Abstract
To identify the scales to assess sleep disorders applied to women with climacteric stage. Bibliographical research without intervention, the available information in scientific databases. Performed in PubMed, ScienceDirect, Scopus, Ebscohos OvidSP and Health Library. The words used in this article: insomnia, adjustment sleep disorder, questionnaires, studies and menopause. Publications of all types were included. Seven scales were identified: Insomnia Severity Index, Athens Insomnia Scale, Pittsburgh Quality of sleep Index, Epworth Sleepiness Scale, Jenkins Sleep Scale, Basic Nordic Sleep Questionnaire and The St Mary's Hospital Sleep Questionnaire. There are validated scales in multiple languages and considered appropriate for studying sleep disorders.
Keywords: Climacteric, Sleep, Quality of life, Menopause
1. Introduction
Several ways to classify sleep disorders (SD) have been proposed. The most important is the International Classification of Sleep Disorders [ICSD-2] with numerous subdivisions and specialized approaches [1]; other one is proposed by the American Psychiatric Association in the Diagnostic and Statistical Manual of Mental Disorders [DSM-5] [2], which is used specially by health professionals who are not experts in sleep medicine. Finally, there is the International Classification of Diseases [ICD] [3] with general medical approach [4].
The SD are widespread and important complaints in the vital climacteric stage [5], [6]. These sleep disorders occur in approximately 30% of the general population increasing with the pass of time; it is estimated in more than 50% of adults over 65 years old [7].
The prevalence of SD varies with the menopausal status, with estimated ranges between 39% and 47% in perimenopause and 35–60% in postmenopausal women [5], [7], [8]. In a study carried out in eleven cities from Latin America, which involved 6.079 women aged 40–59 years, insomnia was reported in 56.6% of the population, with poor quality of sleep or both [9]. Monterrosa [5] has reported that 57.1% of Colombian women in climacteric, who live in the Caribbean and the Pacific coasts, had poor quality of sleep when they were diagnosed by the quality of sleep index of Pittsburgh.
Insomnia is the main SD, being approximately two times more common in women than in men [6], [10]. It is characterized by difficulty to sleep, staying asleep or difficulty to get a restful sleep. It is recommended to specify an episodic, persistent or recurrent event, or whether they are associated with a concurrent mental disorder unrelated to sleep, with a medical condition or another sleep disorder [11], [12].
Among the types of SD, insomnia, in particular, may arise situations that impair the quality of life such as decreased concentration and attention, feelings of fatigue and physical or mental exhaustion, decreased motivation, irritability, difficulty in interpersonal relationships and general complications [11]. It has been noted that SD could often be accompanied by depression, anxiety or cognitive changes, while insomnia and excessive daytime sleepiness are risk factors for the later development of mental illness. The interpretation of SD as an expression of mental diseases allows preventive intervention in mental health [2]. Sleep disorders restrict the appropriate recovery/cell repair that comes during sleep and affect adversely the emotional state of women [11].
The physiology of sleep-wakefulness cycle may be affected as a result of changes in ovarian hormone synthesis. The menopause and the reduction in the availability of endogenous estradiol, that occur within the climacteric, have been considered risk factors for SD. The effect could be directly influenced by changes in the steroid profile, as a result of variations in body temperature by the presence of hot flashes, circadian rhythm disturbances or higher reactivity to stress [11], [12], [13], [14].
There is controversy about whether severity criteria should be assessed in the presence of insomnia or in the functional impairment that this could entailed, which always should be considered due to they could affect the quality of life further than sleep disturbances [15]. The diagnostic method of SD is the polysomnography, considered the gold standard; however, it has some limitations due to it does not evaluate quality of sleep and the impact of SD on daily activities. In additionally, to carry out the procedure is necessary to have a quiet room, as close as possible to the home address, where the patient could sleep and another room where the necessary sleep equipment could be installed [16].
