Abstract
Background
It is critical to recognize high risk patients who are prone to develop stroke in the management of atrial fibrillation (AF). The purpose of this study was to identify the determinants of AF related stroke by assessing the anatomical and functional remodeling of cardiac chambers.
Methods
We compared the cardiac structure and function of 28 consecutive patients with paroxysmal and persistent AF-related stroke with 69 patients with AF and 21 controls without stroke using contrast-enhanced 64-slice multi-detector computed tomography during sinus rhythm.
Results
The volume of left atrium (LA), LA appendage (LAA) and right atrium (RA) were significantly increased across the groups with sinus rhythm (SR), AF and AF-related stroke (p < 0.001 for each, respectively). The emptying fraction and booster-pump function of LA, LAA and RA were decreased across the groups (p < 0.001 for each). In addition, the left ventricular mass index was increased in AF related stroke (p = 0.003). Using multivariate analysis, increased age (p = 0.003), reduced booster-pump function of LA (p = 0.01), LAA (p < 0.001) and RA (p < 0.001) were shown to be independently associated with the occurrence of stroke.
Conclusions
The dilatation and contractile dysfunction of both atria are related to the development of stroke in patients with paroxysmal and persistent AF. Our results suggested that the use of substrate-based assessment may help improve risk stratification of stroke in patients with AF.
Keywords: Atrial fibrillation, Multi-detector computed tomography, Remodeling, Stroke
INTRODUCTION
Atrial fibrillation (AF) carries a substantial risk for cardiovascular mortality and cerebrovascular accident. Up to one-third of first strokes in patients are related to AF. Several risk stratification schemes, including CHADS2 and CHA2DS2-VASc scores, have been proposed to identify low risk patients and to provide recommendations regarding appropriate antithrombotic treatment for moderate to high-risk AF patients.1-3 However, AF-related ischemic stroke in the elderly has still increased significantly in recent years,4 and coping with certain unexpected and complex situations, especially in patients with a CHADS2 score less than 2, remains challenging with the currently available clinical prediction tools.5,6
Structural and functional remodeling of LA is related to the genesis and perpetuation of AF. Furthermore, recent studies also demonstrated that the morphological characteristics of left atrium (LA) and LA appendage (LAA) could be associated with the occurrence of stroke in patients with AF.7-9 Therefore, the assessment of cardiac anatomy and function may play a complementary role to clinical risk stratification schemes, and can improve the quality of care for AF patients. However, there were some echocardiographic studies which showed inconsistent results regarding the interplay between LA remodeling and AF-related stroke.10,11 Multi-detector computed tomography (MDCT) has been widely used prior to AF ablation and is an efficient and reliable imaging tool used to depict the anatomic and dynamic alteration of atria and ventricles simultaneously.12,13 We conducted this study to elucidate the relationship between the cardiac remodeling and AF related stroke using MDCT.
METHODS
The patients with AF and first episode of acute ischemic cerebral infarction who were admitted to our neurological ward were assessed for eligibility. Their stroke mechanism was classified as AF-related stroke based on the definition of the Stop Stroke Study - Trial of Org 10172 in Acute Stroke Treatment (SSS-TOAST) system.14 The AF-related stroke is diagnosed if it is due to embolic stroke in patients with nonvalvular AF, and lacking any other etiologies. The exclusion criteria included AF rhythm at 48 hours after admission, a serum creatinine more than 2 mg/dl, history to contrast allergy and the participants were unconscious or could not provide informed consent. The participants were enrolled after a written informed consent was provided. If the participant could not sign the informed consent, the legally authorized representative should sign the consent with participant’s agreement. The AF group included the AF patients matched for sex and AF type who had no history of stroke and underwent MDCT prior catheter ablation. In addition, the control group enrolled patients without any history of AF and stroke who underwent MDCT for coronary artery disease (CAD) screening. This study and the informed consent procedure were approved by the Institutional Review Board of National Yang Ming University Hospital.
