • Carrier for controlled drug release. |
• low cost • Availability • Can form gel networks and stabilization of emulsions and foams. |
| HSA | The most common protein plasma | 66438 | 5.9 | 585 | • Low blood volume compensation • treatment of related diseases • Drug delivery career • Drug and sample stabilization • Cell culture supplement |
• Availability • Biodegradability • Lack of toxicity |
| BSA | The most common protein plasma | 69323 | 4.7 | 585 | • Drug delivery • Usage in pharmaceutical industry |
• Medical importance • Abundance • Low cost • Ease of purification • Unusual ligand-binding properties |
Abbreviations: OVA: Ovalbumin, HSA: Human serum albumin, BSA: Bovine serum albumin, M.W: Molecular weight, Da: Dalton, pI: isoelectric point, aa No: Amino acid number.
Albumin Structure
Albumin is a single chain protein with low molecular weight (66/5 kDa) which containing 585 amino acids. It is a simple protein, non-glycosylated polypeptide, hydrophobic patches/cavities, and it lacks prosthetic groups. Human albumin gene is located on chromosome 4 q (11-22) and mutations of this gene will end in anomalous protein. This gene has 1691 nucleotide and contains 14 introns and 15 exons.3-6 Albumin structure is composed of three domains which are homologous in structural features (this has been elucidated by using X-ray crystallography) (Figure 1).
Figure 1.
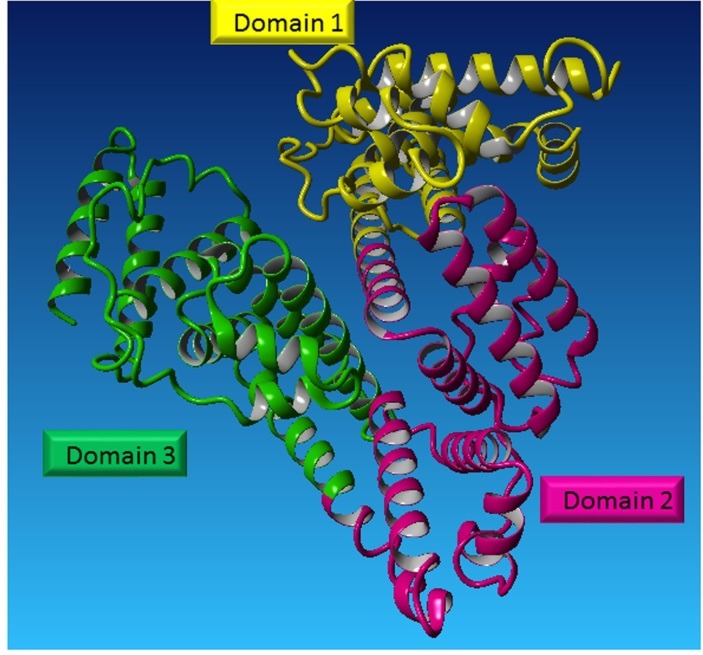
Albumin structure. Albumin protein is composed of three domains (elucidated by X-ray crystallography).
The structure includes domains I in residues 1 to 195, domain II in residues 196 to 383 and domain III residues 384 to 585. Each domain consists of 2 identical subunits (A and B) and is composed of 4 and 6 α-helices consecutively. In X-ray crystallography, albumin was displayed as a heart-shaped tertiary in the human body and is containing 17 pairs of disulfide bridges. In the structure of albumin, there exists only one free cysteine (Cys34 is unpaired). Its approximate dimensions are of 80× 80 × 30 Å and about 68% α–helix (any β-sheet).1,7
Enzymatic properties
Human albumin has interesting enzymatic properties including: esterase activity, enolase activity, effects on eicosanoids, aryl acylamidase activity, stereospecificity, condensation reactions, binding and activation of drug conjugates. Also, some of enzymatic properties of albumin–ligand complexes are as follows: heme- and hemin–human albumin, human albumin and Buckminster fullerene, inactivation of reactive oxygen and nitrogen species, metalloenzymes constructed using albumin, lipid peroxide peroxidase activity and nanoparticles.8
Albumin synthesis
Albumin synthesis occurs in hepatocyte cells, but isn’t stored by the liver. Once produced, it is secreted into the portal circulation.9 The normal concentration of albumin is 3.5- 5 g/dl in healthy adults and 2.9- 5.5 g/dl in children. Nearly 35 percent of the total body albumin is exist in the intravascular compartment. The rate of synthesis is approximately 12-25 grams per day. Its biological half-life is approximately 19 days. Routinely albumin turnover occurred around 14 grams in a normal 70 kg adult which is approximately 50 percent in the muscles and skin.10,11 Among factors that modify albumin metabolism and leads to its reduced synthesis, can be noted to decreased gene transcription (such as trauma, sepsis, hepatic diseases, diabetes, decreased growth hormone, decreased corticosteroids) and ribosome disaggregation (like protein depletion, fasting).12
Hypoalbuminemia, which is described as insufficient low levels of HSA in the blood is a common symptom of ill patients. This symptom is not only a result of reduction in albumin synthesis, but related to the breakdown, protein uptake and leakage to the extravascular space.13,14 These conditions are created in sub-conditions such as liver and kidney, infections, AIDS, lymphoma, cancer, poverty nutrition, surgery, burn damage, chemotherapy, spontaneous bacterial peritonitis, taking medication, prematurity infant, acute respiratory distress syndrome, chronic respiratory diseases, acute necrotizing, infant brain hemorrhage, hydrops fetalis, systemic inflammatory response syndrome, sepsis and infant edema. Moreover, it can be seen in intestinal diseases such as Castleman.15-20
Functions
Albumin is known as a multifunctional plasma protein. It is the main regulator of plasma oncotic pressure. Beside this, any alteration in the oncotic pressure can lead to the stimulation of HSA synthesis. It can bind to different molecules due to its tertiary structure. Such molecules can include metabolites, gases or exogenous substances such as drugs. There has been no evidence about presence of HSA receptor besides only a receptor-mediated endocytosis mechanism is assumed for loaded HSA. It also acts as a chaperone molecule which helps promoting the folding of various proteins and prevents the formation of aggregated proteins related to diseases.8,21,22 Plasma, as a place which is exposed to different types of oxidative stresses, encompasses the HSA. This protein is well-known for its anti-oxidant activity that encounters varieties of reactive oxygen species (ROS) and reactive nitrogen species (RNS). It also reduces the production chance of reactive species by binding to the major cause of free radical production, free Cu (II). This ability will make the HSA an oxido-redox biomarker.23-25 There exist other less-known properties of HSA such as anti-coagulant and anti-thrombotic functions. It also leads to delayed aggregation of platelets by binding to Nitric oxide (NO) and inhibiting its inactivation. Like other plasma proteins, HSA, due to its long half-life, is prone to glycation.26 Glycated HSA will cause irremeable damages in different diseases. Studies also reported that HSA protects other proteins from glycation.HSA is also supposed to be responsible for membrane permeability. The hypothesis of mechanism is due to binding of HSA to subendothelium and interstitial layers and then altering their permeability. HSA is reported to have neuroprotective function on neuronal and glial cells and regulates brain circulation. Experimental ischemic and Alzheimer’s disease model indicates HSA administration, which can play a neuroprotective and anti-oxidant role. This function is mainly related to anti-oxidant properties of HSA. In vitro studies on hepatocytes or liver microsomes of rats indicated that HSA is increased with aging only in male rats. In human, sex-related HSA amount is doubted but there is a fact that before age 14, plasma protein levels are sex-independent and the variation is only occur between ages 18-24. Moreover, HSA level decrease until human being reaches age 55 and after that, it is again age-independent.1,25,27-34
Applications
