Abstract
C1q/TNF-related protein 1 (CTRP1) is a conserved plasma protein of the C1q family with notable metabolic and cardiovascular functions. We have previously shown that CTRP1 infusion lowers blood glucose and that transgenic mice with elevated circulating CTRP1 are protected from diet-induced obesity and insulin resistance. Here, we used a genetic loss-of-function mouse model to address the requirement of CTRP1 for metabolic homeostasis. Despite similar body weight, food intake, and energy expenditure, Ctrp1 knockout (KO) mice fed a low-fat diet developed insulin resistance and hepatic steatosis. Impaired glucose metabolism in Ctrp1 KO mice was associated with increased hepatic gluconeogenic gene expression and decreased skeletal muscle glucose transporter glucose transporter 4 levels and AMP-activated protein kinase activation. Loss of CTRP1 enhanced the clearance of orally administered lipids but did not affect intestinal lipid absorption, hepatic VLDL-triglyceride export, or lipoprotein lipase activity. In contrast to triglycerides, hepatic cholesterol levels were reduced in Ctrp1 KO mice, paralleling the reduced expression of cholesterol synthesis genes. Contrary to expectations, when challenged with a high-fat diet to induce obesity, Ctrp1 KO mice had increased physical activity and reduced body weight, adiposity, and expression of lipid synthesis and fibrotic genes in adipose tissue; these phenotypes were linked to elevated FGF-21 levels. Due in part to increased hepatic AMP-activated protein kinase activation and reduced expression of lipid synthesis genes, Ctrp1 KO mice fed a high-fat diet also had reduced liver and serum triglyceride and cholesterol levels. Taken together, these results provide genetic evidence to establish the significance of CTRP1 to systemic energy metabolism in different metabolic and dietary contexts.
Keywords: adipokine, fatty liver, obesity, diabetes, cholesterol, lipids, C1q/tumor necrosis factor-related protein 1
secreted hormones control energy metabolism via interorgan cross-talk, and their circulating levels are frequently dysregulated in the pathophysiological states of obesity and diabetes. In an effort to uncover novel metabolic regulators, we characterized C1q/TNF-related proteins (CTRP1–CTRP15), a highly conserved family of secreted proteins (4, 56–58, 61–63). Distinct and notable metabolic (4, 5, 10, 38–42, 46, 55, 56), cardiovascular (19, 20, 48, 50, 53, 64, 71, 72, 74), and inflammatory (35, 45) functions have been demonstrated for several members of this protein family based on in vivo functional studies. In vitro studies have also highlighted the involvement of CTRP11 in adipogenesis (58) and the role of CTRP11 in antagonizing lipid-induced insulin resistance (57).
Similar to many CTRP family members, CTRP1 has a distinct expression profile, with the highest expression levels seen in adipose tissue (62). Adipose expression of CTRP1 and its circulating levels are modulated by the metabolic and inflammatory states of animals (21, 62). Its expression is upregulated by the antidiabetic drug rosiglitazone as well as in animals lacking the insulin-sensitizing hormone adiponectin (62). Consistent with a metabolic role, administration of recombinant CTRP1 to wild-type (WT) mice acutely lowers blood glucose (62), and chronic overexpression of CTRP1 in transgenic mice enhances AMP-activated protein kinase (AMPK) activation and skeletal muscle fat oxidation while attenuating insulin resistance induced by high-fat feeding (12, 38).
The physiological relevance of CTRP1 in the context of disease is highlighted by recent studies in humans with metabolic disorders. Circulating levels of CTRP1 are elevated in patients with type 2 diabetes and metabolic syndrome (7, 37, 65) as well as in patients with coronary artery disease (47, 73) and hypertension (16). Whether the observed upregulation of plasma CTRP1 seen in humans is a cause or a consequence of the disease remains to be established. In support of the notion that CTRP1 upregulation represents physiological compensation, mice lacking CTRP1 protein have increased myocardial infarct size, cardiomyocyte apoptosis, and proinflammatory gene expression induced by ischemia-reperfusion injury, whereas systemic delivery of CTRP1 attenuated myocardial damage (72). In contrast, in an apolipoprotein E-deficient mouse model, CTRP1 appears to play an adverse role in promoting atherosclerosis, and its deficiency attenuates disease severity (27). While earlier studies have demonstrated a positive metabolic role for CTRP1 (12, 38), the physiological consequence of its deficiency on glucose and lipid metabolism has not been described. Given the significant caveats and limitations associated with previous recombinant protein infusion and transgenic overexpression studies, we aimed to provide genetic evidence, using a knockout (KO) mouse model, that CTRP1 is indeed required for metabolic homeostasis.
MATERIALS AND METHODS
Animals.
The Ctrp1 KO (−/−) mouse strain used for this research project (B6;129S5-C1qtnf1tm1Lex/Mmucd, identification no. 032164-UCD) was obtained from the Mutant Mouse Regional Resource Center, a National Institutes of Health-funded strain repository, and was donated to the Mutant Mouse Regional Resource Center by Genentech. The Ctrp1 gene is located on mouse chromosome 11 and comprises four exons. The largest exon, exon 4 (which codes for 61% of the full-length protein), was targeted by homologous recombination. A total of 679 bp, spanning the coding region of exon 4 and a portion (162 bp) of the 3′-untranslated region, was deleted. Heterozygous mice were recovered from cryopreserved embryos. Since the embryonic stem cells were derived from the 129S5/Sv mouse strain, we backcrossed Ctrp1 KO mice to the C57BL/6J genetic background for more than six generations. Ctrp1 KO mice were viable and fertile. Genotyping primers for the Ctrp1 WT allele were as follows: forward (DNA063-1) 5′-GGTTCTACAGGTCCCAGGG-3′ and reverse (DNA063-2) 5′-GTGATGTAGGTGTCGAACTCG-3′. The expected size of the WT amplification product was 458 bp. Genotyping primers for the Ctrp1 KO allele were as follows: forward (Neo-3a) 5′-GCAGCGCATCGCCTTCTATCG-3′ and reverse (DNA063-31) 5′-GGAAGTCCCTCTCACGTGTC-3′. The expected size of the KO amplification product was 1,100 bp. To confirm the presence or absence of Ctrp1 mRNA in the adipose tissue of WT and KO mice, we performed semiquantitative PCR analysis using the following primer pair: forward 5′-GTGAGGACCTCCCCACTTCT-3′ and reverse 5′-GACCAGGTAGCCACTGAAGG-3′. The expected size of the amplification product was 632 bp. All Ctrp1 KO (−/−) and WT (+/+) littermate controls used in this study were generated by intercrossing Ctrp1 heterozygous (+/−) mice. Male and female Ctrp1 KO mice and WT littermate controls were housed in polycarbonate cages on a 12:12-h light-dark photocycle with ad libitum access to water and food. Mice were fed a high-fat diet (HFD; 60% kcal derived from fat, D12492, Research Diets) or a matched control low-fat diet (LFD; 10% kcal derived from fat, D12450B, Research Diets). Diet was provided for a period of 24 wk, beginning at 6 wk of age. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University School of Medicine.
CTRP1 ELISA.
An ELISA specific for mouse CTRP1 was obtained from BioVendor R&D. The assay was carried out according to the manufacturer's instructions.
Body composition analysis.
Body composition analyses for fat and lean mass were performed on mice at 19–24 wk using Echo-MRI-100 (Echo Medical Systems, Waco, TX) at The Johns Hopkins University School of Medicine mouse phenotyping core facility. Lean mass was used to normalize the indirect calorimetry data.
Indirect calorimetry.
LFD-fed and HFD-fed WT and Ctrp1 KO mice at 19–24 wk of age were used for simultaneous assessments of daily body weight change, food intake (corrected for spillage), physical activity, and whole body metabolic profile in the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments). Data were collected for 4 days to confirm that mice were acclimated to the calorimetry chambers (indicated by stable body weights, food intake, and diurnal metabolic patterns), and data were analyzed from the fourth day. Rates of oxygen consumption (V̇o2; normalized to ml·lean kg−1·h−1) and carbon dioxide production (V̇co2; in ml·lean kg−1·h−1) in each chamber were measured every 24 min throughout the experiments. The respiratory exchange ratio (RER = V̇co2/V̇o2) was calculated by Comprehensive Laboratory Animal Monitoring System software (version 4.02) to estimate the relative oxidation of carbohydrates (RER = 1.0) versus fats (RER ≈ 0.7), not accounting for protein oxidation. Energy expenditure was calculated as follows: energy expenditure = V̇o2 × [3.815 + (1.232 × RER)] (32) and normalized for lean body mass (in kcal·lean kg−1·h−1) as recommended (3). Physical activities were measured by infrared beam breaks in the metabolic chamber. Average metabolic values were calculated per mouse and averaged across mice for statistical analysis by a Student's t-test.
Intraperitoneal glucose and insulin tolerance test.
Mice were fasted for 6 h before glucose injection. Glucose was injected intraperitoneally into mice at a dose of 1 mg/g body wt. Blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose injection using a glucometer (NovaMax Plus, Billerica, MA). Fasting serum insulin levels were measured using an ELISA kit (Millipore, Billerica, MA). For insulin tolerance tests, food was removed 2 h before insulin injection. Insulin was injected intraperitoneally at a dose of 0.75 U/kg body wt for LFD-fed mice and 1 or 1.5 U/kg body wt for HFD-fed mice, and blood glucose was measured at 0, 15, 30, 60, and 90 min after insulin injection as described above. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated based on fasting glucose and insulin concentrations as follows: HOMA-IR = [fasting glucose (in mM) × fasting insulin (in μU/ml)/22.5] (30). This surrogate index provides a reasonable approximation of the degree of insulin resistance and has been validated against the reference standard glucose clamp for rats (6) and mice (24).
Lipid tolerance test.
For lipid tolerance tests, mice were fasted for 12 h and then gavaged with 20% emulsified Intralipid (soybean oil, Sigma, 10 μl/g body wt). Sera were collected via tail bleed using a Microvette CB 300 (Sarstedt) at 0, 1, 2, 3, and 4 h post-gavage. Serum levels of nonesterified free fatty acids (NEFA) and triglycerides (TG) were quantified using kits from Wako Diagnostics and Infinity Triglycerides (Thermo Scientific), respectively.
Hepatic VLDL-TG quantification.
To measure the hepatic VLDL-TG production rate, a separate cohort of LFD-fed WT and Ctrp1 KO mice was given an intraperitoneal injection of 1,000 mg/kg Poloxamer 407 (Sigma) in saline ∼4 h into the light cycle, as previously described by Millar et al. (33) and our previous study (40). Poloxamer 407 inhibits lipoprotein lipase (LPL) activity and blocks TG hydrolysis, thus allowing VLDL-TG to accumulate over time, and enables the calculation of hepatic VLDL-TG secretion rates (33). Serum samples were collected at 0, 1, 2, 4, and 8 h and analyzed for TG concentration. Serum levels of TG were quantified using the Infinity Triglycerides kit (Thermo Scientific).
Quantification of hepatic and nonhepatic LPL activity.
Experiments were performed as previously described (43). In brief, overnight fasted mice received a retroorbital injection of heparin (300 U/kg body wt). Blood was collected 5 min after heparin injection. Serum was isolated, and postheparin total LPL activity was measured with a kit (BioVision, Milpitas, CA). To distinguish between hepatic and nonhepatic lipase activity, 1 M NaCl (final concentration) was included in the assay. NaCl inhibits all LPL activity except hepatic lipase activity. Nonhepatic LPL activity was determined by subtracting hepatic lipase activity from heparin-displaceable total LPL activity in the blood.
Quantification of intestinal lipid absorption.
Experiments were carried out as previously described (22). In brief, mice were overnight fasted and then injected intraperioneally with Poloxamer 407 to inhibit LPL activity. Inhibition of LPL prevents TG (from gut-derived chylomicrons and liver-derived VLDL) uptake by peripheral tissues. One hour after Poloxamer 407 injection, mice were gavaged with Intralipid (10 μl/g body wt). Blood samples were collected before gavage and at 1, 2, 3, and 4 h after lipid gavage. Serum TG levels were quantified as described above.
