Abstract
AIM
To investigate if pre-treatment platelet counts could provide prognostic information in patients with rectal adenocarcinoma that received neo-adjuvant treatment.
METHODS
Platelet number on diagnosis of stage II and III rectal cancer was evaluated in 51 patients receiving neo-adjuvant treatment and for whom there were complete follow-up data on progression and survival, as well as pathologic outcome at the time of surgery. Pathologic responses on the surgical specimen of patients with lower platelet counts (150-300 × 109/L) were compared with these of patients with higher platelet counts (> 300 × 109/L) by the χ2 test. Overall and progression free survival Kaplan-Meier curves of the two groups were constructed and compared with the Log-Rank test.
RESULTS
A significant difference was present between the two groups in regards to pathologic response with patients with lower platelet counts being more likely to exhibit a good or complete response to neo-adjuvant treatment than patients with higher platelet counts (P = 0.015). Among other factors evaluated, there was also a significant difference between the carcinoembryonic antigen (CEA) at presentation of patients that exhibited a good or complete response and those that had no response or a minimal to moderate response. Patients with a good or complete response were more likely to present with a CEA of less than 5 μg/L (P = 0.00066). There was no significant difference in overall and progression free survival between the two platelet count groups (Log-Rank tests P = 0.42 and P = 0.35, respectively).
CONCLUSION
In this retrospective analysis of stage II and III rectal cancer patients, platelet counts at the time of diagnosis had prognostic value for neo-adjuvant treatment pathologic response. Pre-treatment CEA also held prognostic value in regards to treatment effect.
Keywords: Rectal cancer, Platelets, Prognosis, Treatment response, Neo-adjuvant, Chemoradiation
Core tip: Platelet counts may provide prognostic and treatment efficacy predictive information in various cancers. In this study, platelet number on diagnosis of stage II and III rectal cancer was evaluated in 51 patients before start of neo-adjuvant treatment. A significant difference was present between the two groups, of higher and lower platelets, regarding pathologic response to neo-adjuvant treatment. There was no significant difference in overall and progression free survival between the two platelet count groups. Pre-treatment carcinoembryonic antigen also held prognostic value in regards to treatment effect.
INTRODUCTION
Platelets play a crucial role in maintaining hemostasis and vascular integrity. They are produced from bone marrow precursor cells, megakaryocytes, as fragments breaking off of the megakaryocytes cytoplasm[1]. Abnormalities in platelet number, whether an increase or decrease in their circulating number, are associated with many pathologic conditions[2]. Cancer is a pathology that is often associated with thrombocytosis as the cytokines that stimulate thrombopoiesis are often elevated in cancer[3]. In addition, thrombocytosis has been found to be an adverse prognostic factor in many common cancers[4].
Colorectal cancer is a common malignancy and one of the leading causes of cancer deaths in both men and women[5]. Localized stage rectal cancer is typically treated with neoadjuvant chemoradiotherapy in addition to adjuvant chemotherapy[6]. Pathologic stage is the main prognostic factor and treatment modality determinant. Other prognostic factors include positive surgical margins, pre-treatment elevation of carcinoembryonic antigen (CEA), and high tumour grade[7]. Prognostic markers of positive pathologic response to neo-adjuvant therapy are also important because such response may be associated with survival outcomes in rectal cancer[8]. Moreover, being able to predict which patients would benefit from neo-adjuvant chemoradiation could be important for modification of the treatment plan and sparing of patients predicted not to respond to this therapy and its adverse effects. Thus, additional biomarkers are needed to further promote prognostication of rectal adenocarcinomas. In a previous study in patients with colorectal adenocarcinomas of various stages, pre-treatment thrombocytosis was an independent prognostic factor for overall survival (OS) and progression-free survival (PFS)[9]. Nevertheless, another study in colorectal cancer patients did not observe a difference in survival between patients with thrombocytosis and normal platelet counts[10]. In the current study, we investigated if pre-treatment thrombocytosis provides prognostic information specifically in patients with stage II and III rectal adenocarcinomas that received neo-adjuvant treatment. We also investigated the effect of thrombocytosis on pathologic outcome at the time of surgery.
