Abstract
Background
The safety and efficacy of a China-made polymer-free paclitaxel-eluting microporous stent (Yinyi) at 1-year has been previously reported. However, limited evidence exists regarding the long-term performance of this novel drug-eluting stent (DES). This study investigated the 3-year efficacy and safety of the Yinyi stent in the setting of safety and efficacy registry of the Yinyi stent (SERY-I) clinical trial.
Methods
Between June 2008 and August 2009, a total of 1045 patients undergoing percutaneous coronary intervention (PCI) were implanted with ≥ 1 Yinyi stents at 27 medical centers in mainland China. Thereafter, clinical follow-up was performed for a period of 3 years after enrollment. The primary endpoint was the cumulative rate of composite major adverse cardiac events (MACE) including target lesion revascularization (TLR), the combined incidence of cardiac death, and non-fatal myocardial infarction; the second endpoint was the incidence of stent thrombosis.
Results
Overall, 1376 lesions were treated successfully with 1713 Yinyi stents, and 1019 (98.7%) patients received dual antiplatelet therapy for at least 12 months. At 3 years, a total of 13 (1.33%) patients had suffered cardiac death. The incidence of non-fatal myocardial infarction and TLR was 9 (0.92%) and 58 (5.92%) among the patients. Stent thrombosis occurred in 13 (1.33%) patients, and the rate of Academic Research Consortium (ARC) definite or probable stent thrombosis was 0.82%.
Conclusions
Given the limitations that SERY-I was a single arm, nonrandomized study and only telephone follow-up was performed without angiographic analysis, the safety and efficacy of Yinyi stent observed in this extended follow-up Registry needs further verification.
Keywords: Microporous stent, Paclitaxel-eluting stent, Polymer-free, Stent thrombosis
INTRODUCTION
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) has demonstrated superior performance to bare metal stent (BMS) in terms of antirestenotic efficacy and reduced need for revascularization.1 However, late stent thrombosis (LST) has emerged as a major cause of death and morbidity associated with use of DES, which is related to the pathophysiological spectrum of delayed arterial healing.2-4
Current DESs are now primarily constructed by coating a standard coronary stent with a thin polymer film for drug storage and control of release kinetics.5 But growing evidence has indicated that the “permanent-polymer” used in current DES could trigger chronic inflammatory reactions, leading to delayed arterial healing and increased risks of LST.6-8
Concerns have been raised about the need to develop novel polymer-free DESs with a microporous surface as an alternative to stents with polymer coating for local drug delivery.9,10 A series of Intracoronary Stenting and Angiographic Restenosis (ISAR) studies have demonstrated a novel polymer-free microporous DES is associated with high anti-restenotic efficacy, without reliance on any carrier polymer.11-16
The safety and efficacy registry of the Yinyi stent (SERY-I) clinical trial studied the safety and efficacy of a China-made polymer-free paclitaxel-eluting microporous stent (Yinyi, Dalian, China) in 1045 randomized patients undergoing elective PCI in native vessels. The primary analyses have been previously reported and demonstrated that the Yinyi stent manifests efficacy and safety at 1 year.17 However, it remains unknown whether the efficacy of the Yinyi stent will be maintained over the medium and long-term. The objective of this study was to directly investigate the 3-year safety and efficacy outcomes in patients treated with Yinyi stent in the SERY-I clinical trial.
MATERIALS AND METHODS
Device description and study design
The Yinyi DES platform consists of a 316L stainless steel microporous stent, which is coated on-site with paclitaxel. A detailed description of the coating process has been previously reported, including the paclitaxel elution characteristics and the drug release profile of this novel DES, in associated with the details of the SERY-I study design, methods and patient population.17 Briefly, the SERY-I clinical trial was a prospective, multicenter study for the evaluation of the novel polymer-free Yinyi DES for the prevention of adverse clinical events and in-stent restenosis. Patients who were at least 18 years old, and set to undergo PCI for de novo lesions with ≥ 70% stenosis located in a native coronary artery with a reference-vessel diameter between 2.5 and 4.0 mm were considered eligible for this study. Patients excluded from this study were those with myocardial infarction within 7 days prior to enrollment, a target lesion located in the left main stem or bypass graft, malignancies or other comorbid conditions with life expectancy ≤ 12 months, left ventricular ejection fraction (LVEF) ≤ 35%, glomerular filtration rate (GFR) ≤ 30 ml/min, and known allergy to aspirin, heparin, paclitaxel, or stainless steel (Figure 1). The study protocol was approved by the institutional ethics committee responsible for all participating centers, and all patients gave their written, informed consent for participation in this trial.
