Abstract
Community-dwellers aged ≥60 years enrolled in the Atahualpa Project underwent brain MRI and were interviewed with the Pittsburgh Sleep Quality Index. Of 290 participants, 94 (32%) had poor sleep quality and 143 (49%) had global cortical atrophy (GCA). In a logistic regression model (adjusted for demographics, cardiovascular risk factor, severe edentulism, symptoms of depression, the MoCA score, and neuroimaging signatures of cerebrovascular damage), poor sleep quality was associated with GCA (p=0.004). A multivariate probability model showed that the probability of moderate-to-severe GCA significantly increased in individuals with poor sleep quality aged ≥67 years. This study provides evidence for an association between poor sleep quality and GCA in older adults and the important interaction of age in this association.
Keywords: Global cortical atrophy, Pittsburgh Sleep Quality Index, Population-based study, Sleep quality, Older adults
1. Introduction
Poor sleep quality has been shown to modify cognitive performance [1], [2], but whether this association is related to structural brain damage is controversial. Information about neuroimaging signatures of poor sleep quality is limited to a few studies recruiting small numbers of participants that might not be representative of the population at large [3], [4]. In addition, the independent effect of age has not been properly evaluated since age has only been included as a confounding variable, and no truly interaction models have been constructed.
The Atahualpa Project is an ongoing population-based study designed to reduce the increasing burden of non-communicable diseases – including sleep disorders – in rural Ecuador [5], [6]. Preliminary findings from our cohort showed an association between poor sleep quality and diffuse subcortical damage of vascular origin [7]. In this study, we aimed to assess whether sleep quality independently correlates with global cortical atrophy (GCA) in community-dwelling older adults, and the importance of the interaction of age in this association.
2. Methods
Atahualpa is a village representative of rural coastal Ecuador. More than 95% of the population belong to the Native/Mestizo ethnic group and their living characteristics are homogeneous, as detailed elsewhere [5]. Shift work, as well as light and noise pollution are uncommon during nightly hours in the village.
Atahualpa residents aged ≥60 years identified during yearly door-to-door surveys and prospectively registered in the Atahualpa Project were offered a brain MRI. Out of 385 eligible individuals, 311 (81%) have been enrolled in this imaging sub-study. Reasons for not performing neuroimaging included refusal to participate, severe disability and contraindications for MRI. Out of the 311 individuals with an MRI, 290 were interviewed with the Pittsburgh Sleep Quality Index (PSQI) and the Montreal Cognitive Assessment (MoCA). Subjects with aphasia, blindness or deafness were not interviewed. The Institutional Review Board of Hospital-Clínica Kennedy, Guayaquil, Ecuador (FWA 00006867) approved the study.
2.1. Neuroimaging protocol
MRIs were performed with a Philips Intera 1.5T (Philips Medical Systems, the Netherlands) at Hospital-Clínica Kennedy, Guayaquil. MRI included two-dimensional multi-slice turbo spin echo T1-weighted, fluid attenuated inversion recovery (FLAIR), T2-weighted, and gradient-echo sequences in the axial plane, as well as a T1-weighted sequence oriented in the sagittal plane. We used the pre-established brain imaging package delivered by the manufacturer to homogenize applicability by technicians; slice thickness was 5 mm with 1 mm gap between slices.
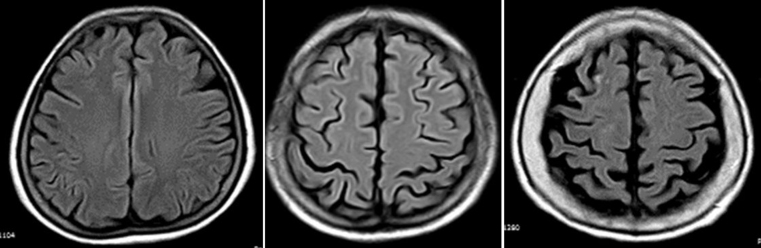
Two readers, blinded to clinical data, independently reviewed all MRIs. Inter-rater agreement was assessed for all findings, and disagreements were resolved by consensus. For rating GCA, we used the visual scale of Pasquier et al. [8], as previously described in detail by our group [9]. In brief, mild GCA was defined as the visualization of sulcal opening peripherally, moderate GCA was characterized by widening along the length of the sulci, and severe GCA was considered to be present when there was gyral thinning (Fig. 1). In addition, the severity of white matter hyperintensities (WMH) of presumed vascular origin – rated according to the modified Fazekas scale [10] – as well as the presence of old strokes and cerebral microbleeds were assessed to be used as confounding variables in multivariate models.
