Abstract
To date, shift workers represent between 15% and 25% of the modern day workforce. Work time poses a great challenge to workers as it requires that they balance productivity and sleep time between shifts. As a result, these workers experience chronic sleep deprivation with increased fatigue and drowsiness due to this sleep deprivation. The impact of this kind of work on the immune system is not yet known. We conducted a literature review with the aim of evaluating articles on this specific type of work's effects on sleep and immunity.
Keywords: Sleep, Immunity, Shift work
1. Introduction
Shift work is work done by an individual whose normal hours of work are outside the traditional 9–5 p.m. work day [1]. It can involve evening or night shifts, early morning shifts, and rotating shifts. It is common in many professions, especially those involving essential services, and today shift workers represent between 15% and 25% of the global workforce [1], [2], [3].
The search for quality sleep has always been a challenge for shift workers, because they must reverse their biological rhythms and conduct activities at nighttime, a time when there is a greater propensity for drowsiness. Furthermore, daytime rest subjects these workers to greater alertness and light [5], [6]. Therefore, they are often chronically sleep deprived [7], [8]. It is known, for example, that sleep deprivation is associated with accidents at work [9], metabolic diseases [10], and cardiovascular diseases [11], [12]. Recent research has also observed an association between sleep loss and viral infections [13], [14], [15].
It is not fully understood how sleep contributes to efficient immunological memory, interferes with the immune system, or predisposes the body to infections [16]. The quiescent period of sleep can serve, for example, to renew functions related to wakefulness and processes affecting the immunological response to infections [17], [18].
The deterioration of sleep in modern society, as observed among shift workers, in turn, is associated with an increasing susceptibility to the development of infectious diseases such as flu [13], [15], airway infections [14] and failure to control immunization against certain diseases [19]. So, sleep debt/ sleep deficit can have a major economic impact on public health policies and probably emerge as an important regulator of the immune system [16].
We conducted a review to evaluate the articles on sleep, immunity and this particular type of work, the so-called shift work.
2. Methods
Research on original articles and review articles about the subject was carried out through PubMed. The general terms used in the search were “sleep,” “immunity or immune system,” and “shift work or shift workers.” The filters were original articles or review articles in English on the adult population. The research was conducted through July 2016.
3. Sleep and immunity
For over 2000 years the relationship between sleep and immunity has been the subject of discussion. Hippocrates, for example, mentioned the presence of sleepiness during the course of an acute infection [18], [20].
Today we know that the interaction between sleep and immunity is established by anatomical and physiological bases [20]. Neurons, glial cells, and immune cells share common intercellular signals, such as hormones, neurotransmitters, cytokines and chemokines [21], [22]. We know, for instance, that all lymphoid tissue receives innervation [23], [24] and pro-inflammatory cytokines, such as IL-1β, TNF-α, and their receptors are expressed in specific brain regions and act in the regulation of many physiological and behavioral processes (17), such as the sleep-wake status [25], [26], [27].
At an experimental level, research regarding TNF-α and IL-1 indicates that these cytokines are also sleep regulators and exert this effect regardless of their pyrogenic effect [28]. For example, administration of IL-1 and TNF-α in vitro produces slow oscillations that resemble NREM sleep in humans [28], [29], [30]. The perfusion of IL-1 reduces excitatory synaptic potentials in rat hippocampus, and the application of TNF in the sensory cortex induces the increase in slow-wave sleep in these animals [31], [32]. IL-1 and serotonergic pathways also interact with each other. 5-HT alters the expression of IL-1 mRNA in brain regions, while IL-1 increases the secretion of 5-HT in the hypothalamus, and a micro-injection of IL-1 in the dorsal raphe nuclei induces sleep NREM [33], [34]. So, it seems obvious that sleep can also influence the immune response.
The role of sleep in modulating the human immune function has been previously tested by observing the prolonged effects of sleep deprivation on various immune parameters or their behavior in diseases that naturally fragment sleep, such as insomnia, disorders of the circadian rhythm and shift work [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48].
