Abstract
Purpose: The aim of this study was to evaluate the antibothropic and anti-inflammatory properties of J. elliptica.
Methods: Phytochemical screening and thin-layer chromatography (TLC) assays were performed on J. elliptica hydroalcoholic extract (TE) in order to observe its main constituents. The antibothropic activity of TE was evaluated by the in vitro neuromuscular blockade caused by Bothrops jararacussu venom (Bjssu), in a mouse phrenic nerve-diaphragm model (PND). A quantitative histological study was carried out to observe a possible protection of TE against the venom myotoxicity. The anti-inflammatory activity was also evaluated in two models, Bjssu-induced paw edema, and carrageenan-induced neutrophils migration in the peritoneal cavity.
Results: TLC analysis revealed several compounds in TE, such as saponins, alkaloids, and phenolic constituents. TE was able to neutralize the blockade and the myotoxicity induced by venom, when it was pre-incubated for 30 min with venom. In addition, it showed anti-inflammatory activity, inducing less neutrophils migration and reducing paw edema.
Conclusion: J. elliptica showed both antibothropic and anti-inflammatory properties.
Keywords: Bothrops jararacussu, Edema, Inflammation, Jatropha elliptica, Myonecrosis, Neurotoxicity
Introduction
The "Cerrado" (tropical savanna) is the second larger biome in Brazil, covering 25% of Brazilian territory. It is characterized by a continuous herbaceous stratum connected to an arboreal stratum, with variable density of woody species. As an ecosystem with limited resources, their native plants can produce and accumulate more bioactive compounds. Some woody species and their permanents parts are most commonly used in traditional medicine.1
Rhyzomes of Jatropha elliptica (Pohl) Oken. (Euphorbiaceae), also known as “batata-de-teiu”, contains diterpenes, esters, coumarins, terpenoids, and steroids.2 In Brazilian communities of Cerrado, J. elliptica is popularly used to treat snakebite, and its rhizomes are prepared by infusion and given orally. They are infused or macerated in hydroalcoholic beverages and used to treat severe itches, syphilis, and snakebites.3 The pharmacological properties including anti-inflammatory activity of genus Jatropha was reviewed by Sharma and Singh.4 Other species from Jatropha genus, such as Jatropha gossypiifolia inhibited enzymatic and biologic activities of the Bothrops jararaca venom in both in vitro and in vivo assays.5
The clinical signs and symptoms of B. jararacussu envenomation were detailed by Milani et al.6, and are similar to other Bothrops species causing ecchymosis, bleeding, skin infection with abscess and local inflammation. This venom has been used to test anti-inflammatory and anti-myotoxic properties, as well as the activity of many substances against in vitro neuromuscular paralysis.
As J. elliptica is popularly used to treat snakebites, we hypothesized that this plant would have an antivenom activity. Therefore, the aim of the present study was to observe the ability of a hydroalcoholic extract, obtained from J. elliptica roots, to neutralize the in vitro neurotoxicity and myotoxicity caused by B. jararacussu venom. In addition, the anti-inflammatory activity was verified through paw-edema and neutrophils migration in mouse models. Thin-layer chromatography (TLC) and other chemical analyses were carried out to observe the main chemical constituents of this extract.(Graphical Abstract)
Materials and Methods
Plant material collection
Roots of Jatropha elliptica were collected in 2013, in Santa Tereza city (S 10° 19.207’ and W 047° 47,954’), at Tocantins state (TO), Brazil. The ethnobotanical study was carried out in the Quilombola Kalunga do Mimoso Community of Arraias city - TO, Brazil. The plant exsiccate was deposited in the Tocantins Federal University (UFT) Herbarium in Porto Nacional - TO, Brazil, voucher specimen #10.681, according to the International Code of Botanical Nomenclature (ICBN).
Preparation of extract
Herbal preparations were prepared in accordance to European Medicines Agency guideline.7 The collected sample was meticulously washed, dried at 37°C for 48 hours. Then it was powdered, ground in a mill, and macerated (531 g during 3 days) in 2 L of 70% ethanol. The suspension was percolated (protected from light) at 20 drops/min, resulting in a 20% (m/v) hydroalcoholic extract.
J. elliptica or “Teiu” extract (TE) was concentrated under reduced pressure and lyophilized, providing 15 g (20% extraction yield). It was stored at room temperature, protected from light and humidity, until the assays were performed.
