Abstract
Sepsis disrupts skeletal muscle proteostasis and mitigates the anabolic response to leucine (Leu) in muscle of mature animals. We have shown that Leu stimulates muscle protein synthesis (PS) in healthy neonatal piglets. To determine if supplemental Leu can stimulate PS and reduce protein degradation (PD) signaling in neonatal muscle during endotoxemia, overnight-fasted neonatal pigs were infused for 8 h with LPS or saline while plasma amino acids, glucose, and insulin were maintained at fasting levels during pancreatic-substrate clamps. Leu or saline was infused during the last hour. Markers of PS and PD were determined in skeletal muscle. Compared with controls, Leu increased PS in longissimus dorsi (LD), gastrocnemius, and soleus muscles. LPS decreased PS in these three muscles by 36%, 28%, and 38%, but Leu antagonized that reduction by increasing PS by 84%, 81%, and 83%, respectively, when supplemented to LPS. Leu increased eukaryotic translation initiation factor (eIF)3b-raptor interactions, eIF4E-binding protein-1, and S6 kinase 1 phosphorylation as well as eIF4E·eIF4G complex formation in LD, gastrocnemius, and soleus muscles of control and LPS-treated pigs. In LD muscle, LPS increased the light chain (LC)3-II-to-LC3 ratio and muscle-specific RING finger (MuRF-1) abundance but not atrogin-1 abundance or AMP-activated protein kinase-α phosphorylation. Leu supplementation to LPS-treated pigs reduced the LC3-II-to-LC3 ratio, MuRF-1 abundance, and AMP-activated protein kinase-α phosphorylation compared with LPS alone. In conclusion, parenteral Leu supplementation attenuates the LPS-induced reduction in PS by stimulating mammalian target of rapamycin complex 1-dependent translation and may reduce PD by attenuating autophagy-lysosome and MuRF-1 signaling in neonatal skeletal muscle.
Keywords: leucine, skeletal muscle, protein synthesis, protein degradation, autophagy
mortality in pediatric patients with acute severe sepsis has decreased significantly in the current era, but in sepsis survivors, the prevalence of residual comorbidities and subsequent disabilities related to the initial inflammatory state impacts late mortality and remains a serious public health problem (11, 52). As part of those comorbidities, acute sepsis can cause >30% loss of muscle mass secondary to skeletal muscle dyshomeotasis in older children and in adults (19, 47), whereas in neonates this imbalance is reflected as growth arrest and may lead to long-term adverse neurological outcomes (6, 19). The erosion in muscle mass seen during sepsis results from a combination of two factors: a decrease in protein synthesis (PS) and an increase in protein degradation (PD) in skeletal muscle (33–35, 43, 46, 47). Thus, innovative therapeutics that attenuate the consequences of skeletal muscle dyshomeostasis during and after sepsis are needed.
Healthy neonates are highly sensitive to nutrient and hormonal stimulation (13) and have a high capacity to synthesize muscle protein in response to the rise in amino acids (AAs) and insulin after a meal (13). Leucine (Leu), an essential and branched-chain AA, is unique in its ability to act not only as a substrate for PS but also as a signaling molecule that stimulates PS (10). Our group and others have demonstrated that Leu administration in healthy neonatal and mature animals stimulates skeletal muscle PS by stimulating mRNA translation initiation (2, 3, 17, 49).
PS is mainly regulated during mRNA translation initiation (23, 25). This phase can be divided into two main steps. The first step leads to the formation of the 43S preinitiation complex, which is controlled and facilitated by eukaryotic translation initiation factor (eIF)2. The second step requires the activation of the mammalian target of rapamycin complex 1 (mTORC1) master kinase. This activation is partly regulated by intracellular protein-protein interactions within mTORC1, such as eIF3b with raptor (21). The eIF3b-raptor complex prompts the phosphorylation of eIF4E-binding protein-1 (4EBP1) and S6 kinase 1 (S6K1) (21, 27) and stimulates mRNA recruitment to the 43S preinitiation complex. This process requires the association of eIF4E with eIF4G to form the eIF4E·eIF4G active complex, which is promoted by the phosphorylation of 4EBP1 (23, 25, 26). Activation of S6K1 also enhances PS by increasing the translation of mRNA families that encode ribosomal proteins (25, 48).
The mTORC1 signaling pathway is downregulated during sepsis (21, 29), and its decreased responsiveness to AAs includes a dampened effectivity of Leu to stimulate translation initiation (“Leu resistance”) in septic adult rats (21, 31, 32, 36, 51). Kazi et al. (27) have suggested that decreased eIF3b binding activity to raptor may underline the sepsis-induced reduction in PS in skeletal muscle of adult rodents. Since the eIF3b-raptor interaction appears to be a crucial step for the stimulation of mRNA translation, its alteration during endotoxemia in mature animals may play an important role in blocking the anabolic response to Leu (21, 27). Sepsis, induced by either cecal ligation and puncture or Esherichia coli lipopolysaccharide (LPS) infusion, in mature rats decreased the phosphorylation of mTORC1, S6K1, and 4EBP1 as well as the amount of eIF3b bound to mTORC1-raptor (21, 27). Sepsis increased the phosphorylation of raptor and AMP-activated protein kinase (AMPK) as well and inhibited the disassociation of 4EBP1 with eIF4E (22, 27, 29, 32), although raptor-mTOR upstream signaling appeared largely unaltered (22, 27). In response to Leu administration, eIF3b-raptor association failed to increase. Accordingly, the disassociation of 4EBP1 from eIF4E remained completely blocked, and the phosphorylation of S6K1 and 4EBP1 was blunted in septic rats (27). Although neonatal muscle is highly sensitive to stimulation by AAs, it is not known whether Leu supplementation will be effective in stimulating muscle in the presence of sepsis in the neonate.
In addition to a reduction in PS, sepsis induces the activation of PD in skeletal muscle. This process aims to release muscle AAs to the liver and to immune system organs to synthesize acute-phase proteins to support the systemic inflammatory response (40, 43). In skeletal muscle during sepsis, reduced activation of PKB, in the presence of low insulin levels and AMPK activation secondary to cellular starvation, will activate forkhead box O (FoxO) nuclear transcription factors to upregulate proteosomal degradation through the ubiquitin ligases MAFbx (atrogin-1) and muscle-specific RING finger (MuRF-1) (28, 40) and the autophagy system through activation of microtubule-associated protein 1 light chain (LC)3 (39, 47). The depression of PKB signaling and activation of AMPK in skeletal muscle also leads to inhibition of the mTORC1 pathway and the activation of the PD pathway (9, 47).
To determine if Leu supplementation can ameliorate the catabolic effects of sepsis on PS and PD in neonatal muscle, neonatal pigs were infused for 8 h with LPS or saline while plasma AAs, glucose, and insulin were maintained at fasting levels during pancreatic-substrate clamps. The endotoxemic-pancreatic-substrate clamp technique was used to control the rise in circulatory insulin and the fall in circulating AA concentrations that occur secondary to LPS infusion (44) and to elucidate the independent action of Leu on PS and PD signaling in neonatal skeletal muscle.
MATERIALS AND METHODS
Animals and experimental design.
Twenty-eight cross-bred piglets (Yorkshire × Landrace × Hampshire × Duroc; 19 male pigs and 9 female pigs) from Rosenbaum farm (Burton, TX) were weaned 24 h postbirth, housed in individual cages in a thermally controlled room (82°F), and fed milk replacement formula ad libitum (Soweena Litter Life, Merrick's, Middletown, WI). At 2 days of age, they were fasted overnight and underwent surgery for jugular and carotid cervical catheter implantation using sterile techniques (15) and isoflurane (PPC, Richmond Hill, ON, Canada). The surgery survival rate was 97.3%. At 6–7 days of age (1.98 ± 0.27 kg), pigs were assigned randomly to control (Con; n = 7), Con+Leu (Con+L)-treated (n = 6), LPS-treated (n = 8), and LPS+Leu (LPS+L)-treated (n = 7) groups. Experiments were performed according to the Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Pancreatic-substrate clamps.
After an overnight fast, animals were placed in a sling system to perform pancreatic-substrate clamps (Fig. 1). Baseline blood samples before the experiment were obtained to target fasting levels of plasma glucose and branched-chain AAs (BCAA) using the glucose oxidase method (YSI model 2300, Yellow Springs Instrument, Yellow Springs, OH) and real-time spectrophotometric measurements of BCAA (5), respectively. The clamp was initiated by infusing 600 μg·kg body wt−1·h−1 somatostatin (Bachem, Torrance, CA) for 9 h after a 600 μg·kg body wt−1 prime dose. Replacement of glucagon (150 ng·kg body wt−1·h−1, Sigma-Aldrich, Milwaukee, WI) to maintain physiological levels and insulin (5 ng·kg body wt−1·min−1, Sigma-Aldrich, St. Louis, MO) to sustain a fasting insulinemic state were initiated 15 min after the start of the somatostatin infusion. Glucose and AAs were clamped ±10th percentile of the individual baseline fasting levels by adjusting the infusion rate of 10% dextrose solution and a balanced mixture of essential [arginine, histidine, isoleucine, Leu, lysine, methionine, phenylalanine (Phe), theonine, tryptophan, and valine] and nonessential (alanine, asparagine, aspartic acid, glutamine, glutamic acid, glycine, ornithine, proline, serine, taurine, and tyrosine) AAs made according to the whole body protein composition of a neonatal piglet (13, 46). One hour after somatostatin infusion was initiated (t0), LPS-treated groups received a continuous intravenous infusion of E. coli endotoxin (10 μg·kg body wt−1·h−1, lyophilized E. coli serotype 0111-B4, Sigma-Aldrich) for 8 h, whereas the control group intravenously received an equal volume of sterile normal saline solution. Rectal temperature was measured every hour to assess signs of a systemic response to LPS. After 7 h of intravenous LPS or saline infusion, Leu was infused for 1 h at 400 μmol·kg body wt−1·h−1 after a 240 μmol/kg body wt prime dose. Saline was intravenously infused in non-Leu-treated groups.