MATERIALS AND METHODS
The case records of 130 patients treated for localized rectal cancer at the Algoma District Cancer Clinic between January 2008 and January 2015 were retrospectively reviewed. Patients were included if they had stage II or III disease, had received neo-adjuvant treatment, and had complete follow-up. Follow-up was considered complete if a patient was followed until his or her death, or was seen within the last six months from data collection. Fifty one patients fulfilled the inclusion criteria and were included in the study. Demographic data, as well as histologic characteristics of tumors, stage, tumor marker CEA, and pathologic response were extracted from the medical records. Platelet number at diagnosis of the 51 patients was also evaluated.
Pathologic response at the time of surgery was categorized in a five tier scale ranging from no response (no evidence of treatment effect on tumor), minimal response (some morphologic effect of treatment evident but no significant regression of tumor areas), moderate response (evident effect of treatment but significant tumor aggregates remaining), good response (only occasional scattered tumor cell aggregates remaining) and complete response (no evidence of tumor in the primary site or the lymph nodes).
OS was defined as the interval from the date of diagnosis to patient death or censored at the date of last contact. PFS was defined as the interval from the date of diagnosis until date of disease progression or censored at the date of last contact without evidence of recurrence. For the purpose of this study, patients with platelet counts of 150-300 × 109/L were included in the lower platelet count group. Patients with counts > 300 × 109/L were included in the higher platelet count group. This value divided patients in two groups with almost equal numbers. Survival plots of the patients with lower and higher platelet counts were constructed using the Kaplan-Meier method and were compared using the Log-Rank test[11]. The χ2 test was used to evaluate differenced in clinical and biologic characteristics of the two groups[12]. The Student’s t test was used for comparison of means. All P values were considered to be significant at a level of P < 0.05. Statistical calculations were performed with online tools available from the Technical University of Denmark (http://www.iscc-serv2.imm.dtu.dk/) and a noncommercial site (http://www.statpages.org/). The study was approved by the Institutional Review Board of our institution. Due to the retrospective nature of the study, no patient consent was required or obtained.
RESULTS
The median age of the patients was 58-year-old. From the 51 patients, 26 patients (51%) were included in the lower platelet (≤ 300 × 109/L) group and had mean platelet counts of 232.5 × 109/L (range, 167-297) at diagnosis of their disease (Table 1). Twenty-five patients (49%) were in the higher platelet (> 300 × 109/L) group and had mean platelet counts of 347 × 109/L (range, 303-693). The median age of the patients with lower platelet counts was 59-year-old (range, 32-79) and those with higher counts was 58-year-old (range, 24-74). In the lower platelet group 38.5% of patients were older than 60-year-old while in the higher platelet group 44% were older than 60-year-old (χ2 test P = 0.69). Forty-four patients in the series received neoadjuvant chemoradiation with continuous infusion of 5-FU or capecitabine as the chemotherapy part. Five additional patients (four in the higher platelet group and one patient in the lower platelet group) received 1-2 cycles of neo-adjuvant mFOLFOX before chemoradiation. Two patients (both in the higher platelet group) received neo-adjuvant radiation alone. No differences in the two groups were noted in the clinical stage at presentation, in the tumor marker CEA or patients’ symptoms of presentation (Table 1). The type of surgery performed after neo-adjuvant therapy (whether an abdominal resection or abdomino-perineal resection (APR)/pelvic exenteration with permanent colostomy) was also not statistically different in the two groups (Table 1). All patients but two had negative pathologic surgical margins at surgery. Both patients with positive pathologic margins (one in the lower and one in the higher platelet group) underwent an APR, had minimal pathologic responses and had a recurrence 12 and 20 mo postoperatively respectively. All patients but three had post-operative 5-fluoropyrimidine-based chemotherapy. Three patients who had complete pathologic response (two in the lower platelet group and one patient in the higher platelet group) elected not to undergo surgery and were placed in close surveillance.
