Abstract
Sleepiness is the condition where for some reason fails to go into a sleep state and will have difficulty in remaining awake even while carrying out activities. Sleep restriction occurs when an individual fails to get enough sleep due to high work demands. The mechanism between sleep restriction and underlying brain physiology deficits is not well assumed. The objective of the present study was to investigate the mental attention (P300) and reaction time [visual (VRT) and auditory (ART)] among night watchmen, at subsequent; first (1st) day, fourth (4th) day and seventh (7th) day of restricted sleep period. After exclusion and inclusion criteria, the study was performed among 50 watchmen (age=18–35 years) (n=50) after providing written informed consent and divided into two group. Group I-(Normal sleep) (n=28) working in day time and used to have normal sleep in night (≥8 h); Group II-(Restricted sleep) (n=22) - working in night time and used to have less sleep in night (≤3 h). Statistical significance between the different groups was determined by the independent student ʻtʼ test and the significance level was fixed at p≤0.05. We observed that among all normal and restricted sleep watchmen there was not any significant variation in Karolinska Sleepiness Scale (KSS) score, VRT and ART, along with latency and amplitude of P300 on 1st day of restricted sleep. However at subsequent on 4th day and 7th day of restricted sleep, there was significant increase in (KSS)score, and prolongation of VRT and ART as well as alteration in latency and amplitude of P300 wave in restricted sleep watchmen when compare to normal sleep watchmen. The present finding concludes that loss of sleep has major impact in dynamic change in mental attention and reaction time among watchmen employed in night shift. Professional regulations and work schedules should integrate sleep schedules before and during the work period as an essential dimension for their healthy life.
Keywords: Attention, P300, Reaction time, Sleep restriction
1. Introduction
Sleep restriction occurs when an individual fails to get sufficient sleep [1]. It mainly refers to the reduced number of hours of sleep that individuals experience from day-to-day or week-to-week. Normally it is suggested that adults should obtain about 8 h sleep per night [2]. The amount of sleep varies considerably from one person to another [3], but on average most adults need about 7–8 h of sleep each night to feel alert and well rested [1], [4]. A person's quality of life can be disrupted due to many different reasons [5]. Lifestyle and occupational factors such as long hours of work, commuting, shift work, family and social commitments [5], [6], [7]. Due to high work demands, sleep restriction is becoming common in modern societies. Extended time awake and/or sleep restriction increase sleep pressure and generate cumulative sleepiness and impair neurobehavioral functioning [8]. Disruption of alertness and risk of professional errors is a major issue, when working under sleep restriction [9], [10]. The nature of brain functioning underlying these behavioral changes remains poorly understood. Because of these conflicts understanding sleep restriction are becoming key issues. Event related potentials; especially the P300 can be used to study information processing during wake and sleep [11]. P300 (also referred to as P3b), is a centro-parietal distribution [12] and wave phenomenon that reflects conscious processes [13], [14]. It is a slower, sustained posterior positivity elicited at around 300 ms [15]and considered as a high-level prediction error signal associated with conscious novelty detection. Event-related potential (ERP) or P300 are neurophysiological measures of arousal and information processing in the brain which may be capable of compensation during sleep loss and it that may be used to investigate the CNS nature of performance during sleepiness [16] by determining the timing of cognitive processes in the order of milliseconds using ERP. The global novelty response, associated with a P300 on EEG, depends on a conscious appraisal of the auditory sequence [17]. It arises from recurrent interactions in a broad set of interconnected areas, including fronto-parietal cortices [18]. The previous research has been done using ERP techniques to examine the neural basis of performance deficits during sleep loss under extreme conditions of total sleep deprivation [16], [17], [19], [20]. The mechanism between sleep restriction and underlying brain physiology deficits is not well assumed among night watchmen. The objective of the present study was to investigate the mental attention and reaction time (auditory and visual) among night watchmen, at subsequent at first (1st) day, fourth (4th) day and seventh (7th) day of restricted sleep period by using electrophysiological techniques.
2. Method
2.1. Inclusion and exclusion criteria
All Participants (watchmen) employed in Peoples University and nearby Community were recruited for the study. Participants meeting the following criteria were included, healthy, good sleeper, non-smoker, and free from medications, no history of depression, neurological disease, or chronic pain and underwent a medical interview to ensure that they had a regular sleep/wake schedule. After signing consent, they were also evaluated for a visual and hearing test to ensure that their visual and hearing range was within an acceptable level for reaction time and to perform auditory ERP tasks. Initially 70 participants volunteer to participate, 20 were eliminated due to exclusion criteria or inability to follow schedule and medical factor. The remaining 50 participants completed the one-week protocol. The study was approved by the research advisory committee of People College of Medical Science and Research Center (PCMS/OD/2015/1056). The participants were divided into two groups. The study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from each subject before the start of the study.