Serum albumin is utilized under various clinical conditions. Restoration of blood volume, emergency treatment of shock, acute management of burns, and other situations associated with hypovolemia are some of the clinical applications of albumin. Knowing this, Serum albumin is a nobility biomarker for liver function synthesis, and also for several diseases, such as, inflammatory disorders, brain tumors, rheumatoid arthritis, myocardial ischemia, cancer, blood brain barrier (BBB) damage, kidney disease, cerebrovascular disease, cardiovascular risk disease, and also in disorders which requires the blood sugar control.35-39 In addition, albumin has different applications in the related research field, including cryopreservation, stabilizer of some of the proteins and as a supplement in cell culture. Novel application of HSA is consisted of a fusion of peptides, a drug nanocarrier and an oxygen transporter.2,11,40
Therapeutic formats
Clinical indication of albumin is done in two forms. The first one is albumin 4-5% (near iso-oncotic solution) which leads to intravascular volume expansion, and the second one is albumin 20-25% (hyper oncotic solution) which is applied in the restoration of COP and fluid balance preservation among compartments.41 Injection of these 5%, 20%, or 25% albumins is used for hypoalbuminemia treatment which is sterilized under 60 ± 0.5°C for 10–11 h to viral inactivation. This sterilization process is likely to induce denatured HSA.42-45
Indications
Clinical indications of albumin 4-5% solution includes emergency treatment of hypovolemic shock, cardiopulmonary bypass, acute liver failure, sequestration of protein rich fluids.46,47 Clinical indications of albumin 20-25% solution may include hypovolemia, hypoproteinemia, adult respiratory distress syndrome (ARDS), hemolytic disease of the newborn (HDN), acute nephrosis, acute liver failure, renal dialysis, hypovolemic shock, burn therapy, cardiopulmonary bypass, sequestration of protein rich fluids, and erythrocyte resuspention.48
Contraindications
Although albumin is a valuable therapeutic product in a broad range of disorders, but its administration in some situations may be hazardous. Precede the prescription of albumin, its circulation amount should be checked, the level of more than 2.5 g/dl (hyperalbuminemia) cause contraindication. Cardiac and renal failures, acute or chronic pancreatitis, pulmonary edema or severe anemia because of the risk of acute circulatory overload are the other situations in which albumin is forbidden. In addition, utilization of albumin in patients with nervous system disorders such as cerebral ischemia and traumatic brain injury (TBI) may be dangerous and life threatening. Also, indication of albumin in conditions is not recommended such as ascites responsive to diuretics, non-hemorrhagic shock, hypoalbuminemia without the incidence of edema or acute hypotension, malnutrition, wound healing, acute normovolemic hemodilution in surgery, ascites responsive to diuretics, protein-losing enteropathies and malabsorption, acute normovolemic hemodilution in surgery.41,49-52
Side effects
Along with the beneficial effects of albumin, it can have a variety of side effects when indicated intravenously. List of these side effects are noted in Table 2.53,54
Table 2. Side effect of intravenous injection of human serum albumin.
| Organ | Side effects |
| Dermatologic | Urticarial, skin rash, pruritus, edema, and erythema |
| Nervous syste | Headache, chills, and febrile reactions |
| Gastrointestinal | Nausea, vomiting and increased salivation |
| Cardiovascular | Hypotension |
| Respiratory | Bronchospasm |
Human albumin purification methods
Plasma fractionation method with ethyl alcohol
Approximately 60 years ago, Cohn and colleagues developed a protein purification method by using plasma fractionation technique for the first time. This approach is based on albumin solubility difference with other plasma proteins. Comparing to other plasma proteins, albumin sediments in lower pH and higher ethanol concentration. In this method there are five available systems for separation of plasma protein. The basic principles includes ethyl alcohol (8-40%), temperature (-3 to -10 °C), pH (4.5-7.2), protein concentration (5.1-.8 %), and ionic strength (0.14-0.01).55-57 Protein separation is a result of the increase in ethyl alcohol concentration occurring in each fraction, so albumin will be seen in the last fraction. This method which is done under bacteriostatic condition, can be used as therapeutic agents. The method is suitable for large-scale and industrial production. But this method has some disadvantages, too; It is not enable to produce high purity product, protein denaturation may occur, and this method needs cold areas.57
The Cohn method combined with chromatography
The Cohn method was modified by Oncley et al. in 1949 and Kistler-Nitschrnan et al. in 1962 in order to improve albumin purification.58 It is shown in Figure 2.
Figure 2.
The Cohn method combined with chromatographic techniques for purification of albumin. Abbrivations: AT III: Antithrombin III, Ch: Chromatography, IEC: Ion exchange chromatography, α1-PI: Alpha1-Proteinase Inhibitor
The Cohn method combined with liquid chromatography
This method at first was described by Tanaka et al. Large volumes of plasma with low price is fractionated in Chon method, so Victoria and colleagues to raise the purity and quality of the product, added liquid chromatography technique. Due to the benefits of both techniques, this method was considered as an integrated method for the purification of plasma proteins. Among advantages of this method one can point to the low cost and high purified albumin which leads to the promoted product quality. The purified product using the present method is yielded approximately 99% which is higher than Cohn’s method. In this method albumin is a by-product and other important therapeutic proteins including factor VIII, intravenous immunoglobulin (IVIG) and some other proteins are isolated from plasma. This method is used for industrial production of plasma proteins in the world.59
Purification from placenta
Another method which has been developed for production of albumin is purification from human placenta. Joaquin Cabrera-Crespo and colleagues described this method which was performed using solvent precipitation (with ethanol) and ion exchange chromatography. Since human placenta collection is available, purification from placenta is one of the most efficient ways of purification of albumin. Among the advantages of the mentioned technique, one can point to the reduction in the volume of resin used in the chromatographic process in comparison with the isolation processes. They have acquired albumin of placenta with 97.1 percent purity.60,61
Affinity precipitation (using a thermo-response polymer attach with an L-thyroxin ligand)
Currently, Cao and Ding reported affinity precipitation process for purification of plasma proteins (such as albumin). It is performed by using reversibly soluble-insoluble polymers combined with an affinity ligand which depends on the polymer features such as temperature, pH and light response.
With using N-methylol acrylamide, butyl acrylate and N-isopropyl acrylamide as monomers, a thermo-response copolymer was synthesized which was called PNBN. Then, L-thyroxin ligand, as an affinity ligand, was coupled to PNBN and was used to purify albumin from human serum.62 One of the advantages of this new production method is tolerable purity HSA production in a single step.