Fig. 1.
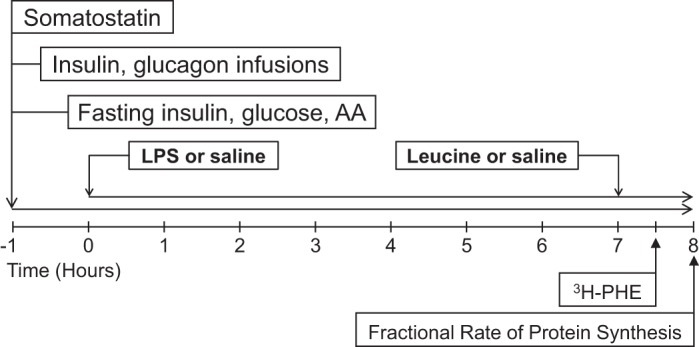
Schematic representation of endotoxemic pancreatic-substrate clamps, indicating the times of hormonal infusion, substrate clamp, LPS infusion, and leucine (Leu) supplementation. AA, amino acid; Phe, phenylalanine.
Circulating insulin, glucagon, and substrate assays.
Blood samples were obtained at baseline levels and every 60 min for later determination of circulating insulin, glucose, and individual AA concentrations. Plasma radioimmunoreactive glucagon and insulin concentrations were analyzed using a porcine insulin radioimmunoassay kit (EMD Millipore, Billerica, MA, and LINCO Research, St. Louis, MO, respectively). Free AA concentrations in plasma were quantified by HPLC (PICO-TAG reverse-phase column, Waters, Mildford, MA) using an analytic method based on deproteinization and derivatization of AAs with phenylisothiocyanate (8). Whole body net AA and glucose disposal rates were calculated from the infusion rates necessary to achieve fasting levels of AAs and glucose (42).
PS rate.
Eight hours and 30 min after the clamp was initiated, animals were injected with an intravenous flooding dose of Phe (10 ml/kg body wt, American Radiolabeled Chemicals, St. Louis, MO), which provided 1.5 mmol Phe/kg body wt and 0.5 mCi of l-[4-3H]Phe/kg body wt. Plasma samples were collected at 5, 15, and 30 min after infusion for measurements of the radioactivity of the Phe blood pool. Piglets were euthanized immediately after the last blood sample, and tissues were collected. Longissimus dorsi (LD), gastrocnemius, and soleus muscles were removed as well as the diaphragm, heart, lung, liver, and ileum. All tissues were frozen in liquid nitrogen and stored at −70°C until analysis; the procedure to process the tissue has been previously described (15).
Fractional rates of PS (KS) or the percentage of protein mass synthesized in a day were calculated (15) using the following formula: KS (%/day) = [(SB/SA) × (1,440/t)] × 100, where SB is the specific radioactivity of the protein-bound Phe, SA is the specific radioactivity of the tissue-free Phe at the time of the tissue collection corrected by the linear regression of the blood-specific radioactivity of the animal against time, and t is the time of labeling (in min). As previously described, after administration of a flooding dose of radioactive labeled Phe, the specific radioactivity of tissue-free Phe is in equilibrium with aminoacyl tRNA-specific radioactivity; thereby, tissue-free Phe is a valid measure of the tissue precursor pool specific radioactivity (14).
Translational capacity and efficiency.
Total tissue protein was quantified using the Pierce BCA assay (37), and RNA content was quantified according to Munro and Fleck (41). Ribosomal abundance or protein synthetic capacity (CS) was estimated as the total RNA-to-protein ratio (i.e., mg RNA/g protein), because the majority of RNA in the tissue is ribosomal (20). Ribosomal translational efficiency or protein synthetic efficiency (KRNA) was estimated as the total protein synthesized per total RNA (i.e., g protein/g RNA).
Immunoblot analysis.
Tissue samples were homogenized and centrifuged at 10,000 g for 10 min at 4°C. Supernatants were diluted in sample buffer and stored at −70°C until analysis (50). Equal amounts of protein samples were electrophoretically separated on polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules CA). These membranes were blocked for 1 h in blocking buffer (5% fat-free milk in Tris-buffered saline with Tween 20). Membranes were incubated overnight with the required primary antibodies. Membranes were next washed three times for 10 min in Tris-buffered saline with Tween 20 followed by 1 h of incubation with the corresponding secondary antibodies (1:5,000; goat anti-mouse and anti-rabbit peroxidase-conjugated antibodies, catalog no. 1721011 and 1706515, Bio-Rad), as previously described (50). Membranes were then washed as indicated above and sequentially exposed to enhanced chemiluminescence reagent (GE Healthcare Bio-Sciences, Pittsburgh, PA). Band quantification was done using the ChemiDoc-It Imaging System (UVP, Upland, CA). The analysis of arbitrary densitometric units of all the bands was in the range of linearity that was previously established. For normalization, immunoblots performed with anti-phospho-specific antibodies were exposed to stripping buffer (Pierce Biotechnology, Rockford, IL) and reprobed with nonphospho-specific antibodies, as previously described (50). The total abundance of the respective protein was used for the normalization of the phosphorylated forms. The abundance of nonphosphorylated proteins was normalized using GAPDH abundance. Primary antibodies used in the immunoprecipitation and Western blot analysis are listed below. Polyclonal antibodies to PKB (catalog no. 9272), phospho-4EBP1 (Thr70) (catalog no. 9455), eukaryotic translation elongation factor (eEF)2 (catalog no. 2332), phospho-eEF2 (Thr56; catalog no. 2331), eIF2α (catalog no. 9722), phospho-eIF2α (Ser51; catalog no. 9721), LC3 (catalog no. 4108), AMPKα (catalog no. 2532), and eIF4E (catalog no. 9742) as well as monoclonal antibodies to phospho-PKB (Ser473) (catalog no. 4051), phospho-S6K1 (Thr389) (catalog no. 9234), phospho-AMPKα (Thr172) (catalog no.2535), and raptor (catalog no. 2280) were supplied by Cell Signaling Technology (Beverly, MA). A polyclonal antibody to S6K1 (catalog no. SC-230) was from Santa Cruz Biotechnology (Dallas, TX). A polyclonal antibody to eIF4G (catalog no. NB100-268) was from Novus Biologicals (Littleton, CO). A polyclonal antibody to MuRF-1 (catalog no. AF5366) was from R&D Systems (Minneapolis, MN). A polyclonal antibody to atrogin-1 (catalog no. AP2041) was from ECM Biosciences (Versailles, KY). A polyclonal antibody to GAPDH (1:8,000, catalog no. AP7873a) was from Abgent (San Diego, CA). Polyclonal antibodies to 4EBP1 (catalog no. A300-501A-M) and eIF3b (catalog no. A301-761A-M) were from Bethyl Laboratories (Montgomery, TX). Unless otherwise stated, these antibodies were used under the same conditions (1:1,000 dilution, in blocking buffer).
Immunoprecipitations.
Immunoprecipitations were performed as previously described (16, 50) with slight modifications. Briefly, tissues homogenates containing 1 mg protein were incubated with 0.5 μg antibody (anti-eIF4E monoclonal antibody, gift of Dr. Leonard Jefferson, Pennsylvania State University, College of Medicine, Hershey, PA; or eIF3b polyclonal antibody) at 4°C overnight with gentle shaking. The next day, 200 μg of BioMag goat anti-rabbit or anti-mouse IgG beads (Qiagen, Valencia, CA), which had been previously blocked with low-salt buffer containing 1% milk, were added to each sample and incubated at 4°C with gentle shaking for 2 h. After incubation, beads were washed twice with 1 ml ice-cold low-salt buffer and once with high-salt buffer. beads were suspended in 100 μl of 1× SDS sample buffer followed by boiling for 5 min. After centrifugation, supernatants were subjected to immunoblot analysis using eIF4G polyclonal antibody or raptor monoclonal antibody. Amounts of eIF4G and raptor were corrected by the immunoprecipitated eIF4E or eIF3b abundance, respectively.
Statistical analysis.