The participants were divided randomly into two groups.
Group I-(Normal sleep) (n=28) – Twenty-eight watchmen (age=18–35 years) working in day time and used to have normal sleep in night (≥8 h).
Group II-(Restricted sleep) (n=22) – Twenty-two watchmen (age=18–35 years) working in night time and used to have less sleep in night (≤3 h).
2.2. Sleep schedule
The participants were instructed to maintain a regular sleep–wake schedule and were monitored. No stimulant of any kind was allowed during the study. For the tests obtained in the normally rested condition, instructed to the subjects to maintain normal sleep in night every day. The participants in the sleep restricted group also had a regular sleep schedule (8 h/night) before the beginning of the experiment and later on participants were instructed to sleep in night less than three hours (<3 h) for one week of their night shift schedule. All the participants were not allowed to sleep in day time. Participants slept at home and completed scheduled sleep diaries, regularly while at home, the duration of sleep was self-monitored. Total time in bed was recorded with a click button by the subject when getting into and out of bed. Participants reported less sleep during study duration which was also confirmed by monitors. After completion of one week study period, participants visited to the laboratory on the morning at 09:00 a.m. for assessment of electrophysiology and RT tasks performance. Each participant was tested after a normal sleep night and after a restricted sleep night in random order. The study was conducted in the department of physiology, Peoples College of medical science and research center, Bhopal, India. All the measurement was assessed at first (1st) day, fourth (4th) day and seventh (7th) day of restricted sleep period. Laboratory assessment of electrophysiology and reaction time took place three times at the end of study period.
2.3. Sleepiness measurement
Participant sleepiness was assessed using a modified version of the Karolinska Sleepiness Scale (KSS) [21]. The Karolinska Sleepiness Scale (KSS) is a 9-point Likert scale based on a self-reported, subjective assessment of the subject's level of drowsiness at the time. These descriptors varied from 1=“very alert” to 9=“very sleepy, fighting sleep, an effort to keep awake”.
2.4. Mood measurement
Participant mood was assessed using the University of Wales Institute of Science and Technology (UWIST) mood adjective checklist [22], which measures three domains of mood: energetic arousal, hedonic tone and tense arousal. Both the KSS and UWIST mood adjective checklist are state assessments and were given prior to reaction time and ERP measurement.
2.5. ERP recording (P300)
The Site-specific changes in the P300 waveform with sleep restriction were examined. P300 was measured in a standard audiometric, sound proof room of neurophysiology laboratory at temperature of 26–30 °C by using Neuroperfect-2000. The “oddball” or “P300” task is commonly used in assessments of sleep restriction, deprivation, and sleep apnea [23], [24], [25]. In common, the task engages attention and generates changes in brain activity across the scalp, especially at central and parietal sites. A simple auditory oddball paradigm was used with 20% rare high tones (40 dB 2 kHz) and with 80% normal low tones (40 dB 1 kHz). The stimulus frequency was 0.5 Hz. Tone frequency (i.e., high vs. low) was used to denote the infrequent and frequent stimuli. Each participant was instructed that they would hear a series of tones. Participant had to press a button when they heard the rare “target” tones. Rare tones were at random mixed with the frequent tones. After the forced awakenings only 200 stimuli were offered in one trial in order to prevent the participant from falling asleep. Signals were derived from Fz, Cz and Pz with linked ears as reference electrode. Signals were averaged with a band pass of 0.1–50 Hz and with an analysis time of 1 s. Responses of the participants were recorded by Neuro machine (Evoked potential measuring system).
2.6. Reaction time (RT) recording
Visual and Auditory RT was measured by Borker and Pednekar [26] by using a simple electrical setup as explained previously by our research group [27].
2.7. Statistical analysis
Data are expressed as Mean±Standard deviation (SD). All data were analyzed with the SPSS for windows statistical package (version 20.0, SPSS Institute Inc., Cary, North Carolina. Statistical significance between the different groups was determined by the independent student ʻtʼ test and repeated measured analysis was performed. The significance level was fixed at p≤0.05.