The cardiac chambers were evaluated with an ECG-gated, 64-slice MDCT scanner (Brilliance CT, 64-slice, Philips, Amsterdam, Netherlands). All the participants underwent a computed tomography (CT) scan during sinus rhythm (SR). We administered 80 ml of the non-ionic contrast medium, iohexol [350 mg of iodine per milliliter (Optiray, Mallinckrodt, Canada)], followed by 20 ml of normal saline with the use of a power injector at a rate of 5.5 ml/second. We used the bolus tracing technique to monitor the signal intensity at the pre-defined region of interest (ROI) in the ascending aorta. When the CT number of the ROI reached the pre-set threshold (Hounsfield unit: 150), the scan automatically started and the patients were instructed to hold their breath to acquire the images, which covered the area from the aortic arch to the cardiac apex (collimation 64 × 0.625 mm, gantry rotation time 400 ms, table speed 19 mm/second, tube voltage 120 kV, effective tube current 500-600 mA).
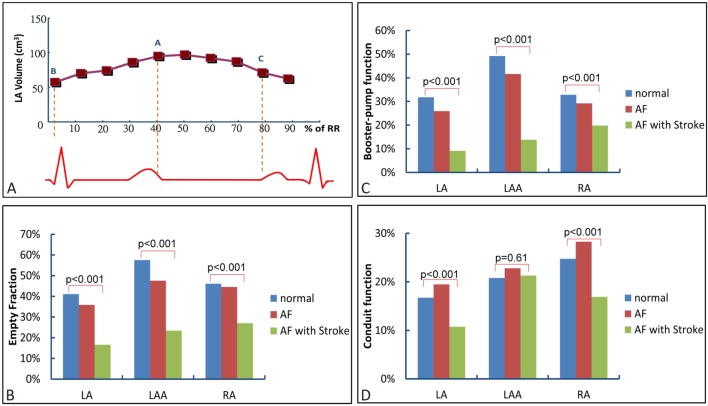
All CT images were analyzed offline with software developed by the Department of Biomedical Engineering, Chung Yuan Christian University (Chung-Li, Taiwan). Axial images were reconstructed at multiple phases covering the cardiac cycle in increments of 10% of the R-R interval. Serial multiphase short-axis images were generated using semi-automated software reformatting to a slice thickness of 0.9 mm. The 10 serial images of LA, LAA, right atrium (RA), left ventricle (LV) and right ventricle (RV) were visually identified (Figure 1). The endocardial border was traced for each slice and, if necessary, manually adjusted in a meticulous fashion. A modified Simpson’s method was used to calculate the volume of the chambers. In addition, the LV epicardial border was depicted in end-diastolic images. Therefore, the left ventricular myocardial mass (LVM) could be obtained by calculating the volume between epicardial and endocardial borders of the LV. Left ventricularmass index (LVMI) was defined as LVM/body surface area. For the cardiac chambers, the ejection fraction (EF) was defined as: (maximal volume – minimal volume)/maximal volume. Dynamic function of LA, LAA and RA, including the active EF (booster-pump) [(volume at P wave beginning – minimal volume)/volume at P wave beginning] and passive EF (conduit) [(maximal volume – volume at P wave beginning)/maximal volume] were assessed (Figure 2A). The left atrial pouch was defined as a cyst-like structure projecting from the heart cavity to outside the plane of the left atrial wall. The size of the pouch was measured by its largest expanse on the axial image.
Figure 1.