Table 1.
Baseline characteristics of all patients in the series and comparison of the groups with lower (≤ 300 × 109/ L) and higher (> 300 × 109/ L) platelet counts n (%)
| Total (%) (n = 51) | ≤ 300 (n = 26) | > 300 (n = 25) | χ2 | |
| Age (yr) | ||||
| > 60 | 21 (41.2) | 10 (38.5) | 11 ( 44.0) | P = 0.69 |
| ≤ 60 | 30 (58.8) | 16 (61.5) | 14 (56.0) | |
| Clinical stage | ||||
| II | 25 (49.0) | 15 (57.7) | 10 (40.0) | P = 0.21 |
| III | 26 (51.0) | 11 (42.3) | 15 (60.0) | |
| CEA (n = 50) | ||||
| > 5 | 25 (50.0) | 11 (44.0) | 14 (56.0) | P = 0.4 |
| < 5 | 25 (50.0) | 14 (56.0) | 11 (44.0) | |
| Symptoms | ||||
| Obstruction/pain | 13 (25.5) | 6 (23.1) | 7 (28.0) | P = 0.69 |
| Bleeding/ asymptomatic | 38 (74.5) | 20 (76.9) | 18 (72.0) | |
| Type of surgery | ||||
| Anterior resection | 27 (52.9) | 13 (50.0) | 14 (56.0) | Anterior resection vs |
| APR | 19 (37.3) | 11 (42.3) | 8 (32.0) | APR/exenteration |
| Pelvic/exenteration | 2 (3.9) | 0 | 2 (8.0) | P = 0.77 |
| None | 3 (5.9) | 2 (7.7) | 1 (4.0) | |
| Pathologic response | ||||
| No response | 15 (29.4) | 8 (30.8) | 7 (28.0) | No/minimal/ |
| Minimal | 7 (13.7) | 4 (15.3) | 3 (12.0) | Moderate resp vs |
| Moderate | 15 (29.4) | 3 (11.6) | 12 (48.0) | Good/complete |
| Good | 5 (9.8) | 5 (19.2) | 0 | P = 0.015 |
| Complete | 9 (17.6) | 6 (23.1) | 3 (12.0) | |
| Lymph nodes at surgery | ||||
| Negative | 31 (60.8) | 15 (57.7) | 16 (64.0) | P = 0.61 |
| Positive | 16 (31.4) | 9 (34.6) | 7 (28.0) | |
| No surgery | 3 (5.9) | 2 (7.7) | 1 (4.0) |
Pre-operative CEA not available in one patient. Lymph node evaluation was not available in the pathology report in one patient. APR: Abdomino-perineal resection; CEA: Carcinoembryonic antigen.
Overall about one third of patients in the series were lymph node positive on pathologic examination at the time of surgery and the percentage did not differ significantly between the two platelet groups (P = 0.61) (Table 1). A complete pathologic response (defined as no pathologic evidence of tumor in either primary site or lymph nodes examined) was obtained after neo-adjuvant treatment in 9 patients (17.6%) in the series and an additional 5 patients (9.8%) had good pathologic responses. No response, minimal or moderate response were observed in 15 (29.4%), 7 (13.7%), and 15 (29.4%) patients respectively. Overall pathologic response differed between the groups. Eleven patients (42.3%) in the lower platelet group had a good or complete pathologic response while only three patients in the higher platelet group (12%) had such a response (P = 0.015). The mean platelet count at diagnosis of good and complete responders was 249.9 (SD = 69.6) while mean platelet count of no/minimal/moderate responders group was 327.0 (SD = 85.6) (t test P = 0.004). Among the 25 patients in the elevated platelet group, 16 patients had converted to a platelet count below 300 × 109/L after the neo-adjuvant treatment, in their pre-operative evaluation, while the remaining nine patients remained with a platelet count above 300 × 109/L. All three pathologic responders were among the 16 converted patients.