Figure 1.
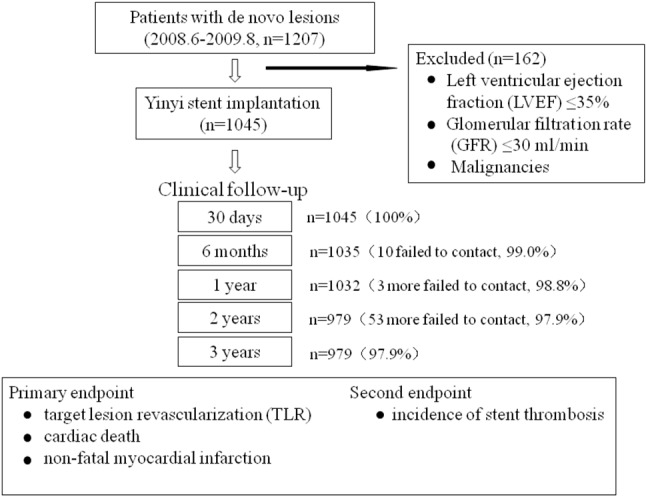
Study flow chart.
Coronary procedures and medication
A 600 mg loading dose of clopidogrel and 200 mg dose of aspirin were given at least 2 h before stent placement. Post-interventional therapy included aspirin 100 mg per day indefinitely, clopidogrel 150 mg per day until hospital discharge but no longer than 3 days, followed by daily administration of 75 mg for at least 12 months as indicated. Other cardiac medications were prescribed as appropriate.
Definitions and endpoints of the study
The primary endpoint for the present analysis was a composite endpoint of major adverse cardiac events (MACE) consisting of target lesion revascularization (TLR), the combined incidence of cardiac death and non-fatal myocardial infarction during a 3-year follow-up. Secondary endpoints were the incidence of stent thrombosis.
TLR was defined as either repeat percutaneous or surgical revascularization involving the target lesion owing to luminal re-narrowing in the presence of symptoms, or objective signs of ischaemia. The diagnosis of myocardial infarction was based on typical chest pain combined with either new pathological Q waves or cardiac troponin I (cTnI) level rising more than 3x the upper limit of normal. Stent thrombosis was classified according to criteria of the Academic Research Consortium (ARC).18 Adverse events were monitored throughout the follow-up period by a clinical visit at 2 and 3 years after the intervention.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), and categorical variables are described with numbers and percentages. An unadjusted cumulative rate of composite MACE was evaluated using the Kaplan-Meier method. The SPSS software package (version 13.0 for Windows; SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
Baseline characteristics and procedural results
A total of 1045 patients were enrolled in this study. Follow-up data were available for 1032 patients at 1 year, and 979 patients at 2 and 3 years, representing 98.8%, 97.9%, and 97.9% of survivors, respectively. The baseline clinical features were shown in Table 1. The procedural characteristics have been shown previously.17 In all, 1376 lesions were treated with 1713 stents, and 1019 (98.7%) patients received dual antiplatelet therapy in the following 12 months. Implantation of the assigned stent was successful in all patients.
Table 1. Baseline characteristics of the analyzable registry sample (N = 1045).
| Age, years | 63.6 ± 10.7 |
| Men, n (%) | 774 (74.1) |
| History of | |
| Myocardial infarction | 225 (21.5) |
| Percutaneous coronary intervention | 79 (7.6) |
| Coronary artery bypass graft surgery | 5 (0.5) |
| Diabetes | 237 (22.7) |
| Non-insulin-dependent | 204 (19.5) |
| Insulin-dependent | 33 (3.2) |
| Hypertension | 629 (60.2) |
| Hyperlipidemia | 376 (36.0) |
| Smoker | 444 (42.5) |
| No. of diseased vessels | |
| 1 | 1013 (73.6) |
| 2 | 290 (21.1) |
| 3 | 73 (5.3) |
| Indications for index procedure | |
| Angina | |
| Stable | 199 (19.0) |
| Unstable | 816 (78.1) |
| Silent myocardial ischemia | 30 (2.9) |
| Left ventricular ejection fraction | 59.7 ± 9.8 |
Values are expressed as mean ± standard deviation (SD) or n (%).