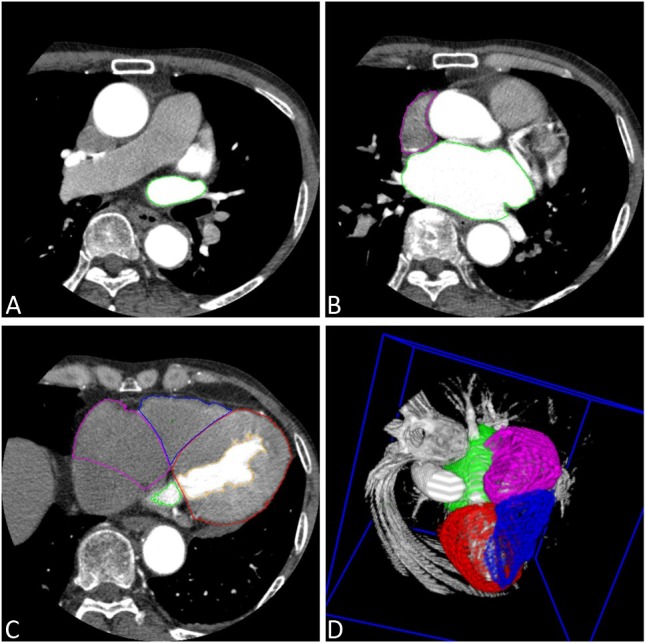
(A-D) Measurement of the volume and function of cardiac chambers, including left atrium (LA) (green), right atrium (RA) (pink), left ventricle (LV) (red) and right ventricle (RV) (blue) by the serial multiphase short-axis images with semiautomated software. (D) Three-dimensional reconstruction of the cardiac chambers.
Figure 2.
(A) Use of volume-time curve to evaluate the functional properties of LA and RA. The volume at A point indicated the maximal volume. The volume at B point indicated the minimal volume and the volume at C point indicated the volume at beginning of P wave. (B-D) Comparison of empty fraction, booster-pump function and conduit function of both atria between the patients with sinus rhythm, atrial fibrillation (AF) and AF related stroke.
Continuous variables with normal distribution are presented as mean ± standard deviation (SD), and categorical variables are presented as absolute values and percentages. A Chi-square test with Yates’ correction or Fisher’s exact test was used for categorical data. A Student’s t test or the Mann-Whitney U test was used for continuous data as appropriate. Multivariate analysis was performed with a stepwise logistic regression model to identify the independent predictors of AF-related stroke. Inclusion criteria for multivariate analysis were set at p < 0.1 with univariate analysis. A value of p < 0.05 was considered statistically significant. Data were analyzed using SPSS for Windows (version 21, SPSS Inc., Chicago, IL, USA).
RESULTS
Fifty-five patients with AF-related stroke were assessed for eligibility. Twenty-seven patients were excluded due to AF rhythm at 48 hours after admission (N = 20), a serum creatinine > 2 mg/dl (N = 4), no informed consent being provided (N = 2) and significant carotid stenosis (N = 1). Finally, we analyzed demographic, laboratory and MDCT data that were prospectively collected on 28 consecutive patients who fulfilled the inclusion and exclusion criteria and provided written informed consent. Sixty-nine patients without any history of stroke and 21 controls were selected for comparison. The gender, history of diabetes mellitus, CAD, hyperlipidemia, hypertension and use of renin-angiotensin aldosterone system blocker, beta blocker were similar among the three groups. However, patients with AF and stroke were older than patients in the other groups (p < 0.001). The percentage of paroxysmal AF was similar between AF and AF-related stroke. Forty-eight patients in the AF group and 16 patients in the AF-related group had a CHADS2 score = 0 or 1 (p = 0.19). Only 7.2% of patients in the AF group and 10.7% in the AF-related stroke group received anticoagulant treatment at enrollment. The patient characteristics were shown in Table 1.
Table 1. The patients’ characteristics and presentation of left atrium (LA) pouch between the three groups.