These studies have produced interesting results [36], [37], [38], [39], [40], [41]. Some studies have shown that acute deprivation (50–64 h) is associated with a temporary increase in the activity of natural killer cells (NK), an increasing count of T-CD4+ lymphocytes, CD8+, monocytes, granulocytes and NK [36], [37], [38], [41]. Other studies regarding partial sleep (early night or late night) and chronic deprivation, which are more common in clinical practice, have, however, shown different results with a decrease in the activity of NK cells and the counts of CD 16 +, CD 56+, CD 57+ and IL-2 levels [35], [39], [40], [45]. We know that these lymphocytes, which participate in innate immunity, are important in the defense against viruses, intracellular bacteria, as well as in response to tumor cells [42], [43], [44].
In one study, Axelsson et al. [35], aiming to investigate partial sleep deprivation for five days and the production of inflammatory cytokines and the Th1/ Th2 balance among healthy subjects, noticed a transient decrease of IL-2 and IL-2/ IL-4 ratio until the fifth day of sleep deprivation.
Fondel et al. [45] conducted a study to evaluate the immune activity in healthy subjects who slept fewer than seven hours (short sleepers), compared to healthy subjects who slept seven to nine hours (normal sleepers). They observed a 30% decrease in the activity of NK cells (p 0.01) and a 49% increase in the activity of T-lymphocytes stimulated by PHA (phytohemagglutinin), independent of plasma cortisol levels.
Sakami et al. [46], in a study that evaluated the immune response in insomniacs and the balance of effector response, observed a change in the Th1/ Th2 immune response in favor of the Th2 response, with a decrease in the secretion of IFN-γ and IFN-γ/IL-4 ratio in insomniacs. They concluded that insomnia causes an alteration of the immune function with a predominance of suppressive Th2 response.
Savarde et al. [48] compared a group of individuals with insomnia to individuals considered to be good sleepers. They observed a significant difference: a greater amount of TCD3+, TCD4+, and TCD8+ lymphocytes and total lymphocytes in the group of good sleepers compared with insomniacs.
Prather [14], in a recent study on 164 healthy subjects experimentally exposed to rhinovirus, observed through pulse actigraphy that those with fewer than six hours of sleep, before exposure, were four times more likely to become sick (<5 h OR=4.50; 95%; 1.08–18.69 and 05–06 h OR=4.24, 95% 1.08–16.71) than those with more than seven hours of sleep (OR=1). This remained significant even after the adjustment for variables, such as smoking, sex, the title of neutralizing antibodies, exercise and alcohol consumption.
The Nurses' Health Study-II [15] was a cohort study conducted from 2001 to 2005 involving more than 56,000 healthy nurses. Researchers found that reduced sleep (<5 h), as well as the poor perception of sleep or excessive sleep (>9 h), presented a risk of 1.39 (95%; 1.06–1.82) and 1.38 (95%; 1.04–1.84) respectively, for the development of pneumonia, even after the adjustment for variables, such as age, body mass index, and smoking.
It was observed that apparent loss of sleep is associated with some immune changes [35–41.45]. In acute deprivation there is a temporary immune activation, although the clinical effects of these findings are unknown. More research is needed to answer such questions [36], [37], [38]. In relation to partial and prolonged deprivation, the aforementioned studies show an increased risk of infection in the airways, especially from viruses [14], [15]. The possible factors involved are impaired innate immunity, expressed by the reduction of the activity of NK cells (CD16+, CD56+, CD57+) and a decrease in Th1 effector cellular response, which is important to the activation of TCD4+ lymphocytes in favor of a more regulatory Th-2 response [35], [39], [40], [45], [46], [48].
3.1. Circadian rhythm, immunity and immunological memory
Human circadian rhythm is controlled by the neurological master clock, suprachiasmatic nucleus (SCN) and peripheral clocks present in almost every cell, including the immune system cells [49], [50], [51]. SCN thus allows all tissues and cells to anticipate and promptly respond to environmental changes, such as light, temperature, and the risk of exposure to pathogens in the environment [52]. It coordinates various behavioral, physiological, and molecular functions.
Cells of the innate and adaptive immune system also show circadian expression as a variation in the blood count or in the peripheral lymphoid organs, as well as in lymphocytic proliferation and in blood cytokine levels [36], [53], [54], [55], [56]. In humans, for example, it is already known that there is a decrease in the T cell count preceding the acrophase of morning cortisol in the blood, while an opposite effect has been observed in the early evening period [53], [55].
In association with the circadian system, sleep is known to regulate immune functions, although it is difficult to distinguish the respective influence of these two regulatory systems [20].