3. Result
3.1. Effect of restricted sleep on sleepiness
The data are summarized in (Table 1) with mean±SD. Among all normal and restricted sleep watchmen there was no significant variation in KSS score on 1st day of restricted sleep. However at subsequent on 4th day and 7th day of restricted sleep, there was significant increase in KSS score in restricted sleep watchmen when compare to normal sleep watchmen, indicating higher levels of sleepiness. In addition to, sleepiness level on 7th day was more significant increase, when compare with 4th day of restricted sleep period.
Table 1.
Effect of restricted sleep on karolinska sleepiness scale (KSS), visual reaction time (VRT) and auditory reaction time (ART). Where *significance change (p≤0.05) compared with normal sleep and #significance change (p≤0.05) compared with 4th day.
| 1st-day | 4th-day | 7th-day | |
|---|---|---|---|
| KSS | |||
| Normal sleep | 2.6±0.6 | 2.4±0.6 | 2.5±0.5 |
| Restricted sleep | 2.5±0.4 | 4.4±0.7* | 6.5±0.9*# |
| VRT(ms) | |||
| Normal sleep | 450±26 | 473±30 | 465±27 |
| Restricted sleep | 460±32 | 569±35* | 715±43*# |
| ART (ms) | |||
| Normal sleep | 243±30 | 250±25 | 260±28 |
| Restricted sleep | 255±27 | 330±44* | 440±40*# |
3.2. Effect of restricted sleep on mood
Among all normal and restricted sleep watchmen there was no significant variation on the UWIST mood adjective checklist on 1st day of restricted sleep. However on 4th day of restricted sleep, restricted sleep watchmen reported significantly lower levels of energetic arousal (16±3) when compare to normal sleep watchmen (25±4). As well as at subsequent 7th day of restricted sleep, restricted sleep watchmen reported significantly lower levels of energetic arousal (09±3) when compare to normal sleep watchmen (26±3).
3.3. Effect of restricted sleep on reaction time
The data are summarized in (Table 1) with mean±SD. The visual and auditory reaction time was comparable in among all normal and restricted sleep watchmen on 1st day of restricted sleep. However at subsequent on 4th day and 7th day of restricted sleep, it was significantly prolonged in restricted sleep watchmen when compare to normal sleep watchmen. As well as, among 4th and 7th day of restricted sleep period, reaction time was more prolonged on 7th day, when compare to 4th day.
3.4. Effect of restricted sleep on site-specific (Fz, Cz, and Pz) event-related potentials (P300)
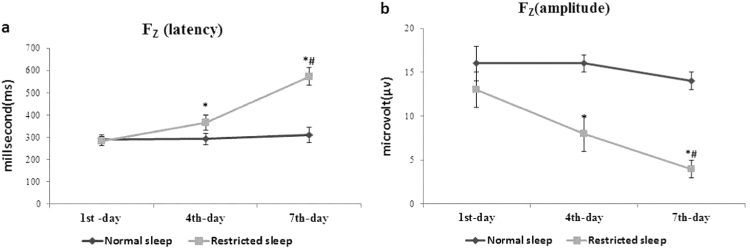
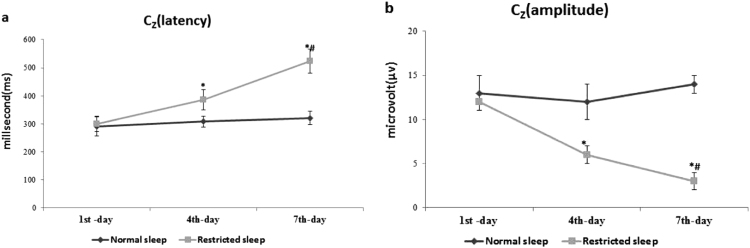
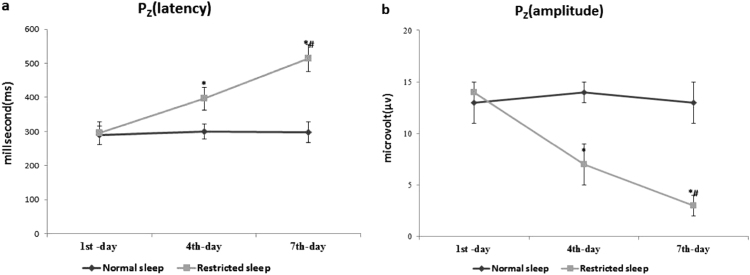
The data are summarized in (Figs. 1a-b, 2a-b, and 3a-b) with mean±SD. The latency and amplitude of P300 wave time was comparable in among all normal and restricted sleep watchmen on 1st day of restricted sleep. However at subsequent on 4th day and 7th day of restricted sleep, the latency of P300 wave was significantly prolonged and amplitude of P300 wave was significantly decreased in restricted sleep watchmen when compare to normal sleep watchmen at all specific site (Fz, Cz, and Pz). As well as among 4th and 7th day of restricted sleep period, prolongation of latency and declining of amplitude was more on 7th day; when compare to 4th day.