Data for each condition are summarized as means ± SE, with 6–8 animals/treatment group. Statistical analysis was performed using ANOVA for a complete randomized design using statistical software (version 19, SPSS). When a significant effect was determined, means were compared using Fisher's protected least-significant-difference post hoc test. Differences were considered significant at P < 0.05. Pearson's correlation was performed between the eIF3b-raptor association and KS, 4EBP1, and S6K1 phosphorylation as well as eIF4E·eIF4G complex formation. Correlations were considered significant at the 0.01 level.
RESULTS
Metabolic response to LPS during pancreatic-substrate clamps.
Pigs that received endotoxin developed fever (P < 0.05; Fig. 2). Circulating insulin concentrations did not increase in response to LPS or Leu and remained below 3 μU/ml, thus achieving fasting levels (2–5 μU/ml) in all groups. During the pancreatic-susbtrate clamps, plasma glucagon concentrations were maintained at fasting levels in saline-infused groups but increased in LPS-infused groups (P ≤ 0.05; Table 1). No differences were found between Leu- and non-Leu-infused groups. Net whole body glucose disposal rates were similar in all groups. LPS increased whole body AA disposal rates in the presence or absence of Leu (P ≤ 0.05; Table 1). Blood glucose and plasma BCAA concentrations were maintained at baseline fasting levels during the study in all groups.
Fig. 2.
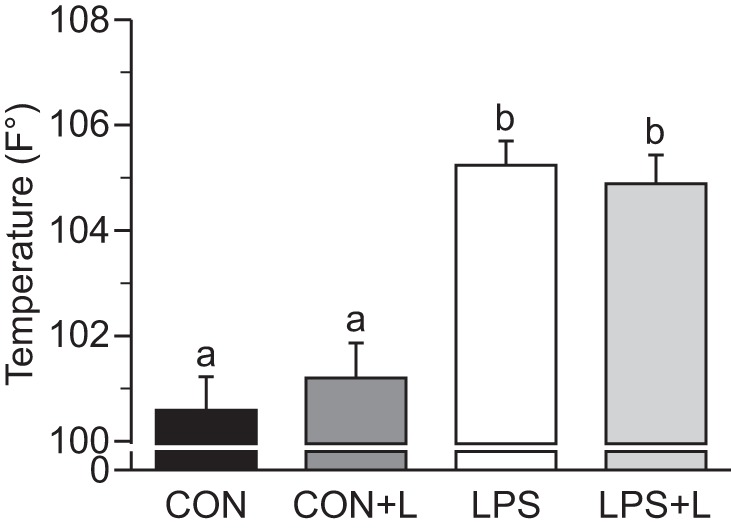
Maximum temperature in piglets infused with Leu during endotoxemic pancreatic-substrate clamps. Con, control group; Con+L, control + Leu-treated group; LPS, LPS-treated group; LPS+L, LPS + Leu-treated group. Values are means ± SE; n = 6–8. Means without a common letter differed (P ≤ 0.05).
Table 1.
Plasma insulin, glucagon, and glucose levels as well as whole body glucose and AA disposal rates in piglets infused with leucine for 1 h at the end of 8-h endotoxemic pancreatic-substrate clamps
| Con | Con+L | LPS | LPS+L | |
|---|---|---|---|---|
| Plasma insulin, μU/ml | ||||
| Baseline | 2.66 ± 0.88 | 1.85 ± 0.22 | 2.30 ± 0.32 | 2.00 ± 0.28 |
| Clamp | 2.79 ± 0.43 | 1.89 ± 0.41 | 2.62 ± 0.77 | 2.30 ± 0.72 |
| Plasma glucagon, pg/ml | ||||
| Baseline | 125 ± 6.0 | 125 ± 16 | 124 ± 9.7 | 126 ± 14 |
| Clamp | 101 ± 6.5a | 119 ± 7.9a | 258 ± 29.5b | 290 ± 44.0b |
| Plasma glucose, mg/dl | ||||
| Baseline | 60.7 ± 4.15 | 66.1 ± 5.63 | 58.5 ± 3.78 | 65.4 ± 6.47 |
| Clamp | 47.8 ± 4.43 | 60.6 ± 6.55 | 51.6 ± 5.63 | 57.4 ± 5.65 |
| Whole blood net glucose disposal rate, mg·kg−1·min−1 | ||||
| Clamp | 14.5 ± 2.07 | 11.0 ± 2.19 | 14.8 ± 1.97 | 15.3 ± 2.07 |
| Whole body net AA disposal rate, mmol·kg−1·h−1 | ||||
| Clamp | 51.7 ± 19.0ab | 40.6 ± 19.8a | 101.2 ± 18.4c | 85.5 ± 19.0bc |
Values are means ± SE; n = 6–8 at baseline or during the clamp at 480 min of infusion. All pigs were provided a balanced amino acid (AA) mixture to achieve fasting AA levels (400–500 nmol branched-chain AA/ml); glucose, glucagon and insulin were infused to maintain basal levels.
Con, control group; Con+L, control + leucine-treated group; LPS, LPS-treated group; LPS+L, LPS + leucine-treated group.
Means without a common superscripted letter differed (P ≤ 0.05).
Total AA and nonessential AA concentrations in plasma were higher in the LPS+L-treated group compared with the Con group (P ≤ 0.05; Table 2). BCAAs were higher in the LPS+L-treated group compared with the LPS-treated group (P ≤ 0.05). Circulating Leu was increased to fed levels (2-to 3-fold of basal level) in Con+L- and LPS+L-treated groups (P ≤ 0.05). Plasma arginine concentrations were higher in the Con+L-treated group compared with the Con group (P ≤ 0.05), and histidine concentrations were higher in the LPS+L-treated group compared with the Con+L-treated group (P ≤ 0.05). Plasma tryptophan concentrations were higher in the LPS+L-treated group compared with the Con group. There were no differences between groups for plasma isoleucine, lysine, methionine, valine, and threonine concentrations. The LPS+L-treated group had higher plasma concentrations of glutamine, glutamate, and taurine than the Con group (P ≤ 0.05). Plasma alanine, asparagine, aspartate, glycine, ornithine, proline, serine, tyrosine, and citrulline concentrations did not differ between groups.
Table 2.
Plasma AA concentrations in piglets infused with leucine at the end of 8-h endotoxemic pancreatic-substrate clamps
| AA | Con | Con+L | LPS | LPS+L |
|---|---|---|---|---|
| EAAs | ||||
| Arginine | 89.7 ± 10.8a | 113 ± 10.7b | 86.6 ± 9.90a | 98.4 ± 10.3a,b |
| Histidine | 55.3 ± 12.7a,b | 45.7 ± 12.5a | 68.8 ± 11.1a,b | 81.1 ± 11.9b |
| Isoleucine | 191 ± 24.4 | 193 ± 24.1 | 200 ± 22.0 | 216 ± 23.2 |
| Leucine | 136 ± 59.9a | 335 ± 53.6b | 133 ± 45.3a | 290 ± 45.3b |
| Lysine | 192 ± 24.0 | 186 ± 23.7 | 153 ± 20.8 | 206 ± 22.2 |
| Methionine | 80.8 ± 14.2 | 76.8 ± 14.0 | 61.1 ± 12.8 | 78.3 ± 13.5 |
| Threonine | 181 ± 25.7 | 213 ± 25.3 | 222 ± 22.6 | 238 ± 24.1 |
| Tryptophan | 27.8 ± 4.50a | 32.6 ± 4.40a,b | 34.3 ± 4.00a,b | 41.8 ± 4.20b |
| Valine | 333 ± 46.4 | 312 ± 45.7 | 316 ± 41.9 | 393 ± 44.1 |
| NEAAs | ||||
| Alanine | 323 ± 82.6 | 362 ± 81.3 | 403 ± 72.8 | 446 ± 77.4 |
| Asparagine | 68.9 ± 10.4 | 78.2 ± 10.2 | 80.4 ± 9.50 | 80.3 ± 9.90 |
| Aspartic acid | 10.0 ± 1.90 | 10.7 ± 1.90 | 9.70 ± 1.60 | 14.5 ± 1.70 |
| Glutamine | 342 ± 52.8a | 406 ± 52.3a,b | 422 ± 45.7a,b | 513 ± 48.9b |
| Glycine | 1,030 ± 116 | 1,090 ± 114 | 1,034 ± 101 | 1,280 ± 108 |
| Ornithine | 77.4 ± 10.4 | 77.9 ± 10.2 | 73.2 ± 9.10 | 83.3 ± 9.70 |
| Proline | 153 ± 21.0 | 162 ± 21.1 | 175 ± 18.3 | 202 ± 19.6 |
| Serine | 134 ± 19.8 | 167 ± 19.8 | 141 ± 17.2 | 184 ± 18.4 |
| Taurine | 77.4 ± 17.7a | 78.6 ± 17.4a | 131 ± 15.4b | 142 ± 16.5b |
| Tyrosine | 149 ± 19.4 | 141 ± 19.1 | 161 ± 17.0 | 177 ± 18.1 |
| Glutamate | 51.0 ± 15.0a | 57.4 ± 15.0a,b | 49.6 ± 13.2a | 82.3 ± 13.7b |
| Citrulline | 92.4 ± 11.8 | 116 ± 11.7 | 103 ± 11.0 | 111 ± 11.4 |
| EAAs | 1,326 ± 156 | 1,440 ± 154 | 1,300 ± 139 | 1,601 ± 148 |
| NEAAs | 2,440 ± 261a | 2,730 ± 261a,b | 2,770 ± 226a,b | 3,330 ± 242b |
| Branched-chain AAs | 708 ± 102a,b | 780 ± 101a,b | 671 ± 93.6a | 892 ± 101b |
| Total AAs | 3,740 ± 380a | 4,160 ± 380a,b | 4,033 ± 329a,b | 4,880 ± 351b |
Values are means ± SE; n = 6–8 at time = 480 min of infusion. AA are shown in mmol/ml. Phenylalanine was used as a tracer to determine protein synthesis rates; its value was elevated at time = 480 min. Consequently, this AA was excluded from total AA and essential AA (EAA) values.