Tissue collection.
Liver, white adipose tissue (perigonadal/visceral and inguinal/subcutaneous), and skeletal muscle samples were immediately harvested from euthanized mice and flash frozen into liquid nitrogen. Unless otherwise stated, tissues were collected from mice in which food was removed for 2 h before euthanization. Homogenized tissue lysates were prepared in RIPA lysis buffer [50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 0.25% deoxycholate] containing protease inhibitors (Complete Mini, Roche) and phosphatase inhibitors (PhosSTOP, Roche). Tisue lysates were centrifuged at 10,000 rpm for 20 min at 4°C for 20 min. Supernatants were collected, and protein content was quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific).
Histology.
WT and Ctrp1 KO mouse tissues were fixed overnight in 10% formalin at 4°C. Fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin at the Histology Reference Laboratory at The Johns Hopkins University School of Medicine.
Serum and blood chemistry analysis.
Mouse serum was harvested by retroorbital bleeding at the time of euthanasia. Blood samples were allowed to clot on ice for 30 min and then centrifuged for 10 min at 10,000 g. Serum samples were stored at −80°C. Glucose concentrations were determined at the time of collection with a glucometer (NovaMax Plus). Serum lipid levels were measured by the Mouse Pathology and Phenotyping Core at The Johns Hopkins University School of Medicine. Serum insulin adiponectin, leptin, TNF-α, macrophage chemotactic protein (MCP)-1, IL-1β, and IL-6 were measured using Millipore kits. Serum transforming growth factor (TGF)-β1 was measured using an Abcam kit, and CTRP1 was measured using a kit from BioVendor R&D. Serum triiodothyronine, thyroxine, thyroid-stimulating hormone, and FGF-21 were measured using kits from Millipore.
Lipid extraction from liver tissue.
Lipid extraction was performed as previously described (40). In brief, the liver (50 mg) was homogenized in 500 μl distilled water. The homogenate (200 μl) was collected for lipid extraction, mixed with 1 ml choloroform-methanol (2:1), and centrifuged at 1,700 rpm for 5 min at 4°C. The chloroform phase was collected and dried in a vacuum. Samples were resuspended in tert-butanol-MeOH-Triton X-100 (3:1:1) before triacylglycerol and cholesterol content were determined using commercially available colorimetric kits (Thermo Scientific).
Western blot analysis.
Western blot analyses were carried out and quantified as previously described (46) using antibodies specific to glucose transporter (GLUT)4, AMPKα, AKT, phospho-AKT (Thr308 and Ser473), and phospho-AMPKα (Thr172) (Cell Signaling Technology). Peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α antibody was obtained from Abcam (catalog no. ab54481). Hsc70 antibody was obtained from Santa Cruz Biotechnology.
Quantitative real-time PCR analysis.
Total RNA was isolated from tissues using TRIzol (Thermo Scientific) and reverse transcribed using the GoScript Reverse transcription system (Promega). Real-time PCR primers for gluconeogenic genes (glucose 6-phosphatase and phosphoenolpyruvate carboxykinase 1) (42), TG synthesis genes [glycerol-3-phosphate acyltransferase (Gpat), acylglycerolphosphate acyltransferase (Agpat), and diacylglycerol acyltransferase (Dgat)] (40), de novo lipogenesis, fat oxidation, and adipokine genes [acetyl-CoA carboxylase, fatty acid synthase (Fasn), sterol regulatory element-binding protein (Srebp)-1, acyl-CoA oxidase 1, carnitine palmitoyltransferase (Cpt)1, Cpt2, long-chain acyl-CoA dehydrogenase, medium-chain CoA dehydrogenase, adiponectin, and leptin] (60), fibrotic genes [collagen type (Col)1, Col3, and Col6] (25), and inflammatory genes (IL-1β, IL-6, and Tgf-β) have been previously published. Other primer sequences used in this study are shown in Table 1. Quantitative real-time PCR analyses were performed on a CFX Connect system (Bio-Rad Laboratories, Hercules, CA). Samples were analyzed in 20-μl reactions with Taq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) per the manufacturer's directions. Data were normalized to 36B4 (adipose tissue), 18S rRNA (skeletal muscle), and β-actin (liver) and expressed as relative mRNA levels using the ΔΔCt method (where Ct is cycle threshold) (26).
Table 1.
Real-time PCR primers used in the study
| Gene | Forward Primer, 5′ to 3′ | Reverse Primer, 5′ to 3′ |
|---|---|---|
| 36B4 | AGATTCGGGATATGCTGTTGGC | TCGGGTCCTAGACCAGTGTTC |
| Hmgcr | CTTGTGGAATGCCTTGTGATTG | AGCCGAAGCAGCACATGAT |
| Sqle | ATAAGAAATGCGGGGATGTCAC | ATATCCGAGAAGGCAGCGAAC |
| Abca1 | GCTGCAGGAATCCAGAGAAT | CATGCACAAGGTCCTGAGAA |
| Apoc2 | AGGTTCCGGCTTGATGAGAA | AGTGGGTTGGCAGGCTTTAT |
| Apoe | CTGACAGGATGCCTAGCCG | CGCAGGTAATCCCAGAAGC |
| Vldlr | GAGCCCCTGAAGGAATGCC | CCTATAACTAGGTCTTTGCAGATATGG |
| Cd36 | ATGGGCTGTGATCGGAACTG | AGCCAGGACTGCACCAATAAC |
| Chrebp-α | CGACACTCACCCACCTCTTC | TTGTTCAGCCGGATCTTGTC |
| Chrebp-β | AGCGGATTCCAGGTGAGG | TTGTTCAGGCGGATCTTGTC |
| Fabp1 | ATGAACTTCTCCGGCAAGTACC | GGTCCTCGGGCAGACCTAT |
| Fatp5 | GTTCTCCCGTCCAAGACCATT | GCTCCGTACAGAGTGTAGCAAG |
| Fxr | GCTTGATGTGCTACAAAAGCTG | CGTGGTGATGGTTGAATGTCC |
| Lxr-α | AGGAGTGTCGACTTCCGCAAA | CTCTTCTTGCCGCTTCAGTTT |
| Lxr-β | ATAGTGGGTCACGAAGCAGC | AGGGCAACAGAGTCGGAGAC |
| Scd1 | CCCAGTCGTACACGTCATTTT | CATCATTCTCATGGTCCTGCT |
| Mlycd | CTCGGGACCTTCCTCATAAAGAGA | GAATAGTTCGTTCCTCCCATGCTC |
| Lipc | ATGGGAAATCCCCTCCAAATCT | GTGCTGAGGTCTGAGACGA |
| Lpl | CCCTGAAGACACAGCTGAGG | GGCTGTACCCTAAGAGGTGG |
| Mcp-1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| Ppar-g | CCAGAGTCTGCTGATCTGCG | GCCACCTCTTTGCTCTGCTC |
| Atgl | TGTGGCCTCATTCCTCCTAC | TCGTGGATGTTGGTGGAGCT |
| Hsl | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| Ccr7 | TGTACGAGTCGGTGTGCTTC | GGTAGGTATCCGTCATGGTCTTG |
| Ccl3 | TTCTCTGTACCATGACACTCTGC | CGTGGAATCTTCCGGCTGTAG |
| Ccl4 | TTCCTGCTGTTTCTCTTACACCT | CTGTCTGCCTCTTTTGGTCAG |
| Nos2 | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| F4/80 | CCCCAGTGTCCTTACAGAGTG | GTGCCCAGAGTGGATGTCT |
| Mgl2 | GCATGAAGGCAGCTGCTATTGGTT | TAGGCCCATCCAGCTAAGCACATT |
| Cd206 | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC |
| Il-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| Arg1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| Cd68 | TTCTGCTGTGGAAATGCAAG | CAATGATGAGAGGCAGCAAG |
| Retnl | CCAATCCAGCTAACTATCCCTCC | ACCCAGTAGCAGTCATCCCA |
| Mcad | GTGCCCAGAGTGGATGTCT | CCCCGCTTTTGTCATATTCCG |
| Lcat | GTAACCACACACGGCCTGTC | TCTTACGGTAGCACATCCAGTT |
| LDLr | CGCGGATCTGATGCGTCGCT | CGGCCCTGGCAGTTCTGTGG |
| Lrp1 | GACCAGGTGTTGGACACAGATG | AGTCGTTGTCTCCGTCACACTTC |
| Pcsk9 | TTGCAGCAGCTGGGAACTT | CCGACTGTGATGACCTCTGGA |
| Sort1 | CCCGGACTTCATCGCCAAG | AGGACGAGAATAACCCCAGTG |
| Idol | AGGAGATCAACTCCACCTTCTG | ATCTGCAGACCGGACAGG |
| Apo-AI | AAGAGGATGTGGAGCTCTACC | TTCTCGCCAAGTGTCTTCAGG |
| ApoAIV | CAGTGAGGAGCCCAGGATGTT | TCTACAGCCTCCTTGGCATT |
| Cyp3a11 | GACAAACAAGCAGGGATGGAC | CCAAGCTGATTGCTAGGAGCA |
| Cyp27a1 | CCAGGCACAGGAGAGTACG | GGGCAAGTGCAGCACATAG |
| Cyp7b1 | GGAGCCACGACCCTAGATG | TGCCAAGATAAGGAAGCCAAC |
| Cyp8b1 | CCTCTGGACAAGGGTTTTGTG | GCACCGTGAAGACATCCCC |
| Abcg1 | CAAGACCCTTTTGAAAGGGATCTC | GCCAGAATATTCATGAGTGTGGAC |
| Abcg5 | AGGGCCTCACATCAACAGAG | GCTGACGCTGTAGGACACAT |
| Abcg8 | CTGTGGAATGGGACTGTACTTC | GTTGGACTGACCACTGTAGGT |
Statistical analysis.
Comparisons between two groups of data were performed using two-tailed Student's t-tests with 95% confidence intervals, and ANOVAs were used to make comparisons involving more than two groups. Values were considered to be statistically significant at P < 0.05. All data are presented as means ± SE.
RESULTS
Generation of the Ctrp1 KO mouse model.
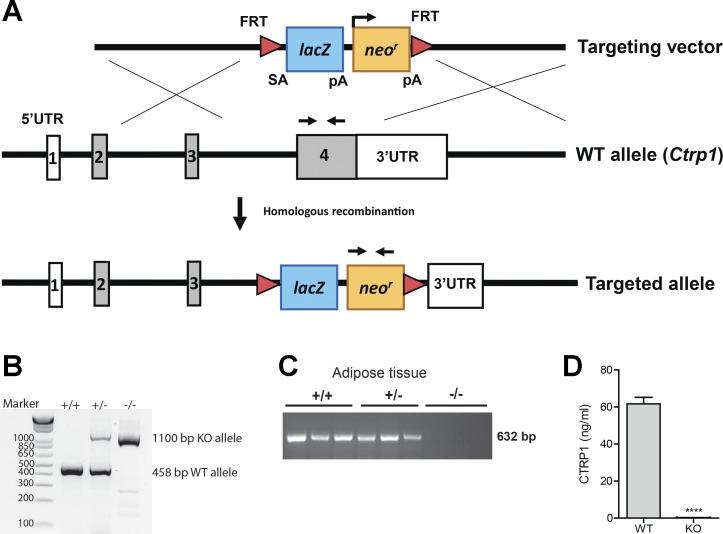
To obtain a null allele for Ctrp1, a 679-bp region of the Ctrp1 gene that spans exon 4 was deleted and replaced with a lacZ reporter and a neomycin resistance cassette (Fig. 1A). Exon 4 encodes 184 amino acids and constitutes 61% of the full-length protein, spanning most of the collagen domain and the entire globular C1q domain. Two sets of primers were designed to amplify a sequence spanning the neomycin-resistant cassette and a sequence within the protein-coding region of WT Ctrp1. This enabled the confirmation of the Ctrp1 heterozygous (+/−) and KO (−/−) alleles (Fig. 1B). Since CTRP1 is abundantly expressed in adipose tissue, we examined its expression in the gonadal fat pad of WT and Ctrp1 KO mice. No Ctrp1 mRNA was detected in the adipose tissue of Ctrp1 KO mice by semiquantitative PCR (Fig. 1C). Ctrp1 mRNA was also not detected in the skeletal muscle, liver, or kidney of KO animals (not shown). Importantly, using an ELISA specific for mouse CTRP1, we demonstrated that KO mice have serum CTRP1 levels approaching the background levels of the assay (Fig. 1D). Therefore, both the mRNA and serum protein data confirmed the lack of CTRP1 in the loss-of-function mouse model. The Ctrp1 KO mice were born at the expected Mendelian ratio. They were viable, fertile, and developed normally with no gross phenotype. Although CTRP1 expression can be detected as early as embryonic day 7 during development (62), it was not required for proper embryonic development.