Fig. 1.
(a-b) Effect of restricted sleep on latency and amplitude of P300 wave. Where *significance change (p≤0.05) compared with normal sleep and #significance change (p≤0.05) compared with 4th day.
Fig. 2.
(a-b) Effect of restricted sleep on latency and amplitude of P300 wave. Where *significance change (p≤0.05) compared with normal sleep and #significance change (p≤0.05) compared with 4th day.
Fig. 3.
(a-b) Effect of restricted sleep on latency and amplitude of P300 wave. Where *significance change (p≤0.05) compared with normal sleep and #significance change (p≤0.05) compared with 4th day.
4. Discussion
Sleepiness is a physiological phenomenon, regulated by two processes: processes S (homeostatic) and C (circadian) [28]. The process S (homeostatic) depends on sleep and wakefulness; the need for sleep increases as wakefulness continues and the process C (circadian) recommends a control of an endogenous circadian pacemaker, which affects thresholds for the onset and offset of a sleep period [28]. The interaction of these two processes determines the sleep/wake cycle and can be used to describe fluctuations in alertness and vigilance. According to this two-process model of sleep regulation, sleep pressure accumulates during wakefulness and dissipates in the course of the following sleep episode [29]. The first hypothesis suggests that sleepiness especially impairs cognitive performances that depend on the prefrontal cortex [30]. These include higher functions, such as language, executive functions, divergent thinking, and creativity.
The more sensitive, approach used to identify cognitive processing abnormalities is to measure event-related potentials (ERPs). In ERP, P300 is an endogenous component that is altered with impairment to attention [31]. The latencies and amplitudes of these characteristic electrical brain responses provide information on cognitive processing. The delayed latency and reduced amplitude have been reported using auditory signal [25]. The impairment of behavioral task has been reported due to result of sleep restriction [32]. These alterations in P300 component reflect delays in the time taken to discriminate sensory stimuli that is allotted to the participants [33]. The electrophysiological measures of ERPs showed dynamic changes in attention as a result of sleep restriction as seen in the reduced amplitude in P300 component of the ERP and a decrease in the amplitude of the P300 waveform has been suggested to be associated with a decrease in attention [34].
We observed at subsequent on 4th day and 7th day of restricted sleep period; the latency of P300 wave was prolonged and decreased amplitude in restricted sleep watchmen. Our finding is consistent with previous studies of sleep restriction, where they have found that four or more days of partial sleep restriction involving less than 7 (<7) hours sleep per night resulted in cumulative adverse effects on neurobehavioral functions and increase lapses of attention on the psychomotor task [35], [36] and whenever there is difficulty in discriminating between stimuli, P300 latency increased with drowsiness preceding sleep onset [37]. We also observed prolongation of reaction time (the failure to respond to a stimulus within a timely fashion) in restricted sleep watchmen; on 4th day and 7th day of restricted sleep period. This supports some of the previous studies on association between sleep loss and reaction time, where the reaction time has been decreased under total or partial sleep loss [38], [39], [40], [41]. It is assumed that reaction tasks would be especially susceptible to sleep restriction because of may be sleepiness [42], [43], short duration and limited capacity of iconic memory [44], obstruction in assignation of spatial attention [45], [46] or decreased oculomotor function[43].
The actual mechanism behind Sleep restriction leads to decreased reaction times and reduced the levels of alertness [47], [48], [49] is still controversial. One hypothesis suggest that sleep restriction influences “bottom-up” attention and arousal processes on a global level, mediated by wake-state instability [50]. These disruptions lead to performance decline on attention and vigilance tasks [40]. Another hypothesis suggest that sleep restriction has domain-specific effects that target specific “top-down” brain areas, notably the prefrontal cortex (PFC) functions, such as flexible thinking, verbal fluency, inhibition, and memory [16], [23], [51], [52], [53]. A more integrative approach encompassing these different theoretical accounts suggests that sleep restriction primarily negatively impacts PFC functions, which influence both top-down and bottom-up processes [54].