Fig. 1.
Fluid attenuated inversion recovery MRIs (TR 9000, TE 120, TI 2500) showing severity of global cortical atrophy (GCA) according to the visual rating scale of Pasquier et al. [8]. From left to right, images represent mild, moderate and severe global GCA respectively. In mild GCA there is sulcal opening peripherally, moderate GCA is characterized by widening along the length of the sulci, and severe GCA is present when there is gyral thinning. Images correspond to three different individuals aged 60–65 years.
2.2. Sleep quality investigation
We used a validated Spanish version of the Pittsburgh Sleep Quality Index (PSQI) [11]. The questionnaire consists of a combination of Likert-type and open ended questions assessing sleep duration (total amount of sleep obtained during the nocturnal sleep episode), sleep disturbances (symptoms that change sleeping habits), sleep latency (length of time that it takes to sleep), day dysfunction due to sleepiness (daytime somnolence), sleep efficiency (ratio of time spent asleep to the amount of time in bed), overall sleep quality (self-perceived satisfaction with sleep), and medications needed to sleep (use of sedatives, sleep inductors, etc). The maximum score is 21 points, and the cutoff value for poor sleep quality is ≥6 points.
2.3. Clinical covariates investigated
Demographics, educational status, and cardiovascular risk factors were assessed through direct interviews and procedures previously described in the Atahualpa Project. Severe edentulism was defined in individuals having less than 10 remaining teeth, symptoms of depression were assessed by the depression axis of the depression-anxiety-stress-21 scale [12], and cognitive performance was assessed by the Spanish version of the Montreal Cognitive Assessment (MoCA) (www.mocatest.org, ©Z. Nasreddine MD, version 07 November 2004).
2.4. Statistical analyses
Analyses were carried out by using STATA version 14 (College Station, TX, USA). In univariate analyses, continuous variables were compared by linear models and categorical variables by x2 or Fisher exact test as appropriate. A multivariate logistic regression model was constructed to assess the association between sleep quality and GCA, after adjusting for demographics, cardiovascular risk factor, severe edentulism, symptoms of depression, the MoCA score, and neuroimaging signatures of cerebrovascular damage (including WMH, strokes and cerebral microbleeds). Due to the potential effect modification of age, a multivariate interaction model for the marginal probabilities of moderate-to-severe GCA was constructed with participants stratified according to the median age of the population. This model was used to get more insights on the importance of the interaction of age in the association between GCA and sleep quality, after adjusting for the other confounders.
3. Results
Mean age of the 290 participants was 69±8 years, 168 (58%) were women and 237 (82%) had primary school education only. Mean values of the PSQI were 5±2 points, with 94 (32%) individuals classified as having poor sleep quality. Neuroimaging studies revealed moderate-to-severe GCA in 143 (49%) individuals, moderate-to-severe WMH in 67 (23%), stroke lesion in 47 (16%) and cerebral microbleeds in 36 (12%). Kappa coefficients for inter-rater agreements of MRI lesions of interest were 0.90 for WMH, 0.76 for cerebral microbleeds, 0.90 for old strokes, and 0.82 for GCA.
The mean MoCA score was 19±5 points. Overall, 20 (7%) individuals had poor physical activity, 12 (4%) had a poor diet, 61 (21%) had a body mass index ≥30 kg/m2, 139 (48%) had blood pressure ≥140/90 mmHg, 93 (32%) had fasting glucose levels ≥126 mg/dL, 34 (12%) had total cholesterol levels ≥240 mg/dL, 142 (49%) had severe edentulism, and 46 (16%) had symptoms of depression.
Table 1 summarizes the characteristics of participants and across categories of sleep quality. In univariate analyses, individuals with poor sleep quality were older, had a lower MoCA score, and the MRI showed more often moderate-to-severe WMH than in those with good sleep quality. There was a non-significant trend for individuals with poor sleep quality to have higher systolic blood pressure levels and to be edentulous than those with good sleep quality. In the univariate analysis, the difference in the prevalence of moderate-to-severe GCA across categories of sleep quality was only marginal (p=0.054).
Table 1.