In the analysis for possible associated factors with a good or complete pathologic response a normal range (< 5 μg/L) CEA level at baseline (P = 0.0004) and lower platelet counts (P = 0.015) were associated with favorable pathologic response (Table 2). Patients that were asymptomatic at presentation (evaluated with a colonoscopy for anemia) or presented with bleeding had a trend towards a better pathologic response than patients presenting with obstruction or pain (P = 0.06). Age and clinical stage at presentation was not statistically associated with the degree of pathologic response. Logistic regression analysis with pathologic response as the outcome variable and platelet counts, CEA and symptoms at presentation as the predictor variables confirmed that lower platelet counts (P = 0.03, odds ratio 0.15, 95%CI: 0.02-0.85) and a normal CEA (P = 0.006, OR = 0.04, 95%CI: 0.004-0.41) but not symptoms at presentation (P = 0.2, OR = 4.7, 95%CI: 0.39-55.89) were significantly associated with a good or complete pathologic response (Table 3).
Table 2.
Comparison of characteristics of patients according to their pathologic response at surgery (n = 48) or at post-neoadjuvant treatment endoscopy (n = 3) n (%)
| No response/minimal/moderate | Good/ complete | χ2P value | |
| Age (yr) | |||
| > 60 | 13 (35.1) | 8 (57.1) | 0.15 |
| ≤ 60 | 24 (64.9) | 6 (42.9) | |
| Clinical stage | |||
| II | 16 (43.2) | 9 (64.3) | 0.18 |
| III | 21 (56.8) | 5 (35.7) | |
| CEA (n = 50) | |||
| > 5 | 24 (64.9) | 1 (7.7) | 0.0004 |
| < 5 | 13 (35.1) | 12 (92.3) | |
| Symptoms | |||
| Obstruction/pain | 12 (32.4) | 1 (7.1) | 0.06 |
| Bleeding/asymptomatic | 25 (67.6) | 13 (92.9) | |
| Platelets | |||
| ≤ 300 | 15 (40.5) | 11 (78.6) | 0.015 |
| > 300 | 22 (59.5) | 3 (21.4) |
Pre-operative CEA not available in one patient. CEA: Carcinoembryonic antigen.
Table 3.
Logistic regression analysis of pathologic response (complete or good vs moderate or minimal or no response) as the outcome variable and platelet counts (≤ vs > 300 × 109/L), carcinoembryonic antigen (≤ 5 μg/L vs > 5 μg/L) and symptoms (obstruction or pain vs bleeding or asymptomatic) at presentation as the predictor variables
| Variable | OR | 95%CI | P value |
| Platelet count | 0.15 | 0.02-0.85 | 0.03 |
| CEA | 0.04 | 0.004-0.41 | 0.006 |
| Symptoms at presentation | 4.7 | 0.39-55.8 | 0.20 |
CEA: Carcinoembryonic antigen.
The median overall survival of patients that had died in the whole cohort (11 of 51 patients) was 21 mo (range 5-68 mo). In the group of patients with lower platelet counts, 4 of the 26 patients (15.4%) had died with a median OS of 20 mo (range 5-24 mo). In the group of patients with higher platelet counts, 7 of the 25 patients (28%) had died with a median OS of 23 mo (range 7-68 mo). Kaplan-Meier survival analysis showed that there was not a statistically significant difference in the OS and PFS between patients in the two platelet groups (Log-Rank test P = 0.42 and 0.35 respectively) (Figure 1A and B). PFS of patients with a good or complete pathologic response was significantly better than that of patients with a lesser pathologic response (Log-Rank test P = 0.01) (Figure 2A). OS curve of pathologic responders clearly separated from lesser responders after 2 years but the difference did not reach statistical significance (Log-Rank test P = 0.15) (Figure 2B).
