Abstract
Purpose: Median septum of Juglans regia L. (Juglandaceae) with anti-diabetic effects has been used in Iranian traditional medicine. The present study estimates both oral acute and subchronic toxicities.
Methods: In the oral acute toxicity study, female Wistar rats were treated with doses of 10, 100, 1000, 1600, 2900 and 5000 mg/ kg of the Juglans regia septum of methanol extract (JRSME), and were monitored for 14 days. In subchronic study, JRSME was administered by gavage at dose of 1000 mg/kg daily in Wistar rats for 28 days. Antioxidant status and biochemical examinations were fulfilled, and the vital organs were subjected to pathological analyses.
Results: The extract did not produce any toxic signs or deaths; the medium lethal dose must be higher than 5000 mg/kg. In subchronic study, No significant morphological and histopathological changes were observed in the studied tissues. There was a significant increase in serum malondialdehyde (MDA) level in treated group compared to control after 4 weeks of JRSME intake. The treatment of rats resulted in a significant reduction of serum urea level (p<0.05), kidney’s xanthine dehydrogenase (XDH) activity (p<0.001) and elevation of aldehyde oxidase (AO) activity (p<0.05) in kidney. In the treated group, the mean diameter of glomerulus and proximal urine tube epithelium stature was slightly greater than control group. A significant increase in serum MDA level is subject for further studies.
Conclusion: This study showed that the extract has no acute or subacute adverse effects with dose of 1000 mg/kg. The administration of JRSME may improve kidney structure and function and help in treatment of some chronic diseases.
Keywords: Juglans regia, Toxicity, Antioxidant enzymes, Oxidative stress, Wistar rats
Introduction
Most of medications used for treatment of different diseases may have various side effects; therefore, alternative approaches with more effectiveness, efficacy and safety are needed. There are only 35% of natural compounds available in current drugs. As plants, in general, are considered to have potential bioactive substances such as antioxidants and other secondary metabolites, there is a great interest in the use of medicinal plants as an alternative to synthesized medications.1-3 According to several studies, medicinal plants and plant-originated products are suggested to be safer and less harmful for human body compared with modern synthetic drugs.4 However, Plants and plant originated medicines like synthetic drugs have adverse effects. Medicinal plants have also been historically used for a long time in traditional treatment of diseases all over the world. As a result, different aspects of medicinal plants have been on the focus of intense scientific researches throughout of the world.
Walnut (Juglans regia) belongs to Juglandaceae family is probably the most widespread tree nut used in the world. It comprises several species and is cultivated commercially worldwide. Apart from nutritional and dietary uses of walnut, various parts of this plant have been used not only in traditional medicine but also in the pharmaceutical and cosmetic industries.5 Walnut leaves are rich in antioxidant compounds such as phenolic acids, flavonoids and naphthoquinones.6,7 These plant-originated antioxidants can protect human body against oxidative DNA damage and several diseases such as cardiovascular diseases and cancer.5-7 Root bark of walnut also contains naphthoquinones like juglone and bisjuglone. It has been shown that bisjuglone may have antitumor property in mouse skin carcinogenesis.5 Moreover, different parts of this plant such as seeds, green husk, walnut male flowers, leaf and root show a broad spectrum of pharmacological activities such as antimicrobial, anthelmintic, keratolytic, antidiarrheal and hypoglycemic effects.8-12
The septum of fruit of Juglans regia has been traditionally used in Iranian traditional medicine to treat diabetic patients.10 Recently, some studies have been conducted to investigate the hypoglycemic activity of Juglans regia septum.10,13 However, the toxicity studies of walnut septum have not received enough attention in the literatures. Therefore, the aim of this study was to investigate the toxic effects of different doses of Juglans regia septum of methanol extract (JRSME) in female Wistar rats. The effects of a single dose (1000 mg/kg bw) of the extract on the activity of some enzymes that are related with reactive oxygen species (ROS) and biochemical parameters were also investigated. The histopathological changes in liver, kidney, heart, brain and eye tissues following administration of the single dose of the extract were also evaluated.
Materials and Methods
Preparation of extract
The walnuts (Juglans regia) were bought from vendors in a market located in Urmia, Iran. Voucher specimens (3747) were deposited at the herbarium of the Department of Medicinal and Industrial Plant, Urmia University. It was authenticated by Dr. Sh. Kazempour Osaloo, Department of Plant Biology, Tarbiat Modares University, Tehran, Iran. The nuts were opened and the median septum of the kernels was removed. The septa were dried and powdered with a blender. Dried septa powder (2 Kg) were immersed in 7 L of methanol (95 %) and land left for 7 days at room temperature on three consecutive occasions. The JRSME was filtered and concentrated using a rotary vacuum evaporator at 40ºC and then lyophilized. The average yield of the lyophilized material as a percentage weight of the starting dried plant was approximately 9% w/w.
