Abstract
Aim
To examine the neural excitability of projections to the tibialis anterior (TA) following blood flow restriction training (BFRT). This is the first study to examine the TA following BFRT.
Methods
Ten subjects performed each experiment. Experiment one consisted of BFRT at 130 mmHg (BFRT-low). Experiment two consisted of BFRT at 200 mmHg (BFRT-high), training (TR-only) and blood flow restriction at 200 mmHg (BFR-only) performed on separate days. Blood flow restriction was applied to the thigh and training consisted of rapid dorsiflexion contractions against gravity every 10 s for 15-min. The motor evoked potential (MEP) peak-to-peak amplitudes were recorded pre-intervention and 1-, 10-, 20- and 30-min post-intervention and expressed relative to the maximal peak-to-peak M-wave at each time-point.
Results
Experiment one revealed no difference in MEP amplitudes for BFRT-low over time (P = 0.09). Experiment two revealed a significant effect of time (P < 0.001), with 1-min post-intervention MEP amplitudes significantly facilitated compared to pre-intervention, but no effect of intervention (P = 0.79) or intervention*time interaction (P = 0.25). Post-hoc power calculations were performed for the intervention*time interaction.
Discussion and conclusions
Corticospinal excitability of projections to the TA did not change following BFRT-low and corticospinal excitability changes between BFRT-high, BFR-only and TR-only interventions were not different over time. In experiment two, there was a significant main effect of time 1-min post-intervention which was mainly due to the BFRT-high intervention. Post-hoc power calculations revealed that 15 subjects were required for a significant interaction effect 80% of the time however, as the changes in corticospinal excitability were not prolonged, a new dataset of ≥ 15 subjects was not acquired.
Keywords: Neuroscience, Physiology
1. Introduction
Blood flow restriction training (BFRT) successfully increases strength, endurance and muscle size compared to conventional (non BFRT) training in the ankle extensors [1, 2, 3] and knee extensors [4, 5, 6, 7] following training regimes for periods of up to 8 weeks. In addition, cortical excitability can increase following single bouts of low load BFRT that are greater than comparable low load training without blood flow restriction [8]. As BFRT is more effective at increasing strength and corticospinal excitability than conventional training, it has been proposed that BFRT would be an effective training regime for patients with weakness due to brain injury. If corticospinal excitability can be increased in patient populations, it may aid to increase training intensity which might result in an increase in exercise-related adaptations.
A recent study [8], using low load BFRT, demonstrated an acute increase in corticospinal excitability following one session of BFRT in the biceps brachii. The corticospinal excitability changes lasted for 20-min for low load training BFR, 40-min following low-load intermittent BFRT at a blood flow restriction (BFR) pressure of 1.3 × systolic blood pressure (sBP) and 60-min following continuous BFRT with a BFR pressure of 0.8 × sBP. Continuous BFR pressure increased the amplitude of the motor evoked potential (MEP) (indicating increased corticospinal excitability) more than the other interventions. This indicates that low load BFRT with continuous pressure can increase corticospinal excitability.
As that study used a continuous BFR at 0.8 × sBP, increasing the BFR pressure and increasing the training time with BFR could increase corticospinal excitability to a greater extent. An acute bout (1 session) of BFR to motor block with high pressure BFR (without training) caused large increases in corticospinal excitability measured using transcranial magnetic stimulation [9]. As training with continuous low pressure BFRT increases cortical excitability, increasing the pressure and duration of training may result in further increases in excitability.
The aim of the current study was to examine the excitability of corticospinal pathways to the tibialis anterior (TA) following high intensity BFRT over two experiments 1) BFRT with cuff occlusion at 130 mmHg (BFRT-low) and 2) BFRT with cuff occlusion at 200 mmHg (BFRT-high) compared to matched BFR-only and training only (TR-only) interventions. As a proof of concept, we have used interventions, that are high intensity with moderate to high pressures so that we can attain the maximal changes to corticospinal excitability from the BFRT paradigm. If effective, we would like to trial a more realistic training protocol in patients with TA weakness. The TA has been chosen as it is frequently weak in patients (causing gait dysfunctions such as drop foot) and being able to increase the effectiveness of corticospinal connections to the TA may increase the training intensity of the TA more than other training regimes. This will be the first study to examine the TA in a BFRT regime.
2. Materials and methods
2.1. Subjects
Ten subjects participated in experiment 1 (age: 34 ± 8 years (mean ± SD); height: 1.79 ± 0.08 m; sBP: 118 ± 6 mmHg; diastolic BP: 69 ± 9 mmHg; 6 males, 4 females) and 10 subjects participated in experiment 2 (age: 29 ± 4 years; height: 1.87 ± 0.09 m; sBP: 117 ± 8 mmHg; diastolic BP: 67 ± 6 mmHg; 10 males). All subjects were right handed. Three subjects participated in both experiments. Written informed consent was obtained from all participants. Approval was granted by the local scientific ethics committee (approval number: 1-16-02-520-14) and adhered to the standards of the Declaration of Helsinki.