Fig. 1.
Generation of C1q/TNF-related protein 1 (Ctrp1) knockout (KO) mice. A: schematic showing the strategy for generating Ctrp1 KO mice by targeted deletion of exon 4 of the mouse Ctrp1 gene and replacement with a lacZ reporter and a neomycin resistance cassette. The red triangle represents the FRT site recognized by Flp recombinase. UTR, untranslated region. B: genotyping results indicate the successful generation of Ctrp1 wild-type (WT; +/+), heterozygous (+/−), and homozygous KO (−/−) alleles using the primer set (forward and reverse arrows) indicated in A. C: semiquantitative PCR analysis indicating the absence of detectable Ctrp1 mRNA in KO mice. D: serum CTRP1 levels of low-fat diet (LFD)-fed WT and KO male mice. All data are expressed as means ± SE. ****P < 0.0001.
Metabolic parameters of WT and Ctrp1 KO mice fed a LFD.
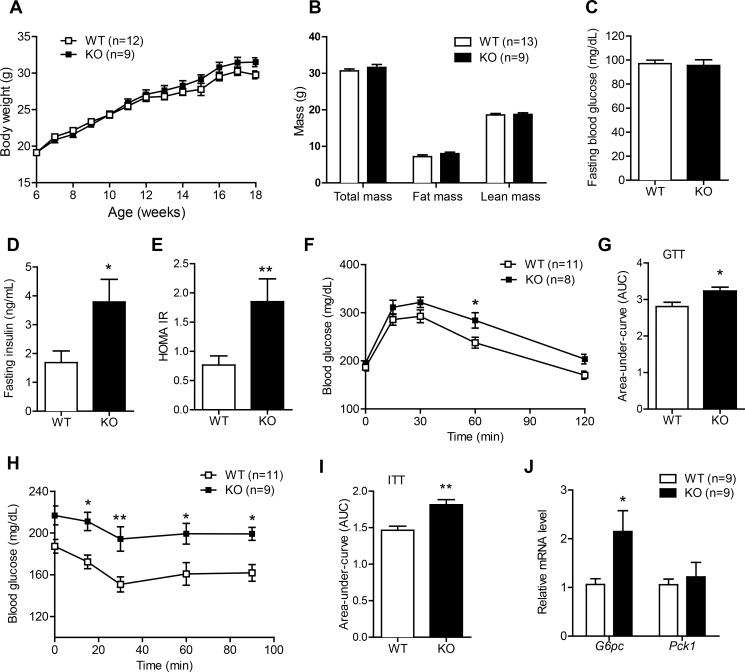
To take into consideration the effects of sex on metabolic outcome (8, 11, 31), we monitored the body weight of both male and female Ctrp1 WT and KO mice. Mice were fed a control LFD that was comparable in fat content to standard laboratory chow. Over a period of 12 wk and beyond, body weights were indistinguishable between Ctrp1 WT and KO mice (Fig. 2A and Table 2). The amount of total fat and lean mass quantified by NMR was similar between Ctrp1 WT and KO male (Fig. 2B) and female (not shown) mice. However, the weight of the gonadal (visceral) but not inguinal (subcutaneous) fat depot was reduced in Ctrp1 KO male mice compared with WT control mice (Table 2). Heart and skeletal muscle weights as well as tibia length were similar between WT and Ctrp1 KO mice (Table 2). Food intake was not different between LFD-fed Ctrp1 WT and KO mice (Table 3). Indirect calorimetry measurements of metabolic rate (V̇o2), RER, energy expenditure, and physical activity levels over a 24-h period revealed no differences between LFD-fed Ctrp1 WT and KO mice of both sexes (Table 3), either during the light or dark phases of the photocycle (not shown).
Fig. 2.
Ctrp1 KO mice fed a LFD develop insulin resistance. A: body weight gain over time of WT (n = 12) and KO (n = 9) male mice fed a control LFD. B: body composition analysis of lean and fat mass of WT (n = 13) and KO (n = 9) male mice. C–E: overnight fasting blood glucose (C), insulin (D), and homeostatic model assessment of insulin resistance (HOMA-IR) index (E) of WT (n = 10) and KO (n = 8) male mice. F: blood glucose levels of WT (n = 11) and KO (n = 8) male mice subjected to a glucose tolerance test (GTT). Glucose was delivered by an intraperitoneal injection. G: area under the curve (AUC) for the GTT data shown in F. H: blood glucose levels of WT (n = 11) and KO (n = 9) male mice subjected to an insulin tolerance test (ITT). I: AUC for the ITT data shown in H. J: quantitative real-time PCR analysis of gluconeogenic gene [glucose 6-phosphatase (G6Pc) and phosphoenolpyruvate carboxykinase 1 (Pck1)] expression in the liver of WT (n = 9) and KO (n = 9) male mice. Expression levels were normalized to β-actin. All data are expressed as means ± SE. *P < 0.05; **P < 0.01.
Table 2.
Tissue weights of LFD-fed and HFD-fed Ctrp1 WT and KO male and female mice
| Male Mice |
Female Mice |
|||||
|---|---|---|---|---|---|---|
| WT | KO | P value | WT | KO | P value | |
| LFD | ||||||
| n | 12 | 8 | 17 | 8 | ||
| Body weight, g | 38.2825 ± 0.694 | 36.92125 ± 0.840 | NS | 29.01 ± 0.780 | 29.72 ± 1.210 | NS |
| Gonadal fat mass, g | 0.955 ± 0.025 | 0.77875 ± 0.036 | *** | 0.5159 ± 0.04220 | 0.4400 ± 0.05910 | NS |
| Gonadal fat mass/body weight | 0.0249 ± 0.0005604 | 0.02104 ± 0.0006512 | *** | 0.01739 ± 0.001096 | 0.01442 ± 0.001489 | NS |
| Inguinal fat mass, g | 0.739 ± 0.052 | 0.645 ± 0.035 | NS | 0.3441 ± 0.02919 | 0.3750 ± 0.03423 | NS |
| Inguinal fat mass/body weight | 0.01926 ± 0.001213 | 0.01746 ± 0.0008242 | NS | 0.01160 ± 0.0007863 | 0.01248 ± 0.0008185 | NS |
| Liver weight, g | 1.983 ± 0.1271 | 2.445 ± 0.1696 | * | 1.405 ± 0.07098 | 1.653 ± 0.1524 | NS |
| Liver weight/body weight | 0.05155 ± 0.002590 | 0.06592 ± 0.003748 | ** | 0.04801 ± 0.001372 | 0.05501 ± 0.003409 | * |
| Gastrocnemius muscle, g | 0.121 ± 0.005 | 0.116 ± 0.005 | NS | ND | ND | N/A |
| Gastrocnemius muscle/body weight | 0.003163 ± 0.0001215 | 0.003148 ± 0.0001273 | NS | ND | ND | N/A |
| Heart, g | 0.169 ± 0.009 | 0.158 ± 0.007 | NS | 0.1488 ± 0.005871 | 0.1475 ± 0.0075 | NS |
| Heart/tibia length | 0.009313 ± 0.0004806 | 0.008790 ± 0.0003024 | NS | 0.008234 ± 0.0003175 | 0.008154 ± 0.0004050 | NS |
| Fed blood glucose (2-h food removal), mg/dl | 192.083 ± 4.914 | 182 ± 6.059 | NS | 158.0 ± 4.263 | 155.4 ± 8.181 | NS |
| Tibia length, mm | 18.147 ± 0.190 | 17.890 ± 0.181 | NS | 18.07 ± 0.09276 | 18.09 ± 0.2816 | NS |
| HFD | ||||||
| n | 11 | 10 | 15 | 10 | ||
| Body weight, g | 55.04 ± 1.089 | 48.85 ± 0.4851 | **** | 53.53 ± 1.816 | 50.20 ± 1.278, n = 10 | NS |
| Gonadal fat mass, g | 0.6082 ± 0.05129 | 0.600 ± 0.6703 | NS | 2.199 ± 0.09796 | 1.916 ± 0.1258 | NS |
| Gonadal fat mass/body weight | 0.01104 ± 0.0009028 | 0.01229 ± 0.001357 | NS | 0.04107 ± 0.001234 | 0.03798 ± 0.001984 | NS |
| Inguinal fat mass, g | 1.256 ± 0.04535 | 0.9150 ± 0.03198 | **** | 1.202 ± 0.04528 | 1.112 ± 0.06734 | NS |
| Inguinal fat mass/body weight | 0.02281 ± 0.0006551 | 0.01874 ± 0.0006571 | *** | 0.02258 ± 0.0007765 | 0.02222 ± 0.001315 | NS |
| Liver weight, g | 3.810 ± 0.1604 | 3.892 ± 0.2103 | NS | 2.149 ± 0.2036 | 2.174 ± 0.1756 | NS |
| Liver weight/body weight | 0.06920 ± 0.002455 | 0.07957 ± 0.004000 | * | 0.03911 ± 0.002605 | 0.04286 ± 0.002777 | NS |
| Heart, g | 0.2291 ± 0.009672 | 0.2190 ± 0.01602 | NS | 0.1540 ± 0.007091 | 0.1470 ± 0.002134 | NS |
| Heart/tibia length | 0.01279 ± 0.0004742 | 0.01223 ± 0.0008627 | NS | 0.008605 ± 0.0003912 | 0.008196 ± 0.0001319 | NS |
| Fed blood glucose (2-h food removal), mg/dl | 175.7 ± 4.357 | 176.5 ± 9.165 | NS | 183.0 ± 4.508 | 194.6 ± 7.162 | NS |
| Tibia length, mm | 17.90 ± 0.2372 | 17.89 ± 0.08167 | NS | 17.89 ± 0.1206 | 17.95 ± 0.1503 | NS |
All data are expressed as means ± SE; n, number of mice. LFD, low-fat diet; HFD, high-fat diet; Ctrp1, C1q/TNF-related protein 1; WT, wild-type; KO, knockout; NS, not significant; ND, not determined; N/A, not applicable.
P < 0.05;
P < 0.01;
P < 0.005;
P < 0.001.
Table 3.