The present study of electrophysiological measures of ERP; showed dynamic changes in attention and prolongation in reaction time; represents variable effect on psychomotor vigilance and cognitive status among watchmen employed for night shift. This may be attributed to the resultant effect of sleep restriction. The importance of maintaining optimal attention, arousal and reaction times in watchmen, will minimize work mistakes while keeping guard at night shift.
5. Conclusion
The present electrophysiological findings revealed that loss of sleep has major impact in dynamic changes of mental attention and prolongation of reaction time among watchmen employed in night shift. Professional regulations and work schedules should integrate sleep schedules before and during the work period as an essential dimension for their healthy life. The most important goal of this finding was to bring awareness that sleeplessness is a major problem that needs to be addressed by every organization.
Limitations
Small number of participants and the study was based only on men, therefore not allowing a study of the differences according to gender and we did not measure the cortisol level to assess the level of mental stress.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We gratefully acknowledge the financial support provided by the People's University, Bhopal, India (Grant no. PCMS/OD/2015/1056) and we thankful to neuro-technician Mr. Kamta Prasad Ahirwar for assisting in recording.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Carskadon M.A., Dement W.C. Normal human sleep: an overview. Princ Pract Sleep Med. 2005;4:13–23. [Google Scholar]
- 2.Groeger J.A., Zijlstra F., Dijk D.J. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res. 2004;13(4):359–371. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 3.Shneerson J.M. Mishawaka, IN, U.S.A.: Wiley-Blackwell; 2000. Handbook of sleep medicine. [Google Scholar]
- 4.Kronholm E. Self-reported sleep duration in finnish general population. J Sleep Res. 2006;15(3):276–290. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 5.Colten H.R., Altevogt B.M. Sleep disorders and sleep deprivation: an unmet public health problem. Washington (DC): National Academies Press (US); 2006. [PubMed] [Google Scholar]
- 6.Hiestand D.M. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. CHEST J. 2006;130(3):780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 7.Monk T.H. Shift work: basic principles. Princ Pract Sleep Med. 2005;4:673–679. [Google Scholar]
- 8.Powell N.B. The road to danger: the comparative risks of driving while sleepy. Laryngoscope. 2001;111(5):887–893. doi: 10.1097/00005537-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Gaba D.M., Howard S.K. Fatigue among clinicians and the safety of patients. New Engl J Med. 2002;347(16):1249–1255. doi: 10.1056/NEJMsa020846. [DOI] [PubMed] [Google Scholar]
- 10.Rajaratnam S.M., Arendt J. Health in a 24-h society. Lancet. 2001;358(9286):999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 11.Colrain I.M., Campbell K.B. The use of evoked potentials in sleep research. Sleep Med Rev. 2007;11(4):277–293. doi: 10.1016/j.smrv.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer K.M., Dien J., Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36(03):409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- 13.Del Cul A., Baillet S., Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5(10):e260. doi: 10.1371/journal.pbio.0050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sergent C., Baillet S., Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8(10):1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 15.Sutton S. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 16.Drummond S.P., Brown G.G. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–S73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 17.Sitt J.D. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137(8):2258–2270. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehaene S., Changeux J.-P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Bekinschtein TA. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. 2009;106(5):1672–1677. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faugeras F. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia. 2012;50(3):403–418. doi: 10.1016/j.neuropsychologia.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Reyner L., Horne J. Falling asleep whilst driving: are drivers aware of prior sleepiness? Int J Leg Med. 1998;111(3):120–123. doi: 10.1007/s004140050131. [DOI] [PubMed] [Google Scholar]
- 22.Matthews G., Jones D.M., Chamberlain A.G. Refining the measurement of mood: the UWIST mood adjective checklist. Br J Psychol. 1990;81(1):17–42. [Google Scholar]
- 23.Cote K.A. Physiological arousal and attention during a week of continuous sleep restriction. Physiol Behav. 2008;95(3):353–364. doi: 10.1016/j.physbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Gosselin A., De Koninck J., Campbell K.B. Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clin Neurophysiol. 2005;116(1):211–222. doi: 10.1016/j.clinph.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.-J. Auditory event-related potentials and psychological changes during sleep deprivation. Neuropsychobiology. 2004;50(1):1–5. doi: 10.1159/000077933. [DOI] [PubMed] [Google Scholar]