2.2. Apparatus and instrumentation
The electromyography activity (EMG) was recorded by surface electrodes (Ambu Neuroline surface electrodes) of the dominant TA and were placed according to Cram et al. [10]. Raw EMG data were amplified and band pass filtered from 10 Hz to 1 kHz.
Single pulse TMS was performed by a magnetic stimulator (Magstim 200, Magstim Company Ltd, United Kingdom) using a 110 mm double cone coil with current applied in the posterior to anterior direction.
Sphygmomanometers (Riester®) were used for measuring brachial blood pressure (55 cm × 14.5 cm) and restricting the blood supply to the lower leg (100 cm × 26 cm). Brachial blood pressure was measured in lying.
2.3. Interventions
All interventions were 15-min unless terminated early. Subjects were lying with a wedge under the knee and lower leg and the foot in the air for the duration of the experiment.
2.3.1. Training protocol
During the training interventions, subjects dorsiflexed their dominant foot as hard and fast as possible and held the contraction for 3 seconds at the maximal active dorsiflexion range. Contractions were performed every 10 seconds.
2.3.2. Blood flow restriction
During the blood occlusion interventions, the cuff was placed around the thigh of the trained leg. The blood pressure cuff was inflated and deflated over a period of 1-min. The 15-min intervention commenced when the blood pressure cuff had been completely inflated and stopped when the blood pressure cuff was deflated.
2.3.3. Experiment 1
Experiment 1 consisted of one intervention; BFRT with the blood pressure cuff inflated to 130 mmHg (BFRT-low).
2.3.4. Experiment 2
As experiment 1 yielded no significant excitability changes (see results), the pressure was increased and control interventions were introduced.
Experiment 2 consisted of three interventions; 1) BFRT with the cuff inflated to 200 mmHg (BFRT-high) 2) BFR with the cuff inflated to 200 mmHg (BFR-only) and 3) training with no BFR (TR-only). All interventions were performed by all subjects. The time between testing sessions was 7 ± 2 days (mean ± SD).
The numeric pain rating score was administered at 4-, 9- and 14-min during the interventions. If the subjects reported a score ≥ 9, the intervention ceased and post-measurements were performed. Towards the end of the BFRT interventions, some subjects were unable to dorsiflex the foot. When this occurred subjects were asked to continue to attempt to dorsiflex the foot.
2.4. Experimental measurements
TMS measurements were collected pre-intervention and 1-, 10-, 20- and 30-min post-intervention. M-max measurements were collected after the TMS measurements at each time-point.
2.4.1. TMS measurements
A cap was placed on the head of the subject and the vertex was marked. The optimal stimulation site for eliciting MEPs in the TA was established by applying stimuli every 4–6 seconds at approx. 50% of the maximal stimulator output (MSO) over the approx. ‘hotspot’. Once a location that elicited frequent MEPs was established, the TMS coil was systematically moved around the approx. ‘hotspot’ until the optimal site (deemed the location that produced the largest peak-to-peak MEP amplitude) had been located. The position of the optimal site was used for all subsequent TMS stimuli in that experimental session. The resting motor threshold was then established with the MSO from approx. 30% MSO in 5% increments until the peak-to-peak MEP amplitude for at least 5/10 MEPs exceeded 50 μV. During testing, 120% of the resting motor threshold was used with stimuli applied every 5–7 seconds for approx. 16 stimuli per time point.
2.4.2. M-wave measurements
For M-wave collection, 100 μs single rectangular pulses were applied to the deep branch of the common peroneal nerve at the fibula head with the cathode positioned proximally. The optimal M-wave location was established and was the location that elicited the highest peak-to-peak M-wave while minimising the stimulus artefact. The optimal position was used for the remainder of the experiment. Following this, the maximal peak-to-peak M-wave (M-max) was established by gradually (5 mA increments) increasing the stimulus intensity until the M-wave peak-to-peak amplitude no longer increased with an increase in stimulus intensity. During testing, 1.5 × of the stimulus intensity used to elicit M-max was used with stimuli applied every 2–2.5 seconds for approx. 28 stimuli per time point.