Animals and experimental design
Female Wistar rats weighing 200 ± 30 g (9-12 weeks old) were used in this study. The experimental protocol for the care and use of laboratory animals was approved by the Ethical Committee of Tabriz University of Medical Sciences. The rats were kept in individual cages under a 12 h light-dark cycle (light from 7 am to 7 pm) at the room temperature (22 ± 3°C). For a week prior to treatment, they were fed with standard laboratory diet and water was given ad libitum.
Toxicity study
For acute toxicity assay, the methanol extract was prepared in 0.9% NaCl (saline) giving a homogeneous suspension of the extract. All samples were always dissolved in a volume of 10 ml/kg and administered by intragastric route, independently of the final concentration to be used. The acute toxicity of the extract was estimated in accordance with Lorke’s method.14 The method estimates dose of a certain compound or extract that kills 50% of the animal population (LD50, 50% lethal dose) by a given administration route. The study was performed in two phases: in the first phase, 12 rats were randomly divided into four groups of 3 rats each. Groups 1, 2 and 3 animals were orally treated with a single dose of 10, 100 and 1000 mg/kg body weight (bw) of the extract, respectively, to possibly establish the range of doses producing any toxic effect. Group 4 was used as the control group and received vehicle alone. In the second phase, 9 rats were randomly divided into three equal groups and further specific doses (1600, 2900 and 5000 mg/kg bw) of the JRSME were administered to each animal to further determine the correct LD50 value. During both phases, the animals were kept under close observation over a period of 14 days and monitored daily for signs of acute toxicity. Restlessness, respiratory distress, convulsions, diarrhea, motor activity, posture and reflexes were qualitatively determined. The weight of the animals was monitored throughout the experiments.
One-fifth of the maximum safe dose of the extract tested for acute toxicity was selected for the subchronic toxicity study.15-18 Thus, for further (subchronic) study of the extract effects on biochemical and histopathological parameters and also antioxidant status of the animals, 12 Wistar rats were randomly divided into two experimental groups each of six animals: group I which served as the control group, and group II which received 20% of the maximum tolerated dose (MTD) of the JRSME (1000 mg/kg bw) by gavage once a day for four weeks. At the same time, the control rats received with the same route of administration an equivalent volume of normal saline.
Blood sampling
After 1-4 weeks of treatment with JRSME, blood samples were taken from retro-orbital plexus of eyes under light anesthesia after 12 h of fasting and placed into heparin-containing tubes to prevent coagulation. Sera samples were prepared by 10 min centrifugation of blood at 3000 g at 4°C and stored at -76°C in accordance with Ghaffari’s method19 for further study.
Tissues sampling
At the end of the experiment, rats were sacrificed between 09.00 and 10.00 am after anesthesia with diethyl ether. To measure the enzymes activity and malondialdehyde (MDA) level, the liver and kidney were removed, weighed and then rapidly washed in 0.9% cold saline and placed in ice-cold isotonic potassium chloride solution (1.15% KCl w/v) containing 0.1 mM EDTA. The livers and kidneys were then chopped separately into 4-5 volumes of 50 mM phosphate buffer (pH 7.4) and homogenized by a homogenizer fitted with a Teflon pestle. The homogenate was then centrifuged at 3000 g for 10 min, the lipid layer was carefully removed, and the resulting supernatant fraction was further centrifuged at 15000 g for 60 min at 4°C. The supernatant was stored at -80°C until use.
In addition, a small piece of the liver, kidney, heart, brain and eye tissue samples were also dissected out and kept in 10% formalin for histological analysis. Then, formalin-fixed tissues were placed into tissue cassettes and processed by tissue-processor (Leica, Jung histokinette 2000, Germany). Six µm sections of the paraffin blocks were prepared and stained with hematoxylin and eosin (H & E). Slides examined by a pathologist under a light microscope (Olympus, CH36 RF200, Japan) and the photographs were taken with a microscope digital camera DP21 (Olympus, U-TVO.5XC-2, Japan).
For quantitative assessment of kidney tissue changes including changes in the urinary tubes (epithelium thickness) and diameter changes of renal glomerulus, 20 glomerulus and cross-section of 5 urinary tubes were randomly selected and counted. Analysis of variance (ANOVA) and post hoc Tukey’s tests were used to analyze data.
Biochemical parameters measurement
The biochemical parameters and antioxidant status of the experimental animals were evaluated at the one week and 4 weeks of the treatment with JRSME. The serum levels of creatinine (Cr), alanine transaminase (ALT), aspartate transaminase (AST) and urea were determined by a test kit provided by the Pars Azmoon Company (Tehran, Iran) using an autoanalyzer (Alcyon, 300, USA).