Characteristics of Atahualpa residents aged ≥60 years across categories of sleep quality (univariate analyses).
| Total series (n=290) | Good sleep quality (n=196) | Poor sleep quality (n=94) | pvalue | |
|---|---|---|---|---|
| Age, mean±SD | 69±8 | 68±7 | 72±10 | 0.001 |
| Women, n (%) | 168 (58) | 112 (57) | 56 (60%) | 0.695 |
| Primary school education, n (%) | 237 (82) | 165 (84) | 72 (77) | 0.118 |
| Poor physical activity, n (%) | 20 (7) | 15 (8) | 5 (5) | 0.463 |
| Poor diet, n (%) | 12 (4) | 8 (4) | 4 (4) | 0.945 |
| Body mass index, mean±SD | 26±5 | 26±5 | 26±5 | – |
| Systolic blood pressure, mean±SD | 144±25 | 142±23 | 148±28 | 0.054 |
| Diastolic blood pressure, mean±SD | 76±12 | 76±11 | 75±12 | 0.482 |
| Fasting glucose mg/dL, mean±SD | 140±83 | 136±79 | 150±90 | 0.178 |
| Total cholesterol mg/dL, mean±SD | 200±33 | 200±33 | 199±34 | 0.811 |
| Severe edentulism, n (%) | 142 (49) | 89 (45) | 53 (56) | 0.080 |
| Symptoms of depression, n (%) | 46 (16) | 28 (14) | 18 (19) | 0.289 |
| MoCA score, mean±SD | 19±5 | 19±5 | 18±5 | 0.019 |
| Moderate-to-severe WMH, n (%) | 67 (23) | 35 (18) | 32 (34) | 0.002 |
| Stroke lesions, n (%) | 47 (16) | 29 (15) | 18 (19) | 0.346 |
| Cerebral microbleeds, n (%) | 36 (12) | 25 (13) | 11 (12) | 0.799 |
| Moderate-to-severe GCA, n (%) | 143 (49) | 89 (45) | 54 (57) | 0.054 |
In the logistic regression model (adjusted for demographics, cardiovascular risk factor, severe edentulism, symptoms of depression, the MoCA score, and neuroimaging signatures of cerebrovascular damage) – with participants stratified by the median age (67 years) – covariates independently associated with GCA in individuals with poor sleep quality were the MoCA score (OR: 0.87; 95% C.I.: 0.81–0.94; p=0.001) and the presence of moderate-to-severe WMH (OR: 0.31; 95% C.I.: 0.13–0.74; p=0.009). In this model, the association of moderate-to-severe GCA with poor sleep quality was highly significant (OR: 0.09; 95% C.I.: 0.02–0.46; p=0.004).
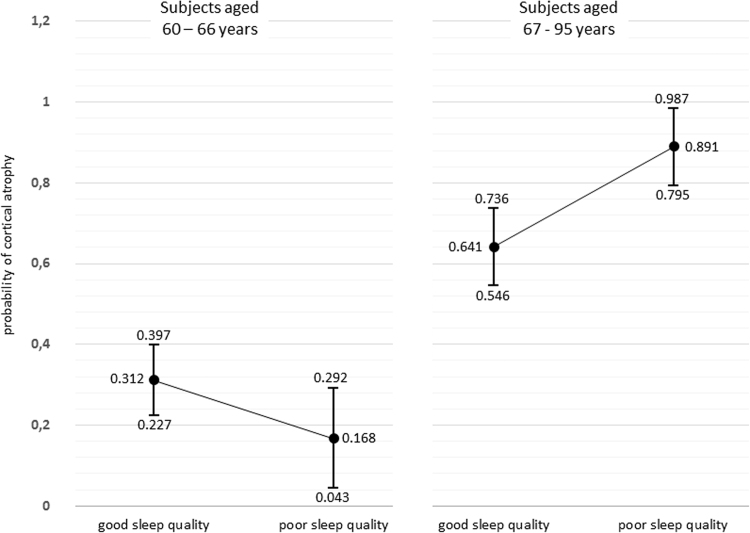
Among subjects aged ≤66 years the probability of moderate-to-severe GCA was significantly lower than among those aged ≥67 years and did not depend on sleep quality. On the other hand, the probability of moderate-to-severe GCA among subjects aged ≥67 years was significantly higher among those with poor sleep quality (Fig. 2).
Fig. 2.
Probabilities (with 95% confidence intervals) of moderate-to-severe global cortical atrophy according to sleep quality, with participants stratified by the median age of the population.
4. Discussion
This study provides evidence for an association between poor sleep quality and GCA in older adults and the important effect of age in this association. In addition, cognitive performance and the presence of diffuse subcortical damage of vascular origin are variables independently associated with GCA in multivariate logistic regression models.