Whole body metabolic parameters of LFD-fed and HFD-fed Ctrp1 WT and KO male and female mice over a 24-h period
| Male Mice |
Female Mice |
|||||
|---|---|---|---|---|---|---|
| WT | KO | P value | WT | KO | P value | |
| LFD | ||||||
| n | 12 | 9 | 13 | 10 | ||
| Food intake, g | 4.690 ± 0.1986 | 4.551 ± 0.2407 | NS | 4.691 ± 0.2746 | 4.808 ± 0.2631 | NS |
| V̇o2, ml·kg lean mass−1·h−1 | 4544 ± 72.35 | 4786 ± 114.9 | NS | 5537 ± 116.9 | 5488 ± 142.6 | NS |
| V̇co2, ml·kg lean mass−1·h−1 | 4309 ± 84.02 | 4484 ± 95.31 | NS | 5252 ± 105.5 | 5252 ± 153.2 | NS |
| RER | 0.9484 ± 0.01153 | 0.9376 ± 0.008531 | NS | 0.9498 ± 0.01204 | 0.9575 ± 0.01583 | NS |
| Energy expanditure, kcal·kg lean mass−1·h−1 | 22.64 ± 0.3630 | 23.78 ± 0.5492 | NS | 27.59 ± 0.5587 | 27.41 ± 0.7088 | NS |
| Physical activity (beam breaks) | 42724 ± 3313 | 39886 ± 2106 | NS | 75971 ± 4388 | 82086 ± 6942 | NS |
| HFD | ||||||
| n | ||||||
| Food intake, g | 2.722 ± 0.1088 | 2.865 ± 0.08533 | NS | 2.388 ± 0.09373 | 2.305 ± 0.08192 | NS |
| V̇o2, ml·kg lean mass−1·h−1 | 4860 ± 75.24 | 4758 ± 58.97 | NS | 5382 ± 90.54 | 5343 ± 99.06 | NS |
| V̇co2, ml·kg lean mass−1·h−1 | 3701 ± 56.74 | 3610 ± 47.73 | NS | 4330 ± 61.52 | 4302 ± 73.03 | NS |
| RER | 0.7616 ± 0.002731 | 0.7587 ± 0.002106 | NS | 0.8050 ± 0.003875 | 0.8055 ± 0.004440 | NS |
| Energy expanditure, kcal·kg lean mass−1·h−1 | 23.10 ± 0.3555 | 22.60 ± 0.2828 | NS | 25.87 ± 0.4191 | 25.68 ± 0.4646 | NS |
| Physical activity (beam breaks) | 27449 ± 1736 | 37192 ± 2563 | ** | 43631 ± 3942 | 57716 ± 5570 | * |
All data are expressed as means ± SE; n, number of mice. V̇o2, O2 consumption; V̇co2, CO2 production; RER, respiratory exchange ratio.
P < 0.05;
P < 0.01
CTRP1 deficiency impairs glucose homeostasis in LFD-fed male mice.
We next determined the impact of Ctrp1 deletion on glucose homeostasis. While fasting (12 h) blood glucose levels were similar between genotypes (Fig. 2C), fasting insulin levels were significantly higher in Ctrp1 KO male mice compared with WT control mice (Fig. 2D), leading to a significantly higher insulin resistance index (HOMA-IR; Fig. 2E). In accordance with these results, Ctrp1 KO mice had significantly impaired glucose and insulin tolerance (Fig. 2, F–I), as well as increased hepatic expression of the gluconeogenic gene glucose-6-phosphatase (Fig. 2J). However, assessment of liver AKT phosphorylations (Thr308 and Ser473) revealed no differences between WT and KO mice (data not shown). These alterations in glucose metabolism, however, were not seen in female KO mice (data not shown).
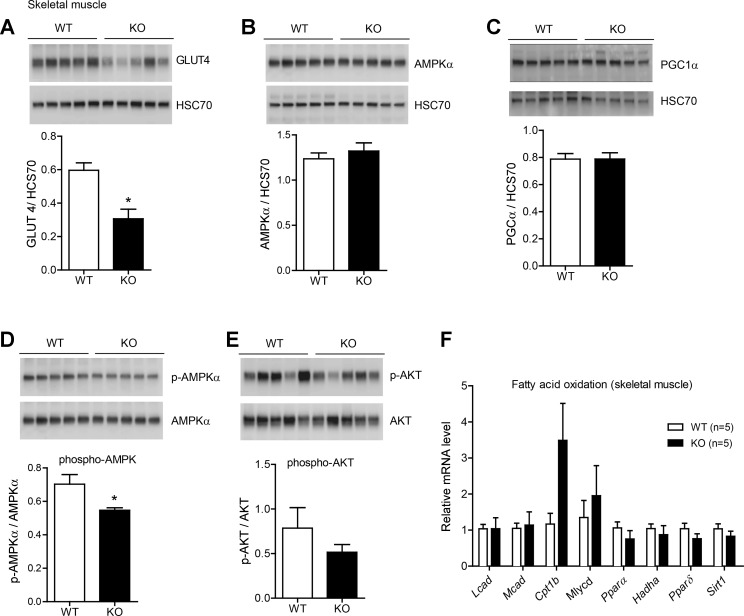
Reduced expression of GLUT4 and AMPK in the skeletal muscle of Ctrp1 KO male mice fed a LFD.
The insulin-responsive glucose transporter GLUT4 and AMPK play important roles in glucose uptake in skeletal muscle (15, 18). Steady-state protein levels of GLUT4 were significantly reduced in Ctrp1 KO mice relative to WT control mice (Fig. 3A). Protein levels of AMPKα and PPAR-γ coactivator-1α, however, were not different between genotypes (Fig. 3, B and C). Phospho-AMPKα (Thr172) levels, a metric of AMPK activation, were also significantly lower in the skeletal muscle of Ctrp1 KO mice (Fig. 3D). Phospho-AKT (Ser473) levels were not different between genotypes (Fig. 3E). Assessment of phosho-AKT at Thr308 also revealed no differences between WT and KO mice (data not shown). The analysis of skeletal muscle genes involved in fat oxidation did not reveal any significant differences between WT and Ctrp1 KO mice (Fig. 3F).
Fig. 3.
Reduced skeletal muscle glucose transporter (GLUT)4 and AMP-activated protein kinase (AMPK)α levels in Ctrp1 KO mice fed a LFD. A–C: quantitative Western blot analysis of skeletal muscle GLUT4 (A), AMPKα (B), peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC1α; C) levels in WT (n = 5) and KO (n = 5) male mice. Data were normalized to HSC70. D and E: quantitative Western blot analysis of phosphorylated (p)AMPKα (Thr172; D) and pAKT (Ser473; E). Data were normalized to total AMPKα or AKT. The total AMPKα blot shown in B is the same as shown in D. F: expression of genes involved in skeletal muscle fat oxidation. Expression levels were normalized to 18S rRNA. Lcad, long-chain acyl-CoA dehydrogenase; Mcad, medium-chain CoA dehydrogenase; Cpt, carnitine palmitoyltransferase; Mlycd, malonyl-CoA decarboxylase; Hadha, hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (Trifunctional Protein) α-subunit; Sirt1, sirtuin 1. All data are expressed as means ± SE. *P < 0.05.
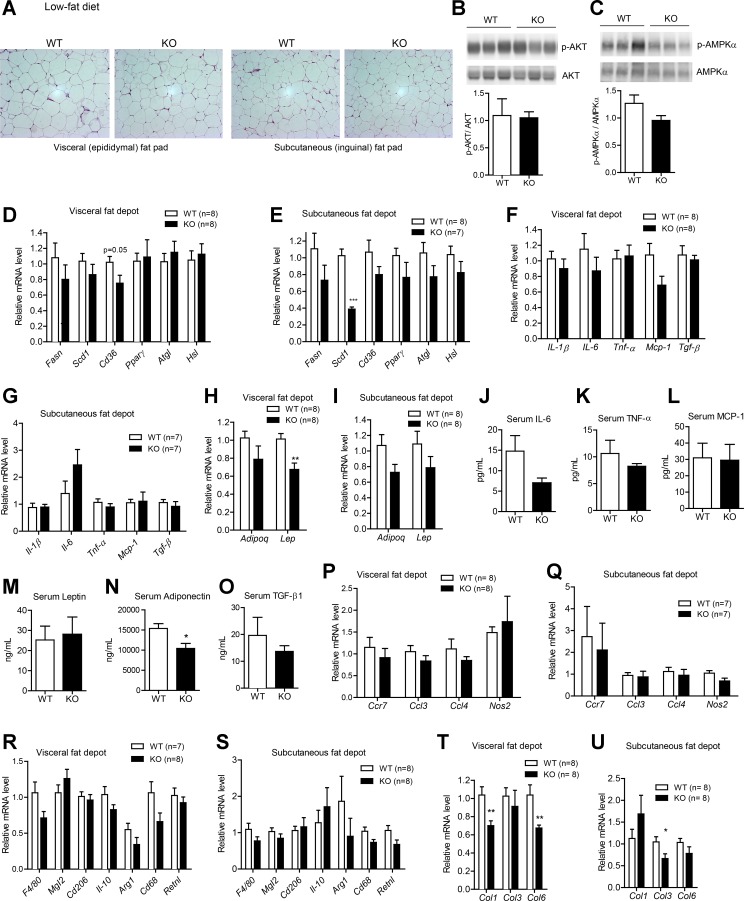
Impact of CTRP1 deficiency on the adipose tissue of LFD-fed male mice.
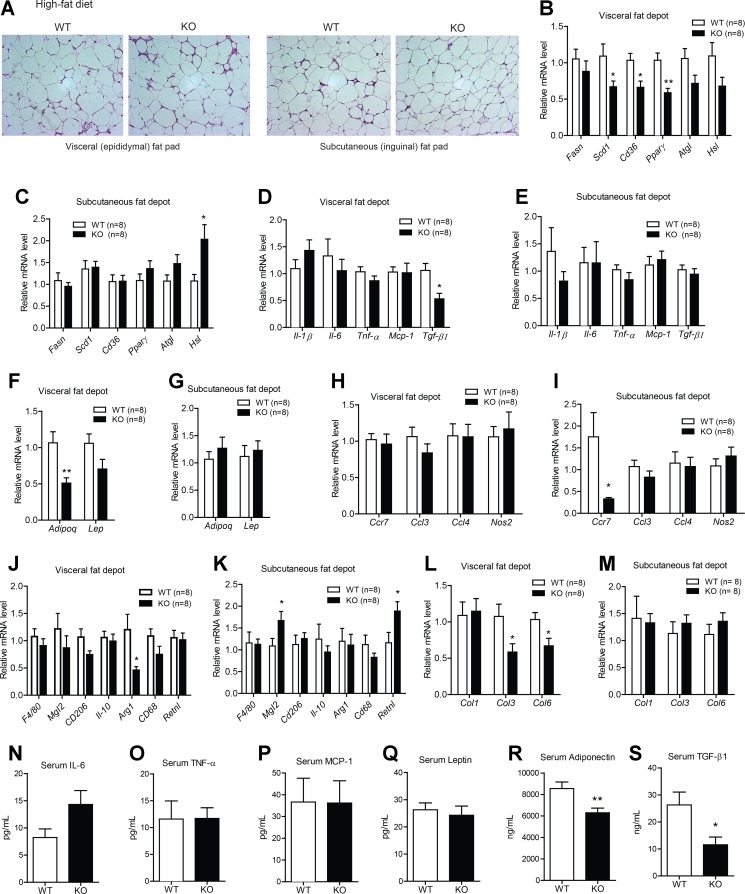
Analysis of adipose tissue histology revealed no gross differences between WT and Ctrp1 KO mice in the visceral or subcutaneous fat depot (Fig. 4A). In contrast to the skeletal muscle of Ctrp1 KO mice, neither phospho-AKT nor phospho-AMPK levels were different between genotypes in the visceral fat depot (Fig. 4, B and C). With the exception of stearoyl-CoA desaturase-1 (Scd1), adipose expression of genes involved in lipid uptake (Cd36), synthesis (Fasn and PPAR-γ), and lipolysis (adipose triglyceride lipase and hormone-sensitive lipase) was similar in both the visceral (epididymal) and subcutaneous (inguinal) fat depot of WT and KO mice (Fig. 4, D and E). Expression of inflammatory cytokines (IL-1β, IL-6, TNF-α, Mcp-1, and TGF-β) and their circulating levels were also not different between genotypes (Fig. 4, F, G, and J–L). While the adipose expression of leptin mRNA was reduced in the visceral fat depot of Ctrp1 KO mice (Fig. 4, H and I), its circulating serum levels were comparable between genotypes (Fig. 4M). Serum levels of adiponectin were significantly lower in KO mice (Fig. 4N), whereas TGF-β levels were not different between genotypes (Fig. 4O). Loss of CTRP1 did not appear to affect the extent or type of macrophages infiltrating the adipose tissue; neither the expression of pan-macrophage marker (F4/80), proinflammatory M1 macrophage markers (macrophage galactose N-acetyl-galactosamine-specific lectin 2, Cd206, IL-10, arginase 1, Cd68, and resistin l), nor anti-inflammatory M2 macrophage markers [chemokine (C-C motif) receptor 7, chemokine (C-C motif) ligand (Ccl)3, Ccl4, and nitric oxide synthase 2] was different in the visceral or subcutaneous fat depot of WT and Ctrp1 KO animals (Fig. 4, P–S). In contrast, the expression of fibrotic collagen genes was significantly reduced in KO animals relative to WT control animals (Fig. 4, T and U).