| Characteristic | SR (N = 21) | AF (N = 69) | AF-related stroke (N = 28) | p-trend |
| Age, years | 58.38 ± 10.86 | 57.12 ± 8.37 | 69.61 ± 12.14 | < 0.001 |
| Female sex, N (%) | 7 (33.3) | 22 (31.9) | 11 (39.3) | 0.78 |
| Diabetes | 3 (14.3) | 12 (17.4) | 8 (28.6) | 0.36 |
| CAD | 3 (14.3) | 21 (30.4) | 5 (17.9) | 0.20 |
| Hyperlipidemia | 8 (38.1) | 19 (27.5) | 8 (28.6) | 0.64 |
| Hypertension | 7 (33.3) | 25 (36.2) | 13 (46.4) | 0.56 |
| ARB/ACEI | 25 (36.2) | 12 (42.9) | 0.71* | |
| Beta blockers | 16 (23.2) | 6 (21.4) | > 0.99* | |
| Anticoagulant | 5 (7.2) | 3 (10.7) | 0.69* | |
| Paroxysmal AF | 35 (50.7) | 14 (50.0) | > 0.99* | |
| CHADS2 < 2 | 49 (71.0) | 16 (57.1) | 0.19* | |
| LA pouch | 6 (28.6) | 22 (31.9) | 8 (28.6) | 0.92 |
| Pouch size, mm2 | 31.50 ± 15.15 | 21.06 ± 11.42 | 26.20 ± 8.51 | 0.13 |
| Location | ||||
| Roof | 6 (100%) | 18 (81.8%) | 8 (100%) | 0.23 |
| Others | 0 (0%) | 4 (18.2%) | 0 (0%) |
AF, atrial fibrillation, ARB/ACEI, angiotensin receptor blocker/angiotensin converting enzyme inhibitor, CAD, coronary artery disease, LA, left atrium, SR, sinus rhythm.
* Indicates the p value compared between patient with AF and AF related stroke.
The anatomic and functional properties of LA and LAA were shown in Table 1 and 2. The incidence, location and size of LA pouch were similar between the patient groups (p = 0.92, 0.13, respectively). The volume of LA and LAA were significantly increased across the patient groups with SR, AF and AF-related stroke (Table 2). The emptying fraction (EF) of LA and LAA were significantly decreased across the patient groups. The booster-pump function (active EF) of LA and LAA were also decreased across the patient groups (p < 0.001 for each, respectively). There was a corresponding decrease in conduit function (passive EF) of LA across the patient groups (p < 0.001). However, the passive EF of LAA was similar between the patient groups (p = 0.61) (Figure 2B-D). The LA function of 20 patients was measured by two investigators who were blinded to the clinical information of the patients. Using the Bland-Altman method, the mean difference between intraobserver and interobserver were 2.4% and 7.2%, respectively.
Table 2. Comparison of structures and function of LA, LAA and RA.
| Characteristic | SR (n = 21) | AF (n = 69) | AF-related stroke (n = 28) | p-trend |
| Left atrial structure and function | ||||
| LAVI, ml/m2 | 54.67 ± 8.26 | 59.63 ± 10.90 | 82.61 ± 14.46 | < 0.001 |
| LAV maximal, ml | 96.34 ± 12.44 | 104.01 ± 20.70 | 137.27 ± 19.96 | < 0.001 |
| LAV minimal, ml | 56.85 ± 10.57 | 67.73 ± 24.21 | 115.94 ± 29.29 | < 0.001 |
| LA EF, % | 41 ± 7 | 36 ± 13 | 17 ± 12 | < 0.001 |
| LA passive EF, % | 17 ± 4 | 19 ± 8 | 11 ± 7 | < 0.001 |
| LA active EF, % | 32 ± 4 | 26 ± 12 | 9 ± 8 | < 0.001 |
| Left atrial appendage structure and function | ||||
| LAAVI, ml/m2 | 3.64 ± 1.25 | 4.47 ± 1.54 | 6.66 ± 3.09 | 0.009 |
| LAA maximal, ml | 5.80 ± 2.09 | 7.70 ± 2.96 | 11.32 ± 4.59 | < 0.001 |
| LAA minimal, ml | 2.50 ± 1.30 | 4.18 ± 2.82 | 8.58 ± 3.78 | < 0.001 |
| LAA EF, % | 58 ± 13 | 48 ± 18 | 23 ± 15 | < 0.001 |
| LAA passive EF, % | 21 ± 10 | 23 ± 11 | 21 ± 7 | 0.61 |
| LAA active EF, % | 49 ± 16 | 42 ± 16 | 14 ± 13 | < 0.001 |
| Right atrial structure and function | ||||
| RAVI, mL/m2 | 47.05 ± 10.65 | 50.90 ± 9.42 | 59.62 ± 12.10 | 0.001 |
| RAV maximal, ml | 88.29 ± 21.51 | 87.91 ± 18.06 | 100.73 ± 24.45 | 0.02 |
| RAV minimal, ml | 47.33 ± 13.57 | 48.48 ± 18.87 | 74.75 ± 26.94 | < 0.001 |
| RA EF,% | 46 ± 8 | 45 ± 13 | 27 ± 15 | < 0.001 |
| RA passive EF,% | 25 ± 7 | 28 ± 10 | 17 ± 8 | < 0.001 |
| RA active EF,% | 33 ± 9 | 29 ± 11 | 20 ± 13 | < 0.001 |
EF, emptying fraction; LAA, left atrial appendage; LAV, left atrial volume; LAVI, left atrial volume index (LAV/body surface area); RA, right atrium; RAV, right atrial volume; RAVI, right atrial volume index. Other abbreviations noted in Table 1.