NEAAs, nonessential AAs.
Means without a common letter differed (P ≤ 0.05).
Skeletal muscle PS rates and translation initiation and PD signaling.
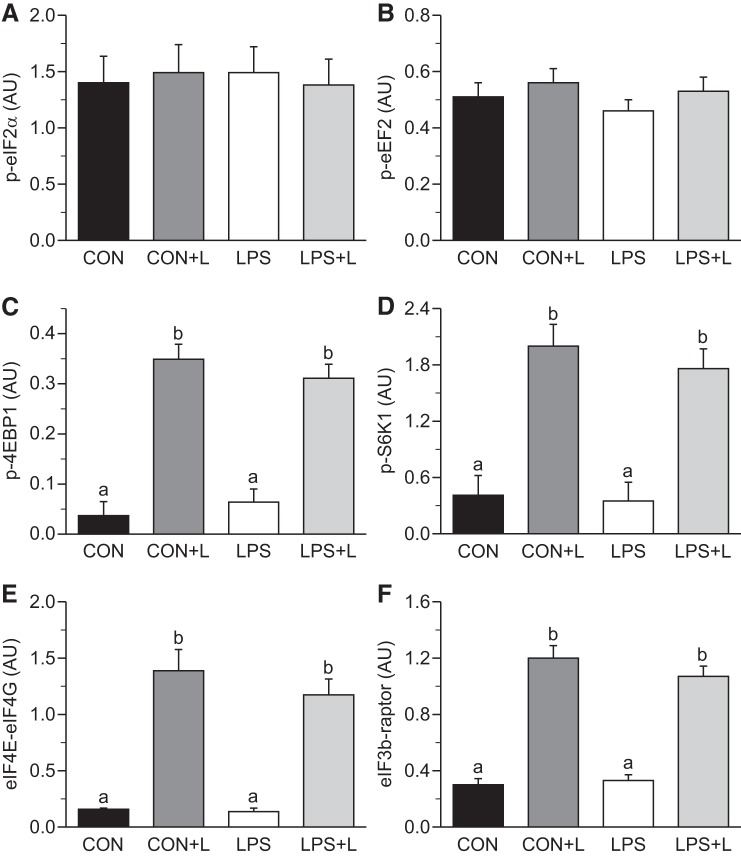
Fractional PS rates, translational efficiency and capacity, and abundance, phosphorylation, and/or protein-protein association of translation initiation and PD signals in LD muscle are shown in Figs. 3–6. The same data for gastrocnemius and soleus muscles are shown in Table 3. LPS reduced PS in LD (−36%, P ≤ 0.05; Fig. 3A), gastrocnemius (−28%, P ≤ 0.05; Table 3), and soleus (−38%) muscles (P ≤ 0.05) compared with controls. Leu infusion alone increased PS in LD muscle by 30%, in gastrocnemius muscle by 66%, and soleus muscle by 30% (P ≤ 0.05). Leu in the presence of LPS increased PS by 84% in LD muscle, 81% in gastrocnemius muscle, and 83% in soleus muscle compared with LPS alone (P ≤ 0.05). No differences in PS rates were observed between sexes in neonatal pigs (P ≥ 0.05).
Fig. 3.
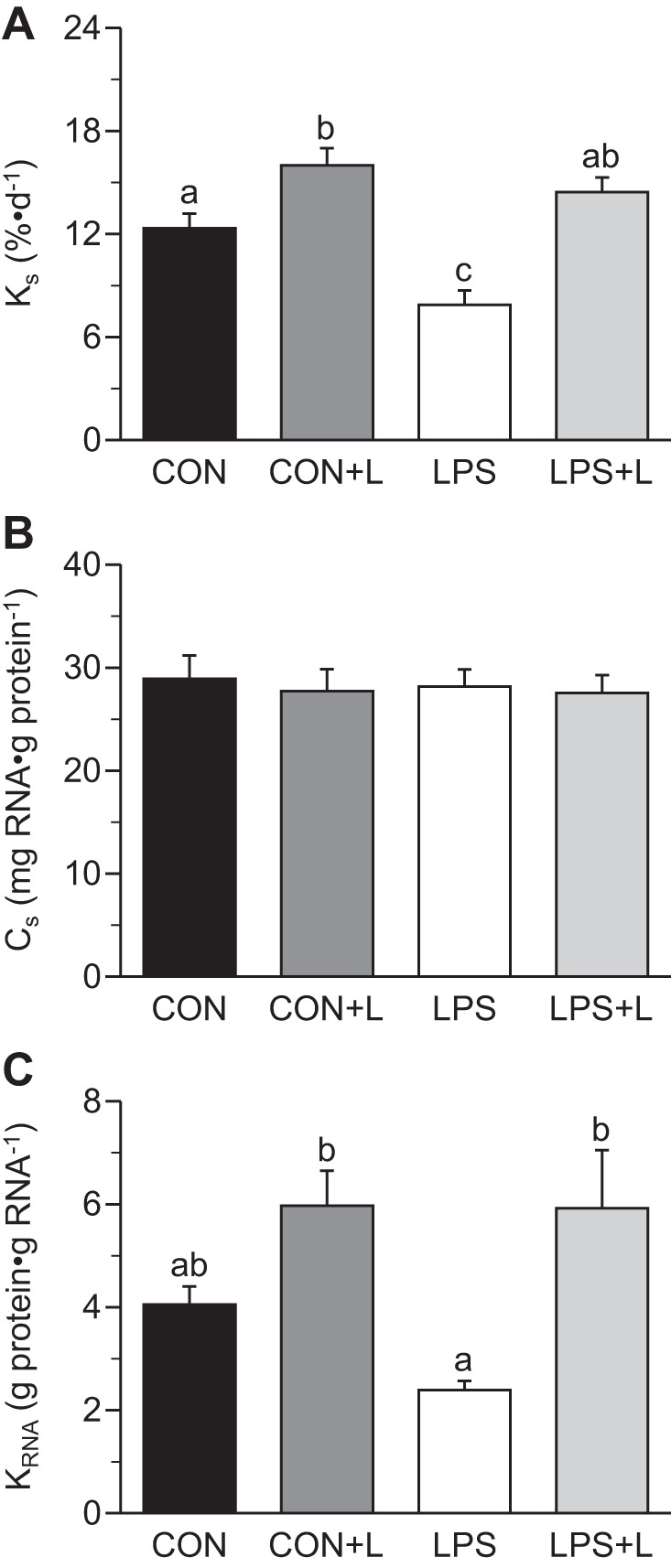
Fractional rates of protein synthesis (KS; A), protein synthetic capacity (CS; B), and protein synthetic efficiency (KRNA; C) in longissimus dorsi muscle of piglets infused with Leu during endotoxemic pancreatic-substrate clamps. Values are means ± SE; n = 6–8. Means without a common letter differed (P ≤ 0.05).
Table 3.