Fig. 4.
Metabolic, inflammatory, and fibrotic gene expression in the adipose tissue of Ctrp1 KO male mice fed a LFD. A: representative histology (hematoxylin and eosin stain; magnification: ×200) of the visceral (epididymal) fat pad of KO male mice and WT littermate controls. B and C: quantitative Western blot analysis of pAKT (B) and pAMPKα (C) in visceral adipose tissue of WT (n = 6) and KO (n = 6) mice. D and E: expression of lipid uptake (Cd36), synthesis [fatty acid synthase (Fasn), stearoyl-CoA desaturase-1 (Scd1), and PPAR-γ] and lipolysis [adipose triglyceride lipase (Atgl) and hormone-sensitive lipase (Hsl)] in the visceral (epididymal; D) and subcutaneous (inguinal; E) fat depot of WT (n = 8) and KO (n = 7–8) mice. F and G: expression of inflammatory [IL-1β, IL-6, TNF-α, and monocyte chemotactic protein (Mcp)-1] and profibrotic [transforming growth factor (TGF)-β1] cytokine genes in the visceral (epididymal; F) and subcutaneous (inguinal; G) fat depot of WT (n = 7–8) and KO (n = 7–8) mice. H and I: expression of adiponectin (Adipoq) and leptin (Lep) in the visceral (epididymal; H) and subcutaneous (inguinal; I) fat depot of WT (n = 8) and KO (n = 8) mice. J–M: serum levels of IL-6 (J), TNF-α (K), MCP-1 (L), leptin (M), adiponectin (N), and TGF-β (O) in WT (n = 10) and KO (n = 8) mice. P and Q: expression of proinflammatory M1 macrophage marker genes in the visceral (epididymal; P) and subcutaneous (inguinal; Q) fat depot of WT (n = 7–8) and KO (n = 7–8) mice. Ccr, chemokine (C-C motif) receptor; Ccl, chemokine (C-C motif) ligand; Nos, nitric oxide synthase. R and S: expression of anti-inflammatory M2 macrophage marker genes in the visceral (epididymal; R) and subcutaneous (inguinal; S) fat depot of WT (n = 7–8) and KO (n = 8) mice. Mgl2, macrophage galactose N-acetyl-galactosamine-specific lectin 2; Arg, arginase; Retn, resistin. T and U: expression of fibrotic collagen (Col) genes in the visceral (epididymal; T) and subcutaneous (inguinal; U) fat depot of WT (n = 8) and KO (n = 8) mice. Expression levels were normalized to 36B4 [also known as ribosomal phosphoprotein P0 (RPLP0)]. All data are expressed as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.005.
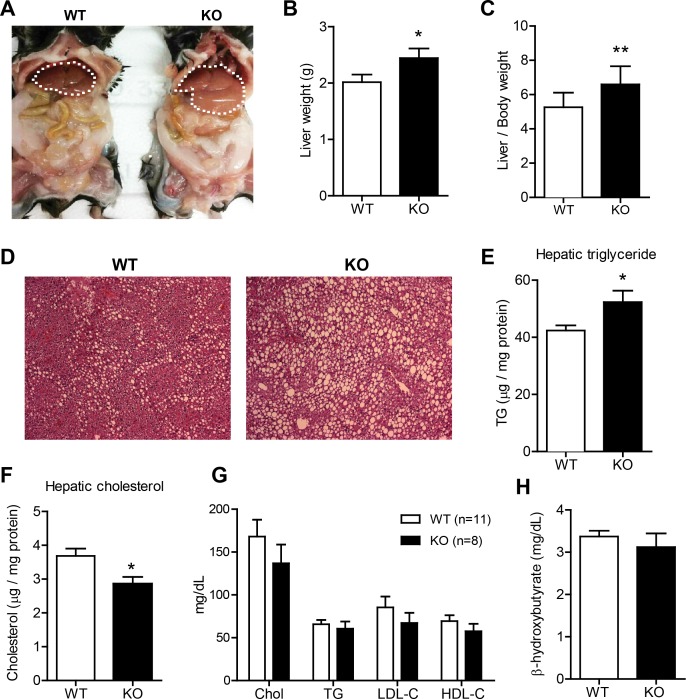
LFD-fed Ctrp1 KO male mice develop liver steatosis.
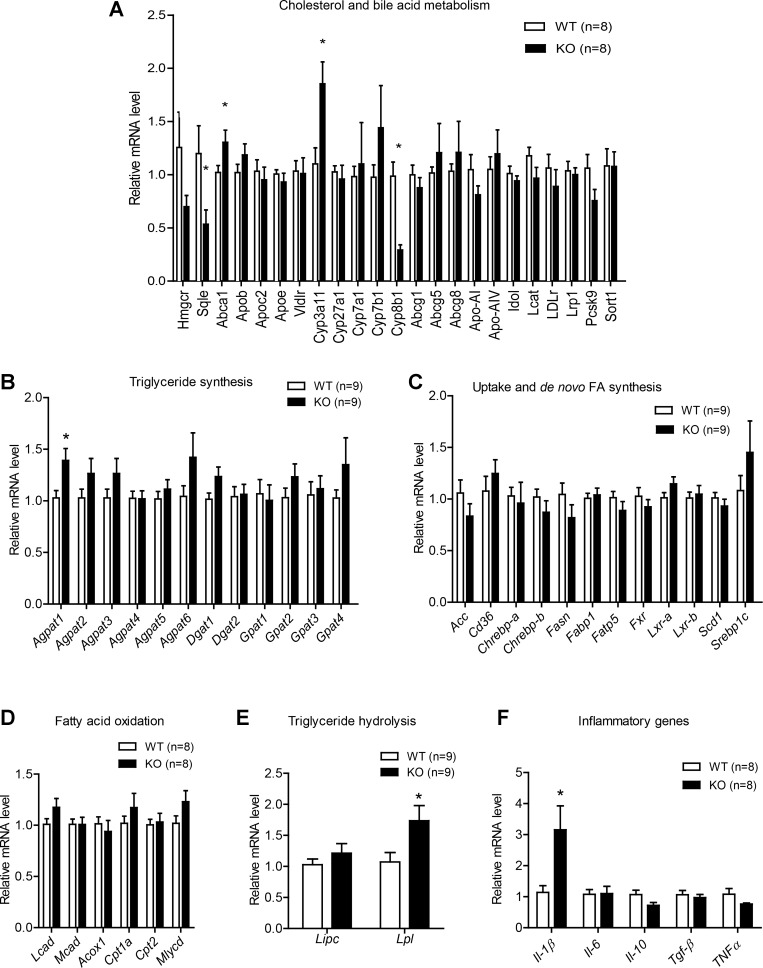
Examination of visceral organs uncovered significant enlargement of the liver in Ctrp1 KO mice relative to WT control mice (Fig. 5, A–C). Histological analysis revealed pronounced steatosis in KO animals (Fig. 5D), with a significant accumulation of hepatic TG in CTRP1-deficient mice compared with WT control mice (Fig. 5E). Total hepatic cholesterol levels, however, were significantly lower in Ctrp1 KO animals (Fig. 5F). In contrast, steady-state serum concentrations of cholesterol, TG, LDL-cholesterol, HDL-cholesterol, and β-hydroxybutyrate were not different between genotypes (Fig. 5, G and H). Quantitative real-time PCR analyses were performed to assess possible changes in the expression of hepatic lipid metabolism genes. Reduced hepatic cholesterol levels were associated with decreased expression of the cholesterol synthesis gene (squalene epoxidase) along with a modestly increased expression of ATP-binding cassette subfamily A member 1, which is involved in cholesterol efflux (Fig. 6A). Interestingly, expression of cytochrome P-450 (Cyp)3a11 and Cyp8b1, which are involved in bile acid metabolism, were also increased and decreased, respectively, in KO mice relative to WT littermates (Fig. 6A). With the exception of Agpat1, hepatic expression of genes involved in TG synthesis (Agpat, Gpat, and Dgat; Fig. 6B), fatty acid uptake and de novo lipogenesis (Fig. 6C), and fat oxidation (long-chain acyl-CoA dehydrogenase, medim-chain acyl-CoA dehydrogenase, acyl-CoA oxidase 1, Cpt1a, Cpt2, and malonyl-CoA decarboxylase; Fig. 6D) was similar between WT and Ctrp1 KO mice. Liver expression of Lpl, however, was significantly increased in KO animals (Fig. 6E). Since fatty liver is frequently associated with inflammation (52), we also determined the hepatic expression of several major inflammatory genes. Expression of IL-1β was significantly upregulated in the liver of Ctrp1 KO mice relative to WT control mice (Fig. 6F).
Fig. 5.
Development of hepatic steatosis in Ctrp1 KO male mice fed a LFD. A: representative image of a KO male mouse and a WT littermate control. The liver is highlighted with a dashed line. B: liver weight of WT (n = 10) and KO (n = 8) mice. C: liver-to-body weight ratio of WT (n = 10) and KO (n = 8) mice. D: representative image of liver histology (hematoxylin and eosin stain; magnification: ×100) of WT and KO male mice. E and F: quantification of liver triglyceride (TG; E) and cholesterol (Chol or C; F) levels in WT (n = 10) and KO (n = 9) mice. G: quantification of serum cholesterol, TG, LDL, and HDL in WT (n = 11) and KO (n = 8) mice. H: quantification of serum β-hydroxybutyrate in WT (n = 11) and KO (n = 9) mice. All data are expressed as means ± SE. *P < 0.05; **P < 0.01.
Fig. 6.
Metabolic gene expression in the liver of Ctrp1 KO male mice fed a LFD. A: expression of cholesterol and bile acid metabolism genes in WT (n = 8) and KO (n = 8) mouse livers. B: expression of genes involved in TG synthesis in WT (n = 9) and KO (n = 9) mouse livers. D–G: expression of genes involved in lipid uptake and de novo fatty acid synthesis (C), fat oxidation (D), TG lipolysis (E), and inflammation (F) in WT (n = 8–9) and KO (n = 8–9) mouse livers. Expression levels were normalized to β-actin. All data are expressed as means ± SE. *P < 0.05. Abca1, ATP-binding cassette subfamily A member 1; Abcg, ATP-binding cassette subfamily G; Acc, acetyl CoA carboxylase; Acox1, acyl CoA oxidase 1; Agpat, 1-acylglycerol-3-phosphate O-acyltransferase; Apob, apolipoprotein B; Apoc2, apolipoprotein C2; Apoe, apolipoprotein E; Chrebp, carbohydrate-responsive element-binding protein; Cpt, carnitine palmitoyltransferase; Cyp, cytochrome P-450; Dgat, diacylglycerol O-acyltransferase; Fabp, fatty acid-binding protein; Fasn, fatty acid synthase; Fxr, farnesoid X receptor; Gpat, glycerol-3-phosphate acyltransferase; Hmgcr, HMG-CoA reductase; Idol, inducible degrader of the LDL receptor; Lcad, long-chain acyl-CoA dehydrogenase; Lcat, lecithin-cholesterol acyltransferase; LDLr, LDLreceptor; Lipc, lipase, hepatic; Lpl, lipoprotein lipase; Lrp1, LDL receptor-related protein 1; Lxr, liver X receptor; Mcad, medium-chain CoA dehydrogenase; Mlycd, malonyl-CoA decarboxylase; Pcsk9, proprotein convertase subtilisin/kexin type 9; Scd1, stearoyl-CoA desaturase-1; Sort1, sortilin; Sqle, squalene epoxidase; Srebp1c, sterol regulatory element-binding protein 1c; Vldlr, VLDL receptor.