Measurement of malondialdehyde
The serum, liver and renal levels of MDA were determined using thiobarbituric acid method as described previously.20
Measurement of GPx and SOD activities
Superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were respectively determined according to the methods reported by Woolliams21 et al. and Paglia and Valentine22 using Randox kits (Randox Laboratories, UK).
Paraoxonase-1 activity determination
Arylesterase activity of paraoxonase 1 (PON-1) toward the substrate phenyl acetate was measured using a UV/Vis spectrophotometer (Shimadzu, UV-2550, Japan) as described before.23
Aldehyde oxidase and xanthine dehydroganase activities determination
Aldehyde oxidase (AO) activity in the liver homogenate was determined by following the oxidation of phenanthridine to phenanthridinone as described elsewhere.24 Due to low activity, kidney AO activity was measured using a sensitive RP-HPLC-fluorescence method (Waters 474,USA).25 Acetonitrile and methanol HPLC (high-performance liquid chromatography) grade were purchased from Merck (Whitehouse Station, NJ, Germany). Phenanthridine, phenanthridinone were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Xanthine dehydrogenase (XDH) activity in the liver and kidney homogenate was determined spectrophotometrically using xanthine as substrate as described elsewhere.26 Xanthine and nicotinamide adenine dinucleotides (NAD+) were respectively purchased from Acros Organics (New Jersey, USA) and Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents used were of analytical grade.
Statistical analysis
The results are expressed as mean±SEM. The statistical analysis was performed using independent Student's t-test (SPSS, version 13). In histopathlogical studies of kidney tissue, differences between the control and the treated groups were assessed by one-way ANOVA followed by post hoc Tukey’s tests. P-values less than 0.05 were considered significant.
Results
Acute toxicity of extract
The LD50 values were calculated using the geometric mean or the doses for which 0.3 and 3.3 deaths could be found. LD50 >5000 mg/kg bw is regarded as non-toxic according to Lorke protocol. During the phase of the evaluation, there was no mortality or any signs of behavioral changes or toxicity following oral administration of 10, 100 or 1000 mg/kg bw of the extract; hence during the second phase, the evaluated doses were selected as 1600, 2900 and 5000 mg/kg bw. Again, no death was recorded among the animals throughout the two weeks experimental period. Therefore, the LD50 in rats for the extract of walnut septum was estimated to be >5000 mg/kg bw. In addition, the animals did not show important changes on behavior (i.e. ataxia, hyperactivity or, hypoactivity) and macroscopic morphology of heart, liver, kidney and lungs during the experimental period compared to the negative control group.
Oxidative stress parameters and enzymes
No significant difference was found in the blood SOD, GPx and the serum PON-1 activities between the control group and the animals treated with JRSME for four weeks (Table 1). There was also no significant difference in these enzymes activities in either the control or the treated animals after one and four weeks. However, in experimental group, the serum activity of AST and ALT in the fourth week was found to be significantly (p<0.05 and p<0.01, respectively) higher than the activity observed in the first week (Table 1). In the control group, only serum ALT activity was changed significantly during four weeks (p<0.05). There was a reduction in both urea and Cr blood levels after four weeks treatment with JRSME extract, although it was only significant for the urea level (p< 0.01). However, the treatment caused a marked elevation in the serum MDA level (p<0.01). There was a slight increase in liver and kidney tissues MDA level in normal group compared with the control group after 4 weeks of the JRSME intake (Table 2). Renal level of AO in the treated rats significantly increased (p<0.05) while kidney XDH activity decreased significantly compared to the control group (p<0.001) (Table 2). In addition, no significant changes were found in the kidney antioxidant enzymes activities, whereas treatment with Juglans regia septum extract did not cause any significant increase in the liver AO, XDH and antioxidant enzymes activities.
Table 1. Comparison of biochemical parameters and antioxidant status of the serum in rats after 1 and 4 weeks of treatment with JRSME.
