Abstract
Purpose: Mesenchymal Stem Cells (MSCs) are the most important members of Bone Marrow (BM) milieu. MSCs affect different kinds of cells, particularly malignant cells of hematologic malignancies, but the effects of MSCs are unclear exactly. Here we analyzed the effects of derived Umbilical Cord Blood-MSCs on proliferation, cell death and some surface markers of U937 cell line in a Co-culture system with MSCs.
Methods: Here we designed Co-culture systems as a model of BM milieu. We cultured U937 cells on UCB-MSCs and MSCs Conditioned Medium (C.M) driven and then treated U937 cells with optimum concentration of chloride cobalt (CoCl2) as a hypoxia-mimetic agent. In addition, we applied suitable concentrations of H2O2 to induce cell death. Proliferation rate, cell death rate and some surface markers of hypoxic U937 cells were analyzed by MTT assay, flow cytometry and Real Time-PCR were flown respectively.
Results: UCB-MSCs showed supportive effects on U937 proliferation rate in normoxia and hypoxia. Lethal effect of H2O2 suppressed in the presence of UCB-MSCs in hypoxia and normoxia. Among CD11a, CD14, CD49d, CD54 and CD116 markers, CD49d was down regulated in presence of UCB-MSCs and CD116 was up regulated in hypoxia. Other markers didn’t show any significant changes.
Conclusion: This work provides evidences that MSCs play critical roles in U937 cells biology. These observations shed new light on MSCs roles and demonstrated that MSCs should be regarded as an important member of BM milieu in several clinical applications such as BM transplantation prognosis and treatment of hematologic malignancies.
Keywords: Hypoxia, Mesenchymal Stem Cells, U937 cell line, Proliferation, CD116, CD49d
Introduction
As well documented, Bone marrow (BM) traditionally contains two systems: hematopoietic cells and the associated supporting stromal part.1 One of the major sections of BM milieu is Mesenchymal Stem Cells (MSCs).2,3 MSCs play critical roles in biology of normal and malignant cells.4-8
Another important factor in BM milieu is physiologic hypoxia. The effects of hypoxia mediated by a significant master key transcription factor is called hypoxia-inducible factor (HIF). HIF, hetrodimeric key transcription factor, contains HIF-α and HIF-β subunits.9 In hypoxia, HIF-α subunits translocate to nucleus and join to HIF-β subunits,10,11 so heterodimers bind to sequences of HIF target genes, which they affect different aspects of cells biology.12,13 In this regard, hypoxia can mediate expression of different kinds of genes in normal and malignant cells.14-17
Several in vitro studies have been reported that HIF is a powerful factor, which improved survival and differentiation of stem cells.18,19 In particular, HIF-1 caused resistance to chemotherapy and radiation approaches.20
Here, we investigated the effects of Umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) on proliferation rate, cell death and some genes expression by U937 cells in hypoxia milieu.
Materials and Methods
Isolation and Culture of UCB-MSCs
UCB-MSCs were collected from umbilical cords, with informed consent, according to the Institute’s human ethical committee guidelines of Tabriz University of Medical Sciences. Cells were cultured in DMEM medium (Gibco, MA, UK) with 10% fetal bovine serum (FBS) (Gibco, MA, UK) and 100 U/ml penicillin as well as 100μg/ml streptomycin (Pen/Strep) (Gibco, MA, UK). Cells were incubated in humidified incubator containing 5% CO2 at 37°C. After incubation, non-adherent cells were discarded and fresh DMEM medium was added to cells. Then, fibroblastoid cells were verified by flowcytometry for MSCs markers including CD29, CD105 (Positive markers) and CD34, CD45 (Negative markers).21
Cell culture
Confirmed U937 cells were purchased from the Pasture Institute of Iran. Thereafter, cells were cultured in RPMI-1640 medium (Sigma-Aldrich, USA) with 10% FBS (Gibco, MA, UK) and Pen/Strep (Gibco, UK) and were incubated. During all steps of the experiments, cell viability was checked by trypan-blue staining and it was more than 86% in all experiments.
UCB-MSCs were seeded at the density of 2×104 cell/well. After 24 hrs, 1×105 U937 cells were added to the UCB-MSCs in RPMI-1640 medium with 10% FBS and Pen/Strep for Co-culturing.