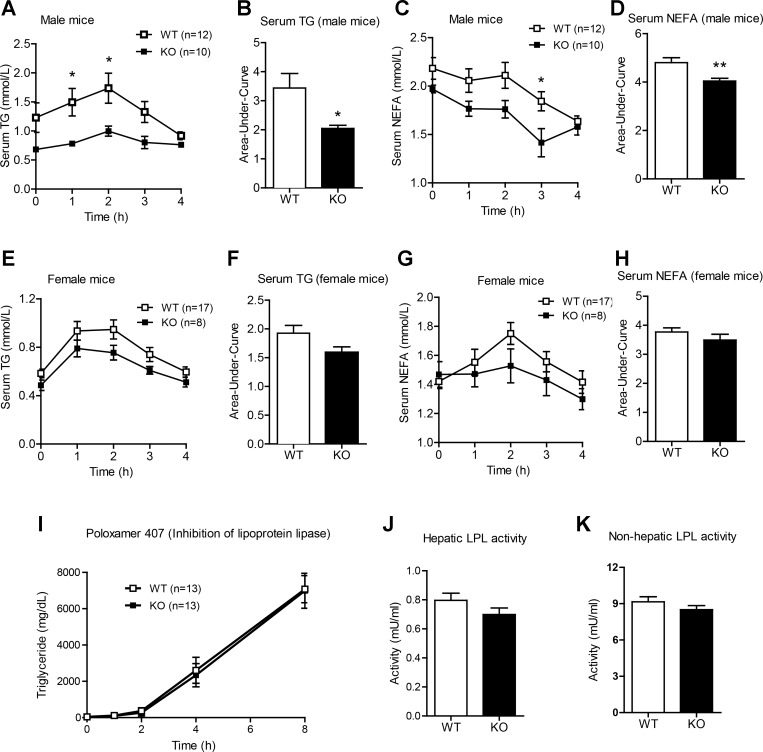
Enhanced lipid tolerance in LFD-fed Ctrp1 KO mice.
In light of hepatic TG accumulation in Ctrp1 KO mice with only modest changes in the expression of hepatic lipid metabolism genes, we decided to examine whether CTRP1 deficiency affects the ability of KO animals to handle acute lipid loading in a lipid tolerance test. After an oral lipid gavage, we observed an enhanced rate of TG and NEFA clearance in Ctrp1 KO male mice compared with WT control mice (Fig. 7, A–D). A similar trend, although not statistically significant, was also observed in female KO animals (Fig. 7, E–H). TG are predominantly secreted from the liver as VLDL particles. Reduced TG secretion may contribute to its retention in the liver, leading to the development of steatosis. To measure hepatic VLDL-TG secretion, LFD-fed WT and Ctrp1 KO mice were injected with Poloxamer 407, an inhibitor of lipoprotein lipase that blocks VLDL-TG hydrolysis and clearance (33). Loss of CTRP1 did not appear to affect the magnitude of hepatic VLDL-TG secretion relative to WT control mice (Fig. 7I). To address if CTRP1 deficiency altered intestinal lipid absorption, we injected another cohort of overnight fasted mice with Poloxamer 407 (inhibitor of LPL) and 1 h later gavaged the mice with Intralipids; the rise in circulating TG (derived from chylomicrons) allowed us to assess lipid absorption in the gut. No differences were observed between WT and KO mice (data not shown). Because intestinal lipid absorption and liver TG secretion were not altered in Ctrp1 KO mice, we next determined if LPL activity, which would dictate the rate of lipid uptake and clearance from the blood, could be different between genotypes. Both hepatic and nonhepatic lipase activity were similar between WT and KO mice (Fig. 7, J and K).
Fig. 7.
Enhanced lipid tolerances in Ctrp1 KO male mice fed a LFD. A: serum TG levels over time in WT (n = 12) and KO (n = 10) male mice challenged with an oral lipid gavage. B: AUC as shown in A. C: serum nonesterified free fatty acids (NEFA) over time in WT (n = 12) and KO (n = 10) male mice challenged with an oral lipid gavage. D: AUC as shown in C. E: serum TG levels over time in WT (n = 17) and KO (n = 8) female mice challenged with an oral lipid gavage. F: AUC as shown in E. G: serum NEFA over time in WT (n = 17) and KO (n = 8) female mice challenged with an oral lipid gavage. H: AUC as shown in G. I: there were no differences in the export of VLDL-TG from the livers of WT (n = 13) and KO (n = 13) male mice. J and K: total LPL (J) and hepatic lipase (K) activity levels in WT (n = 17) and KO (n = 13) male mice. All data are expressed as means ± SE. *P < 0.05; **P < 0.01.
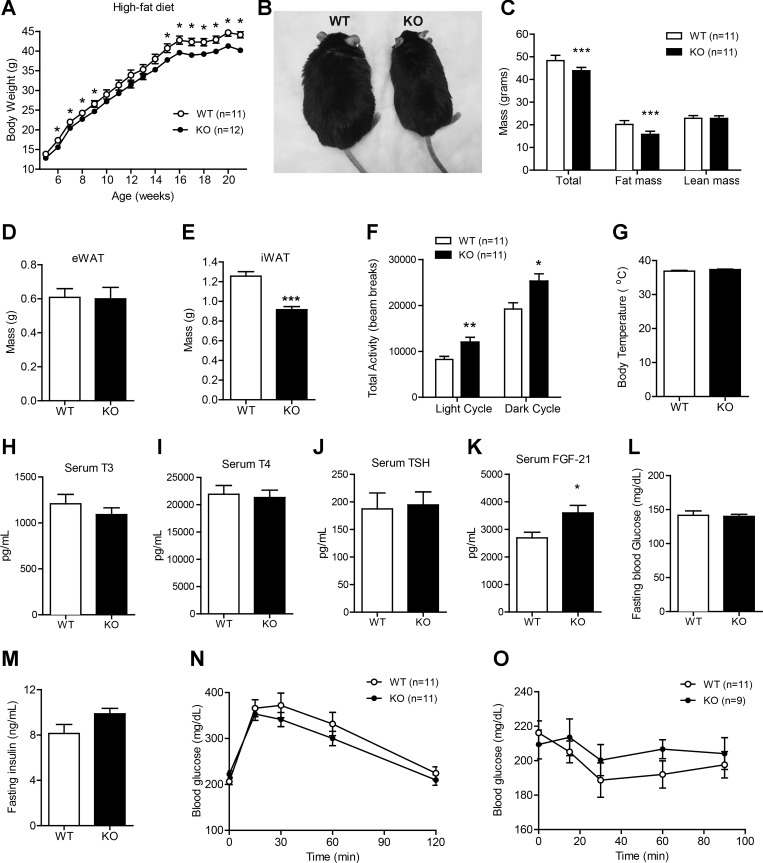
Reduced body weight gain in Ctrp1 KO mice fed a HFD.
To determine the metabolic role of CTRP1 in pathophysiological states, WT and KO mice were fed a HFD to induce obesity and insulin resistance. Unexpectedly, CTRP1 deficiency resulted in significantly less weight gain and lower total fat mass over the course of high-fat feeding (Fig. 8, A–C). Interestingly, a reduction in adiposity was seen in the subcutaneous (inguinal) but not visceral (epididymal) fat depot (Fig. 8, D and E). The decreased weight gain in Ctrp1 KO mice fed a HFD was due, at least in part, to higher physical activity (Fig. 8F). Body temperature was not different between genotypes (Fig. 8G). Given the enhanced physical activity seen in Ctrp1 KO animals, we measured circulating levels of triiodothyronine, thyroxine, and thyroid-stimulating hormone; no differences were observed between genotypes (Fig. 8, H–J). In contrast, circulating levels of FGF-21, a hormone known to promote energy expenditure and physical activity (68), were significantly elevated in KO mice (Fig. 8K). Fasting glucose (12 h) and insulin levels were similar between WT and KO mice (Fig. 8, L–M). When subjected to glucose and insulin tolerance tests, the rate of glucose disposal in the peripheral tissues was comparable between WT and Ctrp1 KO animals (Fig. 8, N and O).
Fig. 8.
Reduced body weight and adiposity and enhanced physical activity levels in Ctrp1 KO male mice fed a high-fat diet (HFD). A: body weight gain over time of WT (n = 11) and KO (n = 12) male mice fed a HFD. B: representative image of WT and KO male mice. C: body composition analysis of lean and fat mass of WT (n = 11) and KO (n = 11) male mice. D and E: visceral (epididymal; eWAT) and subcutaneous (inguinal; iWAT) fat pad weight of WT (n = 11) and KO (n = 10) mice. F: total physical activity levels in the light and dark photocycle of WT (n = 11) and KO (n = 11) mice. G–M: body temperature (G), serum triiodothyronine (T3) levels (H), serum thyroxine (T4) levels (I), serum thyroid-stimulating hormone (TSH) levels (J), serum FGF-21 levels (K), overnight fasting blood glucose levels (L), and fasting insulin levels (M) of WT (n = 11) and KO (n = 10) mice. N: blood glucose levels of WT (n = 11) and KO (n = 11) male mice during the GTT. Glucose was delivered by an intraperitoneal injection. O: blood glucose levels of WT (n = 11) and KO (n = 9) male mice during an ITT. All data are expressed as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.005.
Reduced adipose expression of lipid synthesis and fibrotic genes in HFD-fed Ctrp1 KO male mice.
Given the reduced adiposity seen in Ctrp1 KO mice, we investigated possible changes in various fat depots. Histology of visceral (epididymal) and subcutaneous (inguinal) adipose tissue sections revealed no overt differences between WT and KO mice (Fig. 9A). However, the expression of lipid uptake and synthesis genes (Scd1, Cd36, and PPAR-γ), profibrotic cytokine (TGF-β), and adipokine (adiponectin) were reduced in the visceral (epididymal) but not subcutaneous (inguinal) fat depot of Ctrp1 KO mice (Fig. 9, B–G). In contrast, the expression of hormone-sensitive lipase was found to be upregulated in the subcutaneous fat depot (Fig. 9C). Obesity has been shown to promote macrophage infiltration into adipose tissue (13, 14, 59, 66), but the total number of macrophages, as judged by the expression of the pan-macrophage marker F4/80, was not different between genotypes (Fig. 9, J and K). When we examined marker genes for proinflammatory M1 [chemokine (C-C motif) receptor 7, Ccl3, Ccl4, and nitric oxide synthase 2] or anti-inflammatory M2 (macrophage galactose N-acetyl-galactosamine-specific lectin 2, Cd206, IL-10, arginase 1, Cd68, and resistin l) macrophages, no consistent or overall differences were observed in the visceral and subcutaneous fat depot of WT and Ctrp1 KO mice (Fig. 9, H–K). In accordance with reduced profibrotic Tgfb expression and circulating levels (Fig. 9, D and S), we observed reduced expression of fibrotic collagen genes (Col3 and Col6) in the visceral but not subcutaneous fat depot (Fig. 9, L and M), indicative of reduced adipose fibrosis. Parallel to the mRNA data, we observed no differences in circulating levels of IL-6, TNF-α, MCP-1, or leptin between WT and KO mice (Fig. 9, N–Q). Serum adiponectin levels, however, were significantly lower in KO mice relative to WT control mice (Fig. 9R).
Fig. 9.
Metabolic, inflammatory, and fibrotic gene expression in the adipose tissue of Ctrp1 KO male mice fed a HFD. A: representative histology (hematoxylin and eosin stain; magnification: ×200) of the visceral (epididymal) and subcutaneous (inguinal) fat pad of WT and KO male mice on a HFD. B and C: expression of lipid uptake (Cd36), synthesis (Fasn, Scd1, and PPAR-γ) and lipolysis (Atgl and Hsl) in the visceral (epididymal; B) and subcutaneous (inguinal; C) fat depot of WT (n = 8) and KO (n = 8) mice. D and E: expression of inflammatory (IL-1β, IL-6, TNF-α, and Mcp-1) and profibrotic (TGF-β) cytokine genes in the visceral (epididymal; D) and subcutaneous (inguinal; E) fat depot of WT (n = 8) and KO (n = 8) mice. F and G: expression of Adipoq and Lep in the visceral (epididymal; F) and subcutaneous (inguinal; G) fat depot of WT (n = 8) and KO (n = 8) mice. H and I: expression of proinflammatory M1 macrophage marker genes in the visceral (epididymal; H) and subcutaneous (inguinal; I) fat depot of WT (n = 8) and KO (n = 8) mice. J and K: expression of anti-inflammatory M2 macrophage marker genes in the visceral (epididymal; J) and subcutaneous (inguinal; K) fat depot of WT (n = 8) and KO (n = 8) mice. L and M: expression of fibrotic Col genes in the visceral (epididymal; L) and subcutaneous (inguinal; M) fat depot of WT (n = 8) and KO (n = 8) mice. N–S: serum levels of IL-6 (N), TNF-α (O), MCP-1 (P), leptin (Q), adiponectin (R), and TGF-β1 (S) in WT (n = 11) and KO (n = 10) mice. All gene expression levels were normalized to 36B4. All data are expressed as means ± SE. *P < 0.05; **P < 0.01.