| One week | Four weeks | |||
| Control group (n=6) | Treated group (n=6) | Control group (n=6) | Treated group (n=6) | |
| AST (unit/L) | 166.5 ± 30.3 | 141.1 ± 19.2 | 162.5 ± 21.9 | 177.5 ± 21.7b* |
| ALT (unit/L) | 77.2 ± 15.8 | 65.5 ± 17.3 | 102.8 ± 10.9b* | 122.7 ± 22.8b** |
| SOD (unit/ml) | 242.9 ± 18.9 | 247.5 ± 13.5 | 253.9 ± 13.7 | 255.8 ± 9.7 |
| GPx (unit/L) | 10258.7 ± 695.7 | 11156.4 ± 679.9 | 10569.3 ± 915.7 | 11410.8 ± 536.4 |
| PON-1 (µmol/min/ml) | 108.4 ± 8.6 | 101.1 ± 11.2 | 101.4 ± 9.1 | 110.4 ± 8.0 |
| MDA (nmol/ml) | 2.0 ± 0.4 | 1.9 ± 0.4 | 1.6 ± 0.3 | 3.1 ± 0.7a**b*** |
| Urea (mg/dl) | 48.7 ± 7.1 | 53.3 ± 11.7 | 46.3 ± 7.2 | 36.2 ± 4.4a*b** |
| Cr (mg/dl) | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.4 ± 0.2 |
Data are expressed as mean ± SD for six animals in each group. a: compared with the control group; b: Week 1 vs. Week 4; *p<0.05, **p<0.01, ***p<0.001. JRSME, Juglans regia septum of methanol extract; AST, aspartate transaminase; ALT, alanine transaminase; SOD, superoxide dismutase ; GPx, glutathione peroxidase; PON-1, paraoxonase 1; MDA, malondialdehyde; Cr, creatinine.
Table 2. Comparison of the activities of antioxidant Enzymes and AO, XDH of the liver and Kidney rats after 4 weeks of treatment with JRSME.
| Control group (n=6) | Treated group (n=6) | |||
| Liver | Kidney | Liver | Kidney | |
| SOD (unit/ml) | 13.4 ± 4.6 | 25.3 ± 4.3 | 14.5 ± 3.1 | 25.1 ± 5.1 |
| GPx (unit/L) | 1.50± 0.60 | 1.50 ± 0.20 | 0.99 ± 0.24 | 1.40 ± 0.30 |
| MDA (nmol/ml) | 0.067 ± 0.024 | 0.150 ± 0.036 | 0.085 ± 0.026 | 0.170 ± 0.025 |
| XDH (µmol/min/mg protein) | 0.022 ± 0.068 | 0.007 ± 0.001 | 0.010 ± 0.076 | 0.006 ± 0.001a*** |
| AO (nmol/min/mg protein) | 81.00 ± 17.00 | 0.11 ± 0.02 | 91.00 ± 12.00 | 0.22 ± 0.07a* |
Data are the mean ± SD for six animals in each group. a: compared with the control group
*𝑃 < 0.05,**p<0.01, ***p<0.001. JRSME, Juglans regia septum of methanol extract; SOD, superoxide dismutase ; GPx, glutathione peroxidase; MDA, malondialdehyde; AO, aldehyde oxidase; XDH, xanthine dehydrogenase.
Histopathology of liver, kidney, heart, brain and eye tissues
The effects of JRSME on liver, kidney, heart, brain and eye tissues were also evaluated by histopathologic examinations. The H & E staining of liver specimens are shown in Figure 1A. Livers in the control group had normal lobular architecture with central vein and radiating hepatic but there were some small size areas observed with dispersed inflammation by reaching mononuclear cells mainly lymphocytes and macrophages (mononuclear hepatitis); however, no fatty change and focal necroses were observed (Figure 1A-a). On the other hand, in the treated rats mild degrees of reactive changes along with hypertrophied and proliferated Kupffer cells and inhabitation of neutrophils in disse space were observed, but no fatty change occurred (Figure 1A-b). Furthermore, mild granular degeneration was seen. Lots of small areas with mononuclear hepatitis and congestion in the liver were also observed. Overall, treatment with the extract did not lead to severe pathological effects.
Figure 1.
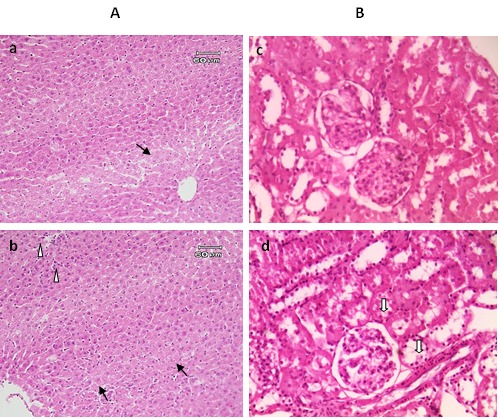
Histological observations of the liver (A) and kidney (B) tissues stained with H & E. Photomicrograph of liver and kidney section from control (a and c, respectively) and treated rat group with JRSME by gavage for 4 weeks (b and d, respectively). Images a-b showing mild granular degeneration (arrows), and in the top left-hand of image (b) mild degrees of reactive changes along with hypertrophied and proliferated Kupffer cells and inhabitation of neutrophils in disse space were observed (arrowheads). Images c-d showing normal view of glomerulus, and in image (d) degrees of degenerative changes of the urinary tract such as fatty change (solid arrow) were seen.