Heat shock method
Since the albumin is stable in thermal fluctuation comparing to the other proteins of the plasma, it can be purified in heat shock purification method. So, albumin protein resists to increasing the temperature up to 60 °C in which potential pathogen may be inactivated. To obtain albumin from serum, 0.04 Ϻ caprylic acid was used to stabilize in pH 5 at 60 °C. In this state other proteins of the serum are denatured and precipitated in solution. Then albumin concentrated by precipitation and ultrafiltration with purity around 98 %.57
Ammonium sulfate precipitation combined with liquid chromatography
Another method for albumin purification is ammonium sulfate precipitation combined with liquid chromatography which yields approximately more than 90 percent purified albumin. In this method albumin is separated from immunoglobulins by 50% ammonium sulfate. Then lipids are removed using ice-cold acetone (in 4 centigrade). In next stage for separating albumin from other proteins (especially transferrin which is the main contaminant), ion exchange and size exclusion chromatography are utilized. DNase and immune detection assays proved that the purified albumin was free of any DNases and immunoglobulins. Eventually, purified albumin was evaluated using western blotting and chemiluminescence.63 Steps of the aforementioned method are shown in Figure 3.
Figure 3.
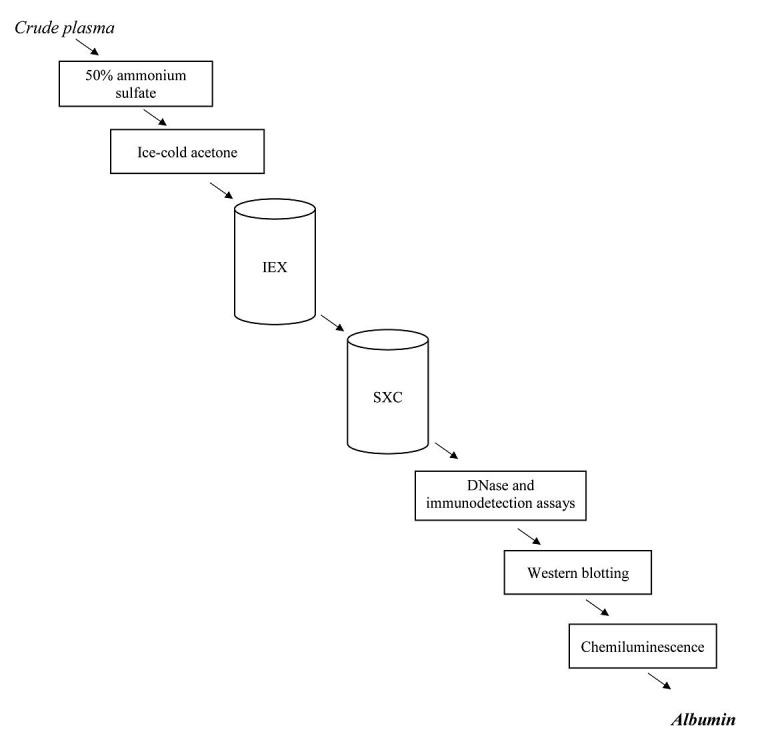
Summary of Ammonium sulfate precipitation combined with liquid chromatography. Abbrivations: IEC: Ion exchange chromatography, SXC: Steric exclusion chromatography.
TCA/Acetone precipitation method
Although many low-abundant serum proteins play an important role in disease detection as a biomarker but some of them such as albumin which exists in high amount is an obstacle for detection. For better detection, we can eliminate albumin and enrich the serum for low-abundant proteins. In order to remove albumin from plasma for disease detection, Chen et al. developed a precipitation method using trichloroacetic acid (TCA)/acetone in 2005.They claim that their method can be a rapid and large scale albumin purification method. The albumin isolated by this method seemed to maintain its native structure beside other characteristics such as solubility, temperature stability, electrophoretic mobility, dye-binging properties, crystallization characteristics, and immunologic behavior.64
Column chromatography for the purification of HSA
Although the ethyl alcohol fractionation method is used in industry for blood protein`s fractionation, the purity is not sufficient enough.57 So, chromatography might be the best choice for albumin and other protein`s purification. To achieve this goal, there have been variable chromatography techniques which some of their most predominant ones include.57
Ion exchange chromatography (IEC)
IEC is widely used for albumin65,66 and other protein production.67,68 Among them the anion exchange method has the most usage.66 Three steps of conventional method for IEC consisting pre-treatment, purification and polishing was shown in Figure 4. Some of the limitations of conventional methods for chromatography such as delayed processing time, low flow rates and etc. which are apparently known for everyone. To cope with these limitations, the new method for ion exchange membrane chromatography (IEM) using ameliorated adsorbers was developed. The simulation studies which compare the IEM with IEC were done by Frerick, et al.69,70 Comparing to column-based separations, the membrane chromatography has some advantages such as high separation efficiencies due to shorter diffusion times,71-73 reduction in buffer usage, small floor space requirements,74 simple method without complex hardware or packing necessities.75 In spite of this advantages, expensive membrane and low efficiency of this technique for industrial purposes is an important drawback which causes the new technology to be replaced.76-78 Moreover, membrane capacity to bind antibodies is relatively low in comparison with advanced resins.78
Figure 4.
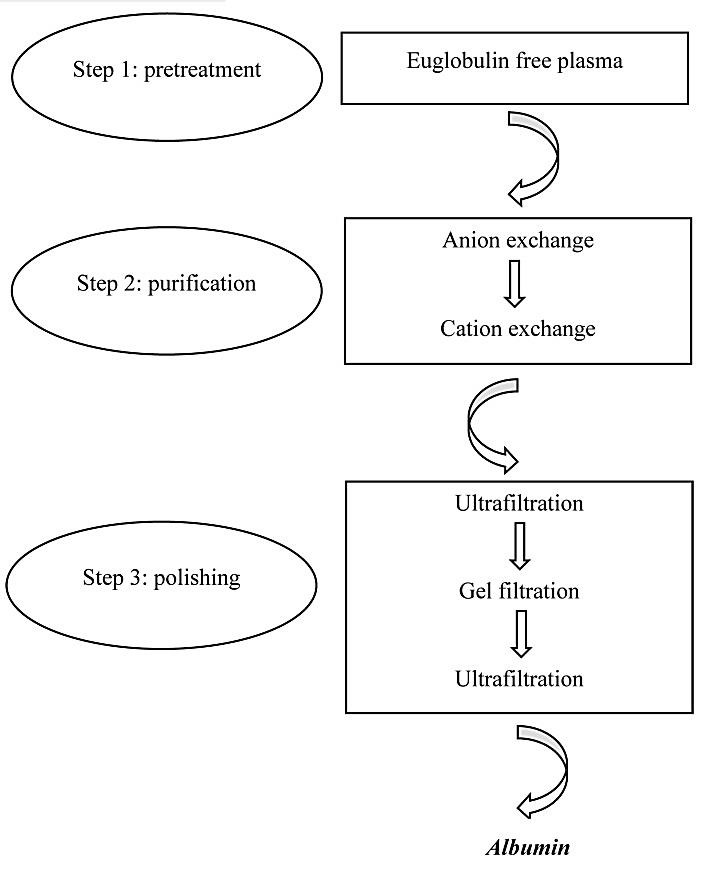
Purification and polishing methods for human serum albumin (HSA). The purification process consists of two anion and cation exchange chromatography steps. The polishing process also consists of three steps containing two ultrafiltration and one gel filtration steps.
