Abstract
Purpose: Triptans are the drug category mostly prescribed for abortive treatment of migraine. Most recent cases of liver toxicity induced by triptans have been described, but the mechanisms of liver toxicity of these medications have not been clear.
Methods: In the present study, we obtained LC50 using dose-response curve and investigated cell viability, free radical generation, lipid peroxide production, mitochondrial injury, lysosomal membrane damage and the cellular glutathione level as toxicity markers as well as the beneficial effects of taurine and/or N-acetyl cysteine in the sumatriptan-treated rat parenchymal hepatocytes using accelerated method of cytotoxicity mechanism screening.
Results: It was revealed that liver toxicity induced by sumatriptan in in freshly isolated parenchymal hepatocytes is dose-dependent. Sumatriptan caused significant free radical generation followed by lipid peroxide formation, mitochondrial injury as well as lysosomal damage. Moreover, sumatriptan reduced cellular glutathione content. Taurine and N-acetyl cysteine were able to protect hepatocytes against sumatriptan-induced harmful effects.
Conclusion: It is concluded that sumatriptan causes oxidative stress in hepatocytes and the decreased hepatocytes glutathione has a key role in the sumatriptan-induced harmful effects. Also, N-acetyl cysteine and/or taurine could be used as treatments in sumatriptan-induced side effects.
Keywords: Oxidative Stress, Mitochondria, N-acetyl cysteine, Sumatriptan, Taurine, Toxicity
Introduction
Primary headache disorder migraine is the 3rd disabling disease in the world and affects more than 10 % of people worldwide.1 It has been estimated that more than thirty million people in the United States suffer from migraine.2 Triptans are a drugs class with proven effect in acute treatment of migraine attacks. The first member of this class of drugs sumatriptan was presented to the market within several formulations. Clearance of organic anions and bile acids from the liver may be affected by sumatriptan.3,4 The major route of elimination of sumatriptan is metabolism in the liver.5 This medication undergoes hepatic metabolic first-pass effects.6 Triptans have various bioavailability and half-life. Oral bioavailability of sumatriptan is low because of first pass metabolism.7,8 Sumatriptan pharmacokinetics is affected by CYP 3A4 inhibitors.9 Sumatriptan is also metabolized by monoamine oxidase enzyme.10
Some studies have suggested that there is little information about triptans poisoning. Toxic doses of triptans could vary depending on some circumstances such as pregnancy.11 On the base of Germany poisons information center, in total, fifty nine cases of triptans’ overdose have been registered. Children constitute high percentages of the patients (forty two cases of al).12 Moreover, ischemic colitis thought to be related to sumatriptan for migraines.13 Moreover there are case reports of adverse effect of triptans on kidney e.g. subacute ischemic injuries of the kidney or renal infarction.14,15 Most recent toxic effects of triptans on liver and the cases of hepatotoxicity of triptans have been reported.16 Another case report was a 17-year-old girl who developed hepatotoxicity during treatment with antimigraine triptans.17 Sumatriptan is in the possible hepatotoxic class of drugs according to its structural moiety.18
In vitro studies performed in tube in absence of cells, suggested that sumatriptan has direct scavenging activity on free radicals,19 but this medication have several metabolites after enzymatic processes in the body, thus, the overall outcome should be clarified.
Water-soluble ROS scavenger N-acetylcysteine (NAC) is naturally formed in garlic and onion.20 This neuroprotective agent is a precursor of the glutathione (GSH) and could interact directly with ROS.21 NAC is anti-inflammatory agent and displays beneficial effects on toxicity induced by HMG-CoA reductase inhibitors, arsenic and CCl4.22-25 2-aminoethanesulfonic acid (taurine) is a cell membrane stabilizer and has shown protective effects against toxicity of antiseizures such as phenytoin and carbamazepinein several organs of the body including testes brain, liver as well as retina.26-29 Beneficial effects of natural flavonol quercetin have been described in cardiovascular disorders, hepatotoxicity and cancer30,31 as well as radiotoxicity.32 This glycoside reduces hepatotoxicity included by sodium fluoride and acetaminophen.33-35 Lipophilic ROS scavenger α-tocopherol (vitamin E) is a supplementary nutrition witch has displayed benefical effects on carcinogenic effects of chemicals specially in combination with sellenium.36 Oxidative stress induced by thallium and mood stabilizers such as valproic acid in hepatocytes has been effectively reduced by vitamin E.37,38
The exact mechanisms of harmful effects of sumatriptan in hepatocytes have not yet been illustrated. The major aim of this work was to determine the cellular mechanisms of harmful effects of sumatriptan in freshly isolated rat parenchymal hepatocytes and to explore the beneficial roles of NAC, taurine, quercetin and/or α-tocopherol.
Materials and Methods
Materials
The materials used in the present study were pure and prepared from Sigma-Aldrich Co. (Taufkirchen, Germany). Becoming stable hepatocytes had been pre-incubated for 30 min before addition of test materials. 1-bromoalkanes have been employed to deplete hepatocytes glutathione.39
Animals
Male Albino rats of Sprague-Dawley strain (250 - 320 g) had been acquired from Medical Sciences University of Tabriz (Tabriz, Iran). Separate plastic cages were employed to keep animals under standard diet of chow and water (ad-lib) with controlled temperature (21 °C – 23 °C). All animals were exposed to photoperiod of light /dark 12:12 h. All tests were fulfilled under ethical standards determined by the local Committee of Animal Experimentation of Medical Sciences University of Tabriz.
Cell Preparation
Collagenase perfusion was performed to isolate hepatocytes as described previously.40 Briefly after removal of Ca2+ with chelator, digestive enzyme collagenase has been employed to prepare singlet and fresh parenchymal hepatocytes. To assess viability of the cells using trypan blue, equal portions of the test hepatocytes were taken at 60, 120 and 180 minutes after incubation.40,41 In all experiments about 80–90 percent of the viable parenchymal hepatocytes were acquired under circulation of combination of 95 % oxygen and 5 % CO2 atmosphere. In all experiments rat parenchymal liver cells have been suspended in Krebs–Henseleit buffer media with the concentration of 106 cells/ml, at pH 7.4 and 37°C in round-bottom flasks 30 min prior to the addition of sumatriptan (5 mM) and/or other materials.