Histopathological examination of kidney tissue in control rats showed that they were mainly normal but some dispersed and small areas of glomerulonephritis and interstitial nephritis were observed (Figure 1B-c). In kidney tissue of the treated rats, there were congestion, mild degenerative changes such as fatty changes in urine tubes; in some areas, damage to the kidney cortex was observed along with interstitial nephritis (Figure 1B-d). In treated group the mean diameter of glomerulus and proximal urine tube epithelium stature was slightly greater than the control group which indicates that the effects of the extract on kidney tissue of treated group were beneficial (Table 3).
Table 3. Comparison of the mean glomerular diameter (µm), height of the epithelium proximal and distal tube (µm) of the Kidney rats after 4 weeks of treatment with JRSME.
| Control group | Treated group | |
| Glomerular diameter (μm) | 106.584 | 110.731 |
| The height of the epithelium proximal tube (μm) | 17.805 | 18.2925 |
| The height of the epithelium distal tube (μm) | 11.707 | 11.707 |
Significantly different from the control by the ANOVA followed by post hoc Tukey’s test. All p>0.05.JRSME, Juglans regia septum of methanol extract.
Regarding the other microscopic examinations of heart, brain and eyes tissues in the control rats, no structural and pathological changes were observed (Figure 2). The treated heart muscular cells of rats had some small degenerative areas with mild lesions of slight damage and more loss of cross-striations muscular cells and more staining (hyalinization) compared with the control group (Figure 2b). No pathological changes were observed in brain and eye tissues in the treated rats (Figure 2d and 2f). Only in microscopic examination (Figure 2d), mild degrees of perineuronal edema and perivascular edema was seen.
Figure 2.
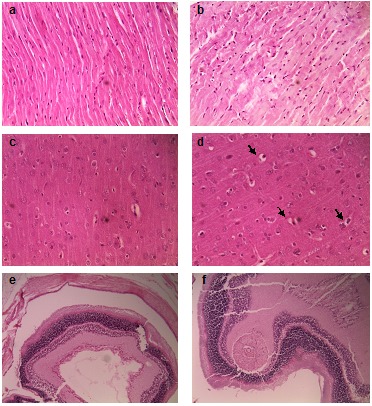
The representative histopathology of various organs of control rat (a, c, and e) versus JRSME-treated rat (b, d, and f) by gavage for 4 weeks and stained with H & E. (a),(b): heart; (c),(d) brain; (e) and (f):eye tissue. Mild degrees of perineuronal edema and perivascular edema in the treated brain of rat (arrows) were seen.
Discussion
Toxicity study is an experimental screening method used to confirm safety of medicinal plants and drugs in animal model trials. Investigation of acute toxicity is usually used as the first step in the toxicological analysis and aimed to understand the biological activity of a chemical and its mechanism of action. The information about acute systemic toxicity acquired in this test is used in hazard identification and risk management regarding with production, handling and use of chemicals.27
In this study, Lorke’s procedure was selected because it offers the advantage that adequate information is obtained using only 24 animals irrespective of the type of material tested and the administration route selected. There were no mortality or acute toxicity in rats treated with 5000 mg/kg JRSME which indicates that the oral LD50 of JRSME is greater than 5000 mg/kg body weight. It has recently been determined in albino mice that the intraperitoneal LD50 values of J. regia aqueous and ethanolic leaves extract were 5.5 g/kg and 3.3 g/kg, respectively,28 whereas in this study, the oral LD50 in rats for the alcoholic extract of walnut septum was determined to be >5000 mg/kg that could be generally considered as nontoxic.
However, as Juglans regia septum could be used for the treatment of chronic diseases like diabetes mellitus, gout and cancer in traditional medicine, its safety should be evaluated through the subchronic toxicity study. Therefore, antioxidant status and also the biochemical and histopathological parameters of the animals were studied following administration of JRSME for four weeks. Almost no changes were observed in the blood SOD, GPx and serum PON-1 activities. There were also no significant alterations in the liver and kidney MDA, SOD and GPx activities. Based on these findings, it could be suggested that normal hepatorenal enzymatic antioxidant mechanisms are maintained during JRSME intake for four weeks.29 However, a significant elevation was observed in the serum level of MDA in the treated rats (Table 1). There was also a slight but not statistically significant increase in the liver and kidney MDA levels. It seems that these tissues encounter with an oxidative stress following treatment with JRSME. Histopathological findings were also indicative of mild pathological changes in these two tissues. On the other hand, the mean diameter of glomerulus and proximal urine tube epithelium stature in the treated animals was slightly greater than the control group which may show that the effects of the extract on kidney tissue of treated group were beneficial. Apart from mild degrees of perineuronal and perivascular edema observed in the brain tissue, there was no pathological change in the eye and brain in the treated rats.