The maximal and minimal volumes of RA were significantly increased across the patient groups with SR, AF and AF-related stroke (p = 0.02, < 0.001, respectively). The EF, conduit function and booster-pump function of RA were significantly reduced across the patient groups (p < 0.001 for each, respectively) (Table 2).
The volumes of LV and RV were similar among the three patient groups (p = 0.31 for minimal LV volume, p = 0.06 for maximal and minimal RV volume). Furthermore, the EF of LV (p = 0.47) and RV (p = 0.34) were also similar across the patient groups. However, the LV mass index (LV mass/body surface area) was significantly increased in patients with AF and stroke (p = 0.003). The associated properties of both LV and RV are presented in Table 3.
Table 3. Comparison of structures and function of left and right ventricles.
| Characteristic | SR (n = 21) | AF (n = 69) | AF-related stroke (n = 28) | p-trend |
| Left ventricular (LV) structure and function | ||||
| LV EF, % | 66 ± 6 | 66 ± 12 | 63 ± 11 | 0.47 |
| LVV maximal, ml | 106.52 ± 18.87 | 107.56 ± 18.70 | 88.55 ± 19.91 | < 0.001 |
| LVV minimal, ml | 35.93 ± 9.34 | 36.67 ± 13.44 | 32.40 ± 11.92 | 0.31 |
| LVMI | 62.95 ± 14.94 | 57.13 ± 12.10 | 71.22 ± 20.97 | 0.003 |
| Right ventricular (RV) structure and function | ||||
| RV EF, % | 42 ± 5 | 45 ± 9 | 45 ± 9 | 0.34 |
| RVV maximal, ml | 153.54 ± 21.85 | 154.51 ± 29.23 | 140.21 ± 26.05 | 0.06 |
| RVV minimal, ml | 89.87 ± 16.84 | 86.30 ± 20.58 | 77.09 ± 21.02 | 0.06 |
EF, ejection fraction; LVMI, left ventricular mass index (LVM/body surface area); LVV, left ventricular volume; RVV, right ventricular volume.
Figure 3 showed the subgroup analysis in AF patients with CHADS2 score < 2. The maximal volume of LA (106.11 ± 21.48 vs. 135.06 ± 18.49 ml, p < 0.001), and LAA (7.68 ± 3.41 vs. 11.36 ± 5.17 ml, p < 0.01) was significantly increased in patients with AF-related stroke compared to those without stroke. The EF of LA (40 ± 10 vs. 16 ± 7%, p < 0.001), LAA (54 ± 12 vs. 30 ± 6%, p < 0.001) and RA (49 ± 12 vs. 31 ± 9%, p < 0.001) was reduced in patients with AF-related stroke (Figure 3). Furthermore, the active EF of LA (23 ± 13 vs. 6 ± 5 %, p < 0.001), LAA (39 ± 15 vs. 10 ± 7%, p < 0.001) and RA (27 ± 12 vs. 19 ± 9%, p < 0.01) was reduced; the passive EF of LA (20 ± 9 vs. 10 ± 4%, p < 0.001), RA (29 ± 10 vs. 16 ± 8%, p < 0.001) was reduced in AF-related stroke group.
Figure 3.