Pharmacognostic Study
Cyanogenic glycosides detection
Picrate papers [filter paper saturated with 0.05 M aqueous picric acid, previously neutralized with NaHCO3 10% (w/v)] were used for this test.8 Color changes from yellow to reddish-brown indicated the enzymatic release of HCN from the plant content. Malus sp. seeds were used as positive control in this assay.
Saponins detection
In a test tube, 100 mg of TE was added to 5 mL of purified water, and the tube was kept in intense agitation during 15 s. Then, the tube was maintained in resting during 15 s, and the result was registered. The test was considered positive when a persistent foam was formed on the liquid surface.9
Foam index determination
A TE solution (0.1g/100 mL of purified water) was distributed in 10 test tubes, in 1, 2, 3 mL and successively until reaching 10 mL. Each tube was adjusted to a final volume of 10 mL with purified water, and shacked for 15 s (twice). After 15 min of resting, the foam height was measured. The tube with remaining dense foam of 1 cm height was registered. The foam index (FI) was calculated as FI = 1000/A, being “A” the volume (in mL) of TE of the higher foam height registered.10
Hemolytic activity
According to the Brazilian Pharmacopeia,10 a diluted series of 0.1% TE was prepared using phosphate buffer pH 7.4, and a 2% sheep-blood suspension. A saponin-rich quillaja (Quillaja saponaria) extract (Sigma-Aldrich, Saint Louis, MO, USA), containing 8 to 10% triterpenoid saponin, was used as positive control. After dilution, all tubes were carefully inverted, avoiding the foam formation. They were inverted again after 30 min, and read for hemolysis after resting for 6 h at 37°C.
Alkaloid acquisition
A solution of 0.1 g TE dissolved in 10 mL of 70% ethanol was basified with 10% NH4OH to pH 9.0, and partitioned four times with 3 mL of ether. The fractions were evaporated, the residue was re-suspended in 5 mL of 1% H2SO4, and basified to pH 9.0 with 10% NH4OH. This entire process was repeated; the residue was re-suspended in 1mL 1% H2SO4, and submitted to the following assays:
Precipitation assay: The reaction was carried out with 2 drops of Dragendorff’s and Mayer’s precipitation reagents. Orange and white color precipitates respectively were considered positive results.11
Thin layer chromatography (TLC): Aliquots of TE and the following standards (all from Sigma-Aldrich, 1 mg/mL, solubilized in methanol): β-sitosterol, gallic acid, tannic acid, chlorogenic acid, caffeic acid, rutin, quercetin, and apigenin were spotted onto 0.3 mm-thick silica gel plates (Merck, Germany).8 The TLC system consisted of ethyl acetate-formic acid-acetic acid-water (100:11:11:24, v/v) as mobile phase. It was visualized by using ammonium vapor and NP/PEG, which consisted of 5% (v/v) ethanol NP (diphenylboric acid 2-aminoethyl ester, Sigma), and 5% (v/v) ethanol PEG 4000 (polyethylene glycol 4000, Synth, Brazil). Visualization was performed under UV light at 365 nm. A second plate was visualized with aluminum chloride. The presence of the phytochemical groups was investigated by comparison with the standards. The retention factor (Rf) of each standard was compared with spots exhibited by TE sample.
Determination of Flavonoids and Polyphenols
Flavonoid content: Flavonoids were determined as previously described by Camargo et al.,12 using quercetin as standard. The percentage of flavonoids (%) was calculated from a standard curve of quercetin (0, 4, 8, 12, and 16 µg/mL) prepared in methanol.
Polyphenol content: Polyphenols were determined by a modified method by Hagerman.13 The percentage of polyphenols was determined by a calibration curve (5, 10, 15, 20, 25, 30, 35, and 40 μg/mL) of gallic acid (Sigma).
Pharmacological Assays
Crude snake venom
Bothrops jararacussu venom was collected from two adult specimens kept in Nature Studies Centre’s Serpentary of University of Vale do Paraiba (Univap), SP, Brazil. The venom was lyophilized and maintained at 4 to 8 °C until the use, being certified by Professor Dr. José Carlos Cogo from University Camilo Castelo Branco, Unicastelo.
Animals
Male Swiss mice (26-32 g) were supplied by Anilab (Animais de Laboratório, Paulínia, SP, Brazil). The animals were housed at 25 ± 3 °C on a 12 h light/dark cycle and had access to food and water ad libitum. The protocol was approved by the institutional Committee for Ethics in Research from Federal University of São Carlos (#070/2012, approved in 2012, April). The experiments were performed in accordance with the guidelines of the Brazilian College for Animal Experimentation and with the guide for the care and use of laboratory animals, Eighth Edition.14
Venom neutralization assays
Mouse phrenic nerve-diaphragm muscle (PND) preparation
The phrenic nerve-diaphragm was obtained from mice anesthetized with halothane (Cristália, Brazil) and killed by exsanguination. The PND myographic recording was performed according to Farrapo et al.15 PND was allowed to stabilize for at least 20 min before the following experiments.