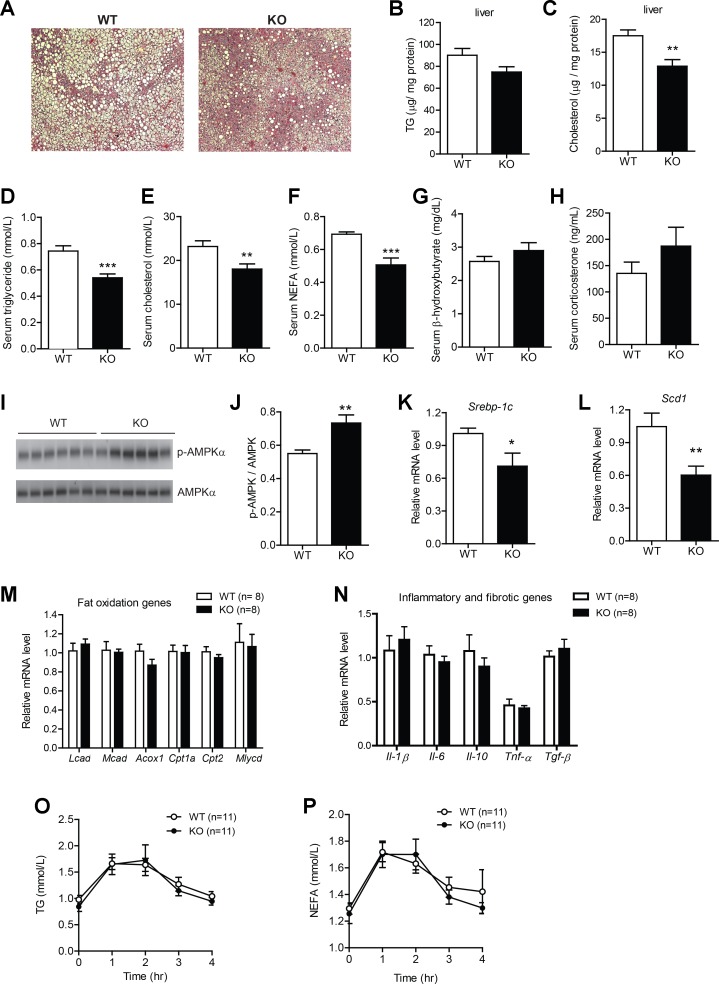
Hepatic and circulating lipid levels are reduced in HFD-fed Ctrp1 KO male mice.
In contrast to the LFD-fed group, HFD-fed Ctrp1 KO mice were found to have reduced liver steatosis in histological sections (Fig. 10A). While hepatic TG content was only modestly lower, cholesterol content was significantly reduced in the KO mouse liver relative to the WT control mouse liver (Fig. 10, B and C). Serum TG, cholesterol, and NEFA levels were also significantly lower in Ctrp1 KO mice compared with WT control mice (Fig. 10, D–F). Serum hydroxybutyrate (ketones) and corticosterone levels, however, were not different between genotypes (Fig. 10, G and H). Reduced hepatic lipid content was associated with increased AMPKα phosphorylation (Fig. 10, I and J) and reduced expression of genes (Srebp-1c and Scd1) involved in lipid synthesis (Fig. 10, K and L). The expression of multiple fat oxidation genes was not different between genotypes (Fig. 10M). The expression of inflammatory (IL-1β, IL-6, IL-10, TNF-α) and fibrotic (TGF-β) cytokine genes was also not different between WT and KO mouse livers (Fig. 10N). No differences in expression of cholesterol and bile acid metabolism genes (except for decreased ATP-binding cassette subfamily A member 1 in KO mice) were noted between genotypes (data not shown). In contrast to the LFD-fed group, oral lipid tolerance testing did not reveal any differences in the rate of TG or NEFA clearance between HFD-fed WT and KO animals (Fig. 10, O and P). Assessment of intestinal lipid absorption and LPL activity (both hepatic and nonhepatic) also revealed no differences between genotypes (data not shown).
Fig. 10.
Reduced hepatic steatosis and serum lipids in Ctrp1 KO male mice fed a HFD. A: representative image of WT littermate and KO mouse liver histology (hematoxylin and eosin stain; magnification: ×100). B and C: quantification of liver TG (B) and cholesterol (C) levels in WT (n = 10) and KO (n = 10) mice. D–H: quantification of serum TG (D), cholesterol (E), NEFA (F), β-hydroxybutyrate (G), and corticosterone (H) in WT (n = 11) and KO (n = 10) mice. I: Western blot analysis of AMPK and pAMPK (Thr172) in the liver of WT (n = 6) and KO mice (n = 6). J: quantification of Western blot results as shown in I. K and L: expression of lipid synthesis genes (Srebp-1c and Scd1) in the liver of WT (n = 8) and KO (n = 8) mice. M: expression of hepatic fat oxidation genes in WT (n = 8) and KO (n = 8) mice. N: expression of inflammatory and fibrotic genes in WT (n = 8) and KO (n = 8) mice. O and P: serum TG (O) and NEFA (P) levels over time in WT (n = 11) and KO (n = 11) male mice challenged with an oral lipid gavage. All data are expressed as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.005.
DISCUSSION
Using a loss-of-function mouse model, we provided critical genetic evidence that CTRP1 is required for metabolic homeostasis. Notably, though, the contributions of CTRP1 to energy metabolism depend on metabolic and dietary contexts. When mice are fed a LFD comparable with standard chow, loss of CTRP1 did not appear to affect body weight or metabolic rate (V̇o2). Its deficiency, however, promoted insulin resistance independent of adiposity. Mice lacking CTRP1 exhibited elevated hepatic gluconeogenic gene expression as well as elevated fasting insulin levels and reduced rates of glucose disposal in response to glucose and insulin challenge compared with WT littermate control mice (Fig. 2). In the absence of CTRP1, we also observed a reduction in the steady-state protein levels of AMPK and GLUT4 in the skeletal muscle relative to WT control mice. Furthermore, relative phospho-AMPKα (a metric of AMPK activation) was also reduced in the skeletal muscle of Ctrp1 KO animals (Fig. 3). Given that both AMPK and GLUT4 are known to play important roles in skeletal muscle glucose uptake (15, 18), the reduction in protein levels, along with decreased insulin action, likely contributes to reduced glucose disposal in response to glucose and insulin injection. In contrast to skeletal muscle, loss of CTRP1 did not alter steady-state AMPKα protein levels or relative phospho-AMPKα protein levels in adipose tissue (Fig. 4).
One of the most striking phenotypes revealed by this study was the enlargement of the liver and the development of prominent steatosis in Ctrp1 KO mice fed a LFD (Fig. 5). Several mechanisms could account for the accumulation of liver TG in Ctrp1 KO animals: 1) decreased hepatic fat oxidation, 2) increased hepatic TG synthesis, 3) decreased TG export from the liver in the form of VLDL-TG particles, or 4) increased lipid flux into the liver. We examined which of these pathways might be altered in the absence of CTRP1. With regard to hepatic fat oxidation, we did not observe any differences in the expression of hepatic fat oxidation genes (Fig. 6), nor did we observe changes in serum ketones (β-hydroxybutyrate acids) levels, a surrogate indicator of hepatic fat oxidation. Furthermore, the RER did not indicate any differences in fat oxidation between WT and KO mice. In the liver, TG are synthesized via the glycerol phosphate pathway (2) through the sequential acylation of glycerol-3 phosphate, lysophosphatidic acid, and diacylglycerol by multiple isoforms of GPAT, AGPAT, and DGAT enzymes (51, 70). With the exception of increased Agpat1 expression, the expression of genes involved TG synthesis or de novo lipogenesis was not found to be different between genotypes. The use of a LPL inhibitor (Poloxamer 407) to block TG hydrolysis and uptake into peripheral tissues allowed us to measure the accumulation of serum TG due to hepatic VLDL-TG export, and no differences in the rate of TG export were observed between WT and Ctrp1 KO mice (Fig. 7). Finally, we performed lipid tolerance tests to determine whether CTRP1 deficiency alters the clearance rate of ingested lipids. Interestingly, loss of CTRP1 enhanced lipid clearance relative to WT control mice (Fig. 7). Ingested lipids (TG and free fatty acids) are normally delivered to the liver from the intestine via the lymphatic system, in the form of chylomicrons, to be repackaged into VLDL-TG particles before being exported out to peripheral tissues. Assessment of intestinal lipid absorption revealed no difference between WT and KO mice. Neither hepatic nor nonhepatic LPL activity, which determined tissue uptake of TG derived from chylomicrons or VLDL, differed between genotypes. Thus, the mechanism that contributes to the accumulation of TG seen in the liver of Ctrp1 KO mice fed a LFD remains to be determined.
In our recent description of the CTRP1 transgenic mouse model, we illustrated that the protective role of CTRP1 was only revealed when mice were challenged with a HFD to induce obesity and insulin resistance (38). We subjected Ctrp1 KO animals to a HFD to determine whether the loss of Ctrp1 might amplify the effects of the HFD. Given that CTRP1 overexpression attenuates metabolic dysfunction induced by a HFD (38) and that Ctrp1 KO mice develop insulin resistance and fatty liver on a LFD, we expected KO animals to develop pronounced glucose intolerance and an even greater degree of liver steatosis when challenged with a HFD. Surprisingly, we observed the opposite. Ctrp1 KO mice that consumed a HFD were leaner, with reduced body weight and adiposity compared with WT littermate controls (Fig. 8). Glucose and insulin tolerance were not significantly different between genotypes, suggesting that the HFD-fed Ctrp1 KO animals were not more insulin resistant than their WT counterparts. An unexpected finding was that Ctrp1 KO mice were significantly more active, during both the light and dark phases of the photocycle compared with HFD-fed WT littermate controls. The activity levels of HFD-fed Ctrp1 KO mice were comparable to KO animals fed a LFD (Table 3); in contrast, HFD-fed WT mice had significantly lower physical activity levels compared with LFD-fed WT animals. Food intake, however, was not different between genotypes on a HFD. The increased physical activity, reduced hepatic steatosis, and improvements in serum lipid profiles seen in HFD-fed Ctrp1 KO mice could be partially attributed to increased circulating levels of FGF-21 (Fig. 8K). FGF-21 is a hormone that known to exert beneficial effects on glucose and lipid metabolism (9, 44). FGF-21 administration has been shown to reduce hepatic steatosis and increase energy expenditure, physical activity, and fat oxidation (1, 67). Thus, the increased physical activity without changes in caloric intake likely contributed, at least in part, to the lower weight gain and adiposity seen in HFD-fed Ctrp1 KO animals relative to WT control animals.