Clinical outcomes
Follow-up data were available for 1045 patients at 30 days, 1035 patients at 6 months, 1032 patients at 1 year, 979 patients at 2 years, and for 979 patients at 3 years, respectively. At 30 days, 2 patients (0.19%) died of sudden cardiac death and another 2 (0.19%) developed non-fatal myocardial infarction. At 6 months, 1 patient died of end stage renal dysfunction, MACE occurred in 36 patients (3.48%), including cardiac death in 6 (0.58%), non-fatal myocardial infarction in 5 (0.48%), and TLR in 25 (2.42%). By 1 year, apart from 1 patient who died of end stage renal dysfunction at 6 months follow-up, another patient died in a traffic accident which counted as a non-cardiac death. During 12 months of follow-up, 8 patients (0.78%) had cardiac death, 6 (0.58%) suffered non-fatal myocardial infarction, and 46 (4.46%) had TLR. By the 2-year interval, 46 patients had aborted the SERY-I trial. During the 24 months of follow-up, 12 patients (1.23%) had cardiac death, 6 (0.61%) suffered non-fatal myocardial infarction, and 53 (5.41%) had TLR. During 36 months of follow-up, 13 patients (1.33%) had cardiac death, 9 (0.92%) suffered non-fatal myocardial infarction, and 58 (5.92%) had TLR. The 30-day, 6-month, 1-year, 2-year and 3-year rates of adverse clinical events are presented in Table 2. Overall, the 3-year MACE-free survival rate was 91.83% (Figure 2).
Table 2. Rates of MACE at 30 days, 6 months, 1-year, 2-year and 3-year follow-up.
| Cumulative 30-day rates (n = 1045) | |
| Death | 2 (0.19) |
| Cardiac | 2 (0.19) |
| Noncardiac | 0 |
| Nonfatal myocardial infarct | 2 (0.19) |
| Target lesion revascularization | 0 |
| All MACE | 4 (0.38) |
| Cumulative 6-month rates (n = 1035) | |
| Death | 7 (0.68) |
| Cardiac | 6 (0.58) |
| Noncardiac | 1 (0.10) |
| Nonfatal myocardial infarct | 5 (0.48) |
| Target lesion revascularization | 25 (2.42) |
| All MACE | 36 (3.48) |
| Cumulative 1-year rates (n = 1032) | |
| Death | 10 (0.97) |
| Cardiac | 8 (0.78) |
| Noncardiac | 2 (0.19) |
| Nonfatal myocardial infarct | 6 (0.58) |
| Target lesion revascularization | 46 (4.46) |
| All MACE | 60 (5.81) |
| Cumulative 2-year rates (n = 979) | |
| Death | 14 (1.43) |
| Cardiac | 12 (1.23) |
| Noncardiac | 2 (0.20) |
| Nonfatal myocardial infarct | 6 (0.61) |
| Target lesion revascularization | 53 (5.41) |
| All MACE | 71 (7.25) |
| Cumulative 3-year rates (n = 979) | |
| Death | 15 (1.53) |
| Cardiac | 13 (1.33) |
| Noncardiac | 2 (0.20) |
| Nonfatal myocardial infarct | 9 (0.92) |
| Target lesion revascularization | 58 (5.92) |
| All MACE | 80 (8.17) |
Values are numbers (%) of patients.
MACE, major adverse cardiac events.
Figure 2.
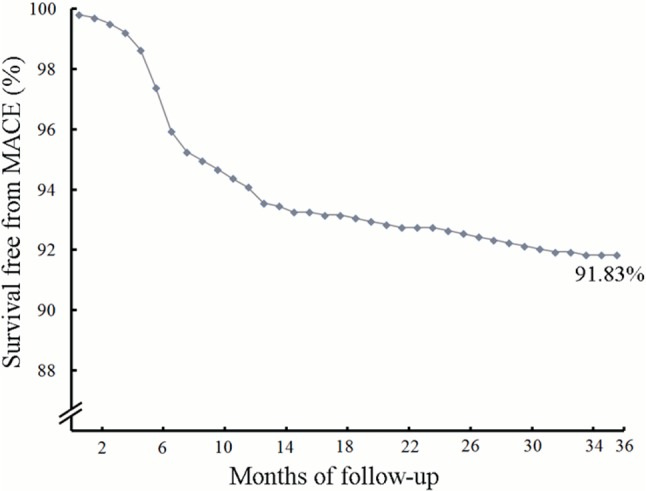
Kaplan-Meier estimates of MACE-free survival at 3-year follow-up in studied populations. MACE, major adverse cardiac events.