A concentration-response curve of TE was carried out using 500 µg (n=8); 750 µg (n=6); 1000 µg (n=4); and 2000 µg (n=2). Control formulation was the Tyrode solution. The 500 µg concentration was further assayed with 40 µg/mL Bjssu venom (n=4), using two models: 1) pre-incubation during 30 min, prior to add into the organ bath (n=5); 2) TE was added after 10 min of venom (n=7).
Quantitative histological study
In order to observe the effect of TE in the myotoxicity (edema, intense myonecrosis characterized by atrophy of the muscle fibers, hyaline aspect, sarcolemmal disruption and lysis of the myofibrils) of Bjssu venom, three resulting preparations from each pharmacological set (Bjssu venom and preincubation) were analyzed by a quantitative morphometric method, and expressed as myotoxicity index (MI), i.e., the percentage of damaged muscle cells number divided by the total number of cells in three non-overlapping, non-adjacent areas of each preparation.16
Anti-inflammatory study
The paw edema and the neutrophils migration in the peritoneal cavity were performed in mice in order to determine the TE’s anti-inflammatory activity. TE was administered orally (V.O.) at doses of 250, 500 or 1000 mg/kg with a gavage needle. For both tests, six animals were used per group (n=6). Phosphate buffered saline (PBS pH 7.4) with 5% dimethylsulfoxide (DMSO) was used to dilute the extract.
Bothrops jararacussu-induced paw edema
The paw edema method proposed by Winter et al.17 was performed using Bjssu venom as the phlogistic agent. First, different doses of Bjssu venom were tested to determine the venom concentration producing similar edema to carrageenan, without causing pronounced bleeding. Formulations consisting of 0.05 mL of 0.9% NaCl with 0.25, 0.5, 1.0 or 2.0 µg of Bjssu were injected into the left hind paw of mice. The contralateral paw received the same volume of sterile saline as a control. A carrageenan group was used as positive control to the venom groups. The edema was measured with a plethysmometer (model 7140, Ugo Basile, Italy) before and at 30 min, 1 h, 2 h, 3 h, 4 h and 5 h after carrageenan or venom injections. Differences between left and right hind-paws volumes were considered as a measure of the edema (mL).
To determine the anti-inflammatory activity of TE, 0.5 µg of the venom was injected into the hind paw. TE (250, 500 and 1000 mg/kg) or vehicle (PBS - 10 mL/kg) were administered orally. As a positive control, dexamethasone (2 mg/kg) was injected intraperitoneally 30 min before the subplantar injection of the phlogistic agent.
Neutrophils migration in the peritoneal cavity
This test was carried out according to Denny et al.18 The neutrophil migration to the peritoneal cavity was stimulated by the intraperitoneal (I.P.) injection of carrageenan at 500 μg per mouse cavity. Vehicle (0.9% NaCl – I.P.) was used as negative control. TE at 250, 500, 1000 mg/kg and vehicle (PBS) were administered orally. Indomethacin (10 mg/kg), used as positive control, was administered I.P. 30 min before the carrageenan stimuli.
Four hours after carrageenan injection, all mice were killed, and their peritoneal cavity content was harvested by washing the cavity with 3 mL of PBS containing EDTA 1.2 mM. Right after the collection, all cells were counted in an optical microscope using a Newbauer chamber. Smears were prepared using a cytocentrifuge (Cytospin 3; Shandon Lipshaw, Pittsburgh, USA), and stained with a Panotic staining kit. The total leucocytes and neutrophils were also counted. The results were represented as number of neutrophils per cavity.
Statistical analysis
Data from neutrophils migration and neuromuscular blockade assay were verified using ANOVA and Tukey as post hoc test. For paw edema assay, ANOVA and Dunnet tests were used. For myographic and histological assays the t-Student test was used. Significance level was set at 5%.
Results and Discussion
Snake envenomation is still an important issue in many rural areas of tropical and subtropical countries. Some medicinal plants, such as J. elliptica are used for treating snakebites in folk medicine. We have confirmed in vivo the anti-inflammatory and antibothropic effects of “teiu extract” (TE) from J. elliptica rhizomes in the present study.