Simulated moving bed chromatography (SMB)
This is a multi-column method based on reversed-phase chromatography in which the sorbent and solvent consumption is decreased. Among the advantages of this method, increased efficiency (due to the countercurrent between solid and liquid phases), higher purity and yielding amount are the most outstanding. Nevertheless it has two disadvantages; on one hand the number of fractions is not more than two and each product stream contains more than one compound. On the other hand, the limitation of isocratic elution mode is a major problem. To overcome this problem, the pressure, pH and other factors can be modified. This four sectioned method is a promoted form of the “triangle method” by Storti et al.79
Steric exclusion chromatography (SXC)
The new method for purification of large proteins is the steric exclusion chromatography (SXC) method which is based on PEG-induced precipitation and it is made using crogel monolith column. The polyethylene glycol (PEG) in the mobile phase helps proteins to restrain on the hydrophilic stationary phase. In 2014, Wang et al. reported that the crogels are very useful for protein separation. The advantage of cryogel monolith column compared to polymethacrylate monoliths is having the large pores of aproximtely 10-100 µm which leads to a high flow rate along with low back pressure. Moreover, the retention capacity is supposed to be higher than adsorption-based chromatography. They could purify albumin and also globulin with high purity from bovine serum sample.80
Expanded bed adsorption chromatography
The traditional fractionation process of plasma (the Cohn`s cold ethanol method) is an isolating method for crucial plasma components. In 2009, Lihme et al. developed a new fractionation process containing five expanded bed adsorption steps using high-density modified agarose/tungsten carbide beads. Through this method, albumin was purified up to 99% purity following a recovery step using 6% agarose–10% tungsten carbide beads coupled with an acidic mixed-mode ligand under the acidic condition (pH 4.5). The EBA chromatography method might be an applicable alternative for core plasma fractionation method.81
Affinity Chromatography
Affinity chromatography is a type of liquid chromatography in which, a biological agent named affinity ligand is used as stationary phase in column. The purpose of this method is to selectively purify analytics and inspect the biological interactions between molecules.82-85 Affinity chromatography was first introduced for the purpose of purification by Cuatecasas, et al. in 1968.86 There are different types of affinity ligands such as protein, enzyme, enzyme substrate or inhibitor, antibody, antigen, hormone, biomimetic dye and etc. Depending on the purpose, selected type of ligand is immobilized within the column and further purification process is done following selective binding.82 Here we describe some:
Dye ligand affinity chromatography
Another important protein purification method is dye-ligand affinity chromatography. The biomimetic monocholoro or dicholorotriazinyl dyes are able to bind most type of proteins, especially enzymes.87,88 It has so many advantages over other methods.89 This method is economically and widely available and its immobilization and scale up is easy. Beside its medium specificity, the capacity is very high. Moreover, the matrix can be stored without losing its activity.90
General dyes used in dye-ligand affinity chromatography can be noted to Reactive Red 120, CB F3GA, Congo Red, R. Green HE4BD, Reactive Blue 4, and Mimetic Blue 1 A6XL.57,91-111
Immobilized Metal-ion Affinity Chromatography (IMAC)
In 2001, Denizli, et al. developed a novel and new method of purification to increase the protein adsorption capacity. They used the PHEMA beads containing 2-methacryloamidohistidine in metal chelate affinity chromatography. This survey indicated a noteworthy growth in the HSA adsorption capacities from human plasma (up to 94.6 mg/g). Moreover, High desorption ratios (up to 98% of the adsorbed HSA) were noted. It was possible to reuse Cu (II) chelated (HEMA-co-MAH) beads without significant decreases in the adsorption capabilities.112
Boronate Affinity Chromatography
Boronate as an affinity ligand is used for affinity chromatography for separation. Targets, like carbohydrate-containing compounds, which have cis-diol groups, can bind to the boronate at a basic pH.82
Synthetic dyes are also used as ligands and the method is known as dye-ligand affinity chromatography. For this purpose, triazine dyes are used in columns to purify albumin and various proteins.113,114
Immunoaffinity chromatography (IAC)
We applied IAC through Cyanogen bromide (CNBr) -activated Sepharose to facilitate albumin purification and improving its purity. Initially, the pure HSA was injected to the three white New Zealand rabbits to produce of polyclonal antibody. Antibody purification was done by IEC and protein G affinity chromatography. Then the purified antibody was attached to the CNBr-activated Sepharose and eventually it used for purification of albumin protein from human serum. Western blotting analysis and heat-induced insolubility were applied for functional and stability assessment of immunoaffinity purified HSA, respectively.11
IAC is mentioned as a robust method for albumin purification while joining with appropriate matrix. CNBr may be a most proper matrices for protein purification due to working as an activation reagent. We produced HSA with a purity of 98% through CNBr-activated sepharose. The purity of prepared albumin was high and no more differences was shown in heat-induced insolubility at 60-90 °C between purified and commercial albumin. Also IAC is a cost-effective and monophase technique which could be a preferred method for plasma proteins purification.11 Albumin purification methods was summarized in Table 3.
Table 3. Comparison of various methods for albumin production.
| Method | Purity (%) | Advantage | Disadvantage | Clinical usage | Large-scale | R |
| Plasma Fractionation | 96 | Low cost, Its accessibility, Bacteriostatic nature of process, Ethanol is accessible and inexpensive, Safety of therapeutic product | Protein denaturation, Need raw material with high quality, Need to refrigerated tanks | ✓ | ✓ | 57 |
| Cohn+ LC | 99 | Low cost, Quality of manufactured product is superior of Cohn method, High purity, High yield, Safety of therapeutic product | Possibility of protein denaturation and/or aggregation during the addition of ethanol | ✓ | ✓ | 59 |
| Placenta | 97.1 | Reduction in the volume of resin used in the chromatographic process, High purity | The yield of product might be lower compared to other method, Difficulties in ensuring donor traceability, Contains a high concentration of a heat-stable alkaline phosphatase, Restriction in blood supply | ✓ | ✓ | 61 |
| Affinity Precipitation | 93.6 | Tolerable purity, Single step | Protein denaturation | × | × | 62 |
| Heat Shock | 98 | High purity | Protein denaturation | ✓ | ✓ | 57 |
| Ammonium sulfate+ LC | N/A | Sufficient purity, Suitable for major laboratory approach, Yield of albumin : ≤ 40 g/liter of serum | Not suitable for major clinical approach | × | ✓ | 63 |
| IEC | ≤ 95 | High yield, Adsorption capacity is high, Better reproducibility, High recovery, Convenient for large-scale production | High media, Capital costs and Ligand leakage, Interactions between albumin and the ion exchange sorbents, Slow separation | ✓ | ✓ | 66 |
| SMB | 96 | Increased efficiency Higher purity and yield |
Higher complexity, Higher maintenance costs | × | × | 79 |
| Dye Ligand affinity chromatography | ≈ 98 | Higher purity and yield, Quick separation, Medium specificity | Adsorption capacity is low, Elution condition is harsh, Incomplete regeneration | × | × | 87 |
Abbreviations: LC: liquid chromatography, IEC: Ion exchange chromatography, SMB: Simulated moving bed chromatography, CH: Chromatography, N/A: not available.