2.5. Data and statistical analysis
The peak-to-peak amplitude of MEPs (μV) and M-max (μV) were extracted for all trials for each intervention for each time point for each subject. The trial-by-trial MEP amplitudes were consistent with log-normal distribution and the trial-by-trial M-max amplitudes were consistent with normal distribution. Both MEP and M-max amplitudes were variance heterogeneous between subjects. To account for the above characteristics the analysis proposed by Pedersen et al. [11] was performed. Briefly, all MEP trials (for each subject, each condition and each time-point) were log transformed, averaged and back-transformed (i.e. the exponential function of the average was taken). This value was expressed as a percentage of the average peak-to-peak amplitude of the 28 M-max trials at the same time-point and the resultant proportion value was log transformed. Data analysis was performed on the log-transformed values. For experiment 1, a linear mixed model was performed with subject as a random factor and time as a fixed factor. To assess the difference in M-max over time, a linear mixed model was performed on absolute peak-to-peak M-max amplitudes with subject as a random factor and time as a fixed factor. For experiment 2, a linear mixed model was performed with subject and subject*intervention interaction as random factors and time, intervention and time*intervention interaction as fixed factors. Contrasts are shown for the amplitudes at post-intervention time-points compared the pre-intervention time-point. These time-points were determined a priori. To assess the difference in M-max over time and between condition, a linear mixed model was performed on absolute peak-to-peak M-max amplitudes with subject and subject*intervention interaction as random factors and time, intervention and time*intervention interaction as fixed factors.
2.6. Data deposition
Data associated with this study has been deposited at Mendeley Data under the DOI http://dx.doi.org/10.17632/j6b9jk56pm.1 [12].
3. Results
For the TR-only, BFR-only and BFRT-low interventions, all subjects successfully completed the 15-min interventions. For BFRT-high, the intervention was terminated early for three subjects (at 11-min, 11-min and 10-min 30 s) due to pain (pain rating of '9', n = 2) and slight nausea (which subsided on removal of the cuff (and reportedly occurs often in this subject during normal exercise), n = 1).
3.1. The effect of the interventions on MEP amplitude
Fig. 1 is an example of 16 MEPs and the corresponding 28 M-wave traces (stimulated at 1.5 × of the stimulus intensity used to elicit M-max) for the same time-point pre, 1-min post, 10-min post, 20-min post- and 30-min post-intervention for one subject for the BFRT-low intervention (experiment 1) and one subject for BFRT-high, BFR-only and TR-only interventions (experiment 2). For analysis, the peak-to-peak amplitude of the raw MEPs were log-transformed, averaged and back transformed before being expressed as a percentage of the average peak-to peak-amplitude of the 28 M-waves at the same time-point. Table 1 and Table 2 show the median and interquartile ranges of the log-transformed, averaged and back transformed peak-to-peak MEP amplitudes as a percentage of the average peak-to-peak M-max amplitudes for all subjects for experiment 1 and all subjects (including the three subjects who terminated the experiment early) for experiment 2, respectively.
Fig. 1.
Individual traces for experiment 1 (A) and experiment 2 (B) of 16 raw motor evoked potentials and 28 maximal peak-to-peak M-waves (stimulated at 1.5 × of the stimulus intensity used to elicit M-max) of the tibialis anterior (TA) as a function of the time since stimulation (ms) pre-intervention, 1-min, 10-min, 20-min and 30-min post-intervention. All interventions were 15-min, blood flow was restricted at the thigh and training consisted of 3 second dorsiflexion contractions every 10 seconds. A: Motor evoked potential traces (left) and M-wave traces for the corresponding time-point (right) for one subject for blood flow restriction training with the cuff pressure at 130 mmHg (BFRT-low (black lines)). B: Motor evoked potential traces (left) and M-wave traces for the corresponding time-point (right) for one subject (tested on different days, 7 days apart) for blood flow restriction training with the cuff pressure at 200 mmHg (BFRT-high (black lines)), blood flow restriction at 200 mmHg without training (BFR-only (blue lines)) and 15-min of training without blood flow restriction (TR-only (red lines)). For this subject the M-wave was smaller post-intervention in the BFRT condition however, this was not observed systematically when all subjects were assessed.
Table 1.
MEP amplitude as a percentage of the M-max for each time point for all subjects for the BFRT-low intervention (experiment 1).
| MEP size (% of M-max) (median (IQR)) |
|
|---|---|
| Time point | BFRT-low |
| Pre-intervention | 10.6 (6.1–20.8) |
| 1-min post | 12.7 (9.5–18.0) |
| 10-min post | 12.1 (5.0–18.3) |
| 20-min post | 12.6 (5.6–16.6) |
| 30-min post | 10.7 (5.6–19.2) |
MEP: Motor evoked potential, M-max: Maximum peak-to-to peak M-wave, IQR: Interquartile range, BFRT-low: Blood flow restriction training with low occlusion pressure.
Table 2.