Pharmacognostic study
The extract was negative for cyanogenic glycosides and the foam index of saponin was 2500, i.e., 2.5 L of water is needed to dilute 1 g of extract to provide a 1 cm-height foam. The hemolytic test was negative for TE and positive to quillaja suspension. Supplementary Figure S1 shows the assays used to evaluate the TE content.
TE did not present cyanogenic glycosides or hemolytic activity, showing that this plant has a potential to be safe for human use. The foam formation is indicative of saponins, which are usually steroidal or triterpene glycoside compounds. Saponins have antimicrobial activity, causing cell rupture on microbial community. In addition, the extract showed alkaloids in its composition, a largest single class of secondary metabolites, which function is still obscure.8 In the alkaloids assay, both colors orange and white show alkaloids (Supplementary Figure S2.1, S2.2, and S2.3) in TE composition. Alkaloids were confirmed by TLC (Supplementary Figure S2.4), using atropine as positive control. Atropine and TE showed similar retention factors (Rf).
The total phenolic content in TE was of 14.5%, which was obtained by using the equation y = 0.1031x + 0.0489 (R= 0.9986), and gallic acid as standard. Flavonoids content was of 0.08% (y = 0.0488x – 0.0025, R= 0.9998; quercetin as standard). Supplementary Figure S3 shows the chromatographic profile of TE (lanes 3 and 9) compared to the following phytochemical standards: 1 - β-sitosterol (Rf=0.9); 2 - gallic acid (Rf=0.92); 4 and 7 - tannic acid (Rf= 0.77); 5 - chlorogenic acid (Rfs=0.6 and 0.75); 6 - caffeic acid (Rf=0.9); 8 - rutin (Rf=0.47 and 0.67); 10 - quercetin (Rf=0.88); and 11 - apigenin (Rf=dragged spot). TE expressed more phenolic constituents (blue colors) than flavonoids (orange colors) in the solvent system used. The chromatographic fingerprint of TE was similar to the ones observed in literature.2 The predominant constitution was phenolic acids (14.5%) and lower flavonoids content (0.08%) were not mentioned previously. Further studies are needed to explore the extract composition.
Venom neutralization assays
Figure 1 shows TE in concentrations of 500 µg (n=8), 750 µg (n=6), 1000 µg (n=4), and 2000 µg (n=2) added into the 5 mL bath containing the preparation. The 500 µg was selected as the concentration for TE in the remaining assays, since it induced fewer changes in the basal response, ideal for the venom neutralization assays. A facilitator effect (increased amplitude of muscular response) was observed in 750 µg TE.
Figure 1.
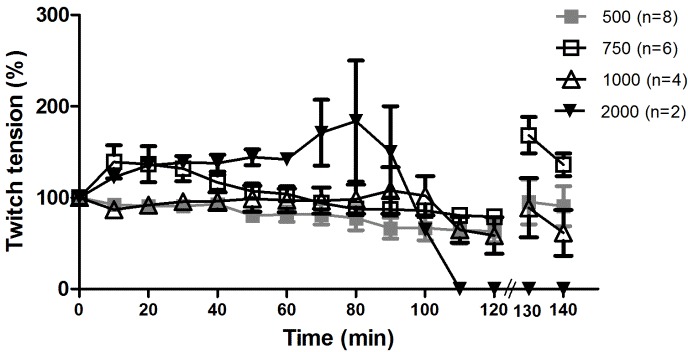
Contractile response of PND incubated with TE without venom
Contractile response (mean ± SEM) of stimulated PND preparations incubated with different TE concentrations and without Bjssu venom. The number of experiments (n) is shown in the legend of figure.
Figure 2 shows the contractile responses of two models. The pre-incubation model (86 ± 14 %) showed a better protective effect than the post venom model (64 ±10 %).
Figure 2.
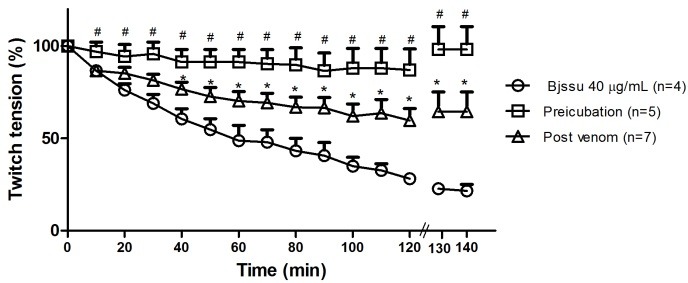
Effect of TE on contractile response of PND in pre-incubated and post venom models
Contractile responses (mean ± SEM) of stimulated PND preparations in pre-incubated (30 min) and post venom models. Statistically significant differences (p<0.05) between post venom model with Bjssu venom alone (*), and pre-incubation model with both Bjssu venom alone and post venom model (#) were indicated. The number of experiments (n) is shown in the legend of figure.