Figure 1.
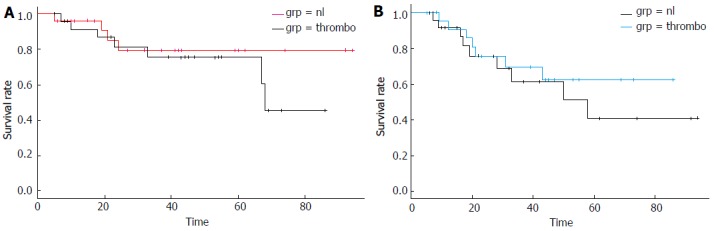
Kaplan-Meier overall survival (A) and progression free survival (B) curves in months from the diagnosis of rectal adenocarcinoma of patients with lower platelet counts (150-300 × 109/L, labeled: nl) vs patients with higher platelet counts (> 300 × 109/L, labeled: thrombo). Log-Rank tests P = 0.47 (A), P = 0.35 (B).
Figure 2.
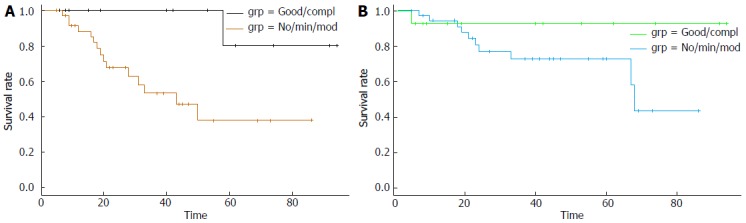
Kaplan-Meier progression-free survival (A) and overall survival (B) curves in months from the diagnosis of rectal adenocarcinoma of patients with a good or complete pathologic response (labeled: Good/compl) vs patients with no response or a minimal or moderate response (labeled: No/min/mod). Log-Rank tests P = 0.01 (A), P = 0.15 (B).
DISCUSSION
Thrombocytosis is associated with several underlying pathologies among which cancer is included[2]. About two out of five patients (40%) with thrombocytosis had an occult cancer in one series[11]. Thrombocytosis or a higher platelet count defined with various cut-offs has been confirmed to be an adverse prognostic factor in several types of cancer including lung, breast, gynecologic and genitourinary[12-16]. It has also been studied and suggested to have prognostic relevance in virtually every type of gastrointestinal carcinoma including gastric, colon and rectal[4].
In a series of Asian patients with colorectal carcinoma, thrombocytosis, defined as platelets counts greater than 300 × 109/L, similarly to our study, was a significant independent prognostic marker for survival[17]. This was confirmed in another large Japanese series and a smaller study of European patients[18,19]. An American study of 1513 patients with localized colorectal cancer that had undergone surgery also evaluated pre-operative thrombocytosis (defined in this study as more than 400 × 109/L) as a prognostic factor for various survival outcomes[20]. Patients with thrombocytosis had a significantly worse overall survival than patients with normal platelets. Distant metastatic recurrence, but not overall recurrence rate or loco-regional recurrence rate, was also worse in patients with thrombocytosis. In contrast, another study of 630 patients showed no correlation of survival with thrombocytosis defined as platelets above 450 × 109/L[10]. Nevertheless this study included patients across stages which may have confounded results.
Two studies from the Far East have specifically examined the prognostic role of platelets in rectal cancer patients[9,21]. Both reports used a similar cut-off for thrombocytosis of 365 to 370 × 109/L and showed a similar percentage of thrombocytosis of about 20%. The authorship of the two publications is overlapping and is not clear if the patients of the smaller study[21] is a sub-set of the patients included in the larger study[9]. Both a lower platelet count, below the cut-off, and a normal CEA were associated with pathologic response[9]. These were the only predictors of such response in logistic regression analysis.
