Abstract
Many patients with hepatocellular carcinoma (HCC) are diagnosed in an advanced stage, so they cannot be offered the option of curative treatments. The results of systemic chemotherapy are unsatisfactory and this has led to molecular targeted approaches. HCC develops in chronically damaged tissue due to cirrhosis in most patients. Several different cell types and molecules constitute a unique microenvironment in the liver, which has significant implications in tumor development and invasion. This, together with genome instability, contributes to a significant heterogeneity which is further enhanced by the molecular differences of the underlying causes. New classifications based on genetic characteristics of the tissue microenvironment have been proposed and key carcinogenic signaling pathways have been described. Tumor and adjacent tissue profiling seem biologically promising, but have not yet been translated into clinical settings. The encouraging first results with molecular - genetic signatures should be validated and clinically applicable. A more personalized approach to modern management of HCC is urgently needed.
Keywords: Systemic, Chemotherapy, Hepatocellular carcinoma, Prognosis
Core tip: The complete failure of chemotherapy in previous years gradually shifted hepatocellular carcinoma (HCC) treatment to the molecular targeted therapies. The initial-albeit limited - effectiveness of the currently approved systemic therapy, sorafenib, is due to the successful combination of targeting cancer cells and their microenvironment. Trials on drugs other than sorafenib, alone or in combination with drugs or transcatheter arterial chemoembolization were disappointing. Recently, genomic based analyses in HCC patients have proposed subclasses, based on molecular characteristics and a proliferative or non-proliferative genotypes. Combined targeted therapies, driven by specific molecular signatures for treatment selection and monitoring, potentially with immunotherapy, could be a future personalized approach.
INTRODUCTION
Hepatocellular carcinoma (HCC) represents globally the fifth most common cancer and is considered the third most frequent cause of cancer related death[1]. In recent years there has been a significant progress in clarifying pathogenesis, etiology, and risk factors for hepatocarcinogenesis. Understanding the importance of underlying cirrhosis in the majority of HCCs led to more integrated approach, as in the majority of cases we have to deal with two diseases, cirrhosis and cancer.
The adoption of the barcelona-clinic liver cancer (BCLC) classification[2,3] offered the opportunity to better categorize HCC patients and select the best treatment option according to tumor stage, degree of liver function impairment and patient characteristics. The outcomes for surgical resection have improved and specific factors, as tumor and liver function characteristics, are being taken into account before the patient is referred for an operation[4]. Moreover, the widespread application of the Milan criteria in the field of transplantation, has changed the transplant procedure from an experimental approach to a standard of care therapy for HCC, which can treat at the same time the tumor and the underlying pre-neoplastic process (namely cirrhosis)[5].
Despite screening patients at risk[6], adopting regular surveillance rules and the impressive improvements in imaging, still many patients with HCCs are diagnosed in an advanced stage, thus being ineligible for radical treatments [transplantation, resection or Radiofrequency ablation (RFA)] or even for ablative techniques [transcatheter arterial chemoembolization (TACE)] that can also provide survival benefit[7].
Patients with advanced HCC, especially if complicated with advanced cirrhosis, have a dismal prognosis. Several therapeutic efforts on this group of patients gave disappointing results in the past. The complete failure of systemic chemotherapy in previous years gradually shifted HCC treatment to the molecular targeted therapies. The first successful trials of sorafenib[8,9] provided a meaningful survival benefit in patients with advanced HCC, leaving at the same time many unresolved issues. This review attempts to present the effort of the scientific research to address the problem of HCC in multiple levels and to critically evaluate the inadequacies of the current trials of systemic treatments.
THE STORY OF NEAR-FAILED SYSTEMIC TREATMENTS
Initial approaches with systemic therapy were ineffective, as HCC is refractory to conventional chemotherapy and poorly tolerable in the context of liver cirrhosis due to altered drug metabolism and toxicity. Initial evidence for some efficacy of the anti-estrogen agent Tamoxifen in small trials were not confirmed in larger clinical trials and the drug has been abandoned[10].
More interesting data came in to light with clinical studies of Somatostatin and its long acting analogues for advanced HCC with very promising initial results[11,12], given the antiproliferative activity of the hormone and the positivity of HCC in somatostatin receptors in roughly 40% of the tumors[13]. Further publications have documented that somatostatin leads to apoptosis and has antineoplastic properties. Nevertheless, randomized trials - mainly from western countries - did not identify a clear survival benefit and this treatment is no longer recommended. There has been criticism for the methodology of these trials and the heterogeneity of selected patient population[14].
Sorafenib, the only currently approved systemic treatment, that demonstrated statistically significant improvement in overall survival and prolonged time to progression in two large randomized controlled trials (Sharp and Asian Pacific)[8,15]. The efficacy of Sorafenib has been attributed to blockade of multiple kinases, most of them involved in the VEGF, PDGF, c-Kit and B-Raf and p38 signaling pathways[16]. Despite the low response rates and the associated toxicity, the drug showed survival benefit in Child’s A patients with a good performance status.