Myographic assays showed that the pre-incubation model was more advantageous than post-venom model, since it permitted to TE constituents to neutralize the venom constituents in vitro before exposing the phrenic nerve preparations. Besides, it showed the ability of the TE to avoid paralysis evolution, suggesting antibothropic properties, such as Casearia gossypiosperma,12 Vellozia flavicans,19 and phenolics from Dipteryx alata.20
Some constituents of our J. elliptica extract, such as flavonoids (apigenin, quercetin, and rutin), probably have a role in the protection against the paralysis induced by Bjssu, although they were found in a very small content (0.08%). Previously, quercetin showed some protection against the neuromuscular blockade of Bjssu in the same experimental conditions.21 In contradiction, another study demonstrated that a commercial rutin formulation did not protect against the paralysis induced by either Bjssu or bothropstoxin-I, which could be related to a large amount used (180 mg/mL).22 Considering these previous studies, we believe that some phenolic compounds of J. elliptica extract may have the effects observed in the present study; however, further studies are needed to prove this hypothesis.
A high extract concentration (2000 µg) produced an intense neurotransmitter release before the complete blockade. From the extract constituents, saponins could be the best candidate to produce this effect, since its neuroprotective effect was previously demonstrated.23 These authors proposed several possibilities for explaining the neuroprotective role of saponins, from which at least three of them can be shared against the venom: modulation of neurotransmitter (intense acetylcholine release followed by a possible depletion); 2) attenuation of Ca2+ influx; and 3) inflammatory inhibition.
The myotoxic index (MI) of venom alone was 44.4% ± 0.86 (n=3), which showed characteristic damage, including myonecrosis, edema, membrane rupture, and presence of ghost cells. In the pre-incubated preparations, MI was reduced to 16.8% ± 0.55 (n=3), showing that TE protected (p<0.05) against the myotoxic effect of Bjssu. Irrespectively of its constituent, J. elliptica extract expressively decreased almost three times the cell damage induced by Bjssu, by an unclear mechanism.
Anti-inflammatory study
Figure 3 describes the variation of paw volume or ∆V (volume of paw edema at each time-interval minus the volume of paw at baseline) after 5 h of injection of Bjssu venom or carrageenan. All Bjssu venom doses were able to induce similar or more pronounced paw edema than carrageenan. The maximum edema was obtained after 2 h of injection for all doses. In doses of 0.5 µg and 0.25 µg, Bjssu venom induced more similar effects to carrageenan than the other doses (p> 0.05). In fact, 0.5 µg of Bjssu venom produced more pronounced edema than carrageenan, 2 h after injection, while 0.25 µg induced less edema at 3 h (p<0.05). Higher venom doses (1 and 2 µg) induced 2 to 3 times higher paw edema than carrageenan, and caused a visible and diffused hemorrhage (p>0.05). Therefore, 0.5 µg of Bjssu venom was chosen for the hind-paw edema assay in order to avoid under-estimated inflammatory effects of the venom. Bjssu at 0.5 µg induced similar paw edema, without a prominent hemorrhage, when compared to 50-µg carrageenan. Bothrops venoms are known to cause edema, local pain, erythema hemorrhage, and necrosis.24
Figure 3.
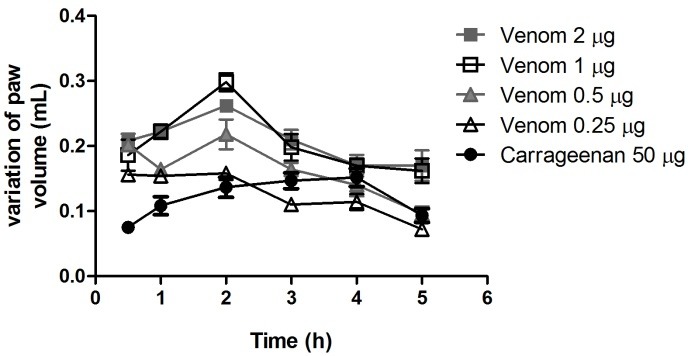
Dose-response of hind-paw edema induced by venom
Dose-response (mean ± SD) profile of hind-paw edema (∆V) induced by B. jararacussu venom. Comparisons were performed between carrageenan and each dose of venom (ANOVA/Dunnet; n=5).