In order to further clarify the prognostic value of platelets in rectal cancer in a predominantly white population, in this retrospective study we investigated the association between pre-treatment thrombocytosis and the effectiveness of neoadjuvant treatment in patients with stage II and III rectal adenocarcinoma. We also explored the relationship between thrombocytosis and both overall and progression free survival. In the current cohort of 51 patients we found no significant differences between the two groups with lower and higher platelet levels regarding the age of patients, pre-treatment tumor CEA, symptoms at presentation, clinical staging at presentation, and the presence of metastatic lymph nodes at the time of surgery. No difference was also observed in the type of surgery in the two groups. In contrast, a significant difference was present regarding the response to neoadjuvant treatment as patients with lower platelet counts were more likely to have a good or complete pathologic response to pre-operative treatment than patients with higher platelet counts (P = 0.015). There was no statistical difference in the OS or PFS of the two platelet groups (Log-Rank test P = 0.47 and 0.35 respectively).
The CEA tumor antigen at presentation was also a prognostic marker for pathologic response (P = 0.0004). Patients with a CEA of less than 5 were more likely to have a good or complete response to neoadjuvant treatment than those that presented with a CEA greater than 5. A higher pre-treatment CEA has been associated with advanced locoregional disease and therefore may be linked to poorer local control[22]. Our data confirm that CEA is prognostic for PFS and predictive for pathologic response to neoadjuvant therapy, as suggested previously[22,23].
The pathogenesis of thrombocytosis in cancer involves production of interleukin-6 (IL-6) at least in some malignancies. In ovarian carcinoma, for example, thrombocytosis was significantly correlated with plasma levels of IL-6[14]. This was investigated in mice bearing ovarian cancer xenografts of human origin, where human IL-6 was found to stimulate hepatocytes via the IL-6 receptor, producing thrombopoietin. Authors proposed that ovarian cancer cells produce IL-6, which functions by stimulating mice liver to produce thrombopoietin, which in turn positively regulates megakaryocyte progenitors in the bone marrow[14]. In renal carcinoma, most examined cases were positive for IL-6 by immunohistochemistry[24]. Serum levels of IL-6 were also elevated in prostate and breast cancer patients[25,26]. Thus cancer cell-derived IL-6 is a trigger of tumor-induced thrombocytosis across various cancers.
The pathophysiology of platelets’ contribution to carcinogenesis involves a protective effect on circulating tumor cells from the attack of the immune system[27]. In addition platelets contribute to the attachment of tumor cells to endothelial cells at sites of metastases. Aggregates of platelets and tumor cells embolize in the microcirculation and facilitate the process of extravasation of tumor cells in metastatic sites. Platelets promote carcinogenesis by their normal function of promoting vascular integrity[28]. They protect the integrity of newly formed tumor vasculature which is prone to hemorrhage and prevent bleeding in tumor beds[29]. Platelets contain several types of active macromolecules and cytokines in their granules. These include vascular endothelial growth factor (VEGF), EGF, platelet-derived growth factor, hepatocyte growth factor, transforming growth factor β (TGFβ), IL-1β, IL-8, CXC motif containing ligand 12 and Sphingosine-1-phosphate[30,31]. These factors have the potential to contribute to metastatic tumor establishment and progression. For example, platelet-derived TGFβ promotes epithelial to mesenchymal transition program in tumor cells through Smad and NF-κB signaling[32]. This program provides epithelial cells with a mesenchymal phenotype that promotes mobility and metastases while protecting them from apoptosis due to lack of adhesion (anoikis)[33]. It has also been found that platelets from cancer patients have a higher VEGF level than platelets from patients without cancer[34]. Interestingly, serum VEGF is not consistently elevated in cancer, with the exception of renal carcinoma, if methods are adequate in order to prevent platelet activation during venipuncture[34]. As a result, platelet levels could provide a better reflection of VEGF concentrations in the primary and metastases sites micro-environment where they are activated and participatete to tumor angiogenesis.