MEP amplitude as a percentage of the M-max for each time point for all subjects for the BFRT-high, BFR-only and TR-only interventions (experiment 2).
| MEP size (% of M-max) (median (IQR)) |
|||
|---|---|---|---|
| Time-point | BFRT-high | BFR-only | TR-only |
| Pre-intervention | 9.5 (4.1–18.7) | 10.9 (6.0–31.4) | 10.6 (6.1–14.7) |
| 1-min post | 21.5 (7.4–50.1) | 10.8 (6.4–36.3) | 13.0 (8.7–21.3) |
| 10-min post | 13.5 (7.3–23.0) | 9.0 (5.8–37.9) | 10.9 (8.5–14.4) |
| 20-min post | 13.8 (4.1–28.9) | 6.8 (4.7–30.9) | 9.4 (3.8–14.5) |
| 30-min post | 11.5 (3.0–30.1) | 8.9 (5.8–35.3) | 9.3 (4.3–14.5) |
MEP: Motor evoked potential, M-max: Maximum peak-to-to peak M-wave, IQR: Interquartile range, BFRT-high: Blood flow restriction training with high occlusion pressure, BFR: Blood flow restriction, TR: training.
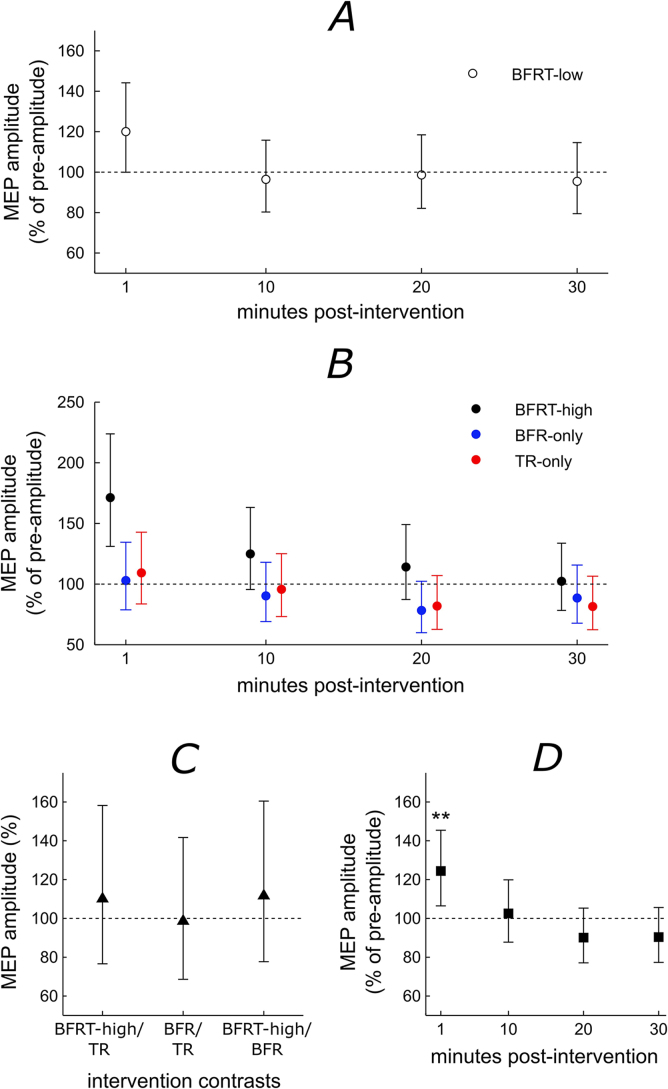
Fig. 2 summarises the differences of median and 95% confidence intervals of the difference of the medians for the linear mixed models for each time-point relative to the pre-intervention amplitude for experiment 1 (Fig. 2A) and experiment 2 (Fig. 2B). Also shown are the modelled amplitude differences for intervention (Fig. 2C) and time relative to pre-intervention (Fig. 2D).
Fig. 2.
A and B: Summary of the median difference and 95% confidence intervals of the magnitude of the response for each time point post-intervention vs. the pre-intervention estimates for the full linear mixed models for experiment 1 and 2 for A: blood flow restriction training at 130 mmHg (BFRT-low, unfilled circles) and B: blood flow restriction training at 200 mmHg (BFRT-high, black filled circles), Blood flow restriction only (BFR-only, blue filled circles) and training only (TR-only, red filled circles. C: The difference of medians and 95% confidence intervals between the different interventions as predicted by the model (black filled triangles) and D: The difference of medians and 95% confidence interval of the magnitude of the response for each time point post-intervention vs. the pre-intervention time-points regardless of intervention (black filled squares). The horizontal line represents a percentage of 100 indicating no difference between the post-intervention estimates and pre-intervention estimate (A, B, D) or between interventions (C). ‘**’ represents a significant difference to the pre-intervention estimate of P = 0.006.