Avoiding very toxic circumstances, in this work, LC50 concentrations of sumatriptan succinate (5 mM) after 2 h of incubation, have been calculated using dose-response curves based on a regression plot of three different concentrations.42
ROS Levels Assay
Hepatocytes were incubated with dichlorofluorescein diacetate (DCFH-DA). DCFH-DA is hydrolyzed to nonfluorescent dichlorofluorescein (DCFH). DCFH reacts with cellular ROS and convert to the highly fluorescent dye dichlorofluorescein (DCF). A FP-750 Jasco fluorescence spectrophotometer (Tokyo, Japan) was used to determine the DCF levels. (Excitation: 500 nm, Emission: 520 nm).43
Mitochondrial Membrane Potential (MMP) Assay
Rhodamine123 were used to assess the MMP.44 The mitochondria uptake this dye and redistribution of rhodamine123 from injured mitochondria to the incubation medium could be measured spectroflourometrically (Excitation: 490 nm, Emission: 520 nm). A FP-750 Jasco fluorescence spectrophotometer (Tokyo, Japan) was used to determine the rhodamine123 levels.45
Lysosomal Damage Assay
In the present study acridine orange were used to assess parenchymal hepatocyte lysosomal damage (Excitation: 495 nm, Emission: 530 nm). The lysosomes uptake this dye and redistribution of acridine orange from injured lysosomes to the medium could be measured using a FP-750 Jasco fluorescence spectrophotometer (Tokyo, Japan).38
Lipid Peroxide Production Assay
Production of lipid peroxides has been measured by assessing the thiobarbituric acid reactive substances (TBARS). Absorbance at 530 nm was measured using UV spectrophotometer at several time intervals as previously described.46
Reduced Glutathione Level Assay
Pure reduced glutathione was used as standards .To measure reduced glutathione by HPLC using a mBondapak NH2 column (Water Associates, Milford, MA) samples were deproteinized (by meta phosphoric acid 5 %) and then derivatized with dinitro- fluorobenzene and iodoacetic acid as previous described.47,48
Statistical Analysis
These data have been compared by ANOVA statistical method followed by post hoc Tukey's test (with a p-value less than 0.05). Results have been presented as mean ± SD of triplicate samples.
Results and Discussion
Hepatocytes Viability
Following incubation of hepatocytes for 3 hours (without any treatment), viability of the control cells was 85 %. In comparison to the control, membrane lysis significantly (p-value <0.05) increased in hepatocytes incubated with sumatriptan concentration-dependently. After 120 min incubation of hepatocytes with sumatriptan, the calculated LC50 (i.e., 50 % membrane lysis within 120 min) was 5 mM (Table 1). This toxicity marker which measured by trypan blue dye exclusion test, was significantly reduced by taurine, NAC, quercetin as well as anti oxidants (BHT, Vitamin E) (p-value <0.05). Moreover, sumatriptan induced hepatocyte lysis has been prevented by l-glutamine (l-Gln) and fructose as ATP generators, cytochrome P450 enzyme inhibitors, endocytosis inhibitors chloroquine and methylamine as well as l-carnitine and trifluoperazine (TFP) as MPT pore sealants (Table 1).
Table 1. Hepatocyte toxicity induced by sumatriptan and protective effect of antioxidants, mitochondrial ATP generators, radical scavengers, lysosomal membrane stabilizers, MPT pore sealing agents and CYP450 inhibitors .
| Treatment | Cytotoxicity % | ||
| Incubation time | |||
| 60 min | 120 min | 180 min | |
| Control (intact hepatocytes) | 15 ± 3 | 17 ± 2 | 19 ± 2 |
| Sumatriptan (5 mM) | 40 ± 3a | 48 ± 3a | 53 ± 4a |
| +Taurine (200 µM) | 27 ± 2b | 29 ± 3b | 32 ± 2b |
| +Quercetin (500 µM) | 17 ± 3b | 18 ± 2b | 23 ± 3b |
| +NAC (200 µM) | 24 ± 3b | 27 ± 3b | 32 ± 4b |
| +Vitamin E (100 µM) | 15± 2b | 17 ± 4b | 22 ± 3b |
| +BHT (50 μM) | 21 ± 2b | 27 ± 3b | 30 ± 2b |
| +Methylamine (30 mM) | 25 ± 3b | 31 ± 1b | 34 ± 2b |
| +Chloroquine (100 μM) | 24 ± 3b | 28 ± 2b | 36 ± 4b |
| +Fructose (10 mM) | 21 ± 4b | 26 ± 3b | 33 ± 2b |
| +L- Gln (1 mM) | 19 ± 2b | 23 ± 2b | 26 ± 2b |
| + TFP (15 µM) | 26 ± 4b | 32 ± 3b | 36 ± 4b |
| +Carnitine (2 mM) | 23 ± 5b | 27 ± 2b | 30 ± 3b |
| +4-MP (500 µM) | 21 ± 3b | 25 ± 4b | 29± 4b |
| +Cimetidine (2 mM) | 22 ± 3b | 25 ± 5b | 28 ± 4b |
| GSH depleted hepatocytes | 20 ± 2 | 22 ± 3 | 25 ± 2 |
| +Sumatriptan (5 mM) (in GSH depleted hepatocytes) |
75 ± 4c | 80 ± 3c | 93 ± 5c |
Cell viability was assessed by trypan blue exclusion test. Results are demonstrated as mean ±S.E. of at least three different experiments.
a Significantly higher than control (p < 0.05).
bSignificantly lower than sumatriptan treated hepatocytes (p < 0.05).
cSignificantly higher than sumatriptan treated hepatocytes (p < 0.05).
Our results showed that hepatocyte lysis notably increased in case of cytochrome P450 induction by pretreatment with phenobarbital for 3 days (Figure 1). In this circumstance hepatocyte lysis decreased by both CYP inhibitors cimetidine (2 mM) and 4-methylpyrazole (4-MP) (500 µM) (p-value < 0.05).
Figure 1.