Consistent studies show that especially during slow-wave sleep, there is an increase of IL-12 by the pre-myeloid dendritic cells, which are the main precursors of mature antigen-presenting cells (APCs). Additionally, a reduction of IL-10 and IL-4 levels by monocytes is seen during slow-wave sleep, thereby facilitating a Th1/ Th2 balance in favor of the Th1 response [57], [58]. It is known that the production of IL-12 by APCs is essential for activation of Helper T cells [59]. This pro-inflammatory sleep pattern during the early portion of the night is then balanced by a Th2 response during the final portion of the night when REM sleep prevails [57]. The induction of Th1 cells, the increase of C3a and the migration of immune cells from the circulatory bed to the secondary lymphoid organs during sleep would increase the chances of interaction between the APCs and the naïve T cells and B lymphocytes (immunological synapse), and these could be mechanisms through which sleep would collaborate on a more efficient immunological memory [36], [57], [60], [61]. This interaction between sleep and adaptive memory has been evaluated in recent studies and has proven the abovementioned hypotheses [19], [62].
Prather et al. [19], in a prospective study of 125 adults aimed to evaluate the effect of sleep on the magnitude of the immune response to a viral antigen (Hepatitis B virus). They observed that fewer than 6 h of sleep per night, measured through a sleep diary and actigraphy, was associated with a reduction of vaccine protection from the B virus, even after three doses, including a catch-up vaccination in the sixth month.
Lange et al. [62], by comparing groups of healthy subjects who slept after immunization with groups that remained awake immediately after immunization for the Hepatitis A virus, observed that sleep in the first group significantly doubled the number of specific Th1 cells, as well as antibodies type IgG1 to the virus from the 8th to the 52nd week of follow-up. They concluded that slow-wave sleep was responsible for this adjuvant effect.
3.2. Shift work
According to the International Classification of Sleep Disorders (ICSD-3), shift work disorder is characterized by insomnia or sleepiness that occurs in association with shift work [2]. Its diagnostic criteria are shown below in Table 1. It is estimated that about 2–5% of the US working population has this disorder [3], [4].
Table 1.
Diagnostic criteria of shift work disorder (A-D must be met)*.
| A | There is a report of insomnia and/or excessive sleepiness, accompanied by a reduction of total sleep time, which is associated with a recurring work schedule that overlaps the usual time for sleep |
| B | The symptoms have been present and associated with the shift work schedule for at least three months. |
| C | Sleep log and actigraphy monitoring (whenever possible and preferably with concurrent light exposure measurement) for at least 14 days (work and free days) demonstrate a disturbed sleep and wake pattern. |
| D | The sleep and/or wake disturbance are not better explained by another current sleep disorder, medical or neurological disorder, mental disorder, medication use, poor sleep hygiene, or substance use disorder. |
[2].
The observed changes in shift work, such as continuous sleep deprivation, stress and a change of the natural circadian pattern can also influence the immune response among individuals [63], [64], [65]. However, there is a scarcity of studies on the subject with even more conflicting results [66], [67] and perhaps their heterogeneity, using different populations and samples and types of shift work evaluated, makes such conclusions difficult (Table 2).
Table 2.
Related articles.
| Articles | Type of study and population | Age | Sample (N) and type of work | Results |
|---|---|---|---|---|
| Nakano et al. [63] | Cross-sectional; Pink and Grey-collar workers | 40/40 | Group A-20 (fixed night shift) / 19 d shift | Reduction of the proliferation of mitogen T Cells in shift workers. The effect was more pronounced in fixed night shift workers. |
| Group B-20 (rotational shift)/20 d shift | ||||
| Mohren et al. [64] | 3-year follow-up of a Cohort Study | 18−66 | 12140 d; three - shifts; five - shifts; irregular shifts | Cold and flu were more common in shift workers than day workers (p<0.005); cold: OR 1.14; CI 0.88–1.46/influenza: OR 1.41; CI 1.07–1.87/gastroenteritis: OR 1.14; 0.84–1.5 |
| Nagai et al. [65] | Cross-sectional; Health Care Professionals | 42 | 57 nurses (shift work) | ↓ ACTV. of NK cells; ↓ ACTV.CD 16+ and CD56+ and ↑ CD 3+, CD 4+ in night workers compared at two times (day-shift/night-shift), *p<0.05. Fatigue was associated with reduced ACTV. of NK cells, p<0.05. |
| Van Mark et al. [66] | Cross-sectional | 36/40 | 225 (shift work) | There was no statistical difference between the lymphocyte count, IL−6 and TNF-α levels between the groups. |
| 137 (day work) | ||||
| Copertaro et al. [67] | 12-month follow-up of a Cohort Study; Health Care Professionals | 35/40 | 96 (shift work) | There was no statistical difference between the levels of IL−1β, IL−6, IFN-γ, TNF and cytotoxicity of NK cells between the groups, at baseline as well as in 12 months. |
| 28 (day work) |
Nakano et al. [63], in one of the first studies on the subject, followed up (fixed and rotational) pink and grey-collar shift workers and daytime shift workers demonstrated that these shift workers showed a reduction in the mitogen proliferation of T lymphocytes to phytohemagglutinin-P (PHA) and concanavalin A, especially those working fixed night shifts.