The linear mixed model for experiment 1 was non-significant (P = 0.09, Fig. 2A). This indicates that the MEP amplitude over time was not altered by the BFRT-low intervention. The linear mixed model for experiment 2 was non-significant for the time*intervention interaction (P = 0.25, Fig. 2B). Therefore, MEP amplitude over time does not differ between BFRT-high, BFR-only and TR-only interventions. There was no significant main effect of intervention (P = 0.79, Fig. 2C) and therefore no difference between BFRT-high, BFR-only or TR only interventions. There was a significant main effect of time (P < 0.001, Fig. 2D) meaning there were significant differences between time points regardless of the intervention. For time, pre-intervention amplitudes were significantly lower than the 1-min post-intervention (P = 0.006) but not 10-min (P = 0.75), 20-min (P = 0.19) or 30-min (P = 0.20) post-intervention.
3.2. The effect of the interventions on M-max amplitude
The linear mixed model assessing M-max amplitude for experiment 1 was non-significant for the main effect of time (P = 0.84). This indicates that the M-max amplitude over time was not altered by the BFRT-low intervention. The linear mixed model for experiment 2 was non-significant for the time*intervention interaction (P = 0.49), main effect of time (P = 0.51) and main effect of intervention (P = 0.17). Therefore, M-max amplitude did not differ over time between BFRT-high, BP-only and TR-only interventions. Table 3 and Table 4 show the mean and SD of the M-max amplitude for experiment 1 and all subjects (including the three subjects who terminated the experiment early) for experiment 2, respectively.
Table 3.
M-max amplitude for each time point for all subjects for the BFRT-low intervention (experiment 1).
| M-max amplitude (μV) (mean ± SD) |
|
|---|---|
| Time point | BFRT-low |
| Pre-intervention | 3618 ± 1292 |
| 1-min post | 3508 ± 1501 |
| 10-min post | 3618 ± 1470 |
| 20-min post | 3535 ± 1519 |
| 30-min post | 3514 ± 1574 |
M-max: Maximum peak-to-to peak M-wave, SD: standard deviation, BFRT-low: Blood flow restriction training with low occlusion pressure.
Table 4.
M-max amplitude for each time point for all subjects for the BFRT-high, BFR-only and TR-only interventions (experiment 2).
| M-max amplitude (μV) (mean ± SD) |
|||
|---|---|---|---|
| Time point | BFRT-high | BFR-only | TR-only |
| Pre-intervention | 3279 ± 644 | 3313 ± 1075 | 2749 ± 624 |
| 1-min post | 3033 ± 852 | 3283 ± 1134 | 2784 ± 645 |
| 10-min post | 3213 ± 434 | 3358 ± 1172 | 2768 ± 681 |
| 20-min post | 3248 ± 409 | 3474 ± 1315 | 2702 ± 576 |
| 30-min post | 3266 ± 453 | 3436 ± 1342 | 2701 ± 670 |
M-max: Maximum peak-to-to peak M-wave, SD: standard deviation, BFRT-high: Blood flow restriction training with high occlusion pressure, BFR: Blood flow restriction, TR: training.
3.3. The effect of the interventions on pain
Table 5 shows the numeric pain rating scales for each intervention at 4-min, 9-min and 14-min during the intervention. For BFRT-high, the two subjects terminated the intervention due to pain were scored '9' at 14-min. The subject that terminated the experiment due to slight nausea was not included. In general, BFRT-high had high pain levels, BFRT-low had moderate pain levels and TR-only/BFR-only had low pain levels.
Table 5.
Pain rating scales for each intervention at 4-min, 9-min and 14-min during the intervention.
| Numeric pain rating score (mean ± SD) |
|||
|---|---|---|---|
| 4-min | 9-min | 14-min | |
| BFRT-low | 4.1 ± 1.7 | 5.3 ± 1.5 | 6.1 ± 1.5 |
| BFRT-high | 4.5 ± 0.9 | 6.6 ± 0.7 | 7.9 ± 0.8 |
| BFR-only | 3.3 ± 0.8 | 3.4 ± 0.8 | 3.4 ± 0.8 |
| TR-only | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.7 ± 0.7 |
BFRT-low: Blood flow restriction training with low occlusion pressure, BFRT-high: Blood flow restriction training with high occlusion pressure, BFR: Blood flow restriction, TR: training.
3.4. Post-hoc power calculations
As the BFRT-high intervention appeared to be more facilitated than the BFR-only and TR-only interventions 1-min post-intervention (see Fig. 2B), a power calculation based on the modelled data in the current study was performed for the statistical test of interaction between time and BFRT-high, BFR-only and TR-only interventions. One thousand datasets were simulated three times for the sample size of n = 10 (the number of subjects used in the current study) and tested for a significant interaction in each dataset. The simulated datasets showed a significant interaction 57% (CI95%: 54–60%), 60% (CI95%: 57–63%) and 58% (CI95%: 55–61%) of the time. Subject numbers were then increased to n = 15 and the power calculation process was repeated. The results of the simulation for the 15 subjects showed a significant interaction 81% (CI95%: 78–83%), 80% (CI95%: 78–83%) and 79% (CI95%: 76–81%) of the time.