During 12-months of follow-up, 10 patients (0.97%) had thrombotic events, including 2 (0.19%) with subacute and 8 (0.78%) with late stent thrombosis. The occurrence of ARC definite or probable stent thrombosis was 0.58%. During 24 months of follow-up, 12 patients (1.23%) had thrombotic events including 2 (0.20%) with subacute, 8 (0.82%) with late stent thrombosis and 2 (0.20%) with very late stent thrombosis. The occurrence of ARC definite or probable stent thrombosis was 0.71%. During 36 months of follow-up, 13 patients (1.33%) had thrombotic events including 2 (0.20%) with subacute, 8 (0.82%) with late stent thrombosis and 3 (0.31%) with very late stent thrombosis. The occurrence of ARC definite or probable stent thrombosis was 0.82%. The 30-day, 6-month, 1-year, 2-year and 3-year rates of stent thrombosis were presented in Table 3.
Table 3. Cumulative occurrence of ARC definite, probable and possible stent thrombosis at 30-day, 6-month, 1-year, 2-year and 3-year follow-up.
| 30 days (n = 1045) | 6-month (n = 1035) | 1-year (n = 1032) | 2-year (n = 979) | 3-year (n = 979) | ||
| Stent thrombosis | ARC definite | 0 (0.00) | 5 (0.48) | 5 (0.48) | 5 (0.51) | 5 (0.51) |
| ARC probable | 1 (0.10) | 1 (0.10) | 1 (0.10) | 2 (0.20) | 3 (0.31) | |
| ARC possible | 1 (0.10) | 1 (0.10) | 4 (0.39) | 5 (0.51) | 5 (0.51) |
Values are numbers (%) of patients. ARC, Academic Research Consortium.
DISCUSSION
The SERY-I trial was designed to assess the extent of side effects of a China-made, polymer-free, paclitaxel-eluting microporous Yinyi stent in patients with coronary artery disease. The present analysis represents the report of extended follow-up with Yinyi stent. The 3-year follow-up results showed the cumulative rates of major adverse cardiovascular events were 8.17%, and the rate of stent thrombosis was 1.33% of all participated patients, indicating this novel DES was safe and effective in real-world clinical practice.
There is ongoing debate about the potential risk of LST regarding long-term outcomes with current DESs.19,20 Apart from prolonged healing and endothelialization due to the antiproliferative drug of choice,6 there is significant evidence that an inflammatory response to the residue from permanent polymers contributes toward LST following DES implantation.6,21,22 Thus, a novel designed polymer-free microporous DES may prevent persistent inflammatory response with vessel wall and reduce the occurence of LST.10 The series ISAR-TEST clinical trial first studied the long-term efficacy and safety of a polymer-free DES (YUKON) when compared with a permanent polymer paclitaxel-eluting stent in 450 randomized patients undergoing PCI. The 5-year follow-up of the ISAR-TEST study finally demonstrated equivalent long-term efficacy and safety between this novel polymer-free DES and a permanent-polymer DES.11-16 Meanwhile, another polymer-free amphilimus-eluting stent (Cre8) was shown to have significantly lower in-stent late lumen loss (LLL) at 6 months than permanent-polymer paclitaxel-eluting stents (Taxus) in de novo lesions clinically, using intravascular ultrasound analysis in 20% of the patients. Other clinical endpoints including cardiac death, myocardial infarction, target lesion revascularization, and stent thrombosis did not differ significantly between the groups in this study.23 However, the 5 years of clinical follow-up deserves additional attention. Other clinical studies of polymer-free DES were also reported elsewhere.24-26 In the present study, we evaluated the technical feasibility of a polymer-free, paclitaxel-eluting stent with microporous surface and assessed the relative efficacy of this novel stent for the prevention of adverse clinical events in a multicenter clinical trial. The results showed that after 3 years of stent implantation, the incidence of adverse clinical events of this novel stent were not demonstrably inferior compared with current standard DES use previously reported.
There were several limitations in this study. First, only a few participating patients underwent follow-up angiography. which can provide useful information regarding lumen renarrowing and stent thrombosis. Although clinical follow-up was performed by specialized personnel of the Clinical Data Management Center, telephone follow-up might not be sufficiently sensitive to capture adverse clinical events. Currently, the Yinyi DES stent platform is undergoing further clinical testing, and results from the SERY-II clinical trial with a focus on LLL, as analyzed by angiography are expected. In addition, the present study was limited by the fact that it is a single arm, nonrandomized study. A head-to-head comparison with other DES are preferred to more reliably confirm the efficacy and safety of this novel DES.
CONCLUSIONS
In conclusion, an extended follow-up multicenter clinical trial supports the elevated safety and efficacy levels noted by the use of the polymer-free paclitaxel-eluting microporous Yinyi stent.
CONFLICTS OF INTEREST
All of the authors declare that they have no conflicts of interest regarding this paper.