Interestingly, unlike XDH activity which decreased significantly, a marked increase was observed in kidney AO activity of the treated animals compared with the control. Both AO and XDH are molybdo-flavoproteins which have a role in oxidation of a wide range of xenobiotics and metabolism of some endogenous compounds.24 In some conditions, XDH can be converted to its oxidative form, xanthine oxidase (XO). The reaction catalyzed by these enzymes result in production of two electrons.
Unlike XDH which utilizes NAD+ as the electron acceptor, AO and XO use molecular oxygen as an electron acceptor. Therefore, the oxidative reactions catalyzed by AO and XO lead to the generation of the reactive oxygen species (ROS) which are involved in many pathological processes such as inflammation, atherosclerosis, cancer, aging and also other ROS-associated diseases.24 Researchers have found high amounts of ROS production during the oxidation of aldehydes by aldehyde oxidase.30 It seems that AO may play an important role in slight increase in liver and particularly renal’s MDA levels and some tissue damages observed in the liver and kidney could be partly attributed to AO activity. However, the effect of JRSME administration on the observed increase in the tissue AO activity is not clear and is subject for further studies.
Any increase in urea, Cr and uric acid levels is observed when there would be substantial damage to functional nephrons.31,32 Therefore, a significant reduction of serum urea level and a slight decrease of Cr serum concentration, as an index of glomerular filtration rate (GFR),33 alongside an increase in glomerulus diameter may be indicative of the improvement of kidney function and structure and the lack of mechanical damage to the renal filtration mechanism due to JRSME intake.
It has been shown that during destruction of liver and heart cells and also inflammatory disorders, the plasma levels of AST and ALT increase.34,35 ALT is considered as an appropriate and more specific parameter to characterize liver injury.36 The serum levels of ALT and AST were not significantly changed following four weeks treatment of the animals with JRSME which may indicate that the JRSME does not cause hepatic impairment or liver damage and has no significant toxic effects on the heart tissue.37
Liver is considered as the main organ for metabolism, and the first organ of exposing the ingested toxins. Losing the integrity of hepatic cell and homeostasis generally lead to failure of energy production and cell membrane rupture, which allow leakage of cell contents and enzymes.38 The present study did not show any destructive effects of JRSME on the stabilization of plasma membrane so that there were no increased in serum levels of AST and ALT nor focal necrosis in the liver section of treated rats. A fatty liver is a common complication resulted from a variety of liver toxicants and represents a possible reversible injury of the hepatocytes.39 In our study, histopathological investigations also did not show fatty changes along with vacuolation. Overall, no significant histopathological changes were observed in the heart, brain and eye tissues of the treated rats. Therefore, median septum of Juglans regia is not likely to make severe toxicological risk.
Conclusion
In summary, no mortality and significant histopathology sign were seen during acute and chronic treatment with alcoholic extract of walnut septum. Furthermore, no morphological changes regarding cell damage and some biochemical disturbances were observed. Moreover, serum biochemical indices used for evaluation of possible changes in liver and kidney tissue confirm the non-toxic effect of the extract. With regard to kidney, the slight reduction of Cr and significant decrease in the urea status and XDH activity due to long-term prescription of JRSME may indicate the effectiveness of the extract in treatment of chronic diseases such as goat, diabetes mellitus and other disorders. However, increase in kidney AO activity alongside the elevation of serum MDA levels is subject for further studies.
Acknowledgments
The authors gratefully acknowledge for the financial support of this project by Iranian National Foundation of elites.
Ethical Issues
The present study is experimental evaluation of the toxic effects of Juglans regia septum of methanol extract (JRSME) in female Wistar rats. In our study, no patients were enrolled, thus no ethical considerations arose over the treatment allocation of individual patients. The animal experimental design was precisely in accordance with the ethical norms approved by animal Care and Use Committee of Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of Interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Abbreviations
JRSME, Juglans regia septum of methanol extract; bw, body weight; ALT, alanine transaminase; AST, aspartate transaminase; Cr, creatinine; SOD, superoxide dismutase ; GPx, glutathione peroxidase; PON-1, paraoxonase 1; MDA, malondialdehyde; GFR, glomerular filtration rate; HPLC, high-performance liquid chromatography; AO, aldehyde oxidase; XDH, xanthine dehydrogenase; XOR, xanthine oxidoreductase; ANOVA, one-way analysis of variance; SD, standard deviation; H & E, hetoxylin and eosin; ROS, reactive oxygen species.