Table 1 shows the ∆V (in percentage) after TE (V.O.) and dexamethasone (I.P.) administration. Both substances reduced the paw edema after 1 h of administration, when compared to a negative control group (NaCl 0.9%, V.O.). In addition, dexamethasone and TE (in all doses) induced maximal edema inhibition between 3 to 5 h after injection in comparison to negative control (p<0.05). TE 1000 mg/kg and dexamethasone induced similar anti-inflammatory effect at 3, 4, and 5 h time-intervals (p<0.05). The edema caused by Bjssu is induced by prostanoids production and neutrophils migration.25 Our results showed that J. elliptica extract, in all concentrations tested, reduced the paw edema similarly to dexamethasone for at least 5 hours.
Table 1. Effect (mean±SD of ΔV in mL, % of reduction) of TE and dexamethasone on paw edema induced by Bjssu venom.
| Groups | Dose (mg/kg) | Mean and Standard deviation of ΔV (mL) and percent of reduction of paw edema (induced by 0.5 μg of Bjssu) | |||||
| 30 min | 1 h | 2 h | 3 h | 4 h | 5 h | ||
| Control | --- | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.02 | 0.08 ± 0.02 |
| Dexamethasone | 2 mg/kg (i.p.) | 0.12 ± 0.04 (10.0%) | 0.07 ± 0.03* (39.5%) |
0.05 ± 0.02* (51%) |
0.04 ± 0.02* (57%) |
0.03 ± 0.02* (60%) |
0.03 ± 0.02* (66%) |
| TE | 250 mg/kg | 0.12 ± 0.04 (13.7%) |
0.06 ± 0.03* (53.5%) |
0.05 ± 0.03* (45.6%) |
0.05 ± 0.02* (44.6%) |
0.04 ± 0.01* (52%) |
0.04 ± 0.02* (54%) |
| TE | 500 mg/kg | 0.13 ± 0.03 (2.5%) |
0.07 ± 0.02* (35%) |
0.07 ± 0.02* (34%) |
0.06 ± 0.01* (39.5%) |
0.05 ± 0.02* (40%) |
0.05 ± 0.02* (44%) |
| TE | 1000 mg/kg | 0.12 ± 0.02 (12.5%) |
0.05 ± 0.03* (53.4%) |
0.06 ± 0.01* (42%) |
0.03 ± 0.02* (66%) |
0.04 ± 0.03* (52%) |
0.03 ± 0.03* (66%) |
Mean and Standard deviation of ΔV represent the mean difference of paw volume between the basal measure and measures at each time point (30 min, 1, 2, 3, 4 and 5 h), n = 5. Percent of reduction of paw edema (previously induced by 0.5 μg of Bjssu) was calculated considering the paw volume for each group in comparison to the control group at each time interval. The symbol * represent significant differences when comparing each group to control (p<0.05; ANOVA - Dunnett’s test).
Figure 4 shows TE (irrespectively of dose) and indomethacin reduced (p<0.05) the neutrophils migration when compared to negative control (vehicle). No significant differences (p>0.05) among indomethacin (positive control), carrageenan (control group), and TE groups (250, 500, and 1000 mg/kg) were observed regarding neutrophils migration. In a dose dependent manner, J. elliptica extract was also able to reduce migration of leucocytes induced by carrageenan similarly to indomethacin. The probable constituents responsible for the extract anti-inflammatory properties were the polyphenols, since these compounds previously showed potential anti-inflammatory properties.26 Thus, in both inflammatory models the extract showed activity similar to the classical anti-inflammatory agents.
Figure 4.
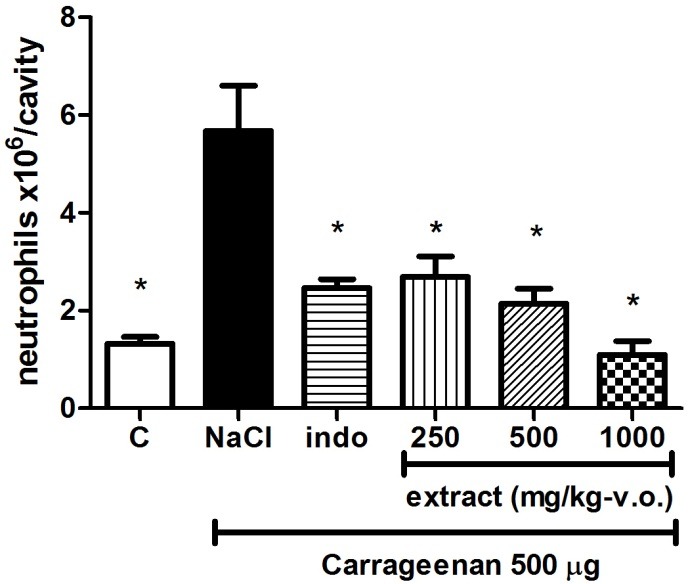
Effect of TE on neutrophils migration
Neutrophils migration (mean ± SD) towards the peritoneal cavity induced by carrageenan injection (i.p.). Control (C) is the intra-peritoneal injection of 0.9% NaCl (no carrageenan). NaCl is the negative control, i.e., 0.9% NaCl administered orally after carrageenan intra-peritoneal injection. Positive control (indo) is indomethacin administered at 10 mg/kg (i.p.). * - statistical significant differences when compared to NaCl group (ANOVA/Tukey, p<0.05; n=6).