Sleep scales are tools to identify, in a subjective way, SD [9], [17]. They offer advantages as easy application and interpretation and the availability to study different disorders [5], [18]. These scales must have been validated in populations and checked in the age groups. Since the high prevalence of SD in climacteric is necessary to know the differences among scales that are available to study adequately these women, and to establish the deterioration of the quality of sleep or the presence of any different types of SD [18], [20], [21]. The aim is to identify and to describe the different scales that have been used to assess SD in climacteric.
2. Methods
Bibliographic research without intervention was used; available data in scientific databases was chosen between January and March in 2016.
2.1. Types of studies
All types of publications were included: clinical trials, meta-analysis, observational studies, thematic or systematic reviews, letters to the editor, comments and editorials related to insomnia and other SD in climacteric women.
2.2. Research strategies
A convenient search was conducted through the databases and electronic resources of the Universidad de Cartagena-Colombia like Pubmed, ScienceDirect, Scopus, Ebscohost, OvidSP and Health Library. It was considered to finish on March 3th, 2016 and was limited to publications between 2005 and 2015, in English and Spanish.
2.3. Keywords
The words used in this article are present in MeSH: insomnia, adjustment sleep disorder, questionnaires, studies and menopause.
2.4. Review methods
A list of the identified titles was done, and then step by step the repeated words were eliminated. The summary was checked in the selected titles to identify those related to insomnia and SD. After that, a second review was carried out to establish those abstracts who were related to women in climacteric. And finally, those available in full text were selected. Type of intervention: Research and thematic review in articles, without intervention in the information obtained from sources.
3. Results
Two thousand eighty-two titles were identified: 567 (27.2%) in Science Direct, 312 (14.9%) in Pubmed, 257 (12.4%) in Ebscohost, 783 (37.7%) in OvidSP and 163 (7.8%) in Virtual Health Library. One thousand two hundred seven (57.9%) titles of the list remained after removing those repeated. Two hundred fifty (20.7%) abstracts were chosen in the first review; after the second review, 82 (32.8%) were relevant and consistent for the goal of this investigation. Finally, 63 (76.8%) articles were selected, of which, 59 (93.6%) were included in the review. Seven scales were identified; they have been used to study different types of SD in climacteric and are presented below [Table 1].
Table 1.
Scales to study sleep disorders in climacteric women.
| Autors (Ref.) | Year | Scale | Type of Sleep Disorders | |
|---|---|---|---|---|
| 1 | Bastien et al. [21]. | 2001 | Insomnia Severity Index (ISI) | Perceived insomnia severity |
| 2 | Soldatos et al. [17] | 2000 | Athens Insomnia Scale (AIS) | Insomnia. |
| Quantification of sleep problems. | ||||
| 3 | Buysse et al. [25] | 1989 | The Pittsburgh Sleep Quality Index: (PSQI) | Quality of sleep |
| Distinguishing in good and poor sleepers. | ||||
| 4 | Johns [29] | 1991 | Epworth Sleepiness Scale (ESS) | Daytime sleepiness |
| 5 | Jenkins et al. [32] | 1988 | Jenkins Sleep Scale (JSS) | High frequency of sleep disorders |
| 6 | Partinem and Gislason [35] | 1995 | Basic Nordic Sleep Questionnaire (BNSQ) | Quality of sleep |
| 7 | Ellis et al. [37] | 1981 | The St Mary's Hospital Sleep Questionnarie (SMHSQ) | Quality of sleep |
3.1. Insomnia Severity Index (ISI)
This scale is specific to study insomnia. It is a reliable, validated and self-applicable instrument, it lets to obtain a quantitative index of perceived severity of insomnia in the last month. The scale has seven questions designed to detect the severity of SD, the relation with the satisfaction experienced, the degree of functional impairment during the day, perception of decline and the concerns related to the sleep problem. Each item is rated on a Likert scale of five points (zero to four) to set a score from zero to 28. The higher scores indicate very severe insomnia; a cutoff point of ten has been proposed as optimal for detecting cases of insomnia in the population. However, the scores could be categorized as follows: Clinically significant insomnia: zero to seven points; some degree of insomnia: eight to 28 points [10], [20], [21].