- 26.Borker A., Pednekar J. Effect of pranayam on visual and auditory reaction time. Indian J Physiol Pharmacol. 2003;47(2):229. [PubMed] [Google Scholar]
- 27.Choudhary A.K. A comparative analysis of dietary habits on sensory motor association and heart rate variability during menstrual cycle. J Clin Diagn Res. 2016;10(1):CC04. doi: 10.7860/JCDR/2016/16421.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004;75(Suppl. 1):A37–A43. [PubMed] [Google Scholar]
- 29.Borbély A.A. A two process model of sleep regulation. Hum Neurobiol. 1982 [PubMed] [Google Scholar]
- 30.Horne J.A. Human sleep, sleep loss and behaviour: Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993 doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 31.Picton T.W., Lins O.G., Scherg M. The recording and analysis of event-related potentials. Handb Neuropsychol. 1995;10:3. 3. [Google Scholar]
- 32.Van Dongen H.P. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–129. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 33.Picton T.W. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Kingshott R.N. The effect of sleep fragmentation on cognitive processing using computerized topographic brain mapping. J Sleep Res. 2000;9(4):353–357. doi: 10.1046/j.1365-2869.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 35.Belenky G. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 36.Drake C.L. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38(6):979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- 37.Colrain I.M., Di Parsia P., Gora J. The impact of prestimulus EEG frequency on auditory evoked potentials during sleep onset. Can J Exp Psychol/Rev Can de Psychol expérimentale. 2000;54(4):243. doi: 10.1037/h0087344. [DOI] [PubMed] [Google Scholar]
- 38.Franzen P.L., Siegle G.J., Buysse D.J. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17(1):34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jugovac D., Cavallero C. Twenty-four hours of total sleep deprivation selectively impairs attentional networks. Exp Psychol. 2012 doi: 10.1027/1618-3169/a000133. [DOI] [PubMed] [Google Scholar]
- 40.Martella D., Casagrande M., Lupiáñez J. Alerting, orienting and executive control: the effects of sleep deprivation on attentional networks. Exp Brain Res. 2011;210(1):81–89. doi: 10.1007/s00221-011-2605-3. [DOI] [PubMed] [Google Scholar]
- 41.Philip P. Fatigue, sleep restriction, and performance in automobile drivers: a controlled study in a natural environment. Sleep. 2003;26(3):277–284. doi: 10.1093/sleep/26.3.277. [DOI] [PubMed] [Google Scholar]
- 42.De Gennaro L. Visual search performance across 40 h of continuous wakefulness: measures of speed and accuracy and relation with oculomotor performance. Physiol Behav. 2001;74(1):197–204. doi: 10.1016/s0031-9384(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 43.Zils E. Differential effects of sleep deprivation on saccadic eye movements. Sleep. 2005;28(9):1109. doi: 10.1093/sleep/28.9.1109. [DOI] [PubMed] [Google Scholar]
- 44.Raidy D.J., Scharff L.F. Effects of sleep deprivation on auditory and visual memory tasks. Percept Mot Skills. 2005;101(2):451–467. doi: 10.2466/pms.101.2.451-467. [DOI] [PubMed] [Google Scholar]
- 45.Bocca M.-L., Denise P. Total sleep deprivation effect on disengagement of spatial attention as assessed by saccadic eye movements. Clin Neurophysiol. 2006;117(4):894–899. doi: 10.1016/j.clinph.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Choo W.-C. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25(2):579–587. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Kopasz M. Sleep and memory in healthy children and adolescents – a critical review. Sleep Med Rev. 2010;14(3):167–177. doi: 10.1016/j.smrv.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Lim J., Dinges D.F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. -, 2005;28(11):1392. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- 50.Doran S., Van Dongen H., Dinges D.F. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital de Biol. 2001;139(3):253–267. [PubMed] [Google Scholar]
- 51.Beebe D.W. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct. 2009;5(1):1. doi: 10.1186/1744-9081-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorsey C. Spectroscopy before and after total sleep deprivation in healthy adult men. Sleep. 2000;23(Suppl. 2):A357–A358. doi: 10.1093/sleep/26.5.573. [DOI] [PubMed] [Google Scholar]
- 53.Szelenberger W. Increased prefrontal event-related current density after sleep deprivation. Acta Neurobiol. Exp. 2005;65(1):19–28. doi: 10.55782/ane-2005-1536. [DOI] [PubMed] [Google Scholar]
- 54.Boonstra T. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. 2007;64(7–8):934–946. doi: 10.1007/s00018-007-6457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]