In conclusion, this retrospective analysis of patients with stage II and stage III localized rectal cancer patients shows that higher platelets counts (defined in the current study as platelets more than 300 × 109/L) at the time of disease diagnosis has prognostic value regarding treatment effect outcome. Additionally, it was shown that pretreatment tumor CEA also has prognostic value regarding treatment effect outcome. Further study is needed in more extensive series to confirm these results, clarify survival prognostication value and to test whether thrombocytosis may be used as a predictive marker for specific therapies. It would be of particular interest to evaluate the role of thrombocytosis as a predictive element of anti-VEGF treatments. In this regard a study of metastatic renal cell cancer patients has shown that those with thrombocytosis have an increased probability for refractoriness to anti-VEGF treatments than patients with normal platelets counts[35]. Moreover, given the presumed role of platelets as protectors of circulating tumor cells from immune attack, an investigation into thrombocytosis as a predictive marker of response to the newly introduced immune checkpoint inhibitors may be worth pursuing.
COMMENTS
Background
Platelet counts are easily measurable laboratory values that are usually measured in all patients with a newly diagnosed cancer as part of a general evaluation.
Research frontiers
This paper proposes the evaluation of this easily available laboratory evaluation as part of the prognostic armamentarium in better defining the therapeutic prospects of rectal cancer patients.
Innovations and breakthrough
This is one of the first studies to specifically evaluate platelets as prognostic factors in neo-adjuvant treatment of newly diagnosed rectal cancer patients to be treated with neo-adjuvant therapy.
Applications
Platelet counts measurement could be used in the clinic to predict the effectiveness of neo-adjuvant cancer treatment.
Terminology
Platelet counts are part of the Complete Blood Count standard laboratory evaluation. Rectal cancers are adenocarcinomas of the terminal part of the colon below the peritoneal fold.
Peer-review
The manuscript is concise, clear, and comprehensive. The purpose, results, and conclusion are clearly stated. The manuscript provides new information and it induce new research.
Footnotes
Supported by the Sault Ste. Marie Academic Medical Association, Ontario, Canada to Voutsadakis IA.
Institutional review board statement: Institution Review Board approval has been obtained for this study.
Informed consent statement: Given that the study was retrospective in nature and the fact that treatments had been provided according to standards of care, no specific informed consents were needed or obtained by the individual patients. Anonymity was guaranteed.
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this study.
Data sharing statement: There are no additional data available for this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: June 29, 2016
First decision: August 10, 2016
Article in press: November 2, 2017
P- Reviewer: Nabi H, Palacios-Eito A S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
References
- 1.Behnke O, Forer A. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur J Haematol Suppl. 1998;61:3–23. doi: 10.1111/j.1600-0609.1998.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 2.Bleeker JS, Hogan WJ. Thrombocytosis: diagnostic evaluation, thrombotic risk stratification, and risk-based management strategies. Thrombosis. 2011;2011:536062. doi: 10.1155/2011/536062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 4.Voutsadakis IA. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol. 2014;6:34–40. doi: 10.4251/wjgo.v6.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 7.Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F. Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum. 1998;41:1033–1049. doi: 10.1007/BF02237397. [DOI] [PubMed] [Google Scholar]
- 8.Molinari C, Matteucci F, Caroli P, Passardi A. Biomarkers and Molecular Imaging as Predictors of Response to Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer. Clin Colorectal Cancer. 2015;14:227–238. doi: 10.1016/j.clcc.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Choi GS, Park JS, Park S, Kawai K, Watanabe T. Clinical significance of thrombocytosis before preoperative chemoradiotherapy in rectal cancer: predicting pathologic tumor response and oncologic outcome. Ann Surg Oncol. 2015;22:513–519. doi: 10.1245/s10434-014-3988-8. [DOI] [PubMed] [Google Scholar]