As previously noticed, there are few studies addressing the association between poor sleep quality and cortical atrophy. In one of them, the authors found a direct association, with the additional advantage of showing progressive cortical atrophy in poor sleepers during the follow-up; however, such study recruited 147 participants through newspaper advertisements, and may not be representative of the population at large [3]. In another study, 66 healthy individuals underwent MRI and were interviewed with the PSQI, with no association at baseline; further follow-up of some of these cases revealed a relationship between short sleep duration and brain atrophy [4]. Other studies have evaluated the relationship of single sleep symptoms with the presence of regional cortical atrophy, thus providing limited information of the whole spectrum of non-breathing sleep-related symptoms and structural brain damage [13]. To our knowledge, no population-based study has addressed the link between poor sleep quality and MRI-documented GCA and the independent effect of age in this association.
Limitations of this study are its cross-sectional design and the fact that we relied on a visual rating scale and not on volumetric assessment to quantify cortical atrophy. However, the population-based design with unbiased inclusion of participants and the high acceptance rate (81%), together with the use of validated field instruments and the models constructed to assess interaction of age and other confounding variables, argue for the strengths of our findings. Further longitudinal studies are warranted to assess whether improvement in sleep quality make individuals less prone to develop cortical atrophy in the follow-up.
Conflicts of interest
Nothing to disclose.
Funding
This study was supported by Universidad Espiritu Santo – Ecuador.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Nebes R.D., Buysee D.J., Halligan E.M., Houck P.R., Monk T.H. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Soc Sci. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyata S., Noda A., Iwamoto K., Kawano N., Okuda M., Ozaki N. Poor sleep quality impairs cognitive performance in older adults. J Sleep Res. 2013;22(5):535–541. doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- 3.Sexton C.E., Storsve A.B., Walhovd K.B., Johansen-Berg H., Fjell A.M. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. doi: 10.1212/WNL.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo J.C., Loh K.K., Zheng H., Sim S.K., Chee M.W. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–1178. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Brutto O.H., Peñaherrera E., Ochoa E. Door-to-door survey of cardiovascular health, stroke, and ischemic heart disease in rural coastal Ecuador – the Atahualpa Project: methodology and operational definitions. Int J Stroke. 2014;9(3):367–371. doi: 10.1111/ijs.12030. [DOI] [PubMed] [Google Scholar]
- 6.Del Brutto O.H., Mera R.M., Farfán R., Castillo P.R. Cerebrovascular correlates of sleep disorders – rational and protocol of a door-to-door survey in rural coastal Ecuador. J Stroke Cerebrovasc Dis. 2014;23(5):1030–1039. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto O.H., Mera R.M., Zambrano M., Lama J., Del Brutto O.H., Castillo P.R. Poor sleep quality and silent markers of cerebral small vessel disease: a population-based study in community-dwelling older adults (the Atahualpa Project) Sleep Med. 2015;16(3):428–431. doi: 10.1016/j.sleep.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Pasquier F., Leys D., Weerts J.G., Mounier-Vehier F., Barkhof F., Scheltens P. Inter and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996;36(5):268–272. doi: 10.1159/000117270. [DOI] [PubMed] [Google Scholar]
- 9.Del Brutto O.H., Mera R.M., Zambrano M., Soriano F., Lama J. Global cortical atrophy (GCA) associates with worse cognitive performance in the Montreal Cognitive Assessment. A population-based study in community-dwelling elders living in rural Ecuador. Arch Gerontol Geriatr. 2015;60(1):206–209. doi: 10.1016/j.archger.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Pantoni L., Basile A.M., Pracucci G. Impact of age-related cerebral white matter changes on the transition to disability: the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24(1–2):51–62. doi: 10.1159/000081050. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Genchi A., Monteverde-Maldonado E., Nenclares-Portocarrero A. Confiabilidad y análisis factorial de la versión en español del índice de calidad de sueño de Pittsburgh en pacientes psiquiátricos. Gac Med Mex. 2008;144(6):491–496. [PubMed] [Google Scholar]
- 12.Osman A., Wong J.L., Bagge C.L., Freedenthal S., Gutierrez P.M., Lozano G. The depression anxiety stress scale – 21 (DASS-21): further examination of dimensions, scale reliability, and correlates. J Clin Psychol. 2012;68(12):1322–1328. doi: 10.1002/jclp.21908. [DOI] [PubMed] [Google Scholar]
- 13.Winkelman J.W., Plante D.T., Schoerning L. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36(7):182–185. doi: 10.5665/sleep.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]