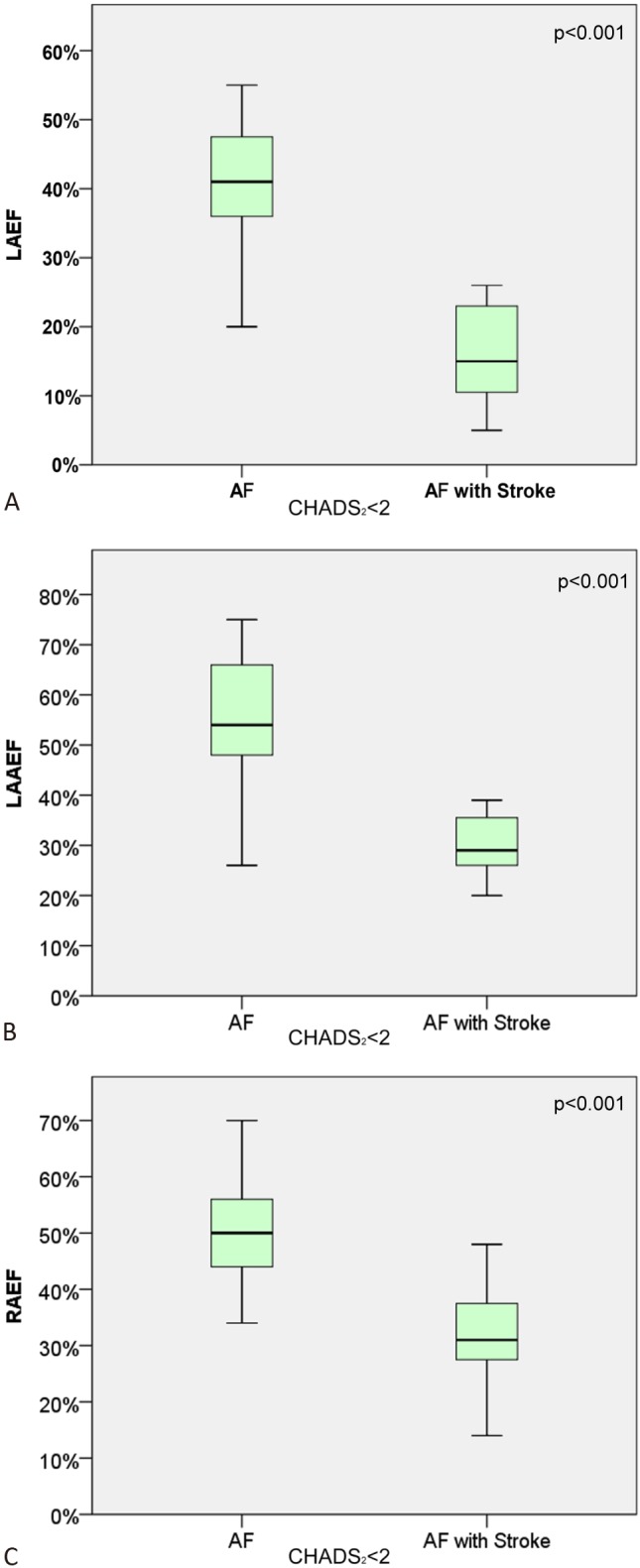
(A-C) Comparison of emptying fraction (EF) of LA, LAA and RA in patients with AF and AF related stroke with a CHADS2 score < 2. The upper and lower margin of the box indicates the upper and lower quartile, and the line within the box indicates the median.
To explore the independent parameters of AF-related stroke, multivariate analysis was performed between patients with AF and AF with stroke. Inclusion criteria for multivariate analysis were set at p < 0.1 with univariate analysis. Stepwise logistic regression involving 11 variables identified four that best distinguished the patients with AF from those with AF-related stroke. The characteristics of older age (p = 0.003), reduced active EF of LA (p = 0.01), LAA (p < 0.001) and RA (p < 0.001) were independently associated with the occurrence of stroke in patients with AF.
DISCUSSION
This study has three major findings. First, structural and functional remodeling of both atria, which manifested as increased atrial volume and decreased triphasic transport function, were more evident not only in patients with AF but in patients with AF-related stroke. Second, the LA pouch is common and is not related to AF and stroke. Third, increased LV mass index could be a determinant of stroke in patients with paroxysmal and persistent AF.