In contrast to the LFD-fed Ctrp1 KO animals that developed fatty liver, KO animals that consumed a HFD unexpectedly had reduced hepatic steatosis compared with WT control animals (Fig. 10). Both hepatic and serum TG levels were reduced in HFD-fed Ctrp1 KO mice. Unlike LFD-fed KO mice, lipid tolerance testing did not reveal any differences in the rate of TG and free fatty acid clearance between HFD-fed WT and KO animals. The reduced body weight and adiposity likely contributed, in part, to the decreased liver steatosis seen in HFD-fed Ctrp1 KO animals. Other factors, such as reduced hepatic expression of lipid synthesis genes (Srebp-1c and Scd1) and an increase in the relative phosphorylation and activation of AMPK (Fig. 10), could also contribute to the lower hepatic lipid content observed in Ctrp1 KO animals. Although less adiposity, a healthier liver, and improved serum lipid levels frequently associate with an improved systemic metabolic profile, the observed reduction in adiposity, hepatic steatosis, and serum lipid levels seen in Ctrp1 KO mice (Figs. 8 and 10) did not appear to affect systemic glucose metabolism, as indicated by lack of differences in glucose and insulin tolerance tests between genotypes (Fig. 8).
Adipose tissue inflammation and fibrosis, particularly in the context of obesity, are known to alter the expression and secretion of adipokines; this, in turn, has systemic effects on energy metabolism and insulin sensitivity (14, 49). Given that CTRP1 is abundantly expressed in adipose tissue (62), we assessed the impact of CTRP1 deficiency on the expression of genes involved in lipid uptake and synthesis, inflammation, macrophage polarization, and tissue fibrosis. With the exception of reduced fibrotic collagen gene expression, loss of CTRP1 had a relatively minor impact on adipose tissue function when mice were fed a LFD (Fig. 4). In the context of HFD-induced metabolic stress, however, the expression of multiple lipid metabolism genes (Scd1, Cd36, and PPAR-γ) was significantly reduced in the adipose tissue of Ctrp1 KO mice (Fig. 9). The adipose expression and circulating levels of profibrotic TGF-β were also reduced in Ctrp1 KO animals. Since TGF-β is a potent inducer of fibrotic collagen gene expression, its reduction in mRNA and circulating levels likely contributed to the decreased expression of Col3 and Col6. While adipose mass and fibrosis are known to impact systemic metabolism (49), their reductions in Ctrp1 KO mice were likely insufficient to alter systemic insulin action (Fig. 8).
Adiponectin is a widely studied insulin-sensitizing adipokine with pleiotropic metabolic function (17, 69). Interestingly, serum adiponectin levels were lower in both LFD and HFD-fed Ctrp1 KO mice compared with WT control mice. Although serum adiponectin levels were reduced in LFD-fed Ctrp1 KO mice (Fig. 4), these changes are unlikely to account for the insulin resistance and fatty liver phenotypes observed in our study. Three independent adiponectin KO mouse lines, when fed a chow diet comparable with the LFD, are largely indistinguishable from WT control mice, with minimum or no detectable metabolic abnormalities (23, 28, 29, 36). When challenged with a HFD, different adiponectin KO mouse lines develop variable, and relatively mild, degrees of insulin resistance compared with WT control mice. In our study, insulin sensitivity was not different between HFD-fed WT and Ctrp1 KO mice despite reduced serum levels of adiponectin (Figs. 8 and 9).
Given the increasing appreciation of sex-dependent differences in metabolic disease phenotypes and severity (34, 54), we included female WT and KO animals in our experiments. Unlike male mice, Ctrp1 KO female mice that consumed a control LFD did not develop insulin resistance, glucose intolerance, or fatty liver. When challenged with a HFD, the metabolic phenotypes (body weight, adiposity, energy expenditure, physical activity, and glucose and insulin tolerance) of Ctrp1 KO female mice were indistinguishable from female WT littermate control mice (Tables 2 and 3 and data not shown). Thus, loss of CTRP1 likely contributes to dysregulated metabolism in a sex-dependent manner. Given the myriad physiological roles of sex hormones, this is neither unexpected nor surprising as the metabolic phenotypes of many loss-of-function mouse models are often manifested in male but not female animals.
In summary, our results support an important role for CTRP1 in metabolic homeostasis. The contribution of CTRP1 to systemic glucose and lipid metabolism is sex dependent and relies on the specific metabolic and dietary context. When fed a LFD, loss of CTRP1 impaired hepatic lipid metabolism and systemic insulin sensitivity. In the context of a HFD, CTRP1 deficiency attenuated diet-induced obesity and fatty liver. Our study underscores the complex regulation of whole body metabolism by secreted regulators of the CTRP family. Future studies will help unravel the cellular mechanisms used by CTRP1 to control various aspects of glucose and lipid metabolism.
GRANTS
This work was supported, in part, by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-084171 to G. W. Wong), a postdoctoral fellowship from the American Heart Association (POST17070119 to X. Lei), a postdoctoral fellowship from the Carlsberg Foundation and Danish Council for Independent Research (DFF-4183-00634 to P. S. Petersen), and predoctoral fellowships from the American Heart Association (PRE16690003 to S. Y. Tan and PRE25710315 to H. C. Little).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.R., X.L., P.S.P., S.Y.T., and H.C.L. performed experiments; S.R., X.L., and G.W.W. analyzed data; S.R. and G.W.W. interpreted results of experiments; S.R. prepared figures; S.R. and G.W.W. drafted manuscript; S.R., X.L., P.S.P., S.Y.T., H.C.L., and G.W.W. edited and revised manuscript; S.R., X.L., P.S.P., S.Y.T., H.C.L., and G.W.W. approved final version of manuscript; G.W.W. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Susan Aja for the help with indirect calorimetry.
REFERENCES
- 1.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem 49: 459–487, 1980. [DOI] [PubMed] [Google Scholar]
- 3.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byerly MS, Petersen PS, Ramamurthy S, Seldin MM, Lei X, Provost E, Wei Z, Ronnett GV, Wong GW. C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J Biol Chem 289: 4055–4069, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byerly MS, Swanson R, Wei Z, Seldin MM, McCulloh PS, Wong GW. A central role for C1q/TNF-related protein 13 (CTRP13) in modulating food intake and body weight. PLoS One 8: e62862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab 295: E1269–E1276, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Chalupova L, Zakovska A, Adamcova K. Development of a novel enzyme-linked immunosorbent assay (ELISA) for measurement of serum CTRP1: a pilot study: measurement of serum CTRP1 in healthy donors and patients with metabolic syndrome. Clin Biochem 46: 73–78, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 361: 1152–1163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, Kahn CR. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest 125: 458–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 functions as an adipokine that improves glucose metabolism. J Biol Chem 286: 34552–34558, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gui Y, Silha JV, Murphy LJ. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res 12: 1481–1491, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Han S, Park JS, Lee S, Jeong AL, Oh KS, Ka HI, Choi HJ, Son WC, Lee WY, Oh SJ, Lim JS, Lee MS, Yang Y. CTRP1 protects against diet-induced hyperglycemia by enhancing glycolysis and fatty acid oxidation. J Nutr Biochem 27: 43–52, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92: 2240–2247, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH, Kim JW, Kim D, Han SH, Lim JS, Kim KI, Yoon do Y, Kim SH, Oh GT, Kim E, Yang Y. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 22: 1502–1511, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem 287: 18965–18973, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kambara T, Shibata R, Ohashi K, Matsuo K, Hiramatsu-Ito M, Enomoto T, Yuasa D, Ito M, Hayakawa S, Ogawa H, Aprahamian T, Walsh K, Murohara T, Ouchi N. C1q/tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-dependent mechanism. Mol Cell Biol 35: 2173–2185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KY, Kim HY, Kim JH, Lee CH, Kim DH, Lee YH, Han SH, Lim JS, Cho DH, Lee MS, Yoon S, Kim KI, Yoon DY, Yang Y. Tumor necrosis factor-α and interleukin-1β increases CTRP1 expression in adipose tissue. FEBS Lett 580: 3953–3960, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, Bullock TM, Tso P. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am J Physiol Gastrointest Liver Physiol 302: G628–G636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277: 25863–25866, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: E261–E270, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Lei X, Li Q, Rodriguez S, Tan SY, Seldin MM, McLenithan JC, Jia W, Wong GW. Thromboxane synthase deficiency improves insulin action and attenuates adipose tissue fibrosis. Am J Physiol Endocrinol Metab 308: E792–E804, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lu L, Zhang RY, Wang XQ, Liu ZH, Shen Y, Ding FH, Meng H, Wang LJ, Yan XX, Yang K, Wang HB, Pu LJ, Zhang Q, Chen QJ, De Caterina R, Shen WF. C1q/TNF-related protein-1: an adipokine marking and promoting atherosclerosis. Eur Heart J 37: 1762–1771, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased β-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277: 34658–34661, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 5: 197–216, 2004. [DOI] [PubMed] [Google Scholar]
- 32.McLean JA, Tobin G. Animal and Human Calorimetry. New York: Cambridge Univ. Press, 1987. [Google Scholar]
- 33.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res 46: 2023–2028, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Miller VM. Why are sex and gender important to basic physiology and translational and individualized medicine? Am J Physiol Heart Circ Physiol 306: H781–H788, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murayama MA, Kakuta S, Maruhashi T, Shimizu K, Seno A, Kubo S, Sato N, Saijo S, Hattori M, Iwakura Y. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun 443: 42–48, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 281: 2654–2660, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Pan X, Lu T, Wu F, Jin L, Zhang Y, Shi L, Li X, Lin Z. Circulating complement-C1q TNF-related protein 1 levels are increased in patients with type 2 diabetes and are associated with insulin sensitivity in Chinese subjects. PLoS One 9: e94478, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson JM, Aja S, Wei Z, Wong GW. C1q/TNF-related protein-1 (CTRP1) enhances fatty acid oxidation via AMPK activation and ACC inhibition. J Biol Chem 287: 1576–1587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson JM, Seldin MM, Tan SY, Wong GW. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS One 9: e88535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol 305: G214–G224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 305: R522–R533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 285: 39691–39701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes 57: 1824–1833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlein C, Talukdar S, Heine M, Fischer Alexander W, Krott Lucia M, Nilsson Stefan K, Brenner Martin B, Heeren J, Scheja L. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab 23: 441–453, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun 452: 8–13, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287: 11968–11980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Y, Lu L, Liu ZH, Wu F, Zhu JZ, Sun Z, Zhang RY, Zhang Q, Hu J, Chen QJ, Wu ZG, Shen WF. Increased serum level of CTRP1 is associated with low coronary collateralization in stable angina patients with chronic total occlusion. Int J Cardiol 174: 203–206, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFα-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol 108: 315–326, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 18: 470–477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, Wang Y, Su H, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation 128: S113–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296: E1195–E1209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52: 1836–1846, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J 27: 25–33, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 5: 241, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab 306: E779–E790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation of AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem 286: 15652–15665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 288: 10214–10229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf RM, Lei X, Yang ZC, Nyandjo M, Tan SY, Wong GW. CTRP3 deficiency reduces liver size and alters IL-6 and TGF-β levels in obese mice. Am J Physiol Endocrinol Metab 310: E332–E345, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 23: 241–258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, Li L, Wu LL. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J Mol Med (Berl) 93: 1311–1325, 2015. [DOI] [PubMed] [Google Scholar]
- 65.Xin Y, Lyu X, Wang C, Fu Y, Zhang S, Tian C, Li Q, Zhang D. Elevated circulating levels of CTRP1, a novel adipokine, in diabetic patients. Endocr J 61: 841–847, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Young Jung D, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab 2: 133–141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49: 2283–2301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation 125: 3159–3169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuasa D, Ohashi K, Shibata R, Mizutani N, Kataoka Y, Kambara T, Uemura Y, Matsuo K, Kanemura N, Hayakawa S, Hiramatsu-Ito M, Ito M, Ogawa H, Murate T, Murohara T, Ouchi N. C1q/TNF-related protein-1 functions to protect against acute ischemic injury in the heart. FASEB J 30: 1065–1075, 2016. [DOI] [PubMed] [Google Scholar]
- 73.Yuasa D, Ohashi K, Shibata R, Takeshita K, Kikuchi R, Takahashi R, Kataoka Y, Miyabe M, Joki Y, Kambara T, Uemura Y, Matsuo K, Hayakawa S, Hiramatsu-Ito M, Ito M, Ikeda N, Murohara T, Ouchi N. Association of circulating C1q/TNF-related protein 1 levels with coronary artery disease in men. PLoS One 9: e99846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol 31: 2616–2623, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]