Conclusion
Regarding to albumin protein priority, different methods were applied for albumin purification; Cohn method which combined with chromatographic techniques is the most well-known among them. In this method other worthwhile proteins of plasma such as coagulation factors, immunoglobulins and etc. would be separated in upstream, and albumin will be seen in the last fraction. Due to the robustness and high purity of plasma in large volumes, this technique, is cost-effective for plasma therapeutic protein production. Hence, this method currently seems to be a major technique in albumin production. The aforementioned methods such as purification from placenta, ammonium sulfate precipitation combined with LC, Heat shock, TCA/Acetone precipitation, different dye ligand affinity chromatographies, Simulated moving bed chromatography, Steric exclusion chromatography, Expanded bed adsorption chromatography and etc for albumin purification are suitable for lab-scale and don’t have enough efficacy.
As respects, the raw material of these techniques is human serum with related safety limitation. Therefore efforts have been done to produce albumin via recombination technology.
Ethical Issues
Not applicable.
Conflict of Interest
The Authors report no declaration of interest.
References
- 1.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: From bench to bedside. Mol Aspects Med. 2012;33(3):209–90. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, He Y, Shi B, Yang D. Human serum albumin from recombinant DNA technology: Challenges and strategies. Biochim Biophys Acta. 2013;1830(12):5515–25. doi: 10.1016/j.bbagen.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Ascenzi P, Fasano M. Allostery in a monomeric protein: The case of human serum albumin. Biophys Chem. 2010;148(1-3):16–22. doi: 10.1016/j.bpc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hirose M, Tachibana A, Tanabe T. Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Mater Sci Eng C. 2010;30(5):664–9. doi: 10.1016/j.msec.2010.02.020. [DOI] [Google Scholar]
- 5.Curry S. Lessons from the crystallographic analysis of small molecule binding to human serum albumin. Drug Metab Pharmacokinet. 2009;24(4):342–57. doi: 10.2133/dmpk.24.342. [DOI] [PubMed] [Google Scholar]
- 6.Varshney A, Sen P, Ahmad E, Rehan M, Subbarao N, Khan RH. Ligand binding strategies of human serum albumin: How can the cargo be utilized? Chirality. 2010;22(1):77–87. doi: 10.1002/chir.20709. [DOI] [PubMed] [Google Scholar]
- 7.Caraceni P, Domenicali M, Tovoli A, Napoli L, Ricci CS, Tufoni M. et al. Clinical indications for the albumin use: Still a controversial issue. Eur J Intern Med. 2013;24(8):721–8. doi: 10.1016/j.ejim.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Kragh-Hansen U. Molecular and practical aspects of the enzymatic properties of human serum albumin and of albumin-ligand complexes. Biochim Biophys Acta. 2013;1830(12):5535–44. doi: 10.1016/j.bbagen.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Quinlan GJ, Martin GS, Evans TW. Albumin: Biochemical properties and therapeutic potential. Hepatology. 2005;41(6):1211–9. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 10.Farrugia A. Albumin usage in clinical medicine: Tradition or therapeutic? Transfus Med Rev. 2010;24(1):53–63. doi: 10.1016/j.tmrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Raoufinia R, Mota A, Nozari S, Aghebati Maleki L, Balkani S, Abdolalizadeh J. A methodological approach for purification and characterization of human serum albumin. J Immunoassay Immunochem. 2016;37(6):623–35. doi: 10.1080/15321819.2016.1184163. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 13.Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001;20(3):271–3. doi: 10.1054/clnu.2001.0439. [DOI] [PubMed] [Google Scholar]
- 14.Lee E, Eom JE, Jeon KH, Kim TH, Kim E, Jhon GJ. et al. Evaluation of albumin structural modifications through cobalt-albumin binding (CAB) assay. J Pharm Biomed Anal. 2014;91:17–23. doi: 10.1016/j.jpba.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Fritz HG, Brandes H, Bredle DL, Bitterlich A, Vollandt R, Specht M. et al. Post-operative hypoalbuminaemia and procalcitonin elevation for prediction of outcome in cardiopulmonary bypass surgery. Acta Anaesthesiol Scand. 2003;47(10):1276–83. doi: 10.1046/j.1399-6576.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 16.Safe Study Investigators , Finfer S, Bellomo R, McEvoy S, Lo SK, Myburgh J. et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: Analysis of data from the saline versus albumin fluid evaluation (safe) study. BMJ. 2006;333(7577):1044. doi: 10.1136/bmj.38985.398704.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eljaiek R, Dubois MJ. Hypoalbuminemia in the first 24h of admission is associated with organ dysfunction in burned patients. Burns. 2013;39(1):113–8. doi: 10.1016/j.burns.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Ryan AM, Hearty A, Prichard RS, Cunningham A, Rowley SP, Reynolds JV. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg. 2007;11(10):1355–60. doi: 10.1007/s11605-007-0223-y. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Nagai H, Yasuda Y, Kanazawa K. The early influence of albumin administration on protein metabolism and wound healing in burned rats. Wound Repair Regen. 2004;12(1):109–14. doi: 10.1111/j.1067-1927.2004.012118.x. [DOI] [PubMed] [Google Scholar]
- 20.Vlahos AL, Crawford RS, Matthew HT, Ledgerwood AM, Lucas CE. Effect of albumin and hespan on rodent hepatocyte function after hemorrhagic shock and sepsis. J Trauma. 2005;59(3):583–8. [PubMed] [Google Scholar]
- 21.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157(2):168–82. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. 2015;12(5):793–812. doi: 10.1517/17425247.2015.993313. [DOI] [PubMed] [Google Scholar]
- 23.Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3(1):4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Martinez R, Andreola F, Mehta G, Poulton K, Oria M, Jover M. et al. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J Hepatol. 2015;62(4):799–806. doi: 10.1016/j.jhep.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–7. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 26.Arasteh A, Farahi S, Habibi-Rezaei M, Moosavi-Movahedi AA. Glycated albumin: An overview of the in vitro models of an in vivo potential disease marker. J Diabetes Metab Disord. 2014;13:49. doi: 10.1186/2251-6581-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent JL, Sakr Y, Reinhart K, Sprung CL, Gerlach H, Ranieri VM. et al. Is albumin administration in the acutely ill associated with increased mortality? Results of the soap study. Crit Care. 2005;9(6):R745–54. doi: 10.1186/cc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wratten ML, Sereni L, Tetta C. Oxidation of albumin is enhanced in the presence of uremic toxins. Renal Fail. 2001;23(3-4):563–71. doi: 10.1081/Jdi-100104738. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki K, Chuang VT, Maruyama T, Otagiri M. Albumin-drug interaction and its clinical implication. Biochim Biophys Acta. 2013;1830(12):5435–43. doi: 10.1016/j.bbagen.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Aubin E, Roberge C, Lemieux R, Bazin R. Immunomodulatory effects of therapeutic preparations of human albumin. Vox Sang. 2011;101(2):131–7. doi: 10.1111/j.1423-0410.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H, Tanase S, Nakajou K, Maruyama T, Kragh-Hansen U, Otagiri M. Role of Arg-410 and Tyr-411 in human serum albumin for ligand binding and esterase-like activity. Biochem J. 2000;349(3):813–9. doi: 10.1042/bj3490813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T. et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18(3):896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 33.Kurono Y, Kushida I, Tanaka H, Ikeda K. Esterase-like activity of human serum albumin. VIII. Reaction with amino acid p-nitrophenyl esters. Chem Pharm Bull (Tokyo) 1992;40(8):2169–72. doi: 10.1248/cpb.40.2169. [DOI] [PubMed] [Google Scholar]
- 34. More J, Bulmer M. Human serum albumin: A multifunctional plasma protein. In: Bertolini J, Goss N, Curling J, editors. Production of plasma proteins for therapeutic use. New Jersey: Wiley; 2012. P. 159-83.