Moher et al. [64] in a cohort study involving more than 10,000 employees in 45 Dutch companies, showed that shift workers, especially those working the night shift, had a higher risk of infections, such as colds, flu and gastroenteritis, compared to those working daytime shifts (p<0.05), even after adjusting for variables such as smoking, exercise, and alcohol consumption (cold: OR 1.14; CI 0.88–1.46/influenza OR 1.41; CI 1.07–1.87/gastroenteritis: OR 1.14; 0.84–1.59).
Nagai et al. [65] demonstrated that shift work is also associated with depression of the innate immune response, exhibiting a decrease in NK cell activity associated with an increase in fatigue compared to day workers (p<0.001). However, in two other studies conducted at the same time [66], [67], different results were observed.
In a recent consensus, the American Academy of Sleep Medicine (AASM) warned that sleep deprivation is associated with a negative impact on the overall health of the population [68] and that shift workers are certainly some of the most affected [68], [69].
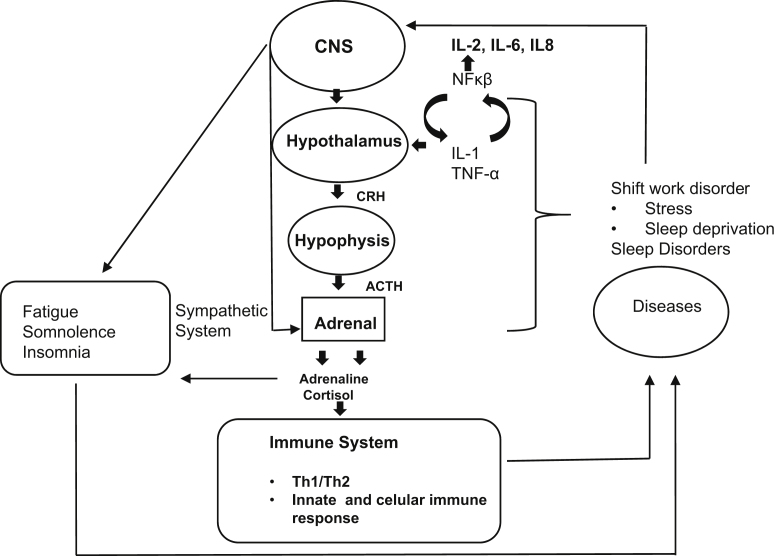
It is still unclear how sleep deprivation negatively affects the immune function. However, sleep can bi-directionally influence the two functions linked to the immune system: the hypothalamic-pituitary-adrenal (HPA) axis [70], [71], [72] and the sympathetic nervous system (SNS) [73] (see Fig. 1). One night of sleep deprivation, for example, results in the activation of the HPA and elevation of plasma cortisol [70], [71], [72]. For instance, we know that cortisol influences the immune system by inducing a reduction of many genes that encode pro-inflammatory cytokines [74], [75] and that catecholamines can also control the migration and activity of immune cells [76], [77]. Moreover, sleep loss, like the various types of sleep disorders, is associated with the activation of inflammatory pathways, such as nuclear factor NF-κβ in regions of the cortex and hippocampus [78], [79] besides the secretion of cytokines such as IL-1 and TNF-α [25], [80], [81]. This may lead to a change of the acquired or innate immune response profile [24], [35], [39] and perhaps justifies the emergence of certain inflammatory diseases [82], [83] and infections [13], [14], [15], [19]. Recent research conducted by Cuesta et al. [84] showed that under a night-oriented schedule, cytokine release was partly altered in response to the change in the sleep–wake cycle, similar to the one that probably occurs in shift workers. However, more work must be carried out to confirm these hypotheses. A model was created for a better general understanding (Fig. 1).