The safety and efficacy of this treatment was further investigated in the Gideon trial (global phase IV, ongoing), focusing on patients with Child’s B that were under represented in the registration trials. The interim analysis showed better outcomes for patients on the full dose (800 mg) as compared to the reduced (400 mg) dose, without significant differences in safety profile[17,18]. However, the median life expectancy of patients under Sorafenib treatment is generally less than one year, and this clearly needs to be improved. For the time being there are no validated factors to predict effectiveness or the possibility of adverse effects[19].
More issues are still open, as what to do when the patient fails to respond or is intolerant to Sorafenib, or if Sorafenib could have a role as adjuvant treatment to other modalities like TACE. More data are expected and towards this direction is a recent study showed that tumor associated neutrophils (TAN) mediate the intratumoral infiltration of macrophages and Tregs by secreting the chemotactic C-C motif ligands CCL2 and CCL17. Thus neovascularization is being stimulated, and HCC growth and metastasis are promoted, all contributing to resistance to Sorafenib[20]. Thus, TAN infiltration is proposed as a potential biomarker.
Sunitinib, a potent multi-targeted receptor tyrosine kinase inhibitor of VEGFR, PDGFR, and c-KIT, reached to phase III study as compared to Sorafenib. The trial was terminated prematurely due to higher incidence of side effects in the sunitinib arm, besides demonstrating no superiority over sorafenib[21].
Brivanib is a potent and selective inhibitor of VEGFR and FGFR and pre-clinical studies have shown in vivo antitumor activity[22]. Three phase III studies have been conducted, yielding negative results. The BRISK-FL study tested the efficacy of Brivanib vs Sorafenib, in patients with advanced HCC without prior systemic treatment[23]. The BRISK-PS study tested Brivanib vs placebo in patients that failed or were intolerant to Sorafenib[24]. In both studies Brivanib failed to improve OS but it did improve time to tumor progression (TTP), indicating some anti-tumor activity. Due to these results, a phase III trial in which Brivanib was used as an adjuvant to TACE was terminated prematurely[25].
Linifanib is a multi-targeted receptor tyrosine kinase inhibitor effective on VEGFR and PDGFR. A phase III trial with 1035 patients comparing Sorafenib with Linifanib, showed similar overall survival in advanced HCC with a more favorable safety profile for Sorafenib; predefined superiority and non-inferiority overall survival boundaries were not met by Linifanib, which was more toxic than Sorafenib[26].
Erlotinib is an orally active inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase. A phase III randomized trial (SEARCH) with 720 HCC patients (Child A cirrhosis) were assigned to Sorafenib/Erlotinib or Sorafenib/placebo[27]. The median OS and TTP were similar in both groups, thus adding Erlotinib to Sorafenib did not improve survival, but increased toxicity instead.
Dovotinib, a VEGFR, PDGFR, FGFR inhibitor was compared head to head with Sorafenib, in a randomized study in the Asian-Pacific in patients with advanced HCC. Although Dovotinib was well tolerated, it failed to show greater efficacy than sorafenib, and thus there will be no phase III trial[28].
In patients who stopped Sorafenib due to disease progression or intolerance, a randomized phase III trial assessed Ramucirumab, a recombinant monoclonal IgG1 and VEGFR-2 blocking antibody (REACH). Despite acceptable safety profile, the study drug did not reached statistically significant survival benefit vs placebo[29]. However, a sub-population with αFP > 400 ng/mL might have benefited from this 2nd line treatment and this is explored in an ongoing trial. Recently Codrituzumab, a humanized monoclonal antibody against Glypican-3 which is expressed in HCC, was studied vs placebo in a phase II randomized trial without showing any clinical benefit[30].
Tivantinib is an oral selective small MET tyrosine kinase inhibitor with antitumor activity in MET-high patients. A phase II randomized placebo-controlled study in patients with advanced HCC, Child’s A score and intolerant or progressing under the first line treatment, showed some promising results on time to progression, but with notable neutropenia in some patients[31]. A phase III study in patients with advanced HCC expressing high levels of c-MET after Sorafenib failure is underway.
Mammalian target of rapamycin (mTOR) regulates cell growth, metabolism and aging in response to nutrients, cellular energy state and growth factors[32]. It is frequently up-regulated in cancer, including HCC, and is associated with poor differentiation and bad prognosis. Blocking this pathway appears an attractive option for HCC treatment. It is well known from the research on transplantation - given its immunosuppressive properties - that mTOR inhibitors (Sirolimus) are associated with better clinical outcomes in patients transplanted for HCC[33,34].
Preliminary data in the non-transplant setting with Sirolimus and Everolimus treatment in HCC patients were encouraging. In the EVOLVE-1 phase III study, patients with advanced HCC and failure/intolerance to Sorafenib, randomized to Everolimus or placebo[35]. Everolimus did not improve OS with no difference to TTP vs placebo. Moreover, Everolimus led to hepatitis B virus (HBV) reactivation in 37% of the cases despite preventive antiviral therapies. A recent phase II randomized trial of the combination of Everolimus with Sorafenib vs Sorafenib alone, in patients with advanced HCC with Child’s score ≤ 7, showed that the combination was not more beneficial; in contrast it was more toxic[36].