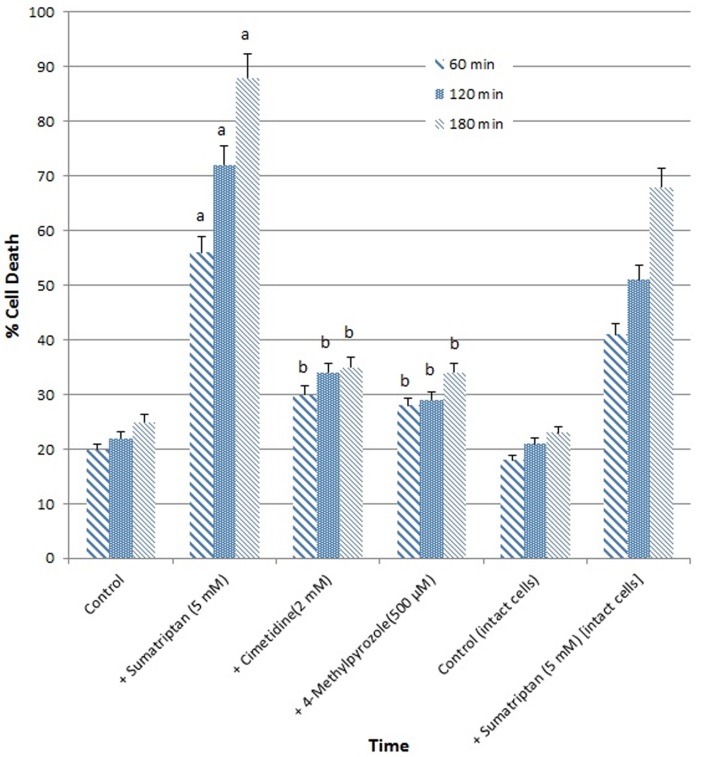
Cell cytotoxicity induction with sumatriptan (5 mM) after 3 days incubation by phenobarbital and the effect of enzyme inhibition by 4- MP (500 µM) and cimetidine (2 mM)
Data are shown as mean±S.E. for at least three different experiments.
aSignificantly higher than control (p < 0.05).
bSignificantly lower than sumatriptan-treated group (p < 0.05).
Scale: 663 × 651.
Membrane lysis was not induced significantly (p-value < 0.05) by the protective agents and cytochrome P450 inhibitors as well as 1-bromoheptane at concentrations used when administered without sumatriptan (data not shown).
ROS Levels
A noticeable increase in ROS generation was observed in hepatocytes exposed to sumatriptan. ROS formation was significantly (p-value <0.05) reduced by incubation of the hepatocytes with taurine and quercetin as well as NAC. Also, ROS formation was significantly (p-value <0.05) reduced by treatment of isolated hepatocytes with aforementioned anti oxidants, l-Gln, fructose, cytochrome 450 inhibitors, l-carnitine, TFP as well as endocytosis inhibitors (Figure 2).
Figure 2.
ROS formation induced by sumatriptan (5 mM) and protective effect of antioxidants, ROS scavengers, lysosomotropic compounds, ATP generators, Mitochondrial permeability transition pore sealing compounds and CYP450 inhibitors
Data are shown as mean±S.E. for at least three different experiments.
aSignificantly higher than control group (p < 0.05).
b Significantly lower than sumatriptan-treated group (p < 0.05).
c Significantly higher than sumatriptan-treated group (p < 0.05).
Scale: 1299 × 794 mm.
Sumatriptan-induced ROS levels was in turn increased by depleting hepatocyte GSH with 1-bromoalkane, demonstrating the impact of glutathione in high ROS levels induced by sumatriptan (Figure 2).
MMP
MMP was reduced by sumatriptan administration to the cells compared to the normal hepatocytes. MMP had been restored by pretreatment of the hepatocytes with anti oxidants, showing the impact of reactive oxygen species in sumatriptan-induced mitochondrial damage (Table 2). As expected, l-carnitine, TFP, l-Gln, fructose, cytochrome P450 enzyme inhibitors and/or endocytosis inhibitors restored MMP too.
Table 2. MMP changes induced by sumatriptan in rat liver hepatocytes and protective effect of antioxidants radical scavengers, lysosomal membrane stabilizers, mitochondrial ATP generators, MPT pore sealing agents and CYP450 inhibitors .
| Treatment |
MMP
(% of control) |
||
| Incubation time | |||
| 15 min | 30 min | 60 min | |
| Control (intact hepatocytes) | 100 | 97 ± 3 | 92 ± 3 |
| Sumatriptan (5 mM) | 72 ± 3a | 65 ± 2a | 53 ± 2a |
| +Taurine (200 µM) | 89 ± 3b | 80 ± 3b | 73 ± 2b |
| +Quercetin (500 µM) | 85 ± 3b | 78 ± 3b | 70 ± 3b |
| +NAC (200 µM) | 90 ± 3b | 81 ± 3b | 75 ± 2b |
| +Vitamin E (100 µM) | 85 ± 3b | 79 ± 2b | 69 ± 3b |
| +BHT (50 μM) | 86 ± 2b | 80 ± 3b | 74 ± 3b |
| +Methylamine (30 mM) | 89 ± 2b | 83 ± 3b | 79 ± 2b |
| +Chloroquine (100 μM) | 84 ± 3b | 76 ± 2b | 68 ± 2b |
| +Fructose (1 mM) | 85 ± 3b | 77 ± 3b | 70 ± 3b |
| +L- Gln (1 mM) | 87 ± 3b | 77 ± 2b | 69 ± 3b |
| + TFP (15 µM) | 87 ± 3b | 81 ± 2b | 77 ± 2b |
| +Carnitine (2 mM) | 86 ± 2b | 77 ± 3b | 65 ± 2b |
| +4- MP (500 µM) | 88 ± 3b | 82 ± 3b | 77 ± 2b |
| +Cimetidine (2 mM) | 86 ± 3b | 81 ± 3b | 76 ± 2b |
| GSH depleted hepatocytes | 98 ± 2 | 95 ± 2 | 93 ± 3 |
| + Sumatriptan (5 mM) (in GSH depleted hepatocytes) | 61 ± 3c | 53 ± 2c | 41 ± 2c |
MMP was determined as the percentage of mitochondrial rhodamine 123 reuptake between control and treated cells. Results are expressed as mean ± S.E. of three separate experiments.
a Significantly lower than control (p < 0.05).
bSignificantly higher than sumatriptan treated hepatocytes (p < 0.05).
cSignificantly lower than sumatriptan treated hepatocytes (in comparison with GSH depleted cells) (p < 0.05).
Sumatriptan-induced mitochondrial damage was in turn increased by depleting hepatocyte GSH with 1-bromoalkane, demonstrating the impact of GSH in sumatriptan induced mitochondrial damage.