4. Discussion
The current study is the first to investigate BFRT targeting the TA. While there were increases in corticospinal excitability immediately after the BFRT-high protocol, these had returned to baseline levels 10-min post-intervention and were not significant within the linear mixed model. When testing for a main effect of time there was a significant increase 1-min post-intervention, which was likely due mainly to the BFRT-high intervention. A post-hoc power calculation determined that the current study was underpowered to detect and interaction between BFRT-high, BFR-only and TR-only over time (power approx. 60%). For an 80% chance to detect a significant interaction effect (and significant difference between protocols), approximately 15 subjects would be required.
4.1. Lack of prolonged corticospinal excitability changes following BFRT
Few studies have investigated cortical excitability following BFRT using TMS. The current study contrasts a recent study [8] investigating continuous BFRT in the biceps brachii that showed immediate increases in corticospinal excitability that remained up to 60-min post-intervention using low load continuous BFRT [8]. The different results could be due to differences in BFR pressure used, training duration and muscle of interest. These will be discussed below.
Brandner et al. [8] used a lower continuous BFR pressure (0.8 × sBP) than the BFRT-high (200 mmHg) and BFRT-low (130 mmHg) paradigms in the current study however it would be expected that this would increase excitability not reduce it. This is supported by the current study, when assessing differences between 1-min post-intervention for BFRT-high and BFRT-low interventions. The BFRT-high intervention had a higher median (and CI95% that did not bisect the ‘no change’ line) compared to the BFRT-low that had a lower median (and CI95% that bisected the ‘no change’ line). For this reason, it is unlikely that a lower pressure could account for the absence of significant models in the current study.
The current study had an increased training time compared to Brandner et al. [8]. It is possible that this is a reason for the differences as some degree of neural fatigue may have occurred during training (15-min) which offset the increase in corticospinal excitability due to BFRT. Reports demonstrate that following training there can be an initial facilitation of MEPs for 3–4-min post-training [13] followed by depression of MEPs that can last from 18- to 30-min [14] in healthy subjects. It is possible that due to the longer duration of training, muscles in the current study became more fatigued during BFRT than the training program in Brandner et al. [8], and therefore the balance of fatigue induced inhibition and BFRT induced excitation of the MEP (as shown in Brandner et al. [8]) was skewed towards inhibition and no increases in corticospinal excitability were observed.
The TA has not been targeted previously in BFRT regimes but as BFRT is successful in increasing strength in most other trialled muscles (such as the ankle [1, 2, 3] and knee extensors [4, 5, 6, 7]) it is unlikely that strength increases do not occur in the TA. Despite this, increased corticospinal excitability is different to increased strength and prolonged corticospinal excitability changes may not occur for the TA. To date, corticospinal excitability, measured using TMS, has only been tested on projections to the biceps brachii following BFRT [8]. In that study, prolonged increases in corticospinal excitability (up to 60-min) were observed following BFRT [8]. Corticospinal excitability can be different between muscles [15], as shown in upper limb muscles, with stronger corticospinal connections to more distal muscles than more proximal muscles [15]. Further, the biceps brachii and TA have different functions (for example the TA is important in walking) and the muscles may respond differently to similar training paradigms. Although the TA may respond to BFRT regimes, the adaptations may be non-neural. A number of studies have reported muscle specific adaptations and changes in hormone levels following BFRT (see Scott et al. [16] for review) and it is possible that these are the only adaptations that occur following BFRT in the TA.
4.2. Increased corticospinal excitability immediately following the interventions
Although there was a lack of prolonged corticospinal excitability following BFRT-high, the linear mixed model for the main effect of time was increased at 1-min post-intervention. Although the BFRT-high intervention was non-significant alone, Fig. 2B suggests that the main contributor to the increase 1-min post-intervention was BFRT-high, although TR-only and BFR-only may have contributed slightly. This study suggests that when combining TR and BFR as one intervention, the two training methods interact (1-min post intervention) to increase corticospinal excitability more than the two individually.