- 10.Nyasavajjala SM, Runau F, Datta S, Annette H, Shaw AG, Lund JN. Is there a role for pre-operative thrombocytosis in the management of colorectal cancer? Int J Surg. 2010;8:436–438. doi: 10.1016/j.ijsu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497–500. doi: 10.1001/archinte.1964.03860100079008. [DOI] [PubMed] [Google Scholar]
- 12.Ji Y, Sheng L, Du X, Qiu G, Su D. Elevated platelet count is a strong predictor of poor prognosis in stage I non-small cell lung cancer patients. Platelets. 2015;26:138–142. doi: 10.3109/09537104.2014.888547. [DOI] [PubMed] [Google Scholar]
- 13.Stravodimou A, Voutsadakis IA. Pretreatment thrombocytosis as a prognostic factor in metastatic breast cancer. Int J Breast Cancer. 2013;2013:289563. doi: 10.1155/2013/289563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot CV, Coward J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Digklia A, Voutsadakis IA. Thrombocytosis as a prognostic marker in stage III and IV serous ovarian cancer. Obstet Gynecol Sci. 2014;57:457–463. doi: 10.5468/ogs.2014.57.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int. 2000;86:203–207. doi: 10.1046/j.1464-410x.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106:887–891. doi: 10.1002/jso.23163. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36:192–200. doi: 10.1007/s00268-011-1329-7. [DOI] [PubMed] [Google Scholar]
- 19.Monreal M, Fernandez-Llamazares J, Piñol M, Julian JF, Broggi M, Escola D, Abad A. Platelet count and survival in patients with colorectal cancer--a preliminary study. Thromb Haemost. 1998;79:916–918. [PubMed] [Google Scholar]
- 20.Wan S, Lai Y, Myers RE, Li B, Hyslop T, London J, Chatterjee D, Palazzo JP, Burkart AL, Zhang K, et al. Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. J Gastrointest Cancer. 2013;44:293–304. doi: 10.1007/s12029-013-9491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai K, Kitayama J, Tsuno NH, Sunami E, Watanabe T. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int J Colorectal Dis. 2013;28:527–535. doi: 10.1007/s00384-012-1594-4. [DOI] [PubMed] [Google Scholar]
- 22.Yang KL, Yang SH, Liang WY, Kuo YJ, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS, Chang SC, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat Oncol. 2013;8:43. doi: 10.1186/1748-717X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han YD, Kim WR, Park SW, Cho MS, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Predictors of Pathologic Complete Response in Rectal Cancer Patients Undergoing Total Mesorectal Excision After Preoperative Chemoradiation. Medicine (Baltimore) 2015;94:e1971. doi: 10.1097/MD.0000000000001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paule B, Belot J, Rudant C, Coulombel C, Abbou CC. The importance of IL-6 protein expression in primary human renal cell carcinoma: an immunohistochemical study. J Clin Pathol. 2000;53:388–390. doi: 10.1136/jcp.53.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 26.Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2:311–315. doi: 10.3816/cbc.2002.n.008. [DOI] [PubMed] [Google Scholar]
- 27.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 28.Ho-Tin-Noé B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9 Suppl 1:56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho-Tin-Noé B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009;175:1699–1708. doi: 10.2353/ajpath.2009.090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunsilius E, Petzer A, Stockhammer G, Nussbaumer W, Schumacher P, Clausen J, Gastl G. Thrombocytes are the major source for soluble vascular endothelial growth factor in peripheral blood. Oncology. 2000;58:169–174. doi: 10.1159/000012095. [DOI] [PubMed] [Google Scholar]
- 32.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niers TM, Richel DJ, Meijers JC, Schlingemann RO. Vascular endothelial growth factor in the circulation in cancer patients may not be a relevant biomarker. PLoS One. 2011;6:e19873. doi: 10.1371/journal.pone.0019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heng DY, Mackenzie MJ, Vaishampayan UN, Bjarnason GA, Knox JJ, Tan MH, Wood L, Wang Y, Kollmannsberger C, North S, et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol. 2012;23:1549–1555. doi: 10.1093/annonc/mdr533. [DOI] [PMC free article] [PubMed] [Google Scholar]