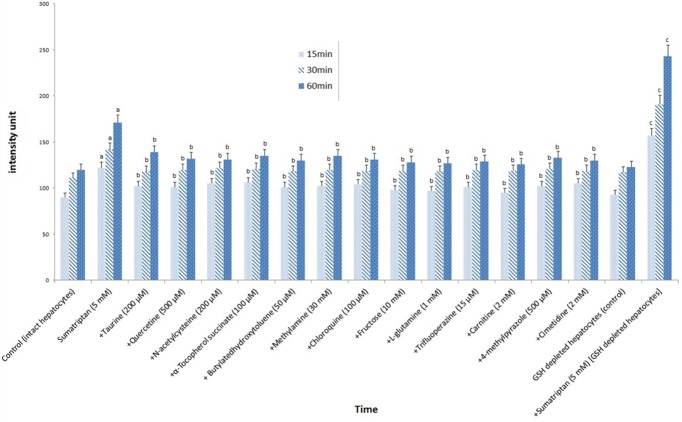
Lysosomal Damage
A significant increase in lysosomal membrane damage was observed in hepatocytes after sumatriptan exposure which is associated with the leakiness of the lysosomal enzymes.
This toxicity marker was significantly (p-value <0.05) inhibited by cytochrome P450 enzyme inhibitors, antioxidants, l-carnitine, TFP, l-Gln and fructose (Table 3). Lysosomal damage was in turn increased by depleting hepatocyte GSH with 1-bromoalkane, demonstrating the impact of glutathione in sumatriptan induced lysosomal damage.
Table 3. Lysosomal membrane damage induced by sumatriptan in rat liver hepatocytes and protective effect of antioxidants, radical scavengers, lysosomal membrane stabilizers, mitochondrial ATP generators, MPT pore sealing agents and CYP450 inhibitors .
| Treatment |
Acridine orange redistribution
(intensity unit of diffused cytosolic green fluorescence) |
||
| Incubation time | |||
| 15 min | 30 min | 60 min | |
| Control (intact hepatocytes) | 7.5 ± 2 | 9.9 ± 3 | 11.5 ± 3 |
| Sumatriptan (5 mM) | 34.3 ± 2a | 39.5 ± 3a | 53.2 ± 3a |
| +Taurine (200 µM) | 14.3 ± 2b | 17.5 ± 3b | 24.6 ± 2b |
| +Quercetin (500 µM) | 17.1 ± 2b | 20.4 ± 2b | 28.6 ± 3b |
| +NAC (200 µM) | 15.3 ± 2b | 18.6 ± 3b | 26.9 ± 3b |
| +Vitamin E (100 µM) | 19.4± 2b | 24.8 ± 2b | 31.5 ± 1b |
| +BHT (50 μM) | 19.3 ± 2b | 27.5 ± 1b | 39.1 ± 1b |
| +Methylamine (30 mM) | 18.4 ± 2b | 21.1 ± 3b | 30.7 ± 1b |
| +Chloroquine (100 μM) | 20.1 ± 2b | 22.2 ± 1b | 36.0 ± 1b |
| +3-Methyladenine (5 mM) | 21.1 ± 3b | 25.5 ± 4b | 34.9 ± 3b |
| +Fructose (10 mM) | 19.8 ± 2b | 27.3 ± 1b | 38.1 ± 2b |
| +L- Gln (1 mM) | 18.3 ± 2b | 26.1 ± 2b | 38.7 ± 3b |
| + TFP (15 µM) | 19.2 ± 3b | 25.5 ± 3b | 32.2 ± 2b |
| +Carnitine (2 mM) | 20.3 ± 3b | 24.9 ± 5b | 37.1 ± 3b |
| +4- MP (500 µM) | 17.2 ± 2b | 22.9 ± 3b | 31.3 ± 4b |
| +Cimetidine (2 mM) | 18.6 ± 2b | 24.7 ± 3b | 33.9 ± 3b |
| GSH depleted hepatocytes | 59.8 ± 2 | 87.4 ± 2 | 121.2 ± 1 |
| + Sumatriptan (5 mM) (in GSH depleted hepatocytes) | 45.6 ± 3c | 53.2 ± 2c | 68.2 ± 2c |
Lysosomal membrane fragility was measured as fluorescent intensity unit of diffused cytosolic green fluorescence induced by acridine orange following the redistribution from lysosomes into cytosol in acridine orange loaded hepatocytes. Results are expressed as mean ±S.E. of three separate experiments (n=3).
a Significantly higher than control (p < 0.05).
bSignificantly lower than sumatriptan treated hepatocytes (p < 0.05).
cSignificantly higher than sumatriptan treated hepatocytes (p < 0.05).
Lipid Peroxides Production
Lipid peroxide induction which determined by measuring thiobarbituric acid reactive substances (TBAR) was significantly (p-value <0.05) increased after administration of sumatriptan. Lipid peroxidation has been significantly reduced by taurine, NAC and/or quercetin.
Also, TBARS generation was significantly (p-value <0.05) prevented by the anti oxidants, endocytosis inhibitors, l-carnitine, TFP, l-Gln, fructose and/or cytochrome P450 enzyme inhibitors. Depletion of hepatocytes GSH with 1-bromoalkane in turn increased the TBARS levels, demonstrating the impact of glutathione in lipid peroxide production by sumatriptan in parenchymal hepatocytes (Table 4).
Table 4. Lipid peroxidation induced by sumatriptan in rat liver hepatocytes and protective effect of antioxidants, radical scavengers, lysosomal membrane stabilizers, mitochondrial ATP generators, mitochondrial permeability transition pore sealing compounds and CYP450 inhibitors .