- 35.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, Tate J. et al. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51(5):810–24. doi: 10.1373/clinchem.2004.046292. [DOI] [PubMed] [Google Scholar]
- 37.Tibbling G, Link H, Ohman S. Principles of albumin and igg analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37(5):385–90. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 38.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L. et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 39.Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with alzheimer's disease is related to vascular factors. Acta Neurol Scand. 1990;81(4):323–6. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 40.He Y, Ning T, Xie T, Qiu Q, Zhang L, Sun Y. et al. Large-scale production of functional human serum albumin from transgenic rice seeds. Proc Natl Acad Sci U S A. 2011;108(47):19078–83. doi: 10.1073/pnas.1109736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossettias G. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7(3):216–34. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kragh-Hansen U, Watanabe H, Nakajou K, Iwao Y, Otagiri M. Chain length-dependent binding of fatty acid anions to human serum albumin studied by site-directed mutagenesis. J Mol Biol. 2006;363(3):702–12. doi: 10.1016/j.jmb.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 43.Dugaiczyk A, Law SW, Dennison OE. Nucleotide sequence and the encoded amino acids of human serum albumin mrna. Proc Natl Acad Sci U S A. 1982;79(1):71–5. doi: 10.1073/pnas.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerety RJ, Aronson DL. Plasma derivatives and viral hepatitis. Transfusion. 1982;22(5):347–51. doi: 10.1046/j.1537-2995.1982.22583017454.x. [DOI] [PubMed] [Google Scholar]
- 45.Fanali G, Ascenzi P, Fasano M. Effect of prototypic drugs ibuprofen and warfarin on global chaotropic unfolding of human serum heme-albumin: A fast-field-cycling 1h-nmr relaxometric study. Biophys Chem. 2007;129(1):29–35. doi: 10.1016/j.bpc.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Tullis JL. Albumin: 1. Background and Use. JAMA. 1977;237(4):355. doi: 10.1001/jama.1977.03270310039005. [DOI] [PubMed] [Google Scholar]
- 47.Clowes GH Jr, Vucinic M, Weidner MG. Circulatory and metabolic alterations associated with survival or death in peritonitis: Clinical analysis of 25 cases. Ann Surg. 1966;163(6):866–85. doi: 10.1097/00000658-196606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boldt J. Use of albumin: An update. Br J Anaesth. 2010;104(3):276–84. doi: 10.1093/bja/aep393. [DOI] [PubMed] [Google Scholar]
- 49.Martelli A, Strada P, Cagliani I, Brambilla G. Guidelines for the clinical use of albumin: Comparison of use in two italian hospitals and a third hospital without guidelines. Curr Ther Res Clin Exp. 2003;64(9):676–84. doi: 10.1016/j.curtheres.2003.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vermeulen LC Jr, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The university hospital consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. 1995;155(4):373–9. doi: 10.1001/archinte.155.4.373. [DOI] [PubMed] [Google Scholar]
- 51.Tarin Remohi MJ, Sanchez Arcos A, Santos Ramos B, Bautista Paloma J, Guerrero Aznar MD. Costs related to inappropriate use of albumin in spain. Ann Pharmacother. 2000;34(10):1198–205. doi: 10.1345/aph.19385. [DOI] [PubMed] [Google Scholar]
- 52.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 53.Clark WG, Brater DC, Johnson AR. Goth's medical pharmacology. 13th ed. St Louis: Mosby; 1992. [Google Scholar]
- 54.Zhou T, Lu S, Liu X, Zhang Y, Xu F. Review of the rational use and adverse reactions to human serum albumin in the people's republic of china. Patient Prefer Adherence. 2013;7:1207–12. doi: 10.2147/ppa.s53484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohn EJ, Strong LE, Hughes WL, Mulford DJ, Ashworth JN, Melin M. et al. Preparation and properties of serum and plasma proteins; a system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–75. doi: 10.1021/ja01207a034. [DOI] [PubMed] [Google Scholar]
- 56.Buchacher A, Iberer G. Purification of intravenous immunoglobulin g from human plasma--aspects of yield and virus safety. Biotechnol J. 2006;1(2):148–63. doi: 10.1002/biot.200500037. [DOI] [PubMed] [Google Scholar]
- 57.Denizli A. Plasma fractionation: Conventional and chromatographic methods for albumin purification. Hacettepe J Biol Chem. 2011;39(4):315–41. [Google Scholar]
- 58.Johnston A, Adcock W. The use of chromatography to manufacture purer and safer plasma products. Biotechnol Genet Eng Rev. 2000;17:37–70. doi: 10.1080/02648725.2000.10647987. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka K, Shigueoka EM, Sawatani E, Dias GA, Arashiro F, Campos TC. et al. Purification of human albumin by the combination of the method of cohn with liquid chromatography. Braz J Med Biol Res. 1998;31(11):1383–8. doi: 10.1590/s0100-879x1998001100003. [DOI] [PubMed] [Google Scholar]
- 60.Cabrera-Crespo J, Goncalves VM, Martins EA, Grellet S, Lopes AP, Raw I. Albumin purification from human placenta. Biotechnol Appl Biochem. 2000;31(2):101–6. doi: 10.1042/ba19990095. [DOI] [PubMed] [Google Scholar]
- 61.Grellet S, Martins EA, Maimoni Goncalves V, Yague Lopes AP, Raw I, Cabrera-Crespo J. An associated process for the purification of immuno globulin g, catalase, superoxide dismutase and albumin from haemolysed human placenta blood. Biotechnol Appl Biochem. 2001;34(Pt 3):135–42. doi: 10.1042/ba20010023. [DOI] [PubMed] [Google Scholar]
- 62.Ding Z, Cao X. Affinity precipitation of human serum albumin using a thermo-response polymer with an l-thyroxin ligand. BMC Biotechnol. 2013;13:109. doi: 10.1186/1472-6750-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odunuga OO, Shazhko A. Ammonium sulfate precipitation combined with liquid chromatography is sufficient for purification of bovine serum albumin that is suitable for most routine laboratory applications. Biochemical Compounds. 2013;1(1):3. doi: 10.7243/2052-9341-1-3. [DOI] [Google Scholar]