TACE is the treatment of choice for intermediate stage HCC. However, following TACE the hypoxic microenvironment promotes up-regulation of proangiogenic factors as VEGF and PDGF. This is the theoretical basis for the combination of TACE with drugs that inhibit angiogenesis, as Sorafenib and Birivanib. A recent review and meta-analysis reported that this combined approach may bring benefit to unresectable HCC in terms of TTP but not OS[37]. Recent studies (START, SOCRATES) that investigated the efficacy and safety of Sorafenib as an adjuvant to TACE displayed good tolerability and interesting response rate[38,39]. Clearly a better defined population of advanced HCC -that might have the maximal benefit from this approach- should be tested in clinical trial. Unfortunately, the recently published SPACE trial[40] showed that despite the combination of DC beads TACE with Sorafenib was feasible, this combination did not actually improve time to tumor progression in intermediate HCC.
Beyond TACE, efficacy and safety of Sorafenib was studied in a randomized phase III trial vs placebo, in patients with HCC after resection or local ablation (STORM trial)[41]. The recurrence free survival was identical in the two arms, whereas side effects were significantly more frequent in patients receiving Sorafenib in whom dose modification was necessary in 90% of the cases.
The combination of Sorafenib with other cytotoxic agents was tested to improve the disappointing results of conventional chemotherapy. In a phase II trial[42] the combination of Sorafenib/Doxorubicin was compared to Doxorubicin alone in Child-A cirrhotic patients with advanced HCC. The trial showed that the combination was better than doxorubicin alone as regards time to progression and overall survival. Whether there is benefit of the combination or this is an effect of sorafenib itself, will be clarified in an on-going phase III trial.
The efficacy and safety of GEMOX (Gemcitabin/Oxaliplatin) plus sorafenib, followed by sorafenib monotherapy was examined in a small trial with 49 patients diagnosed with advanced HCC[43]. This approach was found effective (overall survival 15.7 mo) with manageable toxicity, and these results should be validated in a larger controlled trial. The data of a subsequent phase II randomized study on this combination, as well as the results of a single arm phase II study combining sorafenib with oxaliplatin/capecitabin, showed modest synergistic effect[44]. Further combinations that were tested, such as sorafenib with EGFR inhibitors or with mTOR inhibitors, both failed to show any meaningful antitumor activity.
Finally, the combination of Sorafenib with Octreotide was tested in a phase II study, recruiting 50 patients with advanced HCC and Child-Pugh score A or B[45]. The combination was well tolerated and displayed TTP 7 mo and median overall survival 12 mo. Nevertheless these results have not been confirmed in a larger phase III study as yet. We believe that this combination could provide an option for patients with inadequate response or intolerance to sorafenib (Figure 1).
Figure 1.
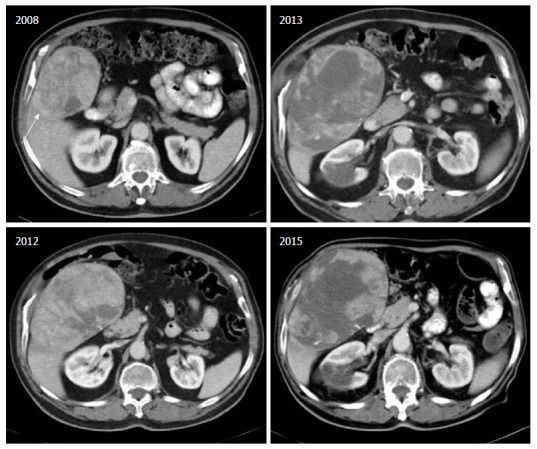
Serial computed tomography scans of a hepatocellular carcinoma patient with multiple co-morbidities precluding radical treatment, surviving 7 years with sequential approach in systemic treatment (Octreotide long acting release, followed by sorafenib). Despite an increase in tumor size, it is evident the central necrosis related to Sorafenib treatment (which was commenced when it became available).
The apparent failure of phase III trials beyond sorafenib, was disappointing but not discouraging for the scientific community (Table 1). Factors contributing to this failure and were related to drug toxicity (especially in cirrhotic patients), lack of significant antitumoural potency, lack of our understanding on diverse mechanisms of tumor progression and metastasis or biomarkers predictive of the efficacy of therapy[16]. Study design was another weak point for some trials. Trials in patients with advanced HCC should also pay attention to specific factors as portal vein invasion, the extrahepatic metastases, and the degree of liver impairment.
Table 1.
Randomized Phase III trials in advanced hepatocellular carcinoma
| Drug | n (patients) | OS (mo) | HR |
| SOR/Exp arm | |||
| First line completed (Sorafenib standard) | |||
| Brivanib | 1155 | 9.9/9.5 | 1.06 |
| Sunitinib | 1074 | 10.2/7.9 | 1.3 |
| Sorafenib/Erlotinib | 720 | 8.5/9.5 | 0.92 |
| Linifanib | 1035 | 9.8/9.1 | 1.04 |
| Second line completed (placebo standard) | |||
| Brivanib | 395 | 8.2/9.4 | 0.89 |
| Everolimus | 546 | 7.3/7.6 | 1.05 |
| Ramucirumab | 565 | 7.6/9.2 | 0.86 |
OS: Overall survival; SOR: Sorafenib arm.