Following BFR over long periods of time (30–50-min) at high pressures (> 200 mmHg), there are significant increases in cortical excitability in muscles proximal to the blood pressure cuff [17, 18, 19]. Additionally, there is a reduction in GABA within the motor cortex to the area supplying the occluded limb following high pressure BFR (200–250 mmHg) for 35-min [20]. As GABA is an inhibitory neurotransmitter, a reduction in GABA could be associated with a disinhibition of the occluded area. That study did not use TMS to assess corticospinal excitability, however another study [9], used TMS to investigate MEPs to the occluded muscle and interneural responses of the nerves supplying the occluded muscles [9]. During/following 40-min of BFR at 210 mmHg, occluded at the forearm, interneural electrodes inserted proximal to the blood pressure cuff at the ulnar and median nerves, recorded the motor volleys elicited using TMS. There was a significant increase in the amplitude of the signals through the interneural recordings in the last 20-min of the ischemic block. This suggests that the change in excitability was in the corticospinal regions proximal to the recording site and altered the motor output to the blood flow restricted areas in the hand. In addition, MEPs elicited following ischemia showed an increase from 0–15-min after BFR (analysed as a single block) which appeared to progressively decrease from immediately post- to 15-min post-training, returning to baseline levels at 15–30-min. Despite this, in the first 15-min of training (time used in the current study), there were no changes in the amplitude of interneurally recorded motor volleys. Therefore, following long term BFR-only, there were corticospinal excitability changes that manifest as increased MEP amplitudes however, changes following short-term BFR-only were not observed.
Training protocols similar to the protocols employed in the current study have not been utilised often. One study [21], investigated MEP amplitudes during and following TA training with contractions performed every 5 seconds for 30-min at 5–10% of the MVC. The study showed no changes in MEP amplitude, assessed at 125% of the resting motor threshold, at 15-min (during) and 30-min (immediately following training). Although that training protocol was different to the training protocol in the current study, it also showed no change in corticospinal excitability following TA training. Another study, assessing biceps brachii corticospinal excitability, showed that low load TR-only elicited short-term (5-min post) changes in MEP amplitude [8]. When this study included BFRT to the low load protocol, there were significant long lasting (60-min) increases in the MEP amplitude.
As short term BFR-only produces few corticospinal excitability changes (although long term BFR-only is effective) and TA training shows minimal changes to corticospinal excitability, it is likely that there is some interaction in the corticospinal system through the combined use of TR-only and BFR-only. In the current study, these changes were evident in the short term (1-min post-training) and in a previous study these were evident in the longer term (60-min post-training) [8].
4.3. Applications of BFRT
The potential for using this BFRT program as a training method is limited. As the changes were only present for a brief period following BFRT, and the changes seemed to be pressure dependent (higher for the BFRT-high than BFRT-low), it is unlikely that the BFRT protocol, in its present form, is suitable to produce long-term increases in corticospinal excitability of the TA. We had expected a prolonged increase in corticospinal excitability that could be utilized in training protocols (to enhance training intensity to increase strength more effectively in the target muscle), however this did not occur. BFRT has been proposed in patients with weakness as it could provide an increased neural drive and training intensity allowing increased exercise-related adaptations. In the current study, in a protocol not suitable for patients, to maximise the increase in corticospinal excitability from BFRT, the TA was trained at a high intensity with a high cuff pressure. However, even with these parameters, we could not elicit long term corticospinal excitability changes. Therefore, protocols that are lower in pressure and intensity, that would be suitable for patients, would likely have less corticospinal adaptations to the TA. Despite this, it is possible that the combination of training paradigm and blood flow restriction pressure used did not lead to a prolonged increase in corticospinal excitability and other training regimes would reveal a prolonged corticospinal excitability increase of the TA.
4.4. Limitations
The current study was underpowered (approx. 60% power) to determine an interaction effect. The study that provided the best estimate for determining subject numbers was Brandner et al. [8] who used 10 subjects and reported long-term significant increases in corticospinal excitability up to 60-min post-intervention. As the current study was a preliminary study investigating an intervention for a new muscle, with a modified but similar training protocol, we felt that Brandner et al. [8] was the best study to provide an estimate for sample size. Given this, we expected to observe significant results using 10 subjects. However, for the TA, and the protocol/interventions used, 10 subjects was insufficient. Post-hoc power calculations based on simulations of the modelled estimates, found that a sample size of 15 subjects would attain a significant interaction effect 80% of the time. As it is incorrect practise to add five subjects to the same dataset as the power calculation was based, a new dataset of 15 subjects would need to be collected. However, as these preliminary results only showed a temporary increase in corticospinal excitability, we decided that the increase was not long-lasting enough to warrant testing a further set of 15 subjects.
5. Conclusion
The current study was the first to investigate high intensity BFRT targeting the TA. The lack of prolonged corticospinal excitability changes demonstrates that for the methodology and training paradigm in the current study, there are no prolonged effects of BFRT training to corticospinal excitability. Although this contrasts the findings of another study [8] there are sufficient differences between the studies that could explain the differences in results.
Declarations
Author contribution statement
Erhard Trillingsgaard Næss-Schmidt, Morten Morthorst, Peter William Stubbs: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Asger Roer Pedersen: Analyzed and interpreted the data; Wrote the paper.
Jørgen Feldbæk Nielsen: Conceived and designed the experiments; Wrote the paper.