KS, CS, KRNA, phosphorylation of insulin and AA signaling regulators, and abundance of eIF4E·eIF4G complex and eIF3b-raptor association in gastrocnemius and soleus muscles of piglets infused with leucine during endotoxemic pancreatic-substrate clamps
| Con | Con+L | LPS | LPS+L | |
|---|---|---|---|---|
| Gastrocnemius muscle | ||||
| KS, %/day | 11.0 ± 0.86b | 18.4 ± 1.02d | 7.93 ± 0.80a | 14.3 ± 0.86c |
| CS, mg RNA/g protein | 27.2 ± 3.41 | 26.9 ± 1.43 | 28.5 ± 1.89 | 28.3 ± 1.59 |
| KRNA, g protein/g RNA | 4.42 ± 0.53b | 5.86 ± 0.74c | 2.83 ± 0.19a | 4.76 ± 0.41bc |
| p-PKB, AU | 0.45 ± 0.70 | 0.40 ± 0.08 | 0.38 ± 0.07 | 0.37 ± 0.07 |
| p-S6K1, AU | 0.11 ± 0.13a | 1.29 ± 0.15b | 0.08 ± 0.13a | 0.98 ± 0.13b |
| p-4EBP1, AU | 0.35 ± 0.23a | 3.45 ± 0.25b | 0.27 ± 0.22a | 2.84 ± 0.23b |
| eIF4E·eIF4G, AU | 0.05 ± 0.09a | 0.89 ± 0.09b | 0.05 ± 0.08a | 0.66 ± 0.09b |
| eIF3b-raptor, AU | 0.28 ± 0.09a | 2.17 ± 0.25b | 0.42 ± 0.09a | 1.60 ± 0.22b |
| p-eIF2α, AU | 0.69 ± 0.15 | 0.66 ± 0.16 | 0.86 ± 0.14 | 0.68 ± 0.15 |
| p-eEF2, AU | 1.18 ± 0.22 | 1.21 ± 0.24 | 1.14 ± 0.21 | 1.02 ± 0.22 |
| Soleus muscle | ||||
| KS, %/day | 10.7 ± 0.86a | 13.9 ± 0.93b | 6.56 ± 0.86c | 12.0 ± 0.86a,b |
| CS, mg RNA/g protein | 18.4 ± 0.41 | 18.7 ± 0.48 | 17.4 ± 1.02 | 20.3 ± 0.99 |
| KRNA, g protein/g RNA | 6.00 ± 0.44b | 7.90 ± 0.78c | 3.66 ± 0.26a | 5.87 ± 0.40b |
| p-PKB, AU | 0.52 ± 0.09 | 0.49 ± 0.10 | 0.56 ± 0.09 | 0.54 ± 0.09 |
| p-S6K1, AU | 0.19 ± 0.30a | 2.39 ± 0.32b | 0.23 ± 0.29a | 1.61 ± 0.30c |
| p-4EBP1, AU | 0.06 ± 0.23a | 0.60 ± 0.25b | 0.04 ± 0.22a | 0.37 ± 0.23b |
| eIF4E·eIF4G, AU | 0.16 ± 0.10a | 1.06 ± 0.10b | 0.12 ± 0.09a | 0.77 ± 0.10b |
| eIF3b-raptor, AU | 0.50 ± 0.07a | 1.91 ± 0.28b | 0.61 ± 0.12a | 1.65 ± 0.26b |
| eIF2α, AU | 1.04 ± 0.18 | 1.06 ± 0.20 | 0.97 ± 0.17 | 0.95 ± 0.18 |
| eEF2, AU | 0.42 ± 0.06 | 0.38 ± 0.06 | 0.39 ± 0.05 | 0.40 ± 0.06 |
Values are means ± SE (n = 6–8).
KS, protein synthesis rate; CS, protein synthetic capacity; KRNA, ribosomal translational efficiency; p, phosphorylated; AU, arbitrary units; S6K1, S6 kinase 1; 4EBP-1, eukaryotic translation initiation factor (eIF)4E-binding protein-1; eEF2, eukaryotic translation elongation factor 2.
Means without a common superscripted letter differed (P ≤ 0.05).
CS in LD, gastrocnemius, and soleus muscles did not differ between groups. LPS tended to decrease KRNA in LD muscle (P ≤ 0.08) and decreased KRNA in gastrocnemius and soleus muscles (P ≤ 0.05). Leu antagonized the LPS-induced decrease in KRNA and restored it to basal values (P ≤ 0.05; Fig. 3C).
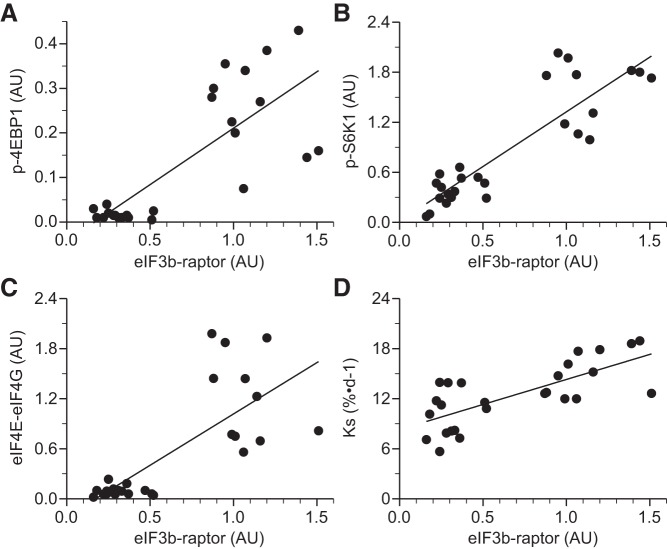
Phosphorylation of eIF2α (Fig. 4A) and eEF2 (Fig. 4B) did not differ between groups. Leu increased 4EBP1 and S6K1 phosphorylation, eIF4E·eIF4G complex formation, and the binding of eIF3b to raptor within mTORC1 in all skeletal muscles in the presence and absence of LPS (P ≤ 0.05; Table 3 and Fig. 4, C–F). The eIF3b-raptor association showed a positive linear correlation, which was independent of treatment, with 4EBP1 and S6K1 phosphorylation, eIF4E·eIF4G complex formation, and PS rate (Fig. 5, A–D). In LD muscle, PKB phosphorylation did not differ between groups in the presence or absence of LPS (Fig. 6A). However, Leu decreased AMPKα activation in the presence of LPS (Fig. 6B). Atrogin-1 abundance was not affected by LPS or Leu (Fig. 7A). LPS augmented MuRF-1 abundance and the LC3II-to-total LC3 ratio (Fig. 7, B and C), but these were restored to values seen in controls in the LPS+L-treated group (P ≤ 0.05).
Fig. 4.
Phosphorylation of eukaryotic translation initiation factor (eIF)2α (A), eukaryotic translation elongation factor (eEF)2 (B), eIF4E-binding protein-1 (4EBP1; C), and S6 kinase (S6K)1 (D) as well as the abundance of eIF4E·eIF4G (E) and eIF3b-raptor (F) complexes in longissimus dorsi muscle of piglets infused with Leu during endotoxemic pancreatic-substrate clamps. AU, arbitrary units; p, phosphorylated form of the protein. Values are means ± SE; n = 6–8. Means without a common letter differed (P ≤ 0.05).
Fig. 5.
Correlations of eIF3b-raptor association with phosphorylation of 4EBP1 (A), S6K1 (B), abundance of the eIF4E·eIF4G complex (C), and KS (D) in longissimus dorsi muscle of piglets infused with Leu during endotoxemic pancreatic-substrate clamps. Continuous line, correlation independent of treatment.
Fig. 6.
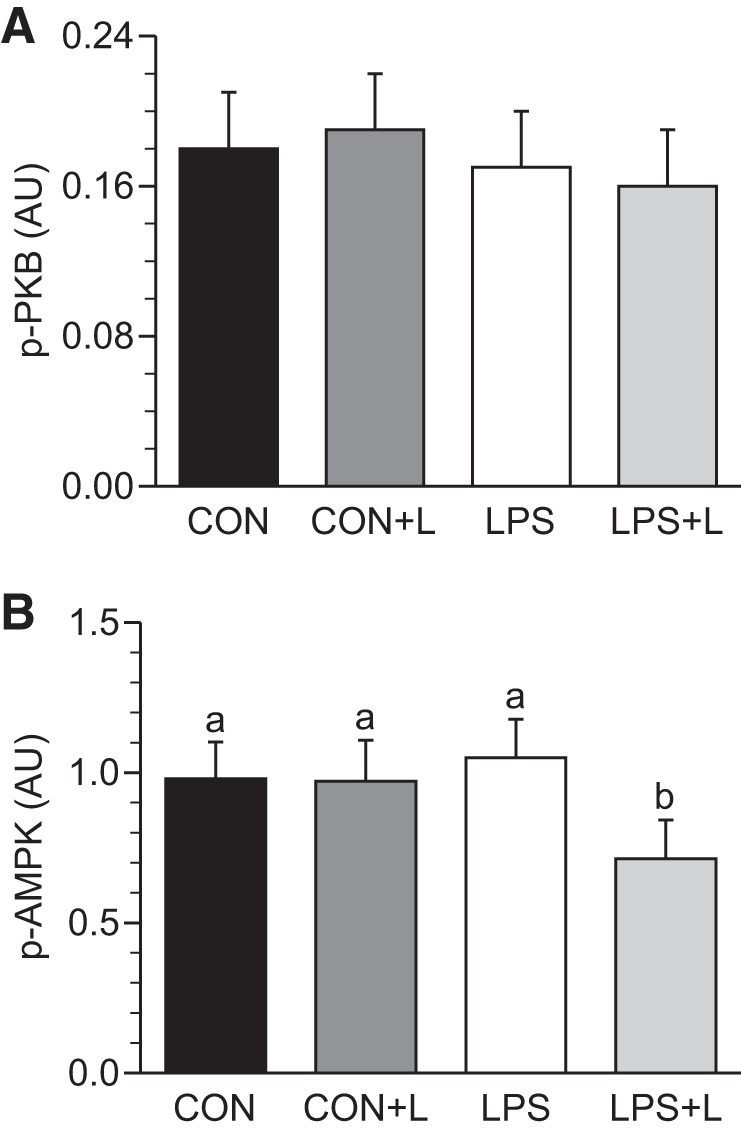
Phosphorylation of PKB (A) and AMP-activated protein kinase (AMPK; B) in longissimus dorsi muscle of piglets infused with Leu during endotoxemic pancreatic-substrate clamps. Values are means ± SE; n = 6–8. Means without a common letter differed (P ≤ 0.05).