| Treatment | TBARS (μM per 10 6 cells) | ||
| Incubation time | |||
| 15 min | 30 min | 60 min | |
| Control (only hepatocytes) | 0.082 ± 0.005 | 0.091 ± 0.005 | 0.144 ± 0.009 |
| Sumatriptan (5 mM) | 0.149 ± 0.012 a | 0.181 ± 0.013 a | 0.245 ± 0.024 a |
| +Taurine (200 µM) | 0.109 ± 0.007 b | 0.127 ± 0.009 b | 0.176 ± 0.011 b |
| +Quercetin (500 µM) | 0.118 ± 0.011 b | 0.141 ± 0.013 b | 0.196 ± 0.010 b |
| +NAC (200 µM) | 0.105 ± 0.010 b | 0.123 ± 0.009 b | 0.178 ± 0.011 b |
| +Vitamin E (100 µM) | 0.115 ± 0.008 b | 0.147 ± 0.011 b | 0.197 ± 0.014 b |
| +BHT (50 μM) | 0.114 ± 0.010 b | 0.140 ± 0.007 b | 0.192 ± 0.011 b |
| +Methylamine (30 mM) | 0.119 ± 0.011 b | 0.141 ± 0.013 b | 0.198 ± 0.013 b |
| +Chloroquine (100 μM) | 0.116 ± 0.007 b | 0.144 ± 0.010 b | 0.202 ± 0.010 b |
| +Fructose (10 mM) | 0.120± 0.007 b | 0.149 ± 0.011 b | 0.191 ± 0.011 b |
| +L-Gln (1 mM) | 0.117 ± 0.007 b | 0.137 ± 0.010 b | 0.197 ± 0.012 b |
| + TFP (15 µM) | 0.113 ± 0.010 b | 0.140 ± 0.014 b | 0.201± 0.010 b |
| +Carnitine (2 mM) | 0.119 ± 0.011 b | 0.146 ± 0.008 b | 0.192± 0.010 b |
| +4- MP (500 µM) | 0.112 ± 0.009 b | 0.135 ± 0.009 b | 0.195 ± 0.011 b |
| +Cimetidine (2 mM) | 0.120 ± 0.009 b | 0.138 ± 0.010 b | 0.204 ± 0.011 b |
| GSH depleted hepatocytes(control) | 0.097 ± 0.008 | 0.124 ± 0.007 | 0.163 ± 0.005 |
| + Sumatriptan (5 mM) (in GSH depleted hepatocytes) | 0.186 ± 0.010 c | 0.239 ± 0.011 c | 0.357 ± 0.019 c |
TBARS formation was expressed as µM concentrations. Results are expressed as mean ±S.E. of three separate experiments.
a Significantly higher than control (p < 0.05).bSignificantly lower than sumatriptan treated hepatocytes (p < 0.05).cSignificantly higher than sumatriptan treated hepatocytes (p < 0.05).
Reduced Glutathione Levels
180 min incubation of the cells with sumatriptan (5 mM) caused significant GSH depletion. As expected, GSH depletion has been significantly (p<0.05) restored with cytochrome P450 enzyme inhibitors, antioxidants, l-carnitine, TFP, l-Gln, fructose as well as lysosomal improver agents (Table 5).
Table 5. GSH depletion induced by sumatriptan in rat liver hepatocytes and protective effect of antioxidants and radical scavengers, lysosomal membrane stabilizers, mitochondrial ATP generators, MPT pore sealing compounds and CYP450 inhibitors .
| Treatment | Intracellular GSH (nmol per 10 6 cell) | ||
| Incubation time | |||
| 60 min | 120 min | 180 min | |
| Control (intact hepatocytes) | 57.1 ± 1.2 | 51.5 ± 2.1 | 48.3 ± 1.6 |
| Sumatriptan (5 mM) | 46.3 ± 2.2a | 38.6 ± 1.9a | 31.6 ± 2.3a |
| +Taurine (200 µM) | 56.4± 2.6b | 49.8± 3.3b | 46.8± 3.1b |
| +Quercetin (500 µM) | 51.3± 2.8b | 48.9± 2.8b | 47.3± 2.1b |
| +NAC (200 µM) | 52.1± 3.1b | 49.3± 3.5b | 47.1± 2.1b |
| +Vitamin E (100 µM) | 51.2± 3.3b | 49.2± 3.6b | 47.3± 2.2b |
| +BHT (50 μM) | 52.8± 2.4b | 48.2± 2.8b | 45.9± 2.7b |
| +Methylamine (30 mM) | 53.5± 2.2b | 47.6± 3.1b | 43.7± 2.1b |
| +Chloroquine (100 μM) | 51.6± 3.2b | 46.5± 3.7b | 43.1± 3.6b |
| +Fructose (10 mM) | 52.2± 3.1b | 48.7± 2.9b | 44.7± 3.5b |
| +L- Gln (1 mM) | 53.4± 3.5b | 48.6± 3.7b | 45.3± 3.9b |
| + TFP (15 µM) | 51.5± 2.8b | 47.9± 3.6b | 44.9± 3.7b |
| +Carnitine (2 mM) | 54.2± 2.5b | 48.3± 3.8b | 45.7± 2.6b |
| +4- MP (500 µM) | 53.3± 3.4b | 48.4± 2.6b | 44.6± 3.3b |
| +Cimetidine (2 mM) | 52.2± 3.3b | 48.1± 2.3b | 43.3± 3.2b |
Results are expressed as the means ± S.E of three separate experiments.
a Significantly lower than control (p < 0.05).b Significantly higher than sumatriptan treated hepatocytes (p < 0.05).
Several organs such as liver are involved in triptans adverse effects.15-17 It is well known that imbalance between antioxidant defense and ROS generation (e.g., glutathione) to removal of ROS lead to oxidative stress.49 GSH is an important antioxidant defense molecule for removal of reactive oxygen species such as lipid hydroperoxides and H2O2.50 It has been demonstrated that sumatriptan affects superoxide release.51 Surprisingly an in vitro study suggested that sumatriptan has scavenging activity on free radicals.19 Also, it has been shown that the scavenging property is dose-dependent.52 Moreover, this medication has several metabolites after enzymatic processes such as indole acetic acid.
Indole acetic acid derivatives are formed by sumatriptan metabolism in the liver53 and one study demonstrated toxic effects of prooxidant radicals of indole acetic acid derivatives.54 On the base of our results sumatriptan induces ROS formation and consequently depletes GSH. Our result also showed that GSH depletion by nontoxic bromoheptane via transferring the heptyl group of potent nontoxic bromoheptane to GSH form heptyl-S-glutathione and39 caused a significant rise in sumatriptan-induced mitochondrial and lysosomal injury and consequently induced cell death.
Migraine induces dysfunctional oxidative phosphorylation and consequently increases ROS formation. Several studies have shown the impact of antioxidants such as vitamin E in migraine.55 Migraine pathogenesis is characterized by an increase in ROS generation. Thus migraine-induced ROS generation was significantly augmented after consumption of sumatriptan. Mitochondria have a key role in migraine. On the base of our results, sumatriptan toxicity is associated with GSH depletion and ROS generation and the antioxidants effectively reduced these toxicity markers. Mitochondrial dysfunction occurred during migraine.56-58 It has been showed that mitochondrial ATP depletion results in MMP drop.59 Normal MPT pore may be affected and opened by ROS formation.60 While MPT is opened, some molecules such as cytochrome C that heretofore could not have crossed the mitochondrial membrane, now can release into cytosol and accelerate apoptosis.61 Surprisingly, our results showed that MMP drop (% DΨm ) ensued after exposure to sumatriptan. This circumstance was prevented by lysosome improver, cytochrome P450 inhibitors, l-Gln, fructose, l-carnitine, TFP. Mitochondrial injury was significantly decreased by glutathione depletion and the MMP drop prevented by anti oxidants, suggesting that the MMP rapidly decreases after GSH depletion which in turn followed ROS formation.