Funding statement
Erhard Trillingsgaard Næss-Schmidt, Asger Roer Pedersen, Jørgen Feldbæk Nielsen, and Peter William Stubbs are supported by Hammel Neurorehabilitation Centre and the University Research Clinic. Peter William Stubbs is also supported by the Health Research Fund of the Central Denmark Region. Morten Morthorst is unfunded.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Patterson S., Ferguson R. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur. J. Appl. Physiol. 2010;108:1025–1033. doi: 10.1007/s00421-009-1309-x. [DOI] [PubMed] [Google Scholar]
- 2.Patterson S., Ferguson R. Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J. Aging Phys. Act. 2011;19:201–213. doi: 10.1123/japa.19.3.201. [DOI] [PubMed] [Google Scholar]
- 3.Takarada Y., Sato Y., Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 2002;86:308–314. doi: 10.1007/s00421-001-0561-5. [DOI] [PubMed] [Google Scholar]
- 4.Ishii N., Madarame H., Odagiri K., Naganuma M., Shinoda K. Circuit training without external load induces hypertrophy in lower-limb muscles when combined with moderate venous occlusion. Int. J. KAATSU Train. Res. 2005;1:24–28. [Google Scholar]
- 5.Shinohara M., Kouzaki M., Yoshihisa T., Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur. J. Appl. Physiol. Occup. Physiol. 1997;77:189–191. doi: 10.1007/s004210050319. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda T., Abe T., Sato Y., Midorikawa T., Kearns C., Inoue K., Ryushi T., Ishii N. Muscle fiber cross-sectional area is increased after two weeks of twice daily KAATSU-resistance training. Int. J. KAATSU Train. Res. 2005;1:65–70. [Google Scholar]
- 7.Yasuda T., Fukumura K., Fukuda T., Uchida Y., Iida H., Meguro M., Sato Y., Yamasoba T., Nakajima T. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand. J. Med. Sci. Sports. 2014;24:799–806. doi: 10.1111/sms.12087. [DOI] [PubMed] [Google Scholar]
- 8.Brandner C., Warmington S., Kidgell D. Corticomotor Excitability is Increased Following an Acute Bout of Blood Flow Restriction Resistance Exercise. Front. Hum. Neurosci. 2015;9:652. doi: 10.3389/fnhum.2015.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNulty P., Macefield V., Taylor J., Hallett M. Cortically evoked neural volleys to the human hand are increased during ischaemic block of the forearm. J. Physiol. 2002;538:279–288. doi: 10.1113/jphysiol.2001.013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cram J., Kasman G., Holtz J. Aspen Publication; Gaithersburg, MA: 1998. Introduction to Surface Electromyography. [Google Scholar]
- 11.Pedersen A., Stubbs P., Nielsen J. Standard assumptions about the trial-by-trial distribution of averaged electromyography data could produce erroneous results. Motor Control. 2013;17:75–94. doi: 10.1123/mcj.17.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Stubbs P. Data for 'Corticospinal excitability changes following blood flow restriction training of the tibialis anterior: a preliminary study'. Mendeley Data. 2016 doi: 10.1016/j.heliyon.2016.e00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samii A., Wassermann E., Ikoma K., Mercuri B., Hallett M. Characterization of postexercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation. Neurology. 1996;46:1376–1382. doi: 10.1212/wnl.46.5.1376. [DOI] [PubMed] [Google Scholar]
- 14.Taylor J., Gandevia S. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve. 2001;24:18–29. doi: 10.1002/1097-4598(200101)24:1<18::aid-mus2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Palmer B., Ashby P. Corticospinal projections to upper limb motoneurones in humans. J. Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott B., Slattery K., Sculley D., Dascombe B. Hypoxia and Resistance Exercise: A Comparison of Localized and Systemic Methods. Sport. Med. 2014;44:1037–1054. doi: 10.1007/s40279-014-0177-7. [DOI] [PubMed] [Google Scholar]
- 17.Brasil-Neto J., Cohen L., Pascual-Leone A., Jabir F., Wall R., Hallet M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- 18.Brasil-Neto J., Valls-Solè J., Pascual-Leone A., Cammarota A., Amassian V., Cracco R., Maccabee P., Cracco J., Hallett M., Cohen L. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- 19.Ziemann U., Corwell B., Cohen L. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J. Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy L., Ziemann U., Chen R., Cohen L. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann. Neurol. 2002;52:755–761. doi: 10.1002/ana.10372. [DOI] [PubMed] [Google Scholar]
- 21.Mrachacz-Kersting N., Fong M., Murphy B., Sinkjaer T. Changes in Excitability of the Cortical Projections to the Human Tibialis Anterior After Paired Associative Stimulation. J. Neurophysiol. 2007;97:1951–1958. doi: 10.1152/jn.01176.2006. [DOI] [PubMed] [Google Scholar]