The last one is subdivided in sub-threshold insomnia or mild: eight to 14, moderate insomnia: 15–21 and severe insomnia: 22–28 [21]. Recently, it has been described in three sub-domains: nocturnal sleep difficulties, sum of questions 1–2–3, impact of insomnia during the day, sum of questions 5–6–7 and dissatisfaction sleep with the sum of questions 1–4–7 [19] [Appendix A].
The ISI could be applied in studies for the population in health care consultation, given that it allows an easy approximation to identify the presence of insomnia.
Arakane [10] found high internal consistency according to Cronbach's alpha coefficient =0.87 in a cross-sectional study carried out in 340 healthy women of a group of inpatients visitors. They were women aged between 40 and 59 years and he showed that 41.5% of those, had some degree of insomnia, taking as cutoff point ≥8. He also demonstrated that 32.0% of them had mild, 7.4% moderate and 2.1% severe insomnia. It was verified the presence of hot flashes, the use of psychotropic substances and physical inactivity, positively and significantly correlated with the total score of the ISI scale, by means of a multiple linear regression model. Likewise, Cuadros [22] found out, in 235 Spanish women aged 40–65 years, a positive correlation between the ISI scale and the Perceived Stress Scale (PSS) scores and the scores in the somatic domain of the Menopause Rating Scale (MRS). This shows a greater insomnia severity, higher perceived stress and somatic deterioration in the quality of life.
3.2. Athens Insomnia Scale (AIS)
This scale arose from the need to develop a tool that could help health professionals when they had to analyze the insomnia severity. It is based on the International Classification of Diseases criteria for insomnia diagnosis [17]. The scale is specific in insomnia cases and it has eight questions, the first four are related to quantitative sleep variables, including sleep induction, nighttime awakenings, early morning awakenings and total sleep duration. The fifth question is about the quality of sleep in general, and the last three ones are about insomnia effects in the efficiency during the day. Each question could be rated from zero (no problem) to three (very serious problem), leaving two intermediate scores. AIS's total score is the sum of the scores on each question and may vary from zero to 24. Scores that are higher than six define the presence of insomnia [17]. There are many publications with AIS, available in many languages including Spanish [23] [Appendix B]. The scale evaluates the events experienced in the last month; its brevity and simplicity allows its application in clinical or community settings.
It has been noted the high degree of internal consistency with a Cronbach's alpha of 0.90 showed by AIS in the general population [23]. It has been demonstrated that daily activities and the presence of menopausal symptoms have an important role in the establishment of insomnia states in Latin America. It was established in this report that the presence of hot flashes, depression, high parity and those whose partners have suffered from premature ejaculation, contributed with high scores on the AIS [24] scale in 204 women aged between 40 and 65 years old.
In a study carried out by Monterrosa [18] et al., in 1.325 Colombian women who belong to three different ethnic groups (afrodescendant, indigenous and mestizo) with ages between 40 and 59 years, it was found by means of a logistic regression, that the severe somatic and psychological deterioration and the current tobacco consumption were risk factors associated to the presence of insomnia. In addition, the insomnia scale was used in this study and a high internal consistency was reported, Cronbach's alpha=0.93.
3.3. Pittsburgh Quality of sleep Index (PSQI)
The Pittsburgh Quality of sleep Index is an instrument published in 1988 by Buysse, which was obtained from three sources: intuition and clinical experience in patients with SD, review of quality of sleep questionnaires proposed in the scientific literature and clinical experience with a pilot instrument applied for 18 months in field experiments. It is a self-report questionnaire that assesses quality of sleep during the last four weeks. The PSQI does not assess the insomnia presence or absence like ISI and AIS do. It distinguishes between bad and good sleep of quality by measuring seven SD components: subjective quality of sleep, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The ISI application allows the orientation about which are the sleep components compromised and which ones allow following the characteristics of the SD. This scale is used to know the quality of sleep [5].
The PSQI has nineteen questions, grouped into ten questions to answer from zero to three in Likert range, a zero score indicates ease, while three indicates severe difficulty, allowing two intermediate scores. The score of the seven SD components are summed to obtain an overall score, from zero to 21 points: zero indicates easy sleep and 21 the higher severity [25] [Appendix C]. The following are the defined components by PSQI scale: qualities of sleep, sleep onset latency, sleep duration and sleep disturbances, medication and daytime dysfunction. It is complex to calculate each component from the form applied, which restricts its use for professional consultations or individual studies. However, it is easy to apply in group evaluations or research studies.