- 64.Chen YY, Lin SY, Yeh YY, Hsiao HH, Wu CY, Chen ST. et al. A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis. 2005;26(11):2117–27. doi: 10.1002/elps.200410381. [DOI] [PubMed] [Google Scholar]
- 65.Kovacs A, Guttman A. Medicinal chemistry meets proteomics: Fractionation of the human plasma proteome. Curr Med Chem. 2013;20(4):483–90. doi: 10.2174/0929867311320040001. [DOI] [PubMed] [Google Scholar]
- 66.Vasileva R, Jakab M, Hasko F. Application of ion-exchange chromatography for the production of human albumin. J Chromatogr. 1981;216:279–84. doi: 10.1016/s0021-9673(00)82356-0. [DOI] [PubMed] [Google Scholar]
- 67.Abdolalizadeh J, Majidi J, Farajnia S. Production and purification of polyclonal antibody against human kappa light chain. J Biol Sci. 2008;8(3):683–6. doi: 10.3923/jbs.2008.683.686. [DOI] [Google Scholar]
- 68.Aghebati Maleki L, Majidi J, Baradaran B, Abdolalizadeh J, Kazemi T, Aghebati Maleki A. et al. Large scale generation and characterization of anti-human cd34 monoclonal antibody in ascetic fluid of balb/c mice. Adv Pharm Bull. 2013;3(1):211–6. doi: 10.5681/apb.2013.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frerick C, Kreis P, Gorak A, Melzner D. Simulation and optimisation of the downstream process for purification of human serum albumin by using ion exchange membrane adsorbers. Desalination. 2006;200(1-3):468–9. doi: 10.1016/j.desal.2006.03.398. [DOI] [Google Scholar]
- 70.Frerick C, Kreis P, Gorak A, Tappe A, Melzner D. Simulation of a human serum albumin downstream process incorporating ion-exchange membrane adsorbers. Chem Eng Process. 2008;47(7):1128–38. doi: 10.1016/j.cep.2007.07.013. [DOI] [Google Scholar]
- 71.Reif OW, Freitag R. Characterization and application of strong ion-exchange membrane adsorbers as stationary phases in high-performance liquid chromatography of proteins. J Chromatogr A. 1993;654(1):29–41. doi: 10.1016/0021-9673(93)83062-W. [DOI] [PubMed] [Google Scholar]
- 72.Tennikova TB, Svec F. High-performance membrane chromatography: Highly efficient separation method for proteins in ion-exchange, hydrophobic interaction and reversed-phase modes. J Chromatogr A. 1993;646(2):279–88. doi: 10.1016/0021-9673(93)83340-X. [DOI] [Google Scholar]
- 73.Camperi SA, Navarro del Canizo AA, Wolman FJ, Smolko EE, Cascone O, Grasselli M. Protein adsorption onto tentacle cation-exchange hollow-fiber membranes. Biotechnol Prog. 1999;15(3):500–5. doi: 10.1021/bp990054g. [DOI] [PubMed] [Google Scholar]
- 74.Phillips M, Cormier J, Ferrence J, Dowd C, Kiss R, Lutz H. et al. Performance of a membrane adsorber for trace impurity removal in biotechnology manufacturing. J Chromatogr A. 2005;1078(1-2):74–82. doi: 10.1016/j.chroma.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Boi C. Membrane adsorbers as purification tools for monoclonal antibody purification. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(1):19–27. doi: 10.1016/j.jchromb.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 76.Charcosset C. Membrane processes in biotechnology: An overview. Biotechnol Adv. 2006;24(5):482–92. doi: 10.1016/j.biotechadv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Low D, O'Leary R, Pujar NS. Future of antibody purification. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(1):48–63. doi: 10.1016/j.jchromb.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 78.Knudsen HL, Fahrner RL, Xu Y, Norling LA, Blank GS. Membrane ion-exchange chromatography for process-scale antibody purification. J Chromatogr A. 2001;907(1-2):145–54. doi: 10.1016/s0021-9673(00)01041-4. [DOI] [PubMed] [Google Scholar]
- 79.Imamoglu S. Simulated moving bed chromatography (smb) for application in bioseparation. Adv Biochem Eng Biotechnol. 2002;76:211–31. doi: 10.1007/3-540-45345-8_6. [DOI] [PubMed] [Google Scholar]
- 80.Wang C, Bai S, Tao SP, Sun Y. Evaluation of steric exclusion chromatography on cryogel column for the separation of serum proteins. J Chromatogr A. 2014;1333:54–9. doi: 10.1016/j.chroma.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 81.Lihme A, Hansen MB, Andersen IV, Burnouf T. A novel core fractionation process of human plasma by expanded bed adsorption chromatography. Anal Biochem. 2010;399(1):102–9. doi: 10.1016/j.ab.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Hage DS, Anguizola JA, Bi C, Li R, Matsuda R, Papastavros E. et al. Pharmaceutical and biomedical applications of affinity chromatography: Recent trends and developments. J Pharm Biomed Anal. 2012;69:93–105. doi: 10.1016/j.jpba.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdolalizadeh J, Nouri M, Zolbanin JM, Barzegari A, Baradaran B, Barar J. et al. Targeting cytokines: Production and characterization of anti-TNF-alpha scFvs by phage display technology. Curr Pharm Des. 2013;19(15):2839–47. doi: 10.2174/1381612811319150019. [DOI] [PubMed] [Google Scholar]
- 84.Abdolalizadeh J, Majidi Zolbanin J, Nouri M, Baradaran B, Movassaghpour A, Farajnia S. et al. Affinity purification of tumor necrosis factor-alpha expressed in raji cells by produced scFv antibody coupled CNBr-activated sepharose. Adv Pharm Bull. 2013;3(1):19–23. doi: 10.5681/apb.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdolalizadeh J, Nouri M, Zolbanin JM, Baradaran B, Barzegari A, Omidi Y. Downstream characterization of anti-TNF-alpha single chain variable fragment antibodies. Hum Antibodies. 2012;21(1-2):41–8. doi: 10.3233/HAB-2012-0260. [DOI] [PubMed] [Google Scholar]
- 86.Cuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968;61(2):636–43. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Denizli A, Piskin E. Dye-ligand affinity systems. J Biochem Biophys Methods. 2001;49(1-3):391–416. doi: 10.1016/s0165-022x(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 88.Denizli A, Kokturk G, Yavuz H, Piskin E. Albumin adsorption from aqueous solutions and human plasma in a packed-bed column with Cibacron Blue F3GA-Zn(II) attached poly(EGDMA-HEMA) microbeads. React Funct Polym. 1999;40(3):195–203. doi: 10.1016/S1381-5148(98)00043-1. [DOI] [Google Scholar]