On the base of their basic properties, chemicals such as drugs containing amine groups can be trapped into lysosomes.62 As a result of this trapping, lysosomal membrane goes instable and consequently lytic enzymes such as proteases release into cytosol. Osmotic injury followed by membrane lysis is a common consequence of releasing of lysosomal content into cytosol.38 Our results showed that hepatocytes incubation with sumatriptan leads to lysosomal injury that could be a result of accumulation of this chemical in lysosomes.
3-methyladenine and chloroquine are inhibitors of hepatocyte autophagy and used as lysosomal improver agents.63 Our results showed that in addition to autophagy inhibitors, ROS scavengers and antioxidants significantly reduced lysosomal injury induced by sumatriptan in hepatocytes, suggesting that sumatriptan induced lysosomal injury can take place not only by direct effects of trapped sumatriptan in lysosomes but also after ROS formation. This was confirmed by an increase in lysosomal injury after GSH depletion. To author’s knowledge, this is the first work which clarifies the impact of sumatriptan in lysosomal injury.
CYP isoenzymes are important factors in ROS formation and can be involved in chemicals toxicity.64 Triptans have several metabolites produced by liver enzymes.65 It has been showed that sumatriptan pharmacokinetics is affected by CYP 3A4 inhibitors.9 Also, quercetin was shown to inhibit the metabolism of chemicals by CYP3A4 in the liver.66 Our results confirmed that pretreatment by CYP inducers phenobarbital caused a significant cell lysis which in turn prevented by cytochrome inhibitors 4-MP and/or cimetidine suggesting that CYP isoenzymes have an important effect in toxicity induced by sumatriptan and this should be considered in coadministration of sumatriptan and medications which induce or inhibit CYP enzymes.
Conclusion
In conclusion our results suggest that anti oxidants and ATP generators seems to be useful medicines for improving triptan efficacy and reducing toxicity induced by these drugs and it is proposed that prescription of appropriate anti oxidants and ATP generators can be included in migraine therapy. Additionally since taurine and N-acetylcysteine are available in drugstores from various pharmaceutical companies, simultaneous prescription of this supplements with sumatriptan is possible.
Moreover, it is suggested that the impact of this medication on cell organelles should be studied by details in animal models of migraine headache and aura regarding the oxidative stress induced by migraine.
Acknowledgments
This article is a part of the thesis number 106 which submitted for PhD degree in Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran and was funded by Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran with grant number: 91/93. The authors thank the Faculty of Pharmacy, Students’ Research Committee, and Biotechnology Research Center of Tabriz University of Medical Sciences, Tabriz, Iran, for financial support and providing facilities to carry out this study.
Ethical Issues
The ethical issue has been stated on page 628, under the section “Animals”.
Conflict of Interest
The authors declared no potential conflicts of interest regarding this research, authorship, and/or publication of this article.
References
- 1.Dussor G. ASICS as therapeutic targets for migraine. Neuropharmacology. 2015;94:64–71. doi: 10.1016/j.neuropharm.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: A 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia. 2015;35(11):996–1024. doi: 10.1177/0333102414566200. [DOI] [PubMed] [Google Scholar]
- 3.Boecxstaens V, Bisschops R, Blondeau K, Vos R, Scarpellini E, De Wulf D. et al. Modulation of the postprandial acid and bile pockets at the gastro-oesophageal junction by drugs that affect gastric motility. Aliment Pharmacol Ther. 2011;33(12):1370–7. doi: 10.1111/j.1365-2036.2011.04664.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z, Liu H, Yu N, Wang F, An G, Xu Y. et al. Hydrophilic anti-migraine triptans are substrates for oatp1a2, a transporter expressed at human blood-brain barrier. Xenobiotica. 2012;42(9):880–90. doi: 10.3109/00498254.2012.675455. [DOI] [PubMed] [Google Scholar]
- 5.Prajapati ST, Patel PB, Patel CN. Formulation and evaluation of sublingual tablets containing sumatriptan succinate. Int J Pharm Investig. 2012;2(3):162–8. doi: 10.4103/2230-973X.104400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tayel SA, El Nabarawi MA, Amin MM, Abou Ghaly MH. Sumatriptan succinate sublingual fast dissolving thin films: Formulation and in vitro/in vivo evaluation. Pharm Dev Technol. 2016;21(3):328–37. doi: 10.3109/10837450.2014.1003655. [DOI] [PubMed] [Google Scholar]
- 7.Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: A comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60(6):1259–87. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Warner PE, Brouwer KL, Hussey EK, Dukes GE, Donn KH, Davis IM. et al. Sumatriptan absorption from different regions of the human gastrointestinal tract. Pharm Res. 1995;12(1):138–43. doi: 10.1023/A:1016211409315. [DOI] [PubMed] [Google Scholar]
- 9.Moore KH, Leese PT, McNeal S, Gray P, O'Quinn S, Bye C. et al. The pharmacokinetics of sumatriptan when administered with clarithromycin in healthy volunteers. Clin Ther. 2002;24(4):583–94. doi: 10.1016/S0149-2918(02)85134-7. [DOI] [PubMed] [Google Scholar]
- 10.Napoletano F, Lionetto L, Martelletti P. Sumatriptan in clinical practice: Effectiveness in migraine and the problem of psychiatric comorbidity. Expert Opin Pharmacother. 2014;15(3):303–5. doi: 10.1517/14656566.2014.858120. [DOI] [PubMed] [Google Scholar]
- 11.Soldin OP, Dahlin J, O’Mara DM. Triptans in pregnancy. Ther Drug Monit. 2008;30(1):5–9. doi: 10.1097/FTD.0b013e318162c89b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasa D, Reinecke HJ, Rauber-Lüthy C, Seidel C, Hoffmann-Walbeck P, Gerber-Zupan G, Overdose of selective serotonin (5HT1) agonists. USA: hypertension; 2012. [Google Scholar]