Fig. 1.
Theoretical model of human chronic deprivation. Activation of the sympathetic system and the hypothalamic adrenal via the central nervous system (CNS); corticotropin release hormone (CRH); adrenocorticotropic hormone (ACTH); and nuclear factor κβ (NFκβ).
4. Conclusion
Sleep is a vital behavioral state of living beings and probably a modulator of the immune function. Both acute and chronic deprivation are associated with immune changes. It is likely that shift workers show an increased risk for viral infections because of a possible compromise of the innate immune response and perhaps also of the immune acquired response. There is a need for more quality studies also evaluate the future risk for the onset of inflammatory or autoimmune diseases among these workers. Future research including the different subtypes of shift workers is necessary to answer many gaps in this interesting theme.
Acknowledgments
The authors thank Antonio Luis Boechat for reviewing this article.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.slsci.2016.10.007.
Appendix B. Supplementary material
Supplementary material
.
References
- 1.National Sleep Foundation. 2016 〈https://sleepfoundation.org/shift-work/content/what-shift-work〉 [accessed 20.08.16] [Google Scholar]
- 2.Darien IL, editor. International classification of sleep disorders. 3rd ed.n. American Academy of Sleep Medicine; 2014. American Academy of Sleep Medicine (AASM) [Google Scholar]
- 3.Costa G., Akerstedt T., Nachwreiner F., Baltieri F., Carvalhais J., Folkard S. Flexible working hours, health, and well-being in Europe: some considerations from a SALTSA project. Chronobio Int. 2004;21:831–844. doi: 10.1081/cbi-200035935. [DOI] [PubMed] [Google Scholar]
- 4.Mcmenamin T.M. A time to work: recent trends in shift work and flexibles chedules. Mon Labor Rev. 2007;130:9–11. [Google Scholar]
- 5.Dijk D.-J., Edgar D.M. Circadian and homeostatic control of wakefulness and sleep. In: Turek F.W., Zee P.C., editors. Regulation of sleep and wakefulness. Marcel Dekker; New York: 1999. pp. 111–147. [Google Scholar]
- 6.Drake C.L., Roehrs T., Richardson G. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 7.Smith M.R., Fogg L.F. Eastman CI.A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. Sleep. 2009;32(11):1481–1489. doi: 10.1093/sleep/32.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honn K.A., Garde A.H., Fischer F.M., Van Dongen H.P.A. 22nd International symposium on shiftwork and working time: challenges and solutions for healthy working hours. Chronobiol Int. 2016;33(6):581–588. doi: 10.1080/07420528.2016.1195632. [DOI] [PubMed] [Google Scholar]
- 9.Laugsand L.E., Strand L.B., Vatten L.J., Janszky I., Bjorngaard H.J. Insomnia symptoms and risk for unintentional fatal injuries—The HUNT study. Sleep. 2014;37(11):1777–1786. doi: 10.5665/sleep.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson B., Knutsson A., Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D.L., Feskanich D., Sanchez B.N., Rexrode K.M., Schernhammer E.S., Lisabeth L.D. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost P., Kolstad H.A., Bode J.P. Shift work and the risk of ischemic heart disease: a systematic review of the epidemiologic evidence. Scand J Work Environ Health. 2009;35:163–179. doi: 10.5271/sjweh.1319. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S., Doyle W.J., Alper C.M., Janicki-Deverts S.D., Turner R.B. Sleep habits and susceptibility to the common cold. Arch Inter Med. 2009;169(1):62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prather A.A., Janicki-Deverts D., Hall M.H., Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S.R., Malhotra A., Gao X., Hu F.B., Neuman M.I., Fawzi W.W. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin M.R. Sleep and infectious disease risk. Sleep. 2012;35(8):1025–1026. doi: 10.5665/sleep.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motivala S., Irwin M.R. Sleep and immunity: cytokine pathways linking sleep and health outcomes. Cur Dir Psychol Sci. 2007;16:21–25. [Google Scholar]