There are many questions for the snakebite managing: the high incidence in several countries, the unsatisfactory emergency health services, the difficult transportation of antiserum and patients, the difficult antiserum handling, the adverse reactions against the antiserum, a lot of clinical complications and the high mortality rates.27 Thus, the bioprospection of new complementary agents for antiserum therapy, such as medicinal plants used in folk medicine, could be essential keys.
Conclusion
J. elliptica extract, which was rich in phenolic compounds, showed medicinal properties as anti-inflammatory, being able to counteract the in vitro neurotoxicity and myotoxicity of B. jararacussu venom.
Acknowledgments
We would like to thank FAPESP for financial support (2004/09705-8; 07/53883-6; 08/52643-4; 12/0871-0) and Mr Demétrio da Costa for sample collection and documentation.
Ethical Issues
The ethical issue has been stated on page 575, under the section “Animals”.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.de Medeiros PM, Ladio AH, Albuquerque UP. Patterns of medicinal plant use by inhabitants of brazilian urban and rural areas: A macroscale investigation based on available literature. J Ethnopharmacol. 2013;150(2):729–46. doi: 10.1016/j.jep.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Goulart MOF, Sant’Ana AEG, Lima RA, Cavalcante SH, Carvalho MG, Braz FR. Fitoconstituintes químicos isolados de Jatropha elliptica. Atribuição dos deslocamentos químicos dos átomos de carbono e hidrogênio dos diterpenos jatrofolonas A e B. Quim Nova. 1993;16(2):95–100. [Google Scholar]
- 3.Pott A, Pott V J. Plantas do Pantanal. Brasília, DF: EMBRAPA-SPI; Corumbá: EMBRAPA-CPAP; 1994. [Google Scholar]
- 4.Sharma SK, Singh H. A review on pharmacological significance of genus Jatropha (Euphorbiaceae) Chin J Integr Med. 2012;18(11):868–80. doi: 10.1007/s11655-012-1267-8. [DOI] [PubMed] [Google Scholar]
- 5.Felix-Silva J, Souza T, Menezes YA, Cabral B, Camara RB, Silva-Junior AA. et al. Aqueous leaf extract of Jatropha gossypiifolia l. (Euphorbiaceae) inhibits enzymatic and biological actions of Bothrops jararaca snake venom. PLoS One. 2014;9(8):e104952. doi: 10.1371/journal.pone.0104952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milani Junior R, Jorge MT, de Campos FP, Martins FP, Bousso A, Cardoso JL. et al. Snake bites by the jararacuçu (Bothrops jararacussu): Clinicopathological studies of 29 proven cases in Sao Paulo State, Brazil. QJM. 1997;90(5):323–34. doi: 10.1093/qjmed/90.5.323. [DOI] [PubMed] [Google Scholar]
- 7. European Medicines Agency (EMA). Guideline on declaration of herbal substances and herbal preparations in herbal medicinal products /traditional herbal medicinal products, 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003272.pdf.
- 8.Harborne AJ. Phytochemical Methods-A guide to modern techniques of plant analysis. 3rd ed. London (UK): Chapman & Hall; 1998. [Google Scholar]
- 9.Costa AF. Farmacognosia Experimental. 3rd ed. Lisboa: Fundação Caloustre Gulbenkian; 1981. [Google Scholar]
- 10. Farmacopeia Brasileira. 5th ed. Brasília: Anvisa; 2010.