Our results showed that the incidence and size of LA pouch was not different among the three patient groups. Although the pouches were suspected to be the potential origin of thrombus, recent reports showed the LA pouch is not associated with the occurrence of stroke15 that is compatible with our observation.
The relationship between increased LA volume and stroke remains to be clarified.16 The present study showed that significant LA dilatation was noted in patients with AF related stroke compared to those without. In addition, we demonstrated that pump and reservoir function of both LA and LAA were impaired in the patients with AF related stroke. Previous anatomic studies had showed that significant fibrosis of the LA and RA can be found in AF. Daccarett et al. discovered a more extensive fibrosis of LA in patients with AF and stroke, using delayed-enhanced magnetic resonance images.17 Park et al. described that electroanatomical remodeling of LA was significantly related to the risk scores or events of stroke in patients with AF.18 The atrial fibrosis could explain, in part, the dysfunction of both atria in patients with AF and AF-related stroke. In addition, atrial stunning after restoration of sinus rhythm from AF also plays a significant role.19 Previous echocardiographic studies had underscored the role of LAA dysfunction in the development of stroke in patients with AF. To our knowledge, this is the first CT study to demonstrate that the severity of the LA/LAA/RA dysfunction may reflect the possibility of stroke in AF patients.
The remodeling process of RA in AF has received substantially less attention as compared to LA. Our group had characterized the RA substrate in patients with atrial tachyarrhythmias arising from RA.20 Furthermore, the present study delineated that the RA enlargement and dysfunction was more evident in patients with AF compared to those without. This finding may reflect the role of bi-atrial remodeling in AF.
The role of RA in AF-related stroke has not been well-studied. Previous investigations have focused on the relationship between RA and stroke due to the paradoxical embolization through the patent foramen ovale. In this study, no RA thrombus was noted by CT scan. The possibility of paradoxical embolization-related stroke is very low. We demonstrated that the patients with impaired mechanical function of both atria are prone to develop stroke, although the causal relationship is hard to define. However, the analysis of RA structure and function can confer additional and important information to grade the risk of stroke in patients with non-permanent AF.
Our result showed the volume and systolic function of both ventricles were not associated with the development of AF and AF related stroke. However, the LV mass index was significantly increased in patients with AF and stroke. A previous echocardiographic substudy of ENGAGE AF-TIMI 48 showed that LV mass index and the incidence of abnormal LV geometry were increased in patients with higher CHADS2 scores.21 The increased LV mass index may cause the LV diastolic dysfunction and elevated end-diastolic pressure of LV which can contribute to the development of AF and atrial fibrosis.22 Because of the anatomic vicinity of LAA and LV, the impaired LV filling attenuates the intracavitary suction effect to influence LA and LAA filling and emptying. Therefore, increased LV mass index can lead to LAA dysfunction and the consequential development of embolic stroke in AF.
Several limitations are noteworthy in interpretation of the study results. First, the patient number was small because we only enrolled those stroke patients with paroxysmal and persistent AF. Further prospective studies are necessary to confirm our results in larger groups. In addition, the study results may not be applied to patients with permanent AF. Second, only 11% of patients with AF-related stroke received anticoagulant (warfarin) at enrollment. This finding may reflect the reality about the physicians’ concerns about bleeding complication of warfarin, and the inability to identify the true low-risk patients at the time of study. Third, we cannot avoid an inherent limitation in terms of the AF burden in such a patient group that had no implanted device. However, our result demonstrated that the presence of atrial dysfunction in the period of stroke was a strong determinant of this neurological consequence in patients with non-permanent AF. In conclusion, the enlargement and reduced transport function of LA, LAA and RA are independently associated with the occurrence of stroke in patients with nonpermanent AF. Use of a comprehensive MDCT study can provide important information regarding the structural and functional remodeling of the cardiac chambers. Substrate-based assessment may confer an additional ability to identify the high-risk stroke patients in patients with AF.