Buysse [25] proposed a cutoff point of 5 to predict poor quality or good quality of sleep. Score ≥5 defines poor sleepers, with sensitivity of 89.6% and 86.5% of specificity. The PSQI has been validated in several languages and in different populations. Macías [26] found high internal consistency identifying Cronbach =0.81. In other study, Escobar-Cordoba [27] found Cronbach's alpha =0.77 in a group aged between 6 and 90 years. Additionally, he reported differences in the score of the PSQI scale according to the characteristics of individuals, with higher scores in elderly people compared to younger people, higher in those with SD; and also, in those patients who took hypnotics and with insomnia.
In a study carried out by Blümel [9], it was found that poor quality of sleep worsened with age and with the change of menopausal status, especially reducing sleep efficiency and sleep latency but with higher use of hypnotics. Vasomotor symptoms (VMS), depressed mood and anxiety were associated with SD in this study. Women who had poor quality of sleep doubled the severity of symptoms, with six or eight times greater risk of deterioration in their quality of life. Anxiety, depression and hot flashes were significant risk factors related to the presence of poor quality of sleep. Better sleep quality was observed in women with better educational levels.
In other study in a group of women from Iran [28], with 52.9±3.3 average age, was found significant correlation between poor quality sleep measured with PSQI and variables like educational and professional level, employment status and partner economic situation.
3.4. Epworth Sleepiness Scale (ESS)
It is a short questionnaire, self-administered proposed by Murray W. Johns in 1991 from Epworth Hospital in Melbourne-Australia. The ESS is a questionnaire that evaluates the tendency to fall asleep in eight sedentary situations: sitting and reading, watching TV, sitting inactive in a public place, as a passenger in a car for an hour of travel, sitting and talking with somebody, lying down and relaxing in the evening, sitting after having lunch without alcohol or in car during a traffic jam. It was designed to be self-administered with questions and many options for each Likert item with scores from zero to three: zero never, one mild, two moderate and three severe. The total score in the scale ranges from zero to 24. The daytime insomnia severity is higher when the score goes up. Thereby, a score from zero to seven indicates normal daytime insomnia, a score from eight to nine shows excessive mild daytime insomnia, a score from 10 to 15 moderate and 16 or more score is indicative of severe daytime sleepiness. These scores are coherent with eight items that has the instrument and total scores measured by Cronbach's alfa, according to specialist, fluctuates from 0.73 to 0.88 [29].
The ESS has been translated to Spanish, Portuguese, Italian, German, Swedish, Finnish, Greek, French, Mandarin, Japanese and Turkish. This tool has not been validated to be used through telephone interviews. It allows knowing how SD affect in the activities of the next day. Appendix D.
Daytime sleepiness is a sleep disorder that has many described risk factors. Chedraui found [30] that postmenopausal status, physical inactivity and hot flashes were the main risk factors in the increase of the daytime sleepiness in a cross-sectional study done in 149 women aged between 40 and 59 years; in accordance with Yazdi [31], who carried out the specific Questionnaire Quality of Life Menopause (MENQOL) in 380 women aged 50 and 60 years. The frequency of severe and moderate insomnia was 8.4% and 11.8%, respectively. Severe daytime sleepiness was present in 27.9% of the participants. Insomnia and daytime sleepiness had an independent negative impact on each domain and the total score of the questionnaire MENQOL. This study showed internal consistency with Cronbach's alpha values for ISI and ESS from 0.82 to 0.78, respectively.