- 11.Bruneton J. Pharmacognosy: phytochemistry medicinal plants. 2nd ed. France (Paris): Lavoisier Publishing; 1999. [Google Scholar]
- 12.Camargo TM, Nazato VS, Silva MG, Cogo JC, Groppo FC, Oshima-Franco Y. Bothrops jararacussu venom-induced neuromuscular blockade inhibited by Casearia gossypiosperma Briquet hydroalcoholic extract. J Venom Anim Toxins incl Trop Dis. 2010;16(3):432–41. doi: 10.1590/S1678-91992010000300009. [DOI] [Google Scholar]
- 13. Hagerman AE. Tannin chemistry and Protein precipitable phenolics. In: The Tannin Handbook. 1998, 2002, 2011. Available from: http://www.users.miamioh.edu/hagermae/. Accessed- 05/15/2015.
- 14.National Research Council of the National Academies. 8th ed. Washington DC: National Academic Press, 2011; 2011. [Google Scholar]
- 15.Nicole MF, Gleidy AAS, Karine NC, Magali GS, Jose CC, Chariston ADB. et al. Inhibition of Bothrops jararacussu venom activities by Plathymenia reticulata Benth extracts. J Venom Res. 2011;2:52–8. [PMC free article] [PubMed] [Google Scholar]
- 16. Oshima-Franco Y, Rosa LJR, Silva GAA, Amaral Filho J, Silva MG, Lopes PS, et al. Antibothropic action of Camellia sinensis extract against the neuromuscular blockade by Bothrops jararacussu snake venom and its main toxin, bothropstoxin-I. In: Gallelli L. ed. Croatia: Intech; 2012.
- 17.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 18.Denny C, Melo PS, Franchin M, Massarioli AP, Bergamaschi KB, de Alencar SM. et al. Guava pomace: A new source of anti-inflammatory and analgesic bioactives. BMC Complement Altern Med. 2013;13:235. doi: 10.1186/1472-6882-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tribuiani N, da Silva AM, Ferraz MC, Silva MG, Bentes AP, Graziano TS. et al. Vellozia flavicans Mart. Ex Schult. Hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complement Altern Med. 2014;14:48. doi: 10.1186/1472-6882-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida EH, Ferraz MC, Tribuiani N, Tavares RVS, Cogo JC, dos Santos MG. et al. Evaluation of the safety of three phenolic compounds from Dipteryx alata Vogel with antiophidian potential. Chin Med. 2015;6(1):1–12. doi: 10.4236/cm.2015.61001. [DOI] [Google Scholar]
- 21.Soares-Silva JO, Oliveira JL, Cogo JC, Tavares RVS, Oshima-Franco Y. Pharmacological evaluation of hexane fraction of Casearia gossypiosperma Briquet: antivenom potentiality. J Life Sci. 2014;8:306–15. doi: 10.17265/1934-7391/2014.04.002. [DOI] [Google Scholar]
- 22.Cintra-Francischinelli M, Silva MG, Andreo-Filho N, Gerenutti M, Cintra AC, Giglio JR. et al. Antibothropic action of Casearia sylvestris Sw. (Flacourtiaceae) extracts. Phytother Res. 2008;22(6):784–90. doi: 10.1002/ptr.2365. [DOI] [PubMed] [Google Scholar]
- 23.Amaral CF, Da Silva OA, Goody P, Miranda D. Renal cortical necrosis following Bothrops jararaca and B. Jararacussu snake bite. Toxicon. 1985;23(6):877–85. doi: 10.1016/0041-0101(85)90379-4. [DOI] [PubMed] [Google Scholar]
- 24.Sun A, Xu X, Lin J, Cui X, Xu R. Neuroprotection by saponins. Phytother Res. 2015;29(2):187–200. doi: 10.1002/ptr.5246. [DOI] [PubMed] [Google Scholar]
- 25.Wanderley CW, Silva CM, Wong DV, Ximenes RM, Morelo DF, Cosker F. et al. Bothrops jararacussu snake venom-induces a local inflammatory response in a prostanoid- and neutrophil-dependent manner. Toxicon. 2014;90:134–47. doi: 10.1016/j.toxicon.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Denny C, Lazarini JG, Franchin M, Melo PS, Pereira GE, Massarioli AP. et al. Bioprospecting of Petit Verdot grape pomace as a source of anti-inflammatory compounds. J Funct Foods. 2014;8:292–300. doi: 10.1016/j.jff.2014.03.016. [DOI] [Google Scholar]
- 27.Gupta YK, Peshin SS. Do herbal medicines have potential for managing snake bite envenomation? Toxicol Int. 2012;19(2):89–99. doi: 10.4103/0971-6580.97194. [DOI] [PMC free article] [PubMed] [Google Scholar]