Conditioned Medium (C.M) preparation
Conditioned Medium (C.M) was prepared by adding 5 ml of RPMI-1640 without FBS to UCB-MSCs (Confelency 60%) and 24 hrs incubation.
Cells treatment
Cobalt chloride (CoCl2) (Sigma, USA) was used to induce hypoxia. CoCl2 dissolved in RPMI-1640 to adjusted 100 μM. Then U937 cells were treated with 100 μM of CoCl2.
Hydrogen peroxide (H2O2) (Merck, Germany) was used for cell death inducing, so H2O2 was diluted to 100 mM with distillated water as a stock solution and cells were treated with 0.5 mM H2O2.
MTT Assay
U937 cells were co-cultured with UCB-MSCs and were incubated with 100 μM of CoCl2 for 96 hrs. After incubation, U937 cells were collected and RPMI-1640, with 5μl MTT solution (0.4 mg/ml), was added to cells pellet and was incubated. Then, Isopropanol/HCl (0.04M) was added and incubated overnight and optical density of solutions were measured by Picodrop (UK) in 540 and 650 nm.
Cell Death Detection with Flowcytometry
U937 cells were incubated with 0.5mM of H2O2 for 24 hrs. Then, live and dead cells were analyzed by forward and side scattering in flowcytometry assay (FACS Calibor, USA).22,23
Real Time-PCR (RT-PCR)
Co-cultured U937 cells with UCB-MSCs (24 hrs) were harvested and RNA was extracted. Thereafter, cDNA was synthesized and expression of genes (CD11a, CD14, CD54, CD49d, and CD116) analyzed (Table 1). The threshold cycle (Ct) value for each gene was normalized Ct number of housekeeping gene (GAPDH) according to ΔΔCT method.
Table 1. Primers sequences used for RT-PCR .
| Primers | 5` 3` → |
| CD14 | F - CTGGAACAGGTGCCTAAAGGAC |
| R - GTCCAGTGTCAGGTTATCCACC | |
| CD11a | F - CTGCTTTTGCCAGCCTCTCTGT |
| R - GCTCACAGGTATCTGGCTATGG | |
| CD49d | F - GCATACAGGTGTCCAGCAGAGA |
| R - AGGACCAAGGTGGTAAGCAGCT | |
| CD54 | F - AGCGGCTGACGTGTGCAGTAAT |
| R - TCTGAGACCTCTGGCTTCGTCA | |
| CD116 | F - CCTGTCAGGATTAACGTCTCGC |
| R - CATTGCTGGGAGGGTTGAATCG |
Statistical Analysis
Data are shown as means ± SD from three separate experiments. Data were evaluated using GraphPad Prism v 5.00 (GraphPad Software, Inc., La Jolla, CA). Student’s t-test (for single comparison) was used. P < 0.05 was regarded statistically significant.
Results and Discussion