3.5. Jenkins Sleep Scale (JSS)
C. David Jenkins designed this instrument in 1988 based on data from two populations that answered self-report scales with three and four questions, to follow common sleep problems in clinical areas. Four items evaluated, in the last month, the difficulty to fall asleep, wake up at night, difficulty to stay asleep and wake up exhausted in the morning instead of sleeping as usual. Each item is rated on a Likert scale from zero to five, where zero is never, 1 is one to three 1–3 days, two is four to seven days, three is eight to 14 days, four is 15–21 days and five is 22–28 days. The scale allows scoring from zero to 20. To get a total score from one to 11 defines a little of SD, while a score ≥12 identifies high frequency of SD [32] [Appendix E].
It was found that SD prevalence was 37.5%, further significant association was observed related to menopausal status, presence of urinary incontinence, being under psychiatric treatment and psychosocial experience like domestic violence, healthy quality perception, educational level attained and regular exercising, in a study in women aged 40–59 years. The JSS scale scores were positively correlated with higher scores of somatic symptoms in menopause, evaluated with the specific scale of menopausal symptoms: Menopause Rating Scale (MRS). It was found that the internal consistency for JSS scale was 0.78 and 0.79 for MRS scale [33].
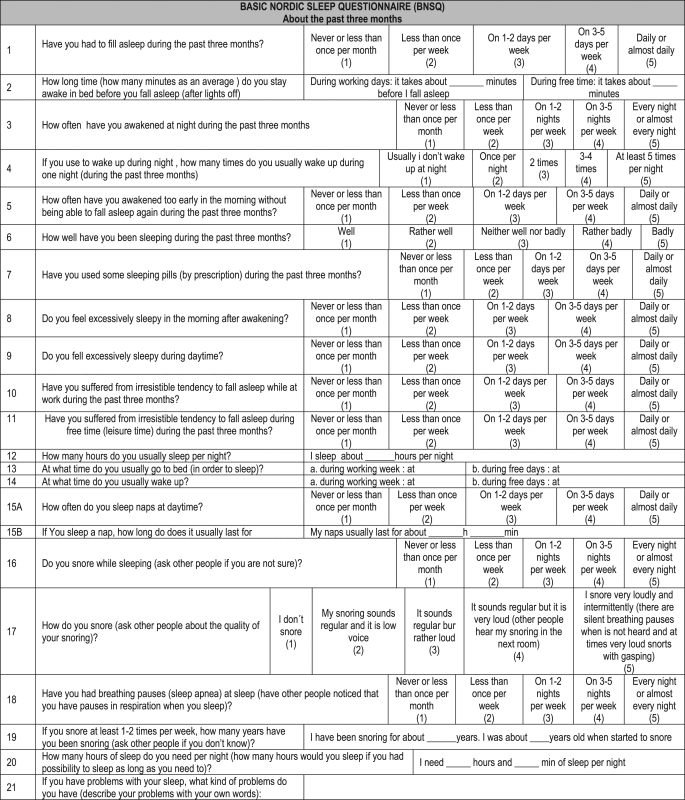
3.6. Basic Nordic Sleep Questionnaire (BNSQ)
The Scandinavian Sleep Research Society formed a working group in 1988 to develop a standardized questionnaire that could be used as a basis for studying the SD. The BNSQ scale has 21 questions that analyzes the most common complaints about sleep in the last three months. Most questions could be immediately answered in each box; some of them must be answered with numbers according to time, some in minutes, hours or days. The last question is open, where SD could be described in free text [34].
The BNSQ was originally developed in English. The basic questions have several possible answers: one is never or less than once a month, two is less than once a week, three is one to two days a week, four is three to five days per week and five is every day or almost every day. Questions about specific rare events could be subdivided in never and less than once a month. Habitual snoring is defined as snoring every night or almost every night. The BNSQ has been widely used in studies in Nordic countries in recent years because it is a valid tool [35] [Appendix F].
Donati [36] checked the quality of sleep with BNSQ scale, quality of life with Short Form-12 questions and hot flashes intensity using a visual scale in 2428 postmenopausal women assessed by a cross-sectional study carried out in Italy. She found that women with lower quality of life reported worse quality of sleep. It also was found that those women, who did not use hormone therapy, reported more frequent forms of poor sleep, more troubles to sleep and sleep problems.