Fig. 7.
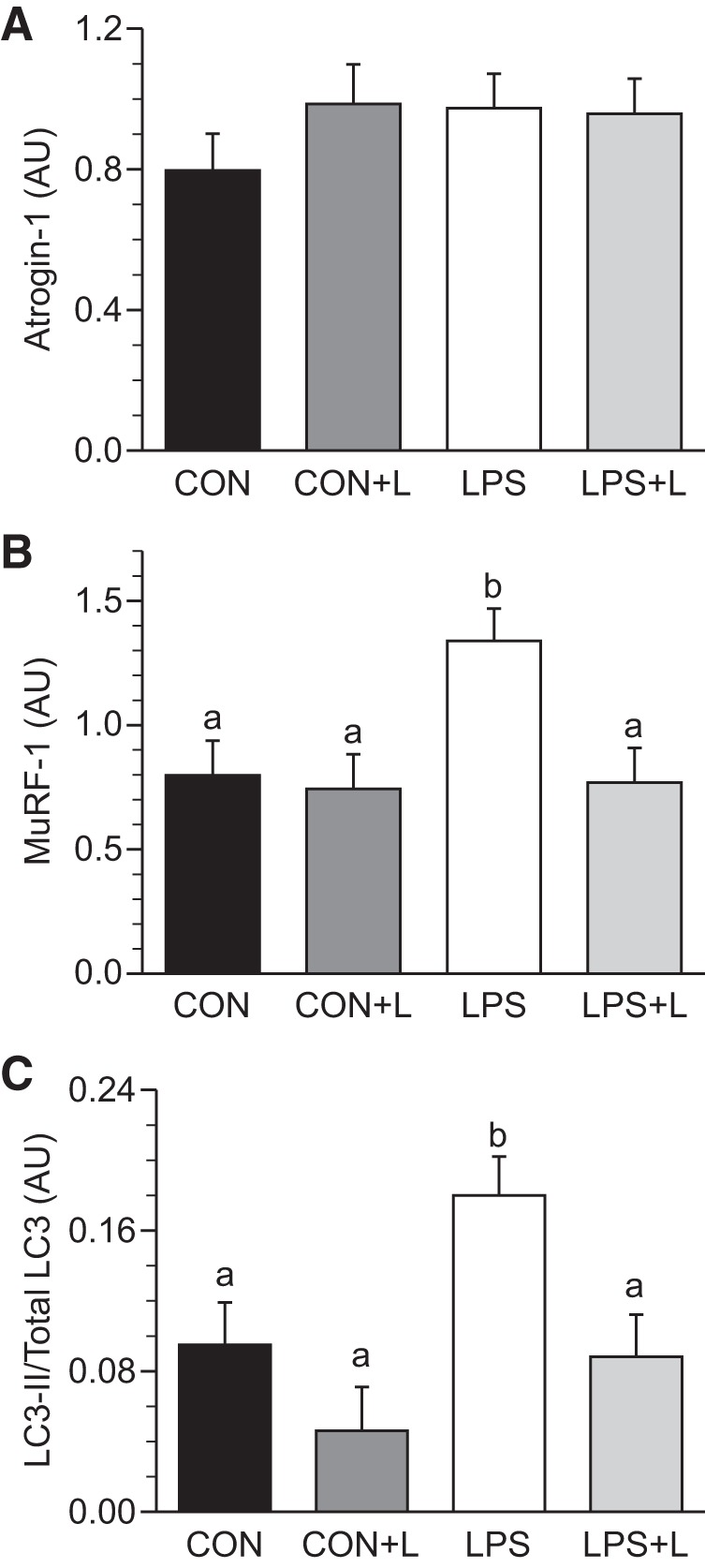
Abundance of atrogin-1 (A), muscle-specific RING finger (MuRF)-1 (B), and the light chain (LC)3-II-to-total LC3 ratio (C) in longissimus dorsi muscle of piglets infused with Leu during endotoxemic pancreatic-substrate clamps. Values are means ± SE; n = 6–8. Means without a common letter differed (P ≤ 0.05).
Diaphragm and heart PS rates and translation initiation and PD signaling.
In the diaphragm, LPS increased the PS rate, KRNA, and 4EBP1 and S6K1 phosphorylation compared with controls (P ≤ 0.05; Table 4). In the LPS+L-treated group, these variables remained elevated. There was no change in CS between groups. Atrogin-1 abundance was increased by LPS compared with controls (Table 5). Leu-supplemented groups did not differ compared with nonsupplemented groups. MuRF-1 did not differ between groups. LPS did not alter the LC3-II-to-total LC3 ratio, which remained unaltered after Leu supplementation. In controls, Leu tended to decrease the LC3-II-to-total LC3 ratio (P ≤ 0.057).
Table 4.
KS, CS, KRNA, and activation of the mTORC1 pathway in the diaphragm and heart in piglets infused with Leu during endotoxemic pancreatic-substrate clamps
| Con | Con+L | LPS | LPS+L | |
|---|---|---|---|---|
| Diaphragm | ||||
| KS, %/day | 14.3 ± 1.97a | 17.7 ± 2.13a,b | 20.1 ± 1.84b | 21.8 ± 1.97b |
| CS, mg RNA/g protein | 20.4 ± 2.32 | 23.6 ± 2.51 | 23.1 ± 2.16 | 19.3 ± 3.25 |
| KRNA, g protein/g RNA | 6.20 ± 0.44a | 7.32 ± 0.81a,b | 8.23 ± 0.42b | 9.09 ± 0.61b,c |
| p-S6K1, AU | 0.28 ± 0.04a | 0.40 ± 0.03a,b | 0.56 ± 0.09b | 0.53 ± 0.05b |
| p-4EBP1, AU | 0.11 ± 0.01a | 0.12 ± 0.02a,b | 0.21 ± 0.05a,b | 0.23 ± 0.04b |
| Heart | ||||
| KS, %/day | 32.9 ± 2.97a | 41.1 ± 3.21a,b | 40.2 ± 2.78a,c | 47.3 ± 2.97b,c |
| CS, mg RNA/g protein | 31.4 ± 3.03 | 32.0 ± 3.27 | 34.5 ± 3.04 | 30.9 ± 4.31 |
| KRNA, g protein/g RNA | 11.1 ± 1.52 | 13.1 ± 1.21 | 11.2 ± 1.91 | 16.9 ± 1.91 |
| p-S6K1, AU | 0.60 ± 0.09 | 0.70 ± 0.08 | 0.63 ± 0.06 | 0.58 ± 0.05 |
| p-4EBP1, AU | 0.44 ± 0.06 | 0.38 ± 0.06 | 0.41 ± 0.06 | 0.46 ± 0.03 |
Values are means ± SE; n = 6–8.
mTORC1, mammalian target of rapamycin complex 1.
Means without a common superscripted letter differ (P ≤ 0.05). Arbitrary Units, AU.
Table 5.
Protein degradation pathway (MuRF-1, atrogin-1, and LC3II/LC3) in the diaphragm and heart in piglets infused with Leu during endotoxemic pancreatic-substrate clamps
| Con | Con+L | LPS | LPS+L | |
|---|---|---|---|---|
| Diaphragm | ||||
| MuRF-1, AU | 0.53 ± 0.11 | 0.35 ± 0.07 | 0.63 ± 0.16 | 0.42 ± 0.09 |
| Atrogin-1, AU | 0.43 ± 0.05a | 0.57 ± 0.14a,b | 0.97 ± 0.22b | 0.75 ± 0.11a,b |
| LC3II/total LC3, AU | 0.31 ± 0.02a,b | 0.19 ± 0.02a | 0.31 ± 0.04b | 0.30 ± 0.05b |
| Heart | ||||
| MuRF-1, AU | 1.27 ± 0.23 | 1.17 ± 0.12 | 1.21 ± 0.25 | 1.23 ± 0.16 |
| Atrogin-1, AU | 1.39 ± 0.32 | 1.37 ± 0.14 | 2.06 ± 0.32 | 1.54 ± 0.28 |
| LC3II/total LC3, AU | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 |
Values are means ± SE; n = 6–8.
MuRF-1, muscle-specific RING finger-1.
Means without a common superscripted letter differed (P ≤ 0.05). The P value was 0.057 for light chain (LC)3-II/total LC3 between Con and Con+L groups in the diaphragm.
In the heart, Leu increased PS in the LPS-treated group compared with controls (P ≤ 0.05; Table 4). CS and ribosomal efficiency as well as S6K1 and 4EBP1 phosphorylation did not differ between groups. There were no differences in the LC3-II-to-total LC3 ratio or MuRF-1 and atrogin-1 abundance between groups (Table 5).
Visceral tissue PS rates and translation initiation and PD signaling.