In normoxia and hypoxia proliferation of U937 was promoted by UCB-MSCs
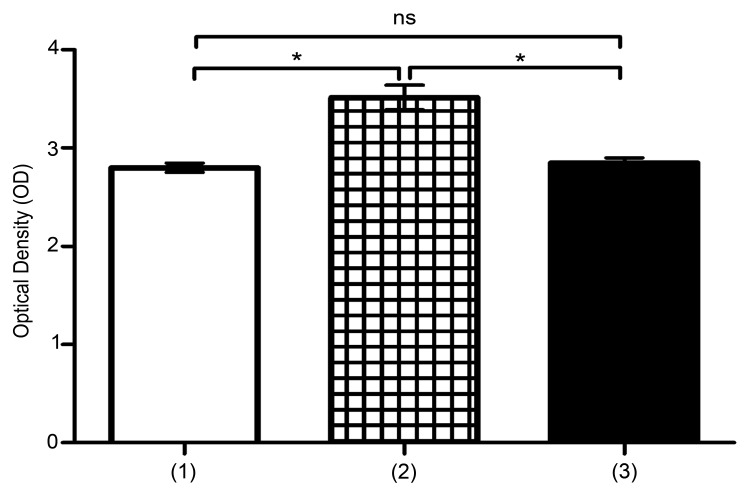
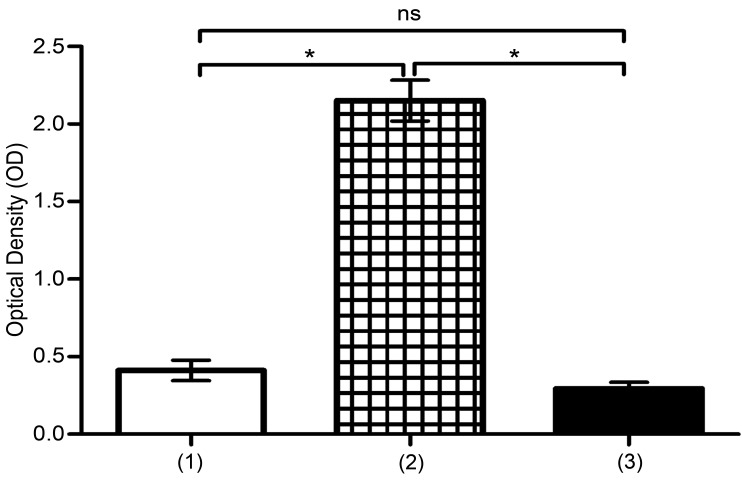
MTT assay showed that proliferation of U937 was significantly high in co-culture with UCB-MSCs, in normoxic and hypoxic conditions (*P<0.05) (Figure 1 and 2). In this regard, several studies have proved that, MSCs play noticeable roles in proliferation of malignant cells models such as U937 cells. Also, hypoxia shows suppressing effects on proliferation of leukemic cells.24,25 Here, we proved that hypoxia reduces proliferation of U937 cells, but in presence of UCB-MSCs, effects of hypoxia have been suppressed.
Figure 1.
Assessment of U937 cells proliferation. Proliferation was analyzed by MTT assay. (1) U937 cells, (2) U937 cells+UCB-MSCs, (3) U937 cells in C.M. ns, non-significant. (*P<0.05, by using student’s t-test).
Figure 2.
The evaluation of proliferation of U937 cells in hypoxia. Proliferation was analyzed by MTT assay. (1) U937 cells+100μM CoCl2, (2) U937 cells+UCB-MSCs+100μM CoCl2, (3) U937 cells in C.M+100μM CoCl2. ns, non-significant. (*P<0.05, by using student’s t-test).
UCB-MSCs supported viability of U937 in normoxia and hypoxia
U937 cells were cultured on UCB-MSCs with and without 100μM of CoCl2, and then incubated for 96 hrs. In the next step, flowcytometry analyzing was performed on 1×105 U937 cells. By comparison with C.M and control group, the results demonstrate that UCB-MSCs support U937 cells viability in hypoxia and normoxia (*P<0.05) (Figure 3 and 4). At the present, supportive roles of MSCs on viability of normal and abnormal cells have been confirmed26 and our results are the same as the others.
Figure 3.

Assessment of U937 cells viability in presence of 0.5mM H2O2. (a) Diagram shows the viability of U937 cells in presence of 0.5mM H2O2. Viability was analyzed by Flowcytometry. (1) U937 cells, (2) U937 cells+UCB-MSCs, (3) U937 cells in C.M. ns, non-significant. (b) Viability of treated U937 cells+0.5mM H2O2 are shown using dot plot. (c) Viability of cultured U937 cells+UCB-MSCs+0.5mM H2O2 are shown using dot plot. (d) Viability of cultured U937 cells in C.M+0.5mM H2O2 are shown using dot plot. ns, non-significant. (*P<0.05, by using student’s t-test).
Figure 4.