References
- 1.Rakh MS, Chaudhari SR. Evaluation of CNS depressant activity of Momordica dioica Roxb willd fruit pulp. Int J Pharm Pharm Sci. 2010;2(4):124–6. [Google Scholar]
- 2.Gill NS, Bajwa J, Dhiman K, Sharma P, Sood S, Sharma PD. et al. Evaluation of therapeutic potential of traditionally consumed Cucumis melo seeds. Asian J Plant Sci. 2011;10(1):86–91. doi: 10.3923/ajps.2011.86.91. [DOI] [Google Scholar]
- 3.Huang C, Zhou T, Chen Y, Zhang S, Chen G. (-)Epicatechin regulation of hydroxysteroid sulfotransferase sta (rsult2a1) expression in female rat steroidogenic tissues. J Pharmacol Toxicol. 2011;6(4):349–60. doi: 10.3923/jpt.2011.349.360. [DOI] [Google Scholar]
- 4.Alam MB, Hossain MS, Chowdhury NS, Mazumder MEH, Haque ME, Islam A. In vitro and in vivo antioxidant and toxicity evaluation of different fractions of Oxalis Corniculata linn. J Pharmacol Toxicol. 2011;6(4):337–48. doi: 10.3923/jpt.2011.337.348. [DOI] [Google Scholar]
- 5.Hasan TN, Grace BL, Shafi G, Al-Hazzani AA, Alshatwi AA. Anti-proliferative effects of organic extracts from root bark of Juglans Regia L. (RBJR) on MDA-MB-231 human breast cancer cells: role of Bcl-2/Bax, caspases and Tp53. Asian Pac J Cancer Prev. 2011;12(2):525–30. [PubMed] [Google Scholar]
- 6.Wilms LC, Hollman PC, Boots AW, Kleinjans JC. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of bpde-DNA adducts in human lymphocytes. Mutat Res. 2005;582(1-2):155–62. doi: 10.1016/j.mrgentox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Nour V, Trandafir I, Cosmulescu S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J Chromatogr Sci. 2013;51(9):883–90. doi: 10.1093/chromsci/bms180. [DOI] [PubMed] [Google Scholar]
- 8.Mirbadalzadeh R, Shirdel Z. Evaluation of the blood-glucose reducing effects of walnut green husk extract in diabetic rats. Int J Pharm Sci Rev Res. 2010;5(3):145–7. [Google Scholar]
- 9.Mohammadi J, Saadipour K, Delaviz H, Mohammadi B. Anti-diabetic effects of an alcoholic extract of Juglans regia in an animal model. Turk J Med Sci. 2011;41(4):685–91. doi: 10.3906/sag-1004-802. [DOI] [Google Scholar]
- 10.Dehghani F, Mashhoody T, Panjehshahin M. Effect of aqueous extract of walnut septum on blood glucose and pancreatic structure in streptozotocin-induced diabetic mouse. Iran J Pharmacol Ther. 2012;11(1):10–4. [Google Scholar]
- 11.Hosseini SE, Karimzadeh K, Vessal M. Effects of a hydroalcoholic extract of walnut male flowers on diabetic rats. Zahedan J Res Med Sci. 2013;15(11):55–8. [Google Scholar]
- 12.Hosseini S, Huseini HF, Larijani B, Mohammad K, Najmizadeh A, Nourijelyani K. et al. The hypoglycemic effect of juglans regia leaves aqueous extract in diabetic patients: a first human trial. DARU. 2014;22(1):19. doi: 10.1186/2008-2231-22-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajikhani R, Solati J. Effects of walnut alcoholic extract (Juglans regia l.) septum on serum glucose, insulin and activities of aminotransferase enzymes. J Appl Chem Res. 2010;12:17–23. [Google Scholar]
- 14.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54(4):275–87. doi: 10.1007/bf01234480. [DOI] [PubMed] [Google Scholar]
- 15.Wills PJ, Asha VV. Acute and subacute toxicity studies of Lygodium flexuosum extracts in rats. Asian Pac J Trop Biomed. 2012;2(1):S200–2. doi: 10.1016/S2221-1691(12)60159-2. [DOI] [Google Scholar]
- 16.Saha P, Mazumder U, Haldar P, Islam A, Kumar RS. Evaluation of acute and subchronic toxicity of Lagenaria siceraria aerial parts. Int J Pharm Sci Res. 2011;2(6):1507–12. [Google Scholar]
- 17.Pieme CA, Penlap VN, Nkegoum B, Taziebou CL, Tekwu EM, Etoa FX. et al. Evaluation of acute and subacute toxicities of aqueous ethanolic extract of leaves of Senna alata (L.) Roxb (Ceasalpiniaceae) Afr J Biotechnol. 2006;5(3):283–9. doi: 10.5897/AJB05.197. [DOI] [Google Scholar]
- 18.Ghosh MN. Fundamentals of Experimental Pharmacology. Kolkata, India: Scientific Book Agency; 1984. [Google Scholar]