In the lung, PS rates, KRNA, as well as S6K1 and 4EBP1 phosphorylation increased with LPS (P ≤ 0.05), but after Leu supplementation, there was no change in the LPS-treated or Con group (Table 6). CS did not differ between groups. In the ileum, there were no differences in KS, CS, or KRNA between groups. LPS increased the phosphorylation of S6K1 and 4EBP1 compared with controls (P ≤ 0.05), but there was no change in Leu-supplemented versus nonsupplemented groups. In the liver, LPS increased KS as well as S6K1 and 4EBP1 phosphorylation compared with controls (P ≤ 0.05). There were no differences in CS or KRNA between groups. Phosphorylation of S6K1 and 4EBP1 increased in the LPS-treated group compared with the Con group (P ≤ 0.05); Leu-supplemented groups were similar to nonsupplemented groups. In the lung, ileum, and liver, the LC3-II-to-total LC3 ratio did not change with LPS- or Leu-infused groups (Table 7).
Table 6.
KS, CS, KRNA, and activation of the mTORC1 pathway in the lung, ileum, and liver in piglets infused with Leu during endotoxemic pancreatic-substrate clamps
| Con | Con+L | LPS | LPS+L | |
|---|---|---|---|---|
| Lung | ||||
| KS, %/day | 33.9 ± 3.78a | 41.3 ± 4.08a,c | 49.2 ± 3.53b,c | 56.6 ± 4.08b |
| CS, mg RNA/g protein | 56.1 ± 4.79 | 56.1 ± 5.18 | 55.5 ± 4.48 | 52.7 ± 6.92 |
| KRNA, g protein/g RNA | 6.50 ± 0.91a | 7.55 ± 0.81a,b | 9.12 ± 0.71b | 10.4 ± 0.41b,c |
| p-S6K1, AU | 0.35 ± 0.03a | 0.41 ± 0.08a,c | 0.83 ± 0.16b | 0.79 ± 0.16b,c |
| p-4EBP1, AU | 0.29 ± 0.04a | 0.38 ± 0.11a,c | 0.64 ± 0.07b | 0.62 ± 0.08b,c |
| Ileum | ||||
| KS, %/day | 56.4 ± 3.23 | 50.1 ± 3.87 | 58.0 ± 3.27 | 60.6 ± 3.27 |
| CS, mg RNA/g protein | 61.5 ± 10.2 | 70.2 ± 11.1 | 75.0 ± 9.55 | 84.0 ± 12.3 |
| KRNA, g protein/g RNA | 8.63 ± 1.11 | 7.33 ± 1.01 | 9.22 ± 0.81 | 9.34 ± 1.91 |
| p-S6K1, AU | 0.31 ± 0.06a | 0.34 ± 0.06a | 0.83 ± 0.16b | 0.87 ± 0.13b |
| p-4EBP1, AU | 0.12 ± 0.02a | 0.12 ± 0.02a | 0.28 ± 0.05b | 0.27 ± 0.04b |
| Liver | ||||
| KS, %/day | 74.4 ± 5.31a | 84.6 ± 5.43a,b | 94.9 ± 3.03b | 78.7 ± 7.38a,b |
| CS, mg RNA/g protein | 65.7 ± 6.62 | 72.6 ± 7.14 | 81.1 ± 6.18 | 83.6 ± 9.40 |
| KRNA, g protein/g RNA | 12.1 ± 1.41 | 11.1 ± 0.92 | 10.3 ± 1.41 | 12.5 ± 1.71 |
| p-S6K1, AU | 0.21 ± 0.04a | 0.25 ± 0.02a | 0.77 ± 0.16b | 0.74 ± 0.09b |
| p-4EBP1, AU | 0.14 ± 0.09a | 0.15 ± 0.02a | 0.49 ± 0.07b | 0.51 ± 0.07b |
Values are means ± SE; n = 6–8. Means without a common superscripted letter differed (P ≤ 0.05).
Table 7.
Protein degradation pathway (LC3-II/LC3) in the lung, ileum, and liver in piglets infused with Leu during endotoxemic pancreatic-substrate clamps
| Con | Con+L | LPS | LPS+L | |
|---|---|---|---|---|
| Lung | ||||
| LC3-II/total LC3, AU | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 |
| Ileum | ||||
| LC3-II/total LC3, AU | 0.16 ± 0.02 | 0.14 ± 0.02 | 0.20 ± 0.01 | 0.17 ± 0.03 |
| Liver | ||||
| LC3-II/total LC3, AU | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.01 |
Values are means ± SE; n = 6–8.
DISCUSSION
Acute systemic inflammatory states, such as those seen in endotoxemia and sepsis, induce a reduction in PS and increase the breakdown of proteins in skeletal muscle, leading to muscle wasting and loss of protein stores (23, 48). In the present study, we aimed to determine if Leu could be used as an agent to minimize such alterations in muscle homeostasis in endotoxemic neonatal pigs. Different from other studies, in this study we neutralized the confounding effect of circulating insulin and AA availability in a large animal model by performing an endotoxemic pancreatic-substrate clamp. Our results demonstrate that during fasting conditions, LPS decreased skeletal muscle PS and that Leu restored PS to levels seen in nonseptic neonates by activation of translational efficiency. In addition, Leu antagonized the LPS-induced increase in MuRF-1 abundance and LC3 activation and decreased AMPK activation, suggesting that Leu ameliorates skeletal muscle PD during systemic inflammation.
In our previous studies, we verified the metabolic and inflammatory responses elicited in neonatal piglets by the titration of different LPS doses and the observation of its systemic effects on piglets of a similar weight and age as those in the present study. The endotoxin dose used in the present experiments increased the temperature, heart rate, and cytokine levels in plasma (TNF-α and IL-1β) as well as increased circulating insulin in previous studies (42, 46). By performing an endotoxemic pancreatic-substrate clamp in the present study, insulin levels were successfully maintained at basal fasting levels in all groups. Locking circulating insulin at fasting levels may explain why glucose disposal rates did not change, in contrast to previous LPS studies (7, 44). Despite similar fasting insulin levels, we found an increase in the whole body AA disposal rates in response to LPS, consistent with Bruins et al. (7) and with our previous studies (44, 45). This higher whole body AA disposal rate was not affected by Leu, suggesting that a short-term Leu infusion does not increase whole body AA requirements during systemic inflammation in the neonatal pig, despite inducing muscle anabolism. Although circulating glucagon concentrations were maintained during the pancreatic-substrate clamp in control pigs, glucagon levels increased in response to LPS despite somatostatin infusion. This elevation could reflect a systemic inflammatory response in which catabolic hormones (catecholamines, glucagon, and cortisol) are necessary for survival and activation of the immunological system (1). The rise in glucagon is unlikely to have impacted protein turnover as glucagon appears to act mainly in the liver, where it can repress mTOR signaling (30), but this response appears to occur only in the presence of an elevation in insulin (4).
During this study, LPS reduced PS in skeletal muscles of glycolytic (LD), mixed (gastrocnemius), and oxidative (soleus) fiber types. This finding is consistent with previous results of our laboratory (46) but differs from that reported in septic mature rats, in which only glycolytic muscles were compromised (33, 51). However, those studies did not control circulating levels of insulin or AAs. This finding suggests that LPS could create a more general negative impact on peripheral muscle in larger animals (35) or that the maintenance of fasting concentrations of insulin, glucose, and AAs may render oxidative muscles more vulnerable to the effect of LPS. In addition, and contrary of what has been described in rodents, we found a robust response of muscle PS to Leu in the presence of LPS, which contradicts the concept of sepsis-induced Leu resistance described in several studies (21, 27, 31, 32). In septic mature rodents, it has been shown that Leu failed to increase the association of raptor with eIF3b, which decreases the disassociation of 4EBP1 from eIF4E and the phosphorylation of S6K1 and 4EBP1 (27). In this regard, we have determined in our studies in healthy neonatal pigs that Leu efficiently increases skeletal muscle protein formation when substrate availability is maintained (18, 53). Therefore, the use of Leu with the infusion of a balanced AA mix to maintain euaminoacidemia in our neonatal endotoxemic animals may account for the discrepancy in findings compared with septic rodents (42). In our study, the increase in eIF3b-raptor association, 4EBP1 and S6K1 phosphorylation, and eIF4E·eIF4G complex formation in response to Leu is consistent with the translation initiation signal activation that we have found in skeletal muscle of healthy neonatal piglets after the administration of Leu (17), and it is similar to the mTORC1 signaling activation that we have found in LPS-infused piglets after the administration of a balanced mixture of AAs (42). Previous studies in our laboratory have demonstrated that healthy neonatal pigs maintain elevated skeletal muscle PS rates and translation initiation signaling compared with older pigs (12), and the response to AAs or Leu supplementation decreases with development (13, 18, 50). Thus, we speculate that our results are a consequence of the high intrinsic anabolic sensitivity of the neonatal skeletal muscle to AA provision and its innate anabolic drive and avidity for rapid growth (12, 13).
In this study, MuRF-1 but not atrogin-1 abundance in skeletal muscle increased in LPS-infused piglets compared with control piglets, suggesting activation of the ubiquitin proteasome system, consistent with a previous study from our laboratory (47). Similarly, LPS increased the LC3-II-to-total LC3 ratio, indicating increased activation of the autophagic system in skeletal muscle (24). Leu supplementation reversed the activation of those PD signals to levels seen in controls and decreased AMPK activation in skeletal muscle in the presence of LPS, suggesting that the restorative effects of this AA in skeletal muscle during endotoxemia antagonize PD signal activation in the neonate.