CONCLUSIONS
Dynamic CT images are effective to delineate the anatomic and functional properties of atria and ventricles. The dilatation and hypokinesia of both atria and increased LV mass are associated with the development of embolic stroke in paroxysmal and persistent AF. Therefore, comprehensive assessment of cardiac chambers may provide incremental value in stratifying the risk of cardioembolic stroke in AF patients.
Acknowledgments
This work was supported by a research grant of the Ministry of Science and Technology, Taiwan (NSC 100-2314-B-010-040).
DISCLOSURES
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 3.Pisters R, Lane DA, Marin F, et al. Stroke and thromboembolism in atrial fibrillation. Circ J. 2012;76:2289–2304. doi: 10.1253/circj.cj-12-1036. [DOI] [PubMed] [Google Scholar]
- 4.Yiin GS, Howard DP, Paul NL, et al. On behalf of the Oxford vascular study. Age-specific incidence, outcome, cost and projected future burden of atrial fibrillation-related embolic vascular events: a population-based study. Circulation. 2014;130:1236–1244. doi: 10.1161/CIRCULATIONAHA.114.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg BA, Kim S, Thomas L, et al. Outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) investigators and patients. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005–2012. doi: 10.1161/CIRCULATIONAHA.114.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao TF, Liu CJ, Chen SJ, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. 2012;43:2551–2555. doi: 10.1161/STROKEAHA.112.667865. [DOI] [PubMed] [Google Scholar]
- 7.Beinart R, Heist EK, Newell JB, et al. Left atrial appendage dimensions predict the risk of stroke/TIA in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:10–15. doi: 10.1111/j.1540-8167.2010.01854.x. [DOI] [PubMed] [Google Scholar]
- 8.Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 9.Kishima H, Mine T, Ashida K, et al. Does left atrial appendage morphology influence left atrial appendage flow velocity? Circ J. 2015;79:1706–1711. doi: 10.1253/circj.CJ-14-1380. [DOI] [PubMed] [Google Scholar]
- 10.McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121:667–674. doi: 10.1161/CIRCULATIONAHA.109.885806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026–2033. doi: 10.1016/j.jacc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Wazni OM, Tsao HM, Chen SA, et al. Cardiovascular imaging in the management of atrial fibrillation. J Am Coll Cardiol. 2006;48:2077–2084. doi: 10.1016/j.jacc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 13.Tsao HM, Hu WC, Wu MH, et al. The impact of catheter ablation on the dynamic function of the left atrium in patients with atrial fibrillation: insights from four-dimensional computed tomographic images. J Cardiovasc Electrophysiol. 2010;21:270–277. doi: 10.1111/j.1540-8167.2009.01618.x. [DOI] [PubMed] [Google Scholar]
- 14.Ay H, Furie KL, Singhal A, et al. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 15.Peng LQ, Yu JQ, Yang ZG, et al. Left atrial diverticula in patients referred for radiofrequency ablation of atrial fibrillation: assessment of prevalence and morphologic characteristics by dual-source computed tomography. Circ Arrhythm Electrophysiol. 2012;5:345–350. doi: 10.1161/CIRCEP.111.965665. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 17.Daccarett M, Badger TJ, Akoum N, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Joung B, Son NH, et al. The electroanatomical remodelling of the left atrium is related to CHADS2/CHA2DS2VASc score and events of stroke in patients with atrial fibrillation. Europace. 2011;13:1541–1549. doi: 10.1093/europace/eur135. [DOI] [PubMed] [Google Scholar]
- 19.Khan IA. Atrial stunning: basics and clinical considerations. Int J Cardiol. 2003;92:113–128. doi: 10.1016/s0167-5273(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Lin YJ, Higa S, Tai CT, et al. Role of the right atrial substrate in different types of atrial arrhythmias. Heart Rhythm. 2009;6:592–598. doi: 10.1016/j.hrthm.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Gupta DK, Shah AM, Giugliano RP, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35:1457–1465. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd AC, McKay T, Nasibi S, et al. Left ventricular mass predicts left atrial appendage thrombus in persistent atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2013;14:269–275. doi: 10.1093/ehjci/jes153. [DOI] [PubMed] [Google Scholar]