EXPLORING THE ETIOPATHOGENESIS
Molecular and phenotypic diversity of HCC - Oncogenic pathways
Beyond the success and wide adoption of the BCLC system on staging and prognosis of HCC[46], recently new molecular classifications based on genetic characteristics of the tissue microenvironment have been proposed. However, HCC is a heterogeneous disease and each tumor is a result of unique combination of several genomic defects that lead to a significant diversity in the pathways of carcinogenesis. It is documented that several differences exist not only amongst different patients, but also between different tumor nodules in the same liver, and even differences in the same nodule.
Cancer cells and stem cells have similar capacity as regards self-renewal, indefinite division, and generation of heterogeneous cell population. The concept of cancer stem cell, referring to a subset of cells bearing stem cell characteristics that is indispensable for tumour development and perpetuation, has been recently adopted[47]. Cancer stem cells are now considered an important target for the eradication of HCC. Furthermore, a 20%-40% of HCC subtypes show progenitor signature suggesting that these tumours derive from liver progenitor cell. These subtypes are highly aggressive and correlate with early recurrence after treatment and metastatic potential, thus correlated to worse prognosis. CD133 antigen (prominin-1) has been identified as a cancer stem cell marker in various cancers, including HCC. Patients with increased CD133 levels have shorter overall survival and higher recurrence rates compared to those with low expression. Recent data showed that IL-6/STAT3 signalling induced CD133 expression, through function co-operation with NF-κΒ and hypoxia-inducible factor 1 alpha (HIF-1α) during hepatocarcinogensis[48] (Figure 2A).
Figure 2.
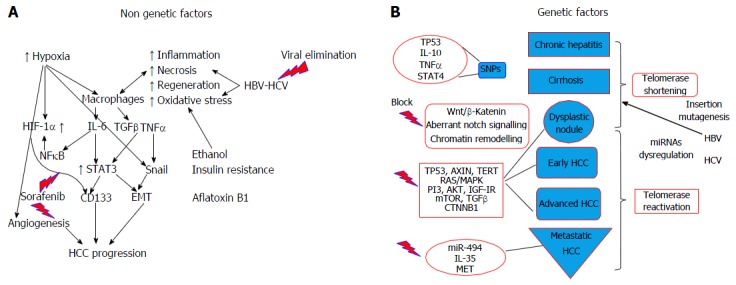
Interplay between genetic and non-genetic factors in the pathogenesis of hepatocellular carcinoma. Potential treatment targets. Hepatocellular carcinoma (HCC) is a complex entity with multifactorial pathogenesis. Control of non-genetic factors (A) (e.g., viral elimination, inhibition of CD133 positive cancer cell overexpression) may lead to alteration of the progress from cirrhosis to HCC. On the other hand, the various genetic irregularities (B) may lead to different HCC profiles with respect to invasiveness (miR-494) or response to treatment. New targeted treatments are also directed against Wnt/β-katenin. HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIF-1α: Hypoxia-inducible factor 1 alpha; TNF: Tumor necrosis factor; IL-6: Interleukin-6; NFκΒ: Nuclear factor-kappa B.
Recently genomic based analyses in HCC patients have identified subclasses, based on molecular characteristics and proliferative and non-proliferative genotypes have been proposed[49]. The proliferative subclass - which is associated with a poor outcome - has been linked to the activation of RAS, mTOR, and/or IGF signaling. This has been further categorized into two phenotypic groups: The Wnt/TGF-β group (activation of these pathways) and the progenitor cell group. In the former, the activation of the Wnt and the TGF-β was the predominant feature, while the latter was enriched in progenitor cell, epithelial cell adhesion molecule and cytoskeletal markers and was associated with increased α-fetoprotein at early stages[50]. On the other hand, the non-proliferative subclass was a heterogeneous one, with patients sharing only β-katenin in their molecular profiles[51]. The prognostic implications of these subclasses have been studied but there is no consensus on it and there is no translational clinical research has been done yet.
The paradigm from the management of other cancers such as colorectal cancer and non-small lung cell carcinoma, where mutations of K-Ras and EGFR drive the therapeutic choices, supports this new approach[52]. Unfortunately, HCC is still away from this path despite the success of sorafenib, a multi kinase inhibitor, which seems a proof towards the right direction. A key point may be that in HCC an average of 30-40 mutations were estimated per tumor, with 5-8 of them being the driver mutations[49] affecting cellular homeostasis and involved in the development of malignant phenotype.
A recent elegant study performing exome sequencing analysis of 243 surgically resected HCCs revealed mutational signatures associated with specific risk factors, as combined tobacco and alcohol use, or aflatoxin B1. The researchers identified 161 putative driver genes associated with 11 recurrent pathways[53]. Moreover, a molecular 5 gene score (based on combined expression level of HN1, RAN, RAMP3, KRT19, and TAF9) was studied in surgical resected samples of 314 HCC, and was found significantly associated with outcomes[54]. Also recent data show that it is possible to modulate gene expression profiles (interfering with histone acetylation) and thus increase the sensitivity to chemotherapeutic agents[55].