REFERENCES
- 1.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 2.Ong AT, Hoye A, Aoki J, et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45:947–953. doi: 10.1016/j.jacc.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 3.McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 4.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 5.Garg S, Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol. 2010;56:S43–S78. doi: 10.1016/j.jacc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47:175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 8.Guagliumi G, Farb A, Musumeci G, et al. Images in cardiovascular medicine. Sirolimus-eluting stent implanted in human coronary artery for 16 months: pathological findings. Circulation. 2003;107:1340–1341. doi: 10.1161/01.cir.0000062700.42060.6f. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama Y, Nishi S, Ishibashi-Ueda H, et al. Development of microporous covered stents: geometrical design of the luminal surface. Int J Artif Organ. 2005;28:600–608. doi: 10.1177/039139880502800609. [DOI] [PubMed] [Google Scholar]
- 10.Rogers CD. Drug-eluting stents: clinical perspectives on drug and design differences. Rev Cardiovasc Med. 2005;6:S3–12. [PubMed] [Google Scholar]
- 11.Ruef J, Storger H, Schwarz F, et al. Comparison of a polymer-free rapamycin-eluting stent (YUKON) with a polymer-based paclitaxel-elutingstent (TAXUS) in real-world coronary artery lesions. Catheter Cardiovasc Interv. 2008;71:333–339. doi: 10.1002/ccd.21326. [DOI] [PubMed] [Google Scholar]
- 12.Mehilli J, Kastrati A, Wessely R, et al. Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen loss. Circulation. 2006;113:273–279. doi: 10.1161/CIRCULATIONAHA.105.575977. [DOI] [PubMed] [Google Scholar]
- 13.Byrne RA, Mehilli J, Iijima R, et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J. 2009;30:923–931. doi: 10.1093/eurheartj/ehp044. [DOI] [PubMed] [Google Scholar]
- 14.Byrne RA, Kastrati A, Tiroch K, et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based Cypher and Endeavor [corrected] drug-eluting stents. J Am Coll Cardiol. 2010;55:2536–2543. doi: 10.1016/j.jacc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Ruef J, Storger H, Schwarz F, et al. Increased restenosis rates 12 months after coronary implantation of the sirolimus-eluting YUKON-choice stent compared to the paclitaxel-eluting TAXUS Stent. Clin Cardiol. 2010;33:E33–E38. doi: 10.1002/clc.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King L, Byrne RA, Mehilli J, et al. Five-year clinical outcomes of a polymer-free sirolimus-eluting stent versus a permanent polymer paclitaxel-eluting stent: final results of the intracoronary stenting and angiographic restenosis-test equivalence between two drug-eluting stents (ISAR-TEST) trial. Catheter Cardiovasc Interv. 2013;81:E23–E28. doi: 10.1002/ccd.24375. [DOI] [PubMed] [Google Scholar]
- 17.Zhang RY, Zhang Q, Zhu JZ, et al. Safety and efficacy of polymer-free paclitaxel-eluting microporous stent in real-world practice:1-year follow-up of the SERY-I registry. Chin Med J (Engl) 2011;124:3521–3526. [PubMed] [Google Scholar]
- 18.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 20.Maisel WH. Unanswered questions--drug-eluting stents and the risk of late thrombosis. N Engl J Med. 2007;356:981–984. doi: 10.1056/NEJMp068305. [DOI] [PubMed] [Google Scholar]
- 21.Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 22.Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 23.Carrie D, Berland J, Verheye S, et al. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J Am Coll Cardiol. 2012;59:1371–1376. doi: 10.1016/j.jacc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Costa JR, Jr., Abizaid A, Costa R, et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc Interv. 2009;2:422–427. doi: 10.1016/j.jcin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Peters S, Behnisch B, Heilmann T, et al. First-in-man use of polymer-free valsartan-eluting stents in small coronary vessels: a comparison to polymer-free rapamycin (2%)-eluting stents. J Renin Angiotensin Aldosterone Syst. 2009;10:91–95. doi: 10.1177/1470320308098591. [DOI] [PubMed] [Google Scholar]
- 26.Costa JR, Jr., Abizaid A, Costa R, et al. Preliminary results of the hydroxyapatite nonpolymer-based sirolimus-eluting stent for the treatment of single de novo coronary lesions; a first-in-human analysis of a third-generation drug-eluting stentsystem. JACC Cardiovasc Interv. 2008;1:545–551. doi: 10.1016/j.jcin.2008.07.003. [DOI] [PubMed] [Google Scholar]