The subjective [11] sleep in 91 premenopausal women aged between 44 and 48 years was checked and compared with the subjective sleep in postmenopausal women aged between 53 and 58 years in another study. It was found that the postmenopausal total sleep time, night time sleep and the number of awakenings on weekdays were shorter compared to premenopausal women. The difference was statistically significant and there were not differences between the two groups in the number of awakenings and the elapsed time to fall asleep in the leisure time. The complexity of the scale limits its use in the consultation of climacteric women.
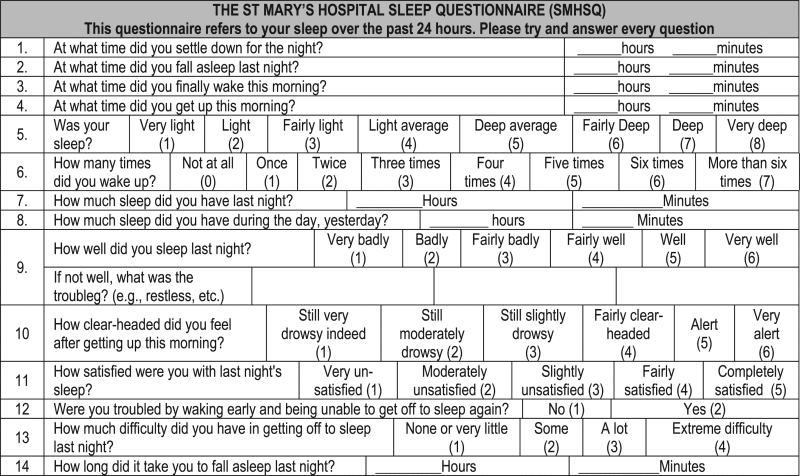
3.7. The St Mary's Hospital Sleep Questionnarie (SMHSQ)
The St Mary's Hospital Sleep Questionnaire is a tool specifically designed to assess sleep in patients, testing the duration and quality of sleep the previous night while they are in the hospital. The items inquire about sleep issues, including latency, restlessness, insomnia and alertness in the morning. This scale has 14 multiple choice questions with short answers. It was applied at the first time in four groups of people, surgical patients, others with medical treatment, the others with psychiatric treatment and the last one with healthy volunteers [37] [Appendix G].
Pien [38] found significant associations between the poor quality of sleep and the presence of hot flashes, smoking, high levels of anxiety, stress and depression in a climacteric population in 2008. He also examined associations among these same risk factors and the ninth question in the questionnaire “How did you sleep last night? ”. The Cronbach's alpha of the scale in this article was 0.76, indicating strong internal consistency.
Other study found a high prevalence of moderate/severe poor sleep in 255 women recruited on Penn Ovarian Again Study Cohort Findings women. Sleep status at premenopausal baseline and concurrent hot flashes predicted strongly and consistently poor sleep in the menopausal transition. Overall, poor sleep does not increase around the final menstrual period and occurs frequently in the absence of hot flashes, indicating that sleep difficulties in the menopausal transition in healthy women are not simply associated with ovarian decline [12].
4. Discussion
The SD are very important problems with many expressions, complex etiology with associated factors [6], [39]. There are many scales that have been developed and validated in several areas of the health; they are available to analyze the sleep behavior and to establish subjectively the presence of SD. To study the general population in specific groups like elderly, children and adolescent patients with dementia and other diseases has been proposed.
The most widely used scales to study climacteric are Insomnia Severity Index (ISI), Athens Insomnia Scale (AIS) and Pittsburgh Quality of Sleep Index (PSQI), with validation in several countries and languages, which make them attractive to assess the prevalence and factors associated with SD. The first two of them allow setting aspects specifically related to insomnia, whose prevalence increases in postmenopausal women, increasing in 60% while in premenopausal women 40%. The four major causes proposed to explain the poor quality of sleep are: sleep disruption associated with hot flashes, increase of sleep apnea, mood disorder and inadequate sleep hygiene leading to chronic insomnia [40].
The PSQI was developed to provide a reliable, valid and standardized measure of quality of sleep, to discriminate between “good” and “poor” sleepers, to procure an easy index to use in patients, clinicians and researchers to understand and to provision a brief, clinical and useful assessment of a variety of sleep disturbances that might affect quality of sleep. It has been widely used in climacteric and it is a proper tool to be applied [5], [9], [28].