Activation of telomerase is the earliest and most frequent alteration in the process of HCC development (mutations in TERT promoter in 60% - most frequently mutated gene - associated with increased telomerase expression)[56]. Genes as TP53 and CTNNB1 are also frequently mutated in HCC, whereas inactivating mutations in TP53 are commonly found (especially with HBV etiology). Recently identified alterations in genes encoding metabolic enzymes, chromatin remodelers and a high rate of mTOR pathway activations could offer potential therapeutic targets[57]. Members of the Wnt pathway (crucial for hepatocarcinogensesis) are involved in the process of cell differentiation, which is frequently altered in cancer cells, whereas failure to control oxidative stress can favor additional DNA mutations and cellular damage.
Key carcinogenic signaling pathways have been described for HCC: Wnt/β-katenin [that can be triggered via both catenin β1 (CTNNB1)-dependent and CTNNB1 independent pathways][58], a proliferation and hepatoblastoma-like pathway[59]. Nevertheless, their molecular signature is broad and for the time being this knowledge is unlikely to have clinical application. Notch signaling is important for normal liver development and aberrant Notch signaling is related to hepatocarcinogenesis (Figure 2B). Chromatin remodeling is important for the maintenance of DNA integrity, which is in turn crucial for cellular homeostasis. Aberrant chromatin remodeling has been implicated with HCC pathogenesis[57] as well as genes that are involved in oxidative stress (which induces mutations).
In respect to the receptor signaling pathways, RAS/MAPK pathway is activated in all patients with advanced HCC and in a large proportion of those with an early stage HCC[60]. PI3/AKT/mTOR and MAPK pathways related to proliferation, apoptosis and survival, as well as pro inflammatory cytokines (IL1, TNFα) and growth factors, such as TGFβ (tumor stroma, progression, metastasis), are potential future clinical targets in HCC therapeutics (Figure 2B).
Very recently, IL-35 expression was found to correlate with HCC aggressiveness, conferring the rational for another novel therapeutic target[61]. IFG-1R signalling is activated in a proportion of patients with HCC and its targeting had demonstrated antitumor activity in experimental models; however a phase II trial with an anti-IFG-1R monoclonal antibody did not show clinical benefit in unselected patients[62]. Finally, dysregulation of MET receptor and its ligand HGF, are crucial for hepatocyte regeneration after liver injury and are common events in HCC patients[49]. Activation of MET is found in half of advanced HCCs, and this pathway is currently tested in clinical trials. A MET inhibitor, cabozantinib, was found to suppress tumour growth and metastasis in a phase II study[63] and is further tested in a phase III second line clinical trial (in patients with high MET expression, treated with tivantinib).
Tissue microenvironment and the role of cirrhosis
Scientific basis: Chronic liver injury triggers a sequence of cell death, inflammation, compensatory regeneration and genetic damage, which drives the development of HCC. In the majority of cases, HCC develops in chronically damaged tissue due to cirrhosis-irrespective of etiology - whereas the other malignancies develop on an otherwise healthy tissue. This, together with genome instability, contributes to a significant heterogeneity which is further enhanced by the molecular differences of the underlying causes, i.e., viral, alcohol, metabolic[52]. Moreover, epithelial plasticity is an important parameter in HCC, as strong inducers of epithelial to mesenchymal transition like TGFβ are able to co-ordinate both fibrogenesis and carcinogenesis, showing rising cytokine levels in cirrhosis as well as late stage HCC[64].
Several different cell types and molecules constitute a microenvironment in the liver, which has significant implications in tumor development and invasion. Myeloid cells, including macrophages and neutrophils are the most abundant cells in the tumor microenvironment[65]. Tumor-associated macrophages acquire protumorigenic properties in primary and metastatic sites and support cancer development and progression, by stimulating cell proliferation and survival, angiogenesis, invasive behavior and suppression of cytotoxic T lymphocytes responses[66]. Tumor-associated neutrophils exhibit both antitumoral and protumoral functions. Dendritic cells, the main type of antigen presenting cells, play an important role in T cell priming. The generation and protective antitumour immunity depends on dendritic cell maturation and antigen presentation[67].
It is generally accepted that dysregulated microenvironment affects tumorigenesis, based on the concept that chronic inflammation is associated with cancer[68]. Moreover, the stromal microenvironment has been recognized as a crucial element for cancer metastasis in general. A reasonable hypothesis is that an altered liver microenvironment, through reprogramming of the inflammatory milieu, may contribute to hepatocarcinogenesis, taking in account that HCC is an inflammation-associated cancer[69]. This microenvironment plays a major role in anti-tumor immunity.
Therapeutic implications: The effectiveness of the currently approved systemic therapy, sorafenib, is due to the successful combination of targeting cancer cells and their microenvironment, as a result of multiple kinases inhibition. Between sorafenib targets, an increasing amount of evidence has suggested that HSC are key regulators of hepatocarcinogenesis through a variety of mechanisms, including direct effects on malignant hepatocytes, and indirect via modulation of the peri-tumoral stroma and immune responses[70]. Moreover, activated stellate cells produce extracellular matrix.
Laminin-332 is produced and excreted by these cells in HCC but not in the surrounding non-neoplastic liver; this stimulates chemotaxis and migration of HCC cells in experimental models and promotes proliferation as well[52]. An association between Ln-332 and Keratin -19 has been documented, the latter being a marker of cholangiocytes[71].