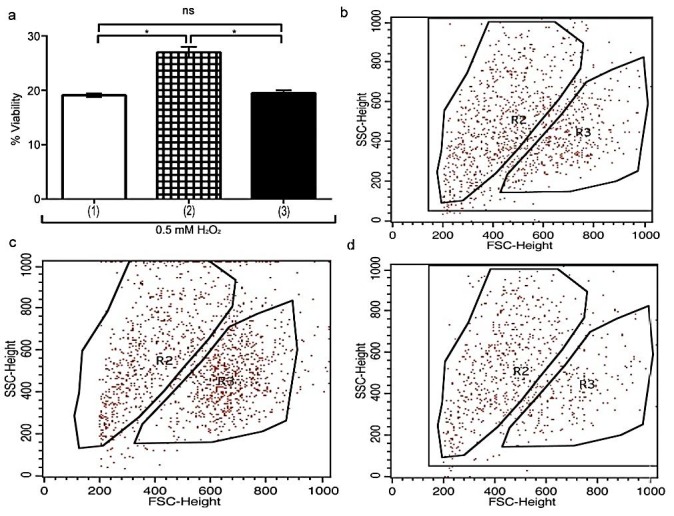
Viability of U937 cells in presence of 0.5 mMH2O2 and hypoxia was analyzed by flowcytometry. (a) Diagram shows the viability of U937 cells. (1) U937 cells+100μM CoCl2, (2) co-cultured U937 cells+UCB-MSCs+100μM CoCl2, (3) cultured U937 cells in C.M+100μM CoCl2. (b) Viability of treated U937 cells+100μM CoCl2 are shown using dot plot. (c) Viability of cultured U937 cells+UCB-MSCs+100μM CoCl2 are shown using dot plot. (d) Viability of cultured U937 cells in C.M+100μM CoCl2 are shown using dot plot. ns, non-significant. (*P<0.05, by using student’s t-test).
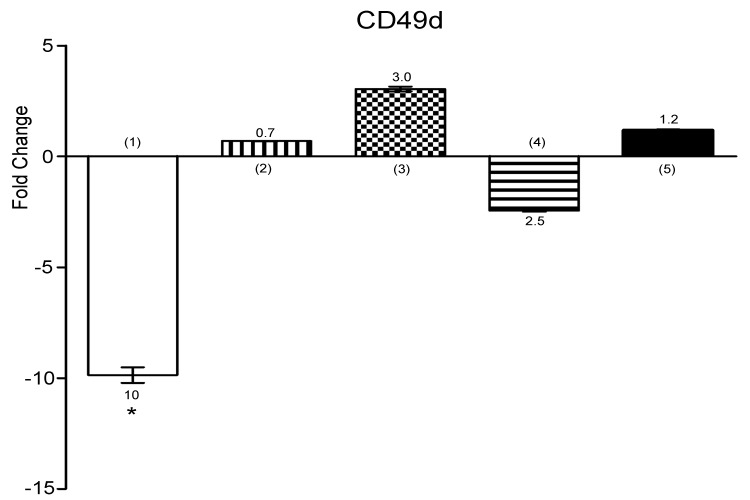
Expression of CD49d down regulated in co-cultured U937 cells
U937 cells were lied down on UCB-MSCs and were treated with 100 μM CoCl2 for 24 hrs. Then, RNAs of U937 cells were extracted, cDNAs were synthesized and RT-PCR was performed.
CD49d is a part of VLA-4 and expresses on monocytes. Naturally, CD49d interacts with VCAM-1 to help migration of monocytes.27 Hypoxia lead to CD49d up-regulation, which lead to transmigration of monocytes.28,29 Contrary, our findings showed, CD49d expression was down-regulated in presence of UCB-MSCs (Figure 5) (*P<0.05).
Figure 5.
Histogram shows the expression of CD49d by U937 cells. Expression of CD49d was analyzed by using RT-PCR. (1) U937 cells+UCB-MSCs, (2) U937 cells in C.M, (3) U937 cells+100μM CoCl2, (4) U937 cells+UCB-MSCs+100μM CoCl2, (5) U937 cells in C.M+100μM CoCl2. ns, non-significant. (*P<0.05, by using student’s t-test).
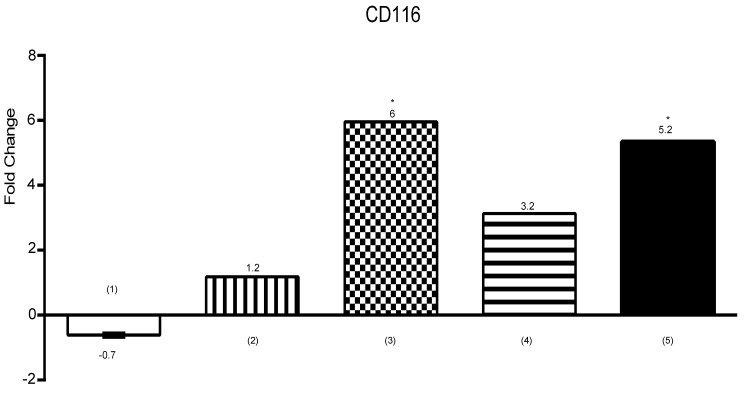
Hypoxia up-regulated CD116 expression
We seeded U937 cells on UCB-MSCs. In the next step, the U937 cells were treated with 100μM CoCl2 and incubated for 24 hrs. Then, we performed RT-PCR on co-cultured U937 cells.