- 89.Zhang S, Sun Y. Further studies on the contribution of electrostatic and hydrophobic interactions to protein adsorption on dye-ligand adsorbents. Biotechnol Bioeng. 2001;75(6):710–7. doi: 10.1002/bit.10067. [DOI] [PubMed] [Google Scholar]
- 90.Hanggi D, Carr P. Analytical evaluation of the purity of commercial preparations of cibacron blue F3GA and related dyes. Anal Biochem. 1985;149(1):91–104. doi: 10.1016/0003-2697(85)90480-4. [DOI] [PubMed] [Google Scholar]
- 91.Akgöl S, Tüzmen N, Denizli A. Porous dye affinity beads for albumin separation from human plasma. J Appl Polym Sci. 2007;105(3):1251–60. doi: 10.1002/app.26178. [DOI] [Google Scholar]
- 92.McLoughlin SB, Lowe CR. Applications of triazinyl dyes in protein purification. Rev Prog Color Relat Top. 1988;18(1):16–28. doi: 10.1111/j.1478-4408.1988.tb00063.x. [DOI] [Google Scholar]
- 93.Tong XD, Sun Y. Agar-based magnetic affinity support for protein adsorption. Biotechnol Prog. 2001;17(4):738–43. doi: 10.1021/bp010054s. [DOI] [PubMed] [Google Scholar]
- 94.Ma ZY, Guan YP, Lu HZ. Affinity adsorption of albumin on cibacron blue F3GA-coupled non-porous micrometer-sized magnetic polymer microspheres. React Funct Polym. 2006;66(6):618–24. doi: 10.1016/j.reactfunctpolym.2005.10.014. [DOI] [Google Scholar]
- 95.Wolman FJ, Grasselli M, Smolko EE, Cascone O. Preparation and characterisation of Cibacron Blue F3G-A poly(ethylene) hollow-fibre affinity membranes. Biotechnol Lett. 2000;22(17):1407–11. doi: 10.1023/A:1005664932413. [DOI] [Google Scholar]
- 96.Jin G, Zhang L, Yao Q. Novel method for human serum albumin adsorption/separation from aqueous solutions and human plasma with Cibacron Blue F3GA-Zn(II) attached microporous affinity membranous capillaries. J Membr Sci. 2007;287(2):271–9. doi: 10.1016/j.memsci.2006.10.047. [DOI] [Google Scholar]
- 97.Qu F, Guan Y, Ma Z, Zhang Q. Synthesis of cibacron blue F3GA-coupled magnetic pmma nanospheres and their use for protein affinity separation. Polym Int. 2009;58(8):888–92. doi: 10.1002/pi.2607. [DOI] [Google Scholar]
- 98. Miyauchi M, Miao J, Simmons TJ, Dordick JS, Linhardt RJ. Flexible electrospun cellulose fibers as an affinity packing material for the separation of bovine serum albumin. J Chromatogr Sep Tech 2011;2(2).
- 99.Wolman FJ, Smolko EE, Cascone O, Grasselli M. Improved hollow-fibre membranes for dye-affinity chromatography. J Sep Sci. 2005;28(1):45–51. doi: 10.1002/jssc.200401915. [DOI] [PubMed] [Google Scholar]
- 100.Denizli A, Tuncel A, Kozluca A, Ecevit K, Piskin E. Cibacron Blue F3G-A Attached Poly(Vinyl Alcohol) Particles for Specific Albumin Adsorption. Sep Sci Technol. 1997;32(5):1003–15. doi: 10.1080/01496399708000942. [DOI] [Google Scholar]
- 101.Denizli A, Salih B, Kozluca A, Piskin E. Comparison of albumin binding capacities of three different reactive dye-derivatized poly(ethylene glycol dimethacrylate-hydroxyethyl methacrylate) microbeads. J Biomater Sci Polym Ed. 1997;8(6):411–20. doi: 10.1163/156856297x00353. [DOI] [PubMed] [Google Scholar]
- 102.Denizli A, Salih B, Kavakli C, Piskin E. Dye-incorporated poly(egdma-hema) microspheres as specific sorbents for aluminum removal. J Chromatogr B Biomed Sci Appl. 1997;698(1-2):89–96. doi: 10.1016/s0378-4347(97)00306-x. [DOI] [PubMed] [Google Scholar]
- 103.Yavuz H, Duru E, Genc O, Denizli A. Cibacron blue f3ga incorporated poly(methylmethacrylate) beads for albumin adsorption in batch system. Colloids Surf Physicochem Eng Aspects. 2003;223(1-3):185–93. doi: 10.1016/S0927-7757(03)00153-5. [DOI] [Google Scholar]
- 104.Odabaşı M, Denizli A. Cibacron Blue F3GA-attached magnetic poly(2-hydroxyethyl methacrylate) beads for human serum albumin adsorption. Polym Int. 2004;53(3):332–8. doi: 10.1002/pi.1305. [DOI] [Google Scholar]
- 105.Yavuz H, Denizli A. Dye affinity hollow fibers for albumin purification. Macromol Biosci. 2004;4(2):84–91. doi: 10.1002/mabi.200300040. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Zhang ZP, Song Y, Cal H. Bovine serum albumin (BSA) adsorption with Cibacron Blue F3GA attached chitosan microspheres. React Funct Polym. 2006;66(9):916–23. doi: 10.1016/j.reactfunctpolym.2005.12.003. [DOI] [Google Scholar]
- 107.Demiryas N, Tüzmen N, Galaev IY, Pişkin E, Denizli A. Poly(acrylamide-allyl glycidyl ether) cryogel as a novel stationary phase in dye-affinity chromatography. J Appl Polym Sci. 2007;105(4):1808–16. doi: 10.1002/app.26187. [DOI] [Google Scholar]
- 108.Uzun L, Yavuz H, Say R, Ersoz A, Denizli A. Poly(ethylene dimethacrylate-glycidyl methacrylate) monolith as a stationary phase in dye-affinity chromatography. Ind Eng Chem Res. 2004;43(20):6507–13. doi: 10.1021/ie040045z. [DOI] [Google Scholar]
- 109.Mccreath GE, Chase HA, Lowe CR. Novel affinity separations based on perfluorocarbon emulsions: Use of a perfluorocarbon affinity emulsion for the direct extraction of glucose-6-phosphate dehydrogenase from homogenised bakers' yeast. J Chromatogr A. 1994;659(2):275–87. doi: 10.1016/0021-9673(94)85069-0. [DOI] [Google Scholar]
- 110.Zhu J, Yang J, Sun G. Cibacron Blue F3GA functionalized poly(vinyl alcohol-co-ethylene) (PVA-co-PE) nanofibrous membranes as high efficient affinity adsorption materials. J Membr Sci. 2011;385-386:269–76. doi: 10.1016/j.memsci.2011.10.001. [DOI] [Google Scholar]
- 111.Uzun L, Odabaşi M, Arıca Y, Denizli A. Poly(styrene‐hydroxyethyl methacrylate) monodisperse microspheres as specific sorbent in dye affinity adsorption of albumin. Sep Sci Technol. 2005;39(10):2401–18. doi: 10.1081/ss-120035934. [DOI] [Google Scholar]
- 112.Odaba M, Garipcan B, Dede S, Denizli A. Methacrylamidohistidine in affinity ligands for immobilized metal-ion affinity chromatography of human serum albumin. Biotechnol Bioprocess Eng. 2001;6(6):402–9. doi: 10.1007/bf02932321. [DOI] [Google Scholar]
- 113.Labrou N, Clonis YD. The affinity technology in downstream processing. J Biotechnol. 1994;36(2):95–119. doi: 10.1016/0168-1656(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 114. Labrou NE, Mazitsos K, Clonis YD. Dye-ligand and biomimetic affinity chromatography. In: Hage DS, editor. Handbook of affinity chromatography. New York: Marcel Dekker, Inc; 2005. P. 231-55.