VEGF not only regulates tumor angiogenesis but also has important immunomodulatory functions. It inhibits dendritic cell maturation in vitro and in vivo, through activation of NFκB. Additionally VEGF may regulate T-cell differentiation and its cytotoxic function and can enhance expression of immune checkpoint molecules[72]. This provides the rational of combining anti-VEGF therapy with checkpoint inhibitors.
Another area of active research is on the effect of mTOR inhibitors on advanced HCC in the non-transplant setting. Recent data showed that mTOR inhibition improves FGFR targeting[73] and reduces the activity level of Golgi protein 73, which is a serum marker for HCC[74]. However, the first results of trials with mTOR inhibitors were less than encouraging. Despite potential applications, the role of the whole tissue microenvironment is difficult to be reduced to the effect of just one molecule or protein.
Immunity and implications
Scientific basis: Inflammation affects every single step of tumourigenesis from initiation, to tumor promotion and metastatic progress. Cancer development and its response to treatment are significantly influenced by innate and adaptive immunity, which either promote or attenuate tumourigenesis[66]. Various types of immune and inflammatory cells are present within tumours; these affect malignant cells through production of cytokines, chemokines, growth factors, prostaglandins and reactive oxygen and nitrogen species[68].
The liver has been considered as an immunologically advantaged organ. A profound clinical paradigm is the development of tolerance in the context of transplantation. It is equipped with several myeloid and non-myeloid cell populations which affect both innate and adaptive responses in physiological conditions as well as in the context of defense against tumors[75].
Kupffer cells represent the largest macrophage population in the human body and together with sinusoidal endothelial and hepatic stellate cells, play a critical role in physiology and disease. Local immunosuppression by these cells is induced by pro-inflammatory cytokines[69] whereas different immune cell subtypes have been related to antitumor immunity in HCC. Kupffer cells in analogy to the two subtypes of macrophages are now characterized as M1 and M2 types. In the case of HCC M2 cells are detrimental and M1 demonstrate anti-tumoral activity, contrary to the opposite effects of those cell subpopulations have in inflammation.
Among immunosuppressive cell populations, myeloid derived suppressor cells and T regulatory cells have the key role in cancer immunosurveillance[76]. A prominent humoral cytokine profile occurs in metastatic liver milieu and a shift towards anti-inflammatory/ immunosuppressive responses is significant for HCC metastases[77].
Therapeutic implications: The liver is a privileged organ with respect to immune function and possesses a unique form of immune regulation: Tolerance is induced to avoid chronic inflammation caused by antigens coming from the portal vein blood. This may hampers an effective immune response against cancer cells[78]. Moreover this is a challenge on the use of conventional immunotherapy is challenged. Immunotherapy trials have so far given suboptimal results. On the other hand, spontaneous immune responses as well as tumor regression have been reported in relation to systemic inflammatory responses[79]. This could as well be a result of M1 effect as previously mentioned.
Adaptive immune responses are well described in various conventional HCC treatments and are related to their effects. This has been extensively investigated in patients undergoing ablative therapies (TACE, RFA), and provide the theoretical basis for combined approaches. This applies to cytotoxic agents as well, and experience with sorafenib in experimental and clinical level is a paradigm.
While growing tumors acquire mutations, some of which create neoantigens that influence the response of patients to immune checkpoint inhibitors[80]. There are other studies supporting that cancers with high rate of somatic mutations respond best to immune check point blockade by triggering tumor rejection via activation of cytotoxic T-lymphocytes, a recent approach with acknowledged success in recent years in melanoma and non-small cell lung cancer[81].
Preclinical and clinical studies have shown potential benefit of modulating immunogenicity of HCC and relevant approaches are currently being tested[82]. The rational to target immune-checkpoints is based on data that HCCs may evade the immune system by expressing molecules as PD-1, CTLA-4, TIM-3, LAG-3 and many more. Despite the fact that the blockade of PD-1 and CTLA-4 is already providing encouraging results in initial trials, overall the therapeutic relevance of blocking these agents is unclear[72].
CONCLUSION
HCC is one of the most lethal cancers and management still deems ineffective. Apart from the problems in prevention or early diagnosis, there are no persuasive answers for those (many) patients with advanced neoplasms. Systemic treatment was disappointing in the past, somehow improved with Sorafenib but with many weaknesses and grey zones, whereas the trials of new compounds beyond Sorafenib provided suboptimal results.
The complexity and heterogeneity of HCC pathogenesis is disregarded in treatment decisions. Is a personalized approach feasible with the limitations of current knowledge? Tumor and adjacent tissue profiling seems biologically significant, but not yet translated into the clinical setting. The role of liquid biopsy, i.e., detection of circulating tumor cells, a hot topic in tumor biology is also inadequately explored in the case of HCC.
Nevertheless, encouraging first results with molecular - genetic signatures are promising towards -at least-prognosis. Additionally, miRNAs which are important regulators of gene expression, have been associated with the occurrence of HCC. In addition, miRNAs are of potential value not only in diagnosis but also in the management of HCC.