CD116 is receptor of GM-CSF and expresses on various myeloid cells.30 Furthermore, GM-CSF/CD116 has a direct role in proliferation and survival of monocyte lineage.31,32 As shown in Figure 6, CD116 expression shows meaningful up-regulation in hypoxia (*P<0.05).
Figure 6.
Expression of CD116 by U937 cells is shown on the diagram. Expression was analyzed by using RT-PCR. (1) U937 cells+UCB-MSCs, (2) U937 cells in C.M, (3) U937 cells+100μM CoCl2, (4) U937 cells+UCB-MSCs+100μM CoCl2, (5) U937 cells in C.M+100μM CoCl2. ns, non-significant. (*P<0.05, by using student’s t-test).
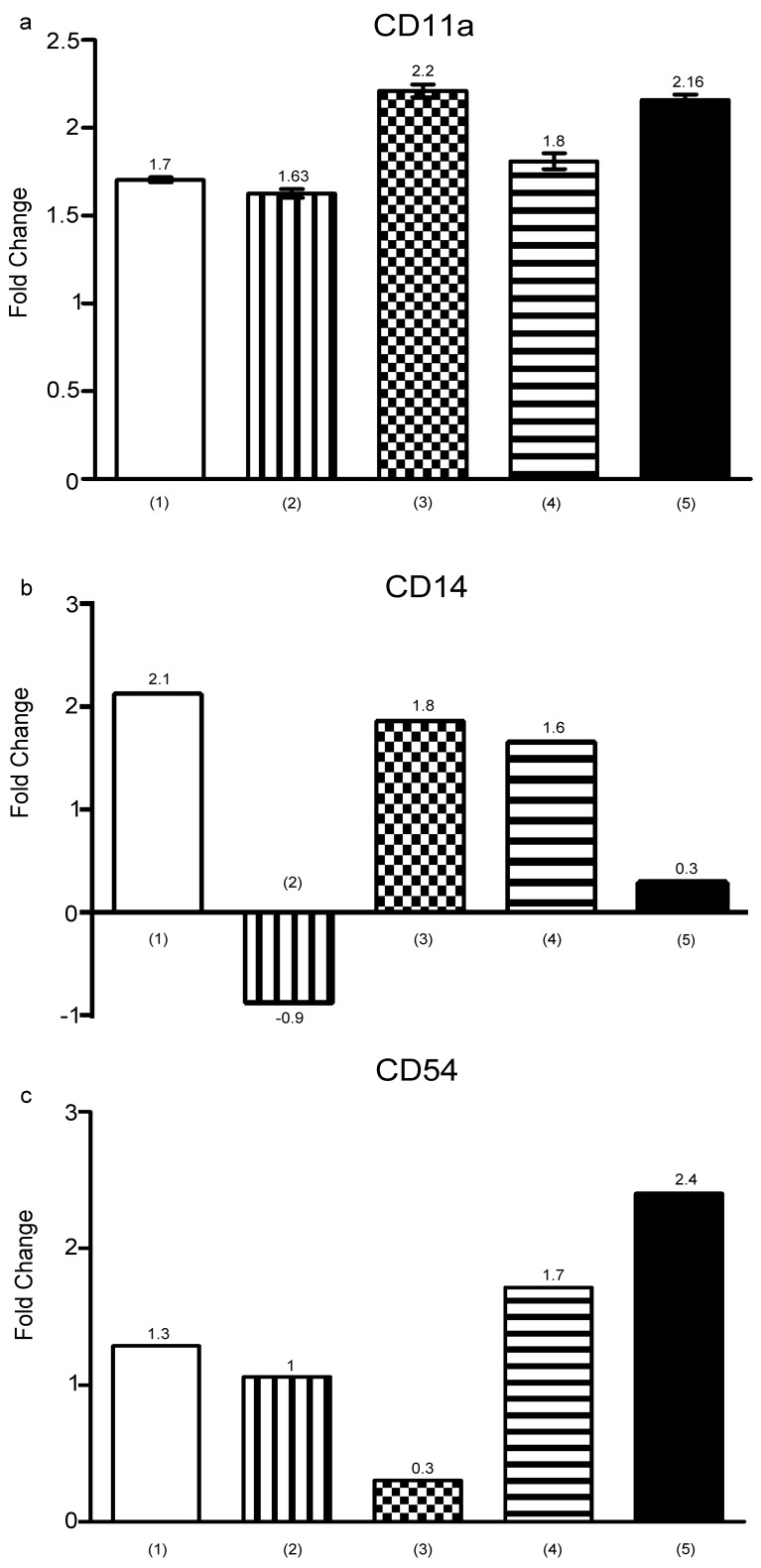
CD11a, CD54 and CD14 status in hypoxia
U937 cells were cultured with UCB-MSCs, C.M and 100 μM CoCl2 for 24 hrs. RT-PCR performed and didn’t show any significant changes in CD11a, CD54 and CD14 markers expression (P>0.05) (Figure 7). CD14 has a role in the phagocytic activity of monocytes33 and CD11a is classical marker of myeloid differentiation that involves in cell adhesion. Additionally, CD11a and CD14 related to differentiation of U937 cells to monocyte/macrophage.34,35 CD54 up-regulation in high pressure of oxygen in endothelial cells documented by recent studies,36 while it should be pointed out that hypoxia enhanced CD54 expression, presumably through NF-kB pathway activation,37 but in our finding CD54 didn’t show any trends in all conditions. Combined with previous findings this discrepancy can be due to differences in cell lines and incubation times.
Figure 7.
Diagrams show expression of (a) CD11a, (b) CD14 and (c) CD54 by U937 cells. RT-PCR performed for assessment of markers expression. (1) U937 cells+UCB-MSCs, (2) cells in C.M, (3) U937 cells+100 μM CoCl2, (4) U937 cells+UCB-MSCs+100 μM CoCl2, (5) U937 cells in C.M+100 μM CoCl2. ns, non-significant. (*P<0.05, by using student’s t-test).
Conclusion
In orchestrate with previous studies, hypoxia suppresses the U937 proliferation in comparison to normoxia. Reciprocally, when U937 co-cultured with the UCB-MSCs, proliferation of U937 was increased. Apoptotic effects of H2O2 on U937 cells were reduced in presence of the UCB-MSCs. Also, CD49d expression was down-regulated and CD116 was up-regulated in the presence of UCB-MSCs and hypoxia respectively. Our findings can be useful in clinical applications and provide a new sight into the roles of MSCs in bone marrow co-transplantation efficacy. We suggest that other experiments should be performing on bone marrow-derived MSCs or animal models.
Acknowledgments
Authors would like to thank East Azerbaijan province Blood Transfusion Research Center for supporting this project.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med (Berl) 2007;85(7):661–72. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Letourneau PA, Menge TD, Wataha KA, Wade CE, S. Cox C J, Holcomb JB. et al. Human bone marrow derived mesenchymal stem cells regulate leukocyte-endothelial interactions and activation of transcription factor nf-kappa b. J Tissue Sci Eng. 2011;Suppl 3:001. doi: 10.4172/2157-7552.S3-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awaya N, Rupert K, Bryant E, Torok-Storb B. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol. 2002;30(8):937–42. doi: 10.1016/s0301-472x(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 5.Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S. et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27(9):1460–6. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 6.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L. et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in nod/scid mice. Exp Hematol. 2003;31(5):413–20. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 7.in 't Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R. et al. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived cd34(+) cells in nod/scid mice. Exp Hematol. 2003;31(10):881–9. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 8.Maitra B SE, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE. et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress t-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 9.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML. et al. Identification of the von hippel-lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 10.Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. 1998;273(5):2895–904. doi: 10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- 11.Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994;45(3):428–38. [PubMed] [Google Scholar]
- 12.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH. et al. Cellular and developmental control of o2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282(2):C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 14.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (hif-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57(10):1009–14. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han SH, Kim M, Park K, Kim TH, Seol DW. Blockade of processing/activation of caspase-3 by hypoxia. Biochem Biophys Res Commun. 2008;375(4):684–8. doi: 10.1016/j.bbrc.2008.08.091. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Park SY, Pai HS, Kim TH, Billiar TR, Seol DW. Hypoxia inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by blocking bax translocation. Cancer Res. 2004;64(12):4078–81. doi: 10.1158/0008-5472.CAN-04-0284. [DOI] [PubMed] [Google Scholar]
- 17.Shroff EH, Snyder C, Chandel NS. Role of bcl-2 family members in anoxia induced cell death. Cell cycle. 2007;6(7):807–9. doi: 10.4161/cc.6.7.4044. [DOI] [PubMed] [Google Scholar]
- 18.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH. et al. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29(1):74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 19.Peterson KM, Aly A, Lerman A, Lerman LO, Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: Role of oxidative stress. Life Sci. 2011;88(1-2):65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E. et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22(21):3213–20. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 21.Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE. et al. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther. 2012;3(6):57. doi: 10.1186/scrt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Z, Otsuyama K, Liu S, Abroun S, Ishikawa H, Tsuyama N. et al. Baicalein, a component of scutellaria radix from huang-lian-jie-du-tang (hljdt), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105(8):3312–8. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 23.Kawano MM, Mihara K, Huang N, Tsujimoto T, Kuramoto A. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood. 1995;85(2):487–94. [PubMed] [Google Scholar]