- 13.Nguyen TQ, Lewis JH. Sumatriptan-associated ischemic colitis: Case report and review of the literature and faers. Drug Saf. 2014;37(2):109–21. doi: 10.1007/s40264-013-0134-7. [DOI] [PubMed] [Google Scholar]
- 14.Fulton JA, Kahn J, Nelson LS, Hoffman RS. Renal infarction during the use of rizatriptan and zolmitriptan: Two case reports. Clin Toxicol (Phila) 2006;44(2):177–80. doi: 10.1080/15563650500514574. [DOI] [PubMed] [Google Scholar]
- 15.Malacarne S, Moll S, Hadaya K, Buhler L, Martin PY. Renal ischaemic injuries during the use of zolmitriptan for treatment of migraines in a transplanted patient under tacrolimus therapy. Nephrol Dial Transplant. 2007;22(11):3341–3. doi: 10.1093/ndt/gfm476. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Atutxa A, Vergara M, Gil M, Dalmau B, Miquel M, Sanchez-Delgado J. et al. Rizatriptan-induced liver toxicity. Report of a case. Gastroenterol Hepatol. 2013;36(4):261–3. doi: 10.1016/j.gastrohep.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Atutxa A. Rizatriptan: First report of toxic hepatitis: Case report. React Wkly. 2013;1453(1):37. doi: 10.1007/s40278-013-3380-7. [DOI] [Google Scholar]
- 18.Liu R, Yu X, Wallqvist A. Data-driven identification of structural alerts for mitigating the risk of drug-induced human liver injuries. J Cheminform. 2015;7:4. doi: 10.1186/s13321-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda Y, Jimbo H, Shimazu M, Satoh K. Sumatriptan scavenges superoxide, hydroxyl, and nitric oxide radicals: In vitro electron spin resonance study. Headache. 2002;42(9):888–92. doi: 10.1046/j.1526-4610.2002.02208.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CC, Lin CC, Liao TS, Yin MC. Protective effect of s-allyl cysteine and s-propyl cysteine on acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44(3):393–7. doi: 10.1016/j.fct.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Yu FY, Wu TS, Chen TW, Liu BH. Aristolochic acid i induced oxidative DNA damage associated with glutathione depletion and erk1/2 activation in human cells. Toxicol In Vitro. 2011;25(4):810–6. doi: 10.1016/j.tiv.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Butterworth RF. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J Clin Exp Hepatol. 2015;5(Suppl 1):S96–S103. doi: 10.1016/j.jceh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdoli N, Azarmi Y, Eghbal MA. Protective effects of n-acetylcysteine against the statins cytotoxicity in freshly isolated rat hepatocytes. Adv Pharm Bull. 2014;4(3):249–54. doi: 10.5681/apb.2014.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy PS, Rani GP, Sainath SB, Meena R, Supriya C. Protective effects of n-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J Trace Elem Med Biol. 2011;25(4):247–53. doi: 10.1016/j.jtemb.2011.08.145. [DOI] [PubMed] [Google Scholar]
- 25.Wong CK, Ooi VE, Wong CK. Protective effects of n-acetylcysteine against carbon tetrachloride- and trichloroethylene-induced poisoning in rats. Environ Toxicol Pharmacol. 2003;14(3):109–16. doi: 10.1016/S1382-6689(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Moneim AM. Effects of taurine against histomorphological and ultrastructural changes in the testes of mice exposed to aluminium chloride. Arh Hig Rada Toksikol. 2013;64(3):405–14. doi: 10.2478/10004-1254-64-2013-2322. [DOI] [PubMed] [Google Scholar]
- 27.Heidari R, Babaei H, Eghbal MA. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arh Hig Rada Toksikol. 2013;64(2):201–10. doi: 10.2478/10004-1254-64-2013-2297. [DOI] [PubMed] [Google Scholar]
- 28. Ahmadian E, Eftekhari A, Fard JK, Babaei H, Nayebi AM, Mohammadnejad D, et al. In vitro and in vivo evaluation of the mechanisms of citalopram-induced hepatotoxicity. Arch Pharm Res 2016. [DOI] [PubMed]
- 29.Eghbal MA, Taziki S, Sattari MR. Mechanisms of phenytoin-induced toxicity in freshly isolated rat hepatocytes and the protective effects of taurine and/or melatonin. J Biochem Mol Toxicol. 2014;28(3):111–8. doi: 10.1002/jbt.21542. [DOI] [PubMed] [Google Scholar]
- 30.Edmondson DE. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: Biological implications. Curr Pharm Des. 2014;20(2):155–60. doi: 10.2174/13816128113190990406. [DOI] [PubMed] [Google Scholar]
- 31.Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem Pharmacol. 2012;83(1):6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Benković V, Knežević A, Đikić D, Lisičić D, Oršolić N, Bašić I. et al. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh Hig Rada Toksikol. 2009;60(2):129–38. doi: 10.2478/10004-1254-60-2009-1908. [DOI] [PubMed] [Google Scholar]
- 33.Padma VV, Baskaran R, Roopesh RS, Poornima P. Quercetin attenuates lindane induced oxidative stress in wistar rats. Mol Biol Rep. 2012;39(6):6895–905. doi: 10.1007/s11033-012-1516-0. [DOI] [PubMed] [Google Scholar]
- 34.Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012;132(2):931–5. doi: 10.1016/j.foodchem.2011.11.070. [DOI] [Google Scholar]
- 35.Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010;48(11):3246–61. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Chiang EC, Shen S, Kengeri SS, Xu H, Combs GF, Morris JS. et al. Defining the optimal selenium dose for prostate cancer risk reduction: Insights from the u-shaped relationship between selenium status, DNA damage, and apoptosis. Dose Response. 2010;8(3):285–300. doi: 10.2203/dose-response.09-036.Chiang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eskandari MR, Pourahmad J, Daraei B. Thallium(I) and thallium(III) induce apoptosis in isolated rat hepatocytes by alterations in mitochondrial function and generation of ROS. Toxicol Environ Chem. 2011;93(1):145–56. doi: 10.1080/02772248.2010.505826. [DOI] [Google Scholar]