Clinical scoring systems incorporating molecular profile characteristics, may better stratify patients at risk for HCC but further prospective validation is needed. The ideal future approach would be combined targeted therapies - driven by specific molecular signatures for the selection and the monitoring during treatment- potentially incorporating immunotherapeutic modalities, such as vaccination and/or check-point blockade.
ACKNOWLEDGMENTS
We would like to thank Professor George Germanidis and Dr. Ioannis Drygiannakis, for reading and providing valuable comments on this paper, and Dr. Maria Daskalogiannaki for providing the computed tomography scans.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflict of interest in relation to this paper.
Peer-review started: July 18, 2016
First decision: August 26, 2016
Article in press: November 22, 2016
P- Reviewer: Gatselis NK, Ratnasari N, Sunami Y S- Editor: Qi Y L- Editor: A E- Editor: Li D
References
- 1.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Au JS, Frenette CT. Management of Hepatocellular Carcinoma: Current Status and Future Directions. Gut Liver. 2015;9:437–448. doi: 10.5009/gnl15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon KV, Hakeem AR, Heaton ND. Review article: liver transplantation for hepatocellular carcinoma - a critical appraisal of the current worldwide listing criteria. Aliment Pharmacol Ther. 2014;40:893–902. doi: 10.1111/apt.12922. [DOI] [PubMed] [Google Scholar]
- 6.Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, Muzikansky A, Clark JW, Kwak EL, Schrag D, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–535. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Nowak AK, Stockler MR, Chow PK, Findlay M. Use of tamoxifen in advanced-stage hepatocellular carcinoma. A systematic review. Cancer. 2005;103:1408–1414. doi: 10.1002/cncr.20963. [DOI] [PubMed] [Google Scholar]
- 11.Kouroumalis E, Skordilis P, Thermos K, Vasilaki A, Moschandrea J, Manousos ON. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998;42:442–447. doi: 10.1136/gut.42.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samonakis DN, Moschandreas J, Arnaoutis T, Skordilis P, Leontidis C, Vafiades I, Kouroumalis E. Treatment of hepatocellular carcinoma with long acting somatostatin analogues. Oncol Rep. 2002;9:903–907. [PubMed] [Google Scholar]
- 13.Abdel-Rahman O, Lamarca A, Valle JW, Hubner RA. Somatostatin receptor expression in hepatocellular carcinoma: prognostic and therapeutic considerations. Endocr Relat Cancer. 2014;21:R485–R493. doi: 10.1530/ERC-14-0389. [DOI] [PubMed] [Google Scholar]
- 14.Samonakis DN, Notas G, Christodoulakis N, Kouroumalis EA. Mechanisms of action and resistance of somatostatin analogues for the treatment of hepatocellular carcinoma: a message not well taken. Dig Dis Sci. 2008;53:2359–2365. doi: 10.1007/s10620-007-0175-9. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 17.Di Marco V, De Vita F, Koskinas J, Semela D, Toniutto P, Verslype C. Sorafenib: from literature to clinical practice. Ann Oncol. 2013;24 Suppl 2:ii30–ii37. doi: 10.1093/annonc/mdt055. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609–617. doi: 10.1111/ijcp.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo M. Biomarkers and Personalized Sorafenib Therapy. Liver Cancer. 2014;3:399–404. doi: 10.1159/000343870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Gao J. Advances in the study of molecularly targeted agents to treat hepatocellular carcinoma. Drug Discov Ther. 2014;8:154–164. doi: 10.5582/ddt.2014.01031. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Yang J, Lu L, Tak WY, Yu X, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60:1697–1707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 26.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 28.Cheng AL, Thongprasert S, Lim HY, Sukeepaisarnjaroen W, Yang TS, Wu CC, Chao Y, Chan SL, Kudo M, Ikeda M, et al. Randomized, Open-Label Phase 2 Study Comparing Frontline Dovitinib vs Sorafenib in Patients With Advanced Hepatocellular Carcinoma. Hepatology. 2016;64:774–784. doi: 10.1002/hep.28600. [DOI] [PubMed] [Google Scholar]
- 29.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 30.Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Ross P, Peron JM, Ebert O, Chan S, et al. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol. 2016;65:289–295. doi: 10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 32.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60:855–865. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411–419. doi: 10.1111/apt.12185. [DOI] [PubMed] [Google Scholar]
- 34.Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 35.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 36.Koeberle D, Dufour JF, Demeter G, Li Q, Ribi K, Samaras P, Saletti P, Roth AD, Horber D, Buehlmann M, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29) Ann Oncol. 2016;27:856–861. doi: 10.1093/annonc/mdw054. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, Han G. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One. 2014;9:e91124. doi: 10.1371/journal.pone.0091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao Y, Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458–1467. doi: 10.1002/ijc.29126. [DOI] [PubMed] [Google Scholar]
- 39.Erhardt A, Kolligs F, Dollinger M, Schott E, Wege H, Bitzer M, Gog C, Lammert F, Schuchmann M, Walter C, et al. TACE plus sorafenib for the treatment of hepatocellular carcinoma: results of the multicenter, phase II SOCRATES trial. Cancer Chemother Pharmacol. 2014;74:947–954. doi: 10.1007/s00280-014-2568-8. [DOI] [PubMed] [Google Scholar]