- 19.Ghaffari T, Nouri M, Saei AA, Rashidi MR. Aldehyde and xanthine oxidase activities in tissues of streptozotocin-induced diabetic rats: effects of vitamin E and selenium supplementation. Biol Trace Elem Res. 2012;147(1-3):217–25. doi: 10.1007/s12011-011-9291-7. [DOI] [PubMed] [Google Scholar]
- 20.Gallou G, Ruelland A, Legras B, Maugendre D, Allannic H, Cloarec L. Plasma malondialdehyde in type 1 and type 2 diabetic patients. Clin Chim Acta. 1993;214(2):227–34. doi: 10.1016/0009-8981(93)90114-J. [DOI] [PubMed] [Google Scholar]
- 21.Woolliams JA, Wiener G, Anderson PH, McMurray CH. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34(3):253–6. [PubMed] [Google Scholar]
- 22.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–69. [PubMed] [Google Scholar]
- 23.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19(1):100–6. [PubMed] [Google Scholar]
- 24.Pirouzpanah S, Hanaee J, Razavieh SV, Rashidi MR. Inhibitory effects of flavonoids on aldehyde oxidase activity. J Enzyme Inhib Med Chem. 2009;24(1):14–21. doi: 10.1080/14756360701841301. [DOI] [PubMed] [Google Scholar]
- 25.Rashidi MR, Amini K, Khani MY, Faridi A, Hanaee J, Sorouraddin MH. A highly sensitive RP-HPLC-fluorescence method to study aldehyde oxidase activity. J AOAC Int. 2011;94(2):550–4. [PubMed] [Google Scholar]
- 26.Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988;254(5 Pt 1):G768–74. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- 27.Walum E. Acute oral toxicity. Environ Health Perspect. 1998;106(Suppl 2):497–503. doi: 10.2307/3433801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseinzadeh H, Zarei H, Taghiabadi E. Antinociceptive, anti-inflammatory and acute toxicity effects of Juglans regia l. Leaves in mice. Iran Red Crescent Med J. 2011;13(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Schlesinger N, Dalbeth N, Perez-Ruiz F. Gout-what are the treatment options? Expert Opin Pharmacother. 2009;10(8):1319–28. doi: 10.1517/14656560902950742. [DOI] [PubMed] [Google Scholar]
- 30.Kundu TK, Hille R, Velayutham M, Zweier JL. Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys. 2007;460(1):113–21. doi: 10.1016/j.abb.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365(9457):417–30. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 32. Sreedharan R, Devarajan P, Van Why S. Pathogenesis of acute renal failure. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, editors. Pediatric Nephrology. Heidelberg, Germany: Springer-Verlag; 2009. P. 1579-602.
- 33.Sugimoto K, Tsuruoka S, Fujimura A. Effect of enalapril on diabetic nephropathy in oletf rats: the role of an anti-oxidative action in its protective properties. Clin Exp Pharmacol Physiol. 2001;28(10):826–30. doi: 10.1046/j.1440-1681.2001.03530.x. [DOI] [PubMed] [Google Scholar]
- 34.Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum: aminotransferases. Scand J Gastroenterol. 1986;21(1):109–13. doi: 10.3109/00365528609034632. [DOI] [PubMed] [Google Scholar]
- 35.Wasan KM, Najafi S, Wong J, Kwong M, Pritchard PH. Assessing plasma lipid levels, body weight, and hepatic and renal toxicity following chronic oral administration of a water soluble phytostanol compound, FM-VP4, to gerbils. J Pharm Pharm Sci. 2001;4(3):228–34. [PubMed] [Google Scholar]
- 36.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 37. Koster J, Wright J. Liver diorders and gallstones. In: Crook MA, editor. Clinical Biochemistry and Metabolic Medicine. 8th ed. London, UK: Hodder and Stoughton Ltd; 2012. P. 252-69.
- 38. Zimmerman HJ. Drug-induced liver disease. In: Schiff ER, Madding WC, Sorell MF, editors. Schiff's Diseases of The Liver. Philadelphia: Lippencourt Raven; 1999. P. 973-1064.
- 39. Treinen-Moslen M. Toxic responses of the liver. In: Klaassen CD, editor. Casarett and Doull’s Toxicology: The Basic Sciences of Poisons. New York: McGraw-Hill; 2001. P. 471-89.