- 18.Opp M.R., Krueger J.M. Sleep and immunity: a growing field with clinical impact. Brain Behav Immun. 2015;47:1–3. doi: 10.1016/j.bbi.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prather A.A., Hall M., Fury J.M., Ross D.C., Muldoon M.F., Cohen S. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012;35(8):1063–1069. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besedovsky L., Lange T., Born J. Sleep and Immune function. Eur J Physiol. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besedovsky H.O., Del Rey A. Immune-neuro-endocrine interations: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 22.Ransohoff R.M. Chemokines and chemokines receptors standing at crossroad of immunology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leposavi´c G., Pilipovi´c I., Radojevi´c K., Pesi´c V., Perisi´cM, Kosec D. Catecholamines as immunomodulators: a rolefor adrenoceptor-mediated mechanisms in fine tuning of T-celldevelopment. Auton Neurosci. 2008;144:1–12. doi: 10.1016/j.autneu.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti B., Morale M.C., Paradis P., Bouvier M. Charac-terization, expression, and hormonal control of a thymic beta2-adrenergic receptor. Am J Physiol. 1994;267:E718–E731. doi: 10.1152/ajpendo.1994.267.5.E718. [DOI] [PubMed] [Google Scholar]
- 25.Obal F., Jr, Krueger J.M. Biochemical regulation of Sleep. Front Biosci. 2003;8:520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 26.Krueger J.M. The role of cytokines in sleep regulation. Cur Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitkovic L., Bockaert J., Jacque C. "Inflammatory" cytokines: neuromodulators in normal brain? J Neurochem. 2000;74(2):457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- 28.Opp M.R. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Moldofsky H., Lue F.A., Eisen J., Keystone E., Gorczynski R.M. The relationship of interleukin-1 and immune functions to sleep in humans. Psychosom Med. 1986;48(5):309–318. doi: 10.1097/00006842-198605000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Manfridi A., Brambilla D., Bianchi S., Mariotti M., Opp M.R., Imeri L. Interleukin-1b enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18(5):1041–1049. doi: 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- 31.Luk W.P., Zhang Y., White T.D., Lue F.A., Wu C., Jiang C.G. Adenosine: a mediator of interleukin-1beta-induced hippocampal synaptic inhibition. J Neurosci. 1999;19(11):4238–4244. doi: 10.1523/JNEUROSCI.19-11-04238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida H., Peterfi Z., Garcia-Garcia F., Kirkpatrick R., Yasuda T., Krueger J.M. State-specific asymmetries in EEG slow wave activity induced by local application of TNF[alpha] Brain Res. 2004;1009(1–2):129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 33.Gemma C., Imeri L., De Simoni M.G., Mancia M. Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol. 1997;272(2):R601–R606. doi: 10.1152/ajpregu.1997.272.2.R601. [DOI] [PubMed] [Google Scholar]
- 34.Gemma C., Imeri L., Opp M.R. Serotonergic activation stimulates the pituitary-adrenal axis and alters interleukin- 1 mRNA expression in rat brain. Psychoneuroendocrinology. 2003;28(7):875–884. doi: 10.1016/s0306-4530(02)00103-8. [DOI] [PubMed] [Google Scholar]
- 35.Axelsson J.M., Rehman J.U.R., Akerstedt T., Ekman R., Miller G.E., Hoglund O., Lekander M. Effects of sustained sleep restriction on mitogen-Stimulated cytokines, chemokines and Thelper1/Thelper2 balance in human. Plos. 2013;8(12):822–891. doi: 10.1371/journal.pone.0082291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Born J., Lange T., Hansen K., MolleM, FehmHL Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- 37.Dinges D.F., Douglas S.D., HamarmanS, Zsugg L., Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- 38.Dinges D.F., Douglas S.D., Zaugg L, Campbell D.E., Mcmann J.M., Whitehouse W.G., Orne E.C., Kapoor S.C., Icaza E, Orne M.T. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64h of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin M., Mascovich A., Gillin J.C., Willoughby R., Pike J., Smith T.L. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–498. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Irwin M., Mcclintick J., Costlow C., Fortner M., White J., Gillin J.C. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB. 1996;10:643–650. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 41.Wilder-Smith A., Mustafa F.B., Earnest A., Gen L., Macary P.A. Impact of partial sleep deprivation on immune markers. Sleep Med. 2013;14:1031–1034. doi: 10.1016/j.sleep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 43.Biron C.A. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2(4):374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 44.Fawzy F.I., Fawzy N.W., Hyun C.S., Elashoff R., Guthrie D., Fahey J.L. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50(9):681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- 45.Fondell E., Axelsson J., Franck K., Ploner A., Lekander M., Balter K. Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behav Immun. 2011;25(7):1367–1375. doi: 10.1016/j.bbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Sakami S., IshikawaT, Kawakami N., HarataniT, Fukui A., Kobayashi F., Fujita O., Araki S., Kawamura N. Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th/2 dominance. Neuroimmunomodulation. 2002;10:337–343. doi: 10.1159/000071474. [DOI] [PubMed] [Google Scholar]
- 47.de Almeida C.M., de Lima T.A., Castro D.B., Torres K.L., da Silva Braga W., Peruhype-Magalhaes V. Immunological/virological peripheral blood biomarkers and distinct patterns of sleeping quality in chronic hepatitis C patients. Scand J Immunol. 2011;73(5):486–495. doi: 10.1111/j.1365-3083.2011.02518.x. [DOI] [PubMed] [Google Scholar]
- 48.Savard J., Laroche L., Simard S., Ivers H., Morin C.M. Chronic Insomnia and Immune Functioning. Psychosom Med. 2003;65(2):211–221. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- 49.Duguay D., Cermakian N. The crosstalk between physiology and circadian clock proteins. Chronobiol Int. 2009;26:1479–1513. doi: 10.3109/07420520903497575. [DOI] [PubMed] [Google Scholar]
- 50.Arjona A., Sarkar D.K. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Haimovich B., Calvano J., Haimovich A.D., Calvano S.E., Coyle S.M., Lowry S.F. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 2010;38:751–758. doi: 10.1097/CCM.0b013e3181cd131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labrecque N., Cermakian N. Circadian Clocks in the Immune System. J Biol Rhythms. 2015;30(4):277–290. doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]
- 53.Lange T., Dimitrov S., Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 54.Young M.R., Matthews J.P., Kanabrocki E.L., Sothern R.B., Roitman-Johnson B., Scheving L.E. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor in men. Chrono- Int. 1995;12:19–27. doi: 10.3109/07420529509064496. [DOI] [PubMed] [Google Scholar]
- 55.Dimitrov S., Benedict C., Heutling D., Westermann J., Born J., Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113(21):5134–5143. doi: 10.1182/blood-2008-11-190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinman L. Elaborate interactions between the immune and nervous system. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 57.Dimitrov S., Lange T., Tiecken S., Fehm H.L., Born J. Sleep associated regulation of T Helper1/T helper 2 cytokines balance in human. Brain Behav Immun. 2004;18:341–348. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Lange T., Dimitrov S., Fehm H.L., Wesermann J., Born J. Shift monocytes Funct Cell Immun Sleep Arch Inter Med. 2006;166:1695–1700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 59.Abbas A.K., Lichtman A.H., Pillai S. 8th ed. Elsevier; USA: 2014. Cellular and Molecular Immunology. [Google Scholar]
- 60.Reis E.S., Lange T., Kohl G., Hermann A., Tschulakow A.V., Naujoks J. Sleep and circadian rhythm regulate circulating complements factors and immunoregulatory properties of C5a. Brain Behav Immun. 2011;25(7):1416–1426. doi: 10.1016/j.bbi.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Bollinger T., Bollinger A., Skrum L., Dimitrov L., Lange T., Solbach W. The influence of regulatory T cells and diurnal Hormones rhythms on T helper cell activity. Immunology. 2010;131:488–500. doi: 10.1111/j.1365-2567.2010.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langet T., Dimitrov S., Bollinger T., Diekelmann S., Born J. Sleep after vaccination boosts immunological memory. J Immunol. 2011;187:283–290. doi: 10.4049/jimmunol.1100015. [DOI] [PubMed] [Google Scholar]