- 40.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim do Y, Chau GY, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 42.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Yue H, Xu S, Wang F, Ma N, Li K, Qiao L, Wang J. First-line gemcitabine and oxaliplatin (GEMOX) plus sorafenib, followed by sorafenib as maintenance therapy, for patients with advanced hepatocellular carcinoma: a preliminary study. Int J Clin Oncol. 2015;20:952–959. doi: 10.1007/s10147-015-0796-5. [DOI] [PubMed] [Google Scholar]
- 44.Hollebecque A, Malka D, Ferté C, Ducreux M, Boige V. Systemic treatment of advanced hepatocellular carcinoma: from disillusions to new horizons. Eur J Cancer. 2015;51:327–339. doi: 10.1016/j.ejca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Prete SD, Montella L, Caraglia M, Maiorino L, Cennamo G, Montesarchio V, Piai G, Febbraro A, Tarantino L, Capasso E, et al. Sorafenib plus octreotide is an effective and safe treatment in advanced hepatocellular carcinoma: multicenter phase II So.LAR. study. Cancer Chemother Pharmacol. 2010;66:837–844. doi: 10.1007/s00280-009-1226-z. [DOI] [PubMed] [Google Scholar]
- 46.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 47.Oishi N, Yamashita T, Kaneko S. Molecular biology of liver cancer stem cells. Liver Cancer. 2014;3:71–84. doi: 10.1159/000343863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiberger T, Chen Y, Ramjiawan RR, Hato T, Fan C, Samuel R, Roberge S, Huang P, Lauwers GY, Zhu AX, et al. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat Protoc. 2015;10:1264–1274. doi: 10.1038/nprot.2015.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 50.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 51.Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell P, Tsai HW, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giannelli G, Rani B, Dituri F, Cao Y, Palasciano G. Moving towards personalised therapy in patients with hepatocellular carcinoma: the role of the microenvironment. Gut. 2014;63:1668–1676. doi: 10.1136/gutjnl-2014-307323. [DOI] [PubMed] [Google Scholar]
- 53.Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nault JC, De Reyniès A, Villanueva A, Calderaro J, Rebouissou S, Couchy G, Decaens T, Franco D, Imbeaud S, Rousseau F, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145:176–187. doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 55.Gillet JP, Andersen JB, Madigan JP, Varma S, Bagni RK, Powell K, Burgan WE, Wu CP, Calcagno AM, Ambudkar SV, et al. A Gene Expression Signature Associated with Overall Survival in Patients with Hepatocellular Carcinoma Suggests a New Treatment Strategy. Mol Pharmacol. 2016;89:263–272. doi: 10.1124/mol.115.101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226–1239.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 57.Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267–1273. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 58.Liu LJ, Xie SX, Chen YT, Xue JL, Zhang CJ, Zhu F. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J Gastroenterol. 2016;22:7486–7499. doi: 10.3748/wjg.v22.i33.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH, Melgar-Lesmes P, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu YP, Yi Y, Cai XY, Sun J, Ni XC, He HW, Wang JX, Lu ZF, Huang JL, Cao Y, et al. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br J Cancer. 2016;114:767–776. doi: 10.1038/bjc.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L, et al. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60:319–324. doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang Q, Chen W, Ren M, Wang J, Zhang H, Deng DY, Zhang L, Shang C, Chen Y. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20:2959–2970. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]
- 64.Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808. doi: 10.1016/j.jhep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut. 2015;64:842–848. doi: 10.1136/gutjnl-2014-307990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson AI, Conroy KP, Henderson NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015;15:63. doi: 10.1186/s12876-015-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, Katoonizadeh A, Wouters J, van Kempen LC, Durnez A, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63:674–685. doi: 10.1136/gutjnl-2012-304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61:1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheller T, Hellerbrand C, Moser C, Schmidt K, Kroemer A, Brunner SM, Schlitt HJ, Geissler EK, Lang SA. mTOR inhibition improves fibroblast growth factor receptor targeting in hepatocellular carcinoma. Br J Cancer. 2015;112:841–850. doi: 10.1038/bjc.2014.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, Wang Y, Tao J, Shi Y, Gai X, Huang F, Ma Q, Zhou Z, Chen H, Zhang H, et al. mTORC1 Up-Regulates GP73 to Promote Proliferation and Migration of Hepatocellular Carcinoma Cells and Growth of Xenograft Tumors in Mice. Gastroenterology. 2015;149:741–752.e14. doi: 10.1053/j.gastro.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Wirth TC. Spontaneous and therapeutic immune responses in hepatocellular carcinoma: implications for current and future immunotherapies. Expert Rev Gastroenterol Hepatol. 2014;8:101–110. doi: 10.1586/17474124.2014.862497. [DOI] [PubMed] [Google Scholar]
- 76.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 78.Aerts M, Benteyn D, Van Vlierberghe H, Thielemans K, Reynaert H. Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J Gastroenterol. 2016;22:253–261. doi: 10.3748/wjg.v22.i1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB (Oxford) 2012;14:500–505. doi: 10.1111/j.1477-2574.2012.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]