- 24.Hass R, Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun Signal. 2012;10(1):26. doi: 10.1186/1478-811X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G. et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YM, Zhang GZ, Leng ZX, Lu ZX, Bu LS, Gao S. et al. Study on the bone marrow mesenchymal stem cells induced drug resistance in the u937 cells and its mechanism. Chin Med J (Engl) 2006;119(11):905–10. [PubMed] [Google Scholar]
- 27.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin vla-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 28.Binsky I, Lantner F, Grabovsky V, Harpaz N, Shvidel L, Berrebi A. et al. Tap63 regulates vla-4 expression and chronic lymphocytic leukemia cell migration to the bone marrow in a cd74-dependent manner. J Immunol. 2010;184(9):4761–9. doi: 10.4049/jimmunol.0904149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Audoy-Remus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallieres L. Rod-shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci. 2008;28(41):10187–99. doi: 10.1523/JNEUROSCI.3510-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 31.Conti L, Cardone M, Varano B, Puddu P, Belardelli F, Gessani S. Role of the cytokine environment and cytokine receptor expression on the generation of functionally distinct dendritic cells from human monocytes. Eur J Immunol. 2008;38(3):750–62. doi: 10.1002/eji.200737395. [DOI] [PubMed] [Google Scholar]
- 32.Stanley ER, Berg KL, Einstein DB, Lee PS, Pixley FJ, Wang Y. et al. Biology and action of colony-stimulating factor-1. Mol Reprod Dev. 1997;46(1):4–10. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzmaier D, Foell D, Weinhage T, Varga G, Dabritz J. Peripheral monocyte functions and activation in patients with quiescent crohn's disease. PLoS One. 2013;8(4):e62761. doi: 10.1371/journal.pone.0062761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hass R, Bartels H, Topley N, Hadam M, Kohler L, Goppelt-Strube M. et al. Tpa-induced differentiation and adhesion of u937 cells: Changes in ultrastructure, cytoskeletal organization and expression of cell surface antigens. Eur J Cell Biol. 1989;48(2):282–93. [PubMed] [Google Scholar]
- 35.Prudovsky I, Popov K, Akimov S, Serov S, Zelenin A, Meinhardt G. et al. Antisense cd11b integrin inhibits the development of a differentiated monocyte/macrophage phenotype in human leukemia cells. Eur J Cell Biol. 2002;81(1):36–42. doi: 10.1078/0171-9335-00219. [DOI] [PubMed] [Google Scholar]
- 36.Willam C, Schindler R, Frei U, Eckardt KU. Increases in oxygen tension stimulate expression of icam-1 and vcam-1 on human endothelial cells. Am J Physiol. 1999;276(6 Pt 2):H2044–52. doi: 10.1152/ajpheart.1999.276.6.H2044. [DOI] [PubMed] [Google Scholar]
- 37.Combe C, Burton CJ, Dufourco P, Weston S, Horsburgh T, Walls J. et al. Hypoxia induces intercellular adhesion molecule-1 on cultured human tubular cells. Kidney Int. 1997;51(6):1703–9. doi: 10.1038/ki.1997.235. [DOI] [PubMed] [Google Scholar]