- 63.Nakano Y., Miura T., Hara I., Aono H., Miyano N., Miyajima K. The effects of shift work on the cellular immune function. J Hum Ergol. 1982;11:131–137. [PubMed] [Google Scholar]
- 64.Mohren D.C., Jansen N.W., Kant I.J., Galama J., van den Brandt P.A., Swaen G.M. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 2002;44(11):1003–1011. doi: 10.1097/00043764-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Nagai M., Morikawa Y., Kitaoka K., Nakamura K., Sakurai M., Nishijo M. Effects of fatigue on Immune function in nurses performing shift work. J Occup Health. 2011;53:312–319. doi: 10.1539/joh.10-0072-oa. [DOI] [PubMed] [Google Scholar]
- 66.Van Mark A., Weiler S.W., Schroder M., Otto A., Jauch-Chara K., Groneberg D.A. The impact of shift work induced chronic circadian disruption on IL-6 and TNF-alpha immune responses. J Occup Med Toxicol. 2010;5:18. doi: 10.1186/1745-6673-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Copertaro A., Bracci M., Gesuita R., Carle F., Amati M., Baldassari M. Influence of shift-work on selected immune variables in nurses. Ind Health. 2011;49(5):597–604. doi: 10.2486/indhealth.ms1210. [DOI] [PubMed] [Google Scholar]
- 68.Dregan A., Armstrong D. Cross-country variation in sleep disturbance among working and older age groups: an analysis based on the European Social Survey. Int Psycho Geriatr. 2011:1–8. doi: 10.1017/S1041610211000664. [DOI] [PubMed] [Google Scholar]
- 69.Kessler R.C., Berglund P.A., Coulouvrat C., Hajak G., Roth T., Shahly V. Insomnia and the performance of US workers: results from the America Insomnia Survey. Sleep. 2011;34(9):1161–1171. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 71.Leproult R., Copinschi G., Buxton O., Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 72.D'Aurea C., Poyares D., Piovezan R.D., Passos G., Tufik S., Mello M.T. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr. 2015;73(6):516–519. doi: 10.1590/0004-282X20150053. [DOI] [PubMed] [Google Scholar]
- 73.Molina P.E. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis intraumatic injury. Shock. 2005;24:3–10. doi: 10.1097/01.shk.0000167112.18871.5c. [DOI] [PubMed] [Google Scholar]
- 74.Barnes P.J. Anti-inflammatory actions of glucocorticoids:molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 75.Hou N., Zhang X., Zhao L., Zhao X., Li Z., Song T., Huang C. A novel chronic stress-induced shift in the Th1 to Th2response promotes colon cancer growth. Biochem Biophys Res Commun. 2013;439:471–476. doi: 10.1016/j.bbrc.2013.08.101. [DOI] [PubMed] [Google Scholar]
- 76.Leposavi´c G., Pilipovi´c I., Radojevi´c K., Pesi´c V., Perisi´cM, Kosec D. Catecholamines as immunomodulators: a rolefor adrenoceptor-mediated mechanisms in fine tuning of T-cell development. Auton Neurosci. 2008;144:1–12. doi: 10.1016/j.autneu.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Sanders V.M. The beta2-adrenergic receptor on T and B lym-phocytes: do we understand it yet? BrainBehav. Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z., Gardi J., Kushikata T., Fang J., Krueger J.M. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276(6 Pt 2):R1812–R1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- 79.Basheer R., Rainnie D.G., Porkka-Heiskanen T., Ramesh V., McCarley R.W. Adenosine, prolonged wakefulness, and A1-activated NF-kappaB DNA binding in the basal forebrain of the rat. Neuroscience. 2001;104(3):731–739. doi: 10.1016/s0306-4522(01)00111-7. [DOI] [PubMed] [Google Scholar]
- 80.Chennaoui M., Sauvet F., Drogou C., Van B.P., Langrume C., Guillard M. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine. 2011;56:318–324. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 82.Kinnucan J.A., Rubin D.T., Ali T. Sleep and inflammatory bowel disease: exploring the relationship between Sleep disturbances and inflammation. Gastroenterol Hepatol. 2013;9:718–727. [PMC free article] [PubMed] [Google Scholar]
- 83.Grandner M.A., Sands-Lincoln M.R., Pak V.M., Garland S.N. Sleep duration,cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cuesta M., Boudreau P., Dubeau-Laramee B., Cermakian N., Boivian D.B. Simulated night shift disrupts circadian rhythms of immune functions in humans. J Immunol. 2016;196:2466–2475. doi: 10.4049/jimmunol.1502422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material