The Insomnia Severity Index, Athens Insomnia Scale, Pittsburgh Quality of sleep Index (PSQI) and Basic Nordic Sleep Questionnaire (BNSQ) have been used to check modifications in the prevalence of SD in women with hormonal therapy for the treatment of menopause symptoms, demonstrating its efficacy in these scenes [5], [9], [18], [36].
The PSQI could be difficult to apply as a self-administered instrument in patients with low educational levels. The internal consistency determined by Cronbach's alpha was appropriated for the 19 items and 7 components. The scores of the items, the components and the total value remained stable during the time of Test-retest. The cutoff point of five has been used to define the population with poor quality of sleep [25].
Perimenopausal and postmenopausal women usually sleep less; they have more insomnia and more necessity of medication to sleep than those premenopausal ones [8]. However, they usually do not talk to their doctors voluntarily and the health providers do not ask about aspects related to it. Insomnia, nonrestorative sleep and excessive daytime sleepiness are frequent after menopause [6]. Both situations contribute to raise the alteration, to create empirical measures and to favor the dependence on drugs.
The application of these scales that assess sleep, especially the Athens Insomnia Scale (AIS) to assess the insomnia and Jenkins Sleep Scale (JSS) to check the general condition of sleep could be applied before or after the consultation. This could be the beginning to identify the sleep disorders in women during climacteric.
The Sleep is essential for a healthy immune, metabolic, physical and cognitive system and also for a good quality of life. The Jenkins Sleep Scale (JSS) is helpful due to its brevity and easy application, which offers a panoramic view of the sleep condition [6].
Freeman [12] has proposed that lack of sleep, classified as moderate or severe before menopause, could be predictor of severe SD in postmenopause, OR:3.5 [CI95%: 2.5–5.1]. Other factors also could indicate the presence of SD: Moderate or severe hot flushes: OR:1.7[CI95%:1.5–2.1]; anxiety: OR:1.1 [CI95%:1.08–1.12]; depression: OR:2.1[CI95%:1.7–2.6] and previous clinical profile of depression: OR:2.0[CI95:1.51–2.86]. It was not found statistical significance with obesity, perceived stress, to smoke, estradiol levels, FSH, and inhibin B. These were risk factors to SD; but there are controversies and different positions about the role of reproductive hormones in the balance between sleep and sleeplessness, and the presence or complication in some SD.
The incidence of Sleep-disordered breathing (SDB) is other cause of bad sleep in perimenopausal women with incidence that increases in postmenopause [12]. After controlling the age, the body max index and other factors of quality of life, postmenopausal women had 2.6 times more SDB than premenopausal women. The suggested mechanisms to this apparent increment include the changes in the distribution of body fat with a rise in the waist and hip measurements; as well as the decrease of sexual hormones [41], [42], [43]. The clinical profile has been named sleep apnea syndrome or hypopnea and is frequent in women in middle-age; its symptoms are loud snoring, awakenings during sleep, reduction in the saturation of oxygen, to feel restless sleep and excessive daytime sleepiness. Severe episodes and the occurrence of SD for a long period have been associated with cardiovascular diseases [8], [44].
The STOPBang Sleep Apnea Questionnaire is a specific tool to estimate the SDB presence. It has eight questions to answer “yes” or “no”. Do you snore loudly (louder than talking or loud enough to be heard through closed doors)? Do you often feel tired, fatigued, or sleepy during daytime? Has anyone observed you stop breathing during your sleep? Do you have or are you being treated for high blood pressure? BMI more than 35 kg/m2? Age over 50 years old? Neck circumference >16 in. (40 cm)? or Male? [45].
These are the evaluation criteria for general population: low risk of obstructive sleep apnea: affirmative answers for none or two questions. Intermediate risk: affirmative answer for three or four questions. High risk: it is set by the four following considerations. First: affirmative answer for five or eight questions. Second: affirmative answer for two or more of the first four