- 38.Pourahmad J, Eskandari MR, Kaghazi A, Shaki F, Shahraki J, Fard JK. A new approach on valproic acid induced hepatotoxicity: Involvement of lysosomal membrane leakiness and cellular proteolysis. Toxicol in Vitro. 2012;26(4):545–51. doi: 10.1016/j.tiv.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Khan S, O'Brien PJ. 1-bromoalkanes as new potent nontoxic glutathione depletors in isolated rat hepatocytes. Biochem Biophys Res Commun. 1991;179(1):436–41. doi: 10.1016/0006-291X(91)91389-T. [DOI] [PubMed] [Google Scholar]
- 40.Moldéus P, Högberg J, Orrenius S. [4] isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/S0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- 41.Khalili Fard J, Jafari S, Eghbal MA. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull. 2015;5(4):447–54. doi: 10.15171/apb.2015.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidari R, Babaei H, Eghbal MA. Ameliorative effects of taurine against methimazole-induced cytotoxicity in isolated rat hepatocytes. Sci Pharm. 2012;80(4):987–99. doi: 10.3797/scipharm.1205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskandari MR, Fard JK, Hosseini MJ, Pourahmad J. Glutathione mediated reductive activation and mitochondrial dysfunction play key roles in lithium induced oxidative stress and cytotoxicity in liver. Biometals. 2012;25(5):863–73. doi: 10.1007/s10534-012-9552-8. [DOI] [PubMed] [Google Scholar]
- 44.Andersson BS, Aw TY, Jones DP. Mitochondrial transmembrane potential and pH gradient during anoxia. Am J Physiol. 1987;252(4 Pt 1):C349–55. doi: 10.1152/ajpcell.1987.252.4.C349. [DOI] [PubMed] [Google Scholar]
- 45.Eskandari MR, Rahmati M, Khajeamiri AR, Kobarfard F, Noubarani M, Heidari H. A new approach on methamphetamine-induced hepatotoxicity: Involvement of mitochondrial dysfunction. Xenobiotica. 2014;44(1):70–6. doi: 10.3109/00498254.2013.807958. [DOI] [PubMed] [Google Scholar]
- 46.Fard JK, Hamzeiy H, Sattari M, Eftekhari A, Ahmadian E, Eghbal MA. Triazole rizatriptan induces liver toxicity through lysosomal/mitochondrial dysfunction. Drug Res (Stuttg) 2016;66(9):470–8. doi: 10.1055/s-0042-110178. [DOI] [PubMed] [Google Scholar]
- 47.Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 48.Taziki S, Sattari MR, Dastmalchi S, Eghbal MA. Cytoprotective effects of melatonin against amitriptyline-induced toxicity in isolated rat hepatocytes. Adv Pharm Bull. 2015;5(3):329–34. doi: 10.15171/apb.2015.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Meister A. Selective modification of glutathione metabolism. Science. 1983;220(4596):472–7. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 51.Read SJ, Manning P, McNeil CJ, Hunter AJ, Parsons AA. Effects of sumatriptan on nitric oxide and superoxide balance during glyceryl trinitrate infusion in the rat. Implications for antimigraine mechanisms. Brain Res. 1999;847(1):1–8. doi: 10.1016/S0006-8993(99)01985-X. [DOI] [PubMed] [Google Scholar]
- 52.Olesen J. Nitric oxide-related drug targets in headache. Neurotherapeutics. 2010;7(2):183–90. doi: 10.1016/j.nurt.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silberstein SD, Marcus DA. Sumatriptan: Treatment across the full spectrum of migraine. Expert Opin Pharmacother. 2013;14(12):1659–67. doi: 10.1517/14656566.2013.810209. [DOI] [PubMed] [Google Scholar]
- 54.Tafazoli S, O'Brien PJ. Prooxidant activity and cytotoxic effects of indole-3-acetic acid derivative radicals. Chem Res Toxicol. 2004;17(10):1350–5. doi: 10.1021/tx034217t. [DOI] [PubMed] [Google Scholar]
- 55.Bütün A, Nazıroğlu M, Demirci S, Çelik Ö, Uğuz AC. Riboflavin and vitamin e increase brain calcium and antioxidants, and microsomal calcium-atp-ase values in rat headache models induced by glyceryl trinitrate. J Membr Biol. 2015;248(2):205–13. doi: 10.1007/s00232-014-9758-5. [DOI] [PubMed] [Google Scholar]
- 56.Fried NT, Moffat C, Seifert EL, Oshinsky ML. Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am J Physiol Cell Physiol. 2014;307(11):C1017–30. doi: 10.1152/ajpcell.00332.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hershey AD, Powers SW, Vockell AL, LeCates SL, Ellinor PL, Segers A. et al. Coenzyme q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47(1):73–80. doi: 10.1111/j.1526-4610.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 58.Yorns WR Jr, Hardison HH. Mitochondrial dysfunction in migraine. Semin Pediatr Neurol. 2013;20(3):188–93. doi: 10.1016/j.spen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Sharma J, Johnston MV, Hossain MA. Sex differences in mitochondrial biogenesis determine neuronal death and survival in response to oxygen glucose deprivation and reoxygenation. BMC Neurosci. 2014;15:9. doi: 10.1186/1471-2202-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong R, Steenbergen C, Murphy E. Mitochondrial permeability transition pore and calcium handling. Methods Mol Biol. 2012;810:235–42. doi: 10.1007/978-1-61779-382-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Parrish AB, Kurokawa M, Matsuura K, Freel CD, Andersen JL. et al. Rsk-mediated phosphorylation and 14-3-3ε binding of apaf-1 suppresses cytochrome c-induced apoptosis. EMBO J. 2012;31(5):1279–92. doi: 10.1038/emboj.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: Analysis and therapeutic implications. J Pharm Sci. 2007;96(4):729–46. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 63.Pliyev BK, Menshikov M. Differential effects of the autophagy inhibitors 3-methyladenine and chloroquine on spontaneous and tnf-α-induced neutrophil apoptosis. Apoptosis. 2012;17(10):1050–65. doi: 10.1007/s10495-012-0738-x. [DOI] [PubMed] [Google Scholar]
- 64.Priyadarsini RV, Nagini S. Quercetin suppresses cytochrome P450 mediated ros generation and NFκB activation to inhibit the development of 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinomas. Free Radic Res. 2012;46(1):41–9. doi: 10.3109/10715762.2011.637204. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong SC, Cozza KL. Triptans. Psychosomatics. 2002;43(6):502–4. doi: 10.1176/appi.psy.43.6.502. [DOI] [PubMed] [Google Scholar]
- 66.Egert S, Rimbach G. Which sources of flavonoids: Complex diets or dietary supplements? Adv Nutr. 2011;2(1):8–14. doi: 10.3945/an.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]