During this study, and consistent with a previous study (44), we found an increase in PS in the diaphragm, lung, and liver but not the heart secondary to endotoxemia. This could be a physiological adaptation related to the augmented respiratory work and immune response that the animals experience during this inflammatory state (46). Leu supplementation did not demonstrate an effect on PS in these tissues. In contrast to previous findings in our study (46), PS in the ileum, another important organ in terms of cellular immunity, did not increase with LPS, although the mTORC1 signaling pathway was activated in response to LPS. The reason for this discrepancy in mTORC signaling and PS is unknown.
In regard to PD pathways, LPS increased atrogin-1 abundance but not MuRF-1 abundance in the diaphragm compared with controls, consistent with a recent study in septic rats (38). This response could be associated with the respiratory failure that is seen during acute inflammatory states (38). Leu tended to decrease atrogin-1 abundance in the LPS-treated group, but this was not statistically significant. The LC3-II-to-total LC3 ratio did not change in Con or LPS-treated groups before or after Leu supplementation in the lung, ileum, and liver.
Perspectives
The results of the present study demonstrate that Leu supplementation attenuates the short-term catabolic effect induced by endotoxemia in skeletal muscle of neonatal pigs. This finding is contrary to the resistance of skeletal muscle to the anabolic action of Leu found in septic mature rodents (21, 31, 32, 35). Differences in the anabolic potential of neonatal and mature animals may be at least in part responsible for such dissimilar Leu responses during systemic inflammation. AA availability provided during the endotoxemic pancreatic-substrate clamp may also have been permissive to the anabolic action of Leu.
GRANTS
This work is a publication of the Children's Nutrition Research Center, United States Department of Agriculture (USDA)/Agricultural Research Service, and Department of Pediatrics, Baylor College of Medicine. Financial support for this project was provided by National Institutes of Health Grants HD-072891 (to T. A. Davis), AR-044474 (to T. A. Davis), and HD-085573 (to T. A. Davis) as well as USDA NIFA 2013-67015-20438 (to T. A. Davis) and USDA CRIS 6250-51000-055 (to T. A. Davis).
DISCLAIMER
The contents of this publication do not necessarily reflect the views or policies of the United States Department of Agriculture, nor does the mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.D.H.-G., R.A.O., and T.A.D. conception and design of research; A.D.H.-G., D.A.C., R.M., H.V.N., A.S., and R.A.O. performed experiments; A.D.H.-G., R.M., A.S., and T.A.D. analyzed data; A.D.H.-G., R.A.O., and T.A.D. interpreted results of experiments; A.D.H.-G. and R.M. prepared figures; A.D.H.-G. drafted manuscript; A.D.H.-G., D.A.C., R.M., H.V.N., A.S., R.A.O., and T.A.D. edited and revised manuscript; A.D.H.-G., D.A.C., R.M., H.V.N., A.S., R.A.O., and T.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank M. L. Fiorotto for helpful discussions, O. E. Smith for statistical analysis, A. C. Gillum for optimization of figures, and R. D. Almonaci for expert technical assistance.
REFERENCES
- 1.Alberti KG, Batstone GF, Foster KJ, Johnston DG. Relative role of various hormones in mediating the metabolic response to injury. J Parenter Enteral Nutr 4: 141–146, 1980. [DOI] [PubMed] [Google Scholar]
- 2.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130: 139–145, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Baum JI, Kimball SR, Jefferson LS. Glucagon acts in a dominant manner to repress insulin-induced mammalian target of rapamycin complex 1 signaling in perfused rat liver. Am J Physiol Endocrinol Metab 297: E410–E415, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem 240: 48–53, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Briend A, Khara T, Dolan C. Wasting and stunting–similarities and differences: policy and programmatic implications. Food Nutr Bull 36: S15–S23, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Bruins MJ, Deutz NE, Soeters PB. Aspects of organ protein, amino acid and glucose metabolism in a porcine model of hypermetabolic sepsis. Clin Sci (Lond) 104: 127–141, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37: 593–539, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Carling D, Viollet B. Beyond energy homeostasis: the expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab 21: 799–804, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 47: 259–270, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after a pediatric severe sepsis. Pediatrics 123: 849–857, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol Endocrinol Metab 277: E103–E109, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 286: 8287–8296, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288: E914–E921, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293: E1615–E1621, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 27: 793–799, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Fiorotto ML, Davis TA, Reeds PJ, Burrin DG. Nonnutritive factors in colostrum enhance myofibrillar protein synthesis in the newborn pig. Pediatr Res 48: 1–7, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Frost RA, Lang CH. mTOR signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 26: 83–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 32: 416–426, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45: 2147–2157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-García A, Manjarín R, Suryawan A, Nguyen HV, Davis TA, Orellana RA. Amino acids, independent of insulin, attenuate skeletal muscle autophagy in neonatal pigs during endotoxemia. Pediatr Res 80: 448–451, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hershey JW, Sonenberg N, Mathews MB. Principles of translational control: an overview. Cold Spring Harb Perspect Biol 1: 4, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinnebusch AG, Lorsch JR. The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol 1: 4, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazi AA, Pruznak AM, Frost RA, Lang CH. Sepsis-induced alterations in protein-protein interactions within mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. Shock 35: 117–125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Kim JY, Yu BP, Chung HY. The activation of NF-κB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology 9: 33–47, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Kimball SR, Orellana RA, O'Connor PM, Suryawan A, Bush JA, Nguyen HV, Thivierge MC, Jefferson LS, Davis TA. Endotoxin induces differential regulation of mTOR-dependent signaling in skeletal muscle and liver of neonatal pigs. Am J Physiol Endocrinol Metab 285: E637–E644, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem 279: 54103–54109, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab 287: E721–E730, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol 203: 144–155, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism 561: 49–57, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Lang CH, Frost RA, Jefferson LS, Kimball SR, Vary TC. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol Endocrinol Metab 278: E1133–E1143, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 46: 2787–2798, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 38.Maes K, Stamiris A, Thomas D, Cielen N, Smuder A, Powers SK, Leite FS, Hermans G, Decramer M, Hussain SN, Gayan-Ramirez G. Effects of controlled mechanical ventilation on sepsis-induced diaphragm dysfunction in rats. Crit Care Med 42: e772–e782, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Mattick JS, Kamisoglu K, Ierapetritou MG, Androulakis IP, Berthiaume F. Branched- chain amino acid supplementation: impact on signaling and relevance to critical illness. Wiley Interdiscip Rev Syst Biol Med 5: 449–460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munro HN, Fleck A. The determination of nucleic acids. Meth Biochem Anal 14: 173–176, 1966. [DOI] [PubMed] [Google Scholar]
- 42.Orellana RA, Jeyapalan A, Escobar J, Frank JW, Nguyen HV, Suryawan A, Davis TA. Amino acids augment muscle protein synthesis in neonatal pigs during acute endotoxemia by stimulating mTOR-dependent translation initiation. Am J Physiol Endocrinol Metab 293: E1416–E1425, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Orellana RA, Kimball SR, Nguyen HV, Bush JA, Suryawan A, Thivierge MC, Jefferson LS, Davis TA. Regulation of muscle protein synthesis in neonatal pigs during prolonged endotoxemia. Pediatr Res 55: 442–449, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Orellana RA, Kimball SR, Suryawan A, Escobar J, Nguyen HV, Jefferson LS, Davis TA. Insulin stimulates muscle protein synthesis in neonates during endotoxemia despite repression of translation initiation. Am J Physiol Endocrinol Metab 292: E629–E636, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Orellana RA, O'Connor PM, Bush JA, Suryawan A, Thivierge MC, Nguyen HV, Fiorotto ML, Davis TA. Modulation of muscle protein synthesis by insulin is maintained during neonatal endotoxemia. Am J Physiol Endocrinol Metab 291: E159–E166, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Orellana RA, O'Connor PM, Nguyen HV, Bush JA, Suryawan A, Thivierge MC, Fiorotto ML, Davis TA. Endotoxemia reduces skeletal muscle protein synthesis in neonates. Am J Physiol Endocrinol Metab 283: E909–E916, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Orellana RA, Suryawan A, Wilson FA, Gazzaneo MC, Fiorotto ML, Nguyen HV, Davis TA. Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs. Am J Physiol Regul Integr Comp Physiol 302: R682–R690, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Späte U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 265–269, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Suryawan A, Nguyen HV, Almonaci RD, Davis TA. Differential regulation of protein synthesis in skeletal muscle and liver of neonatal pigs by leucine through an mTORC1-dependent pathway. J Anim Sci Biotechnol 3: 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 281: E908–E915, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol Cell Physiol 262: C1513–C1519, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, Singhi SC, Erickson S, Roy JA, Bush JL, Nadkarni VM, Thomas NJ. Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 191: 1147–1157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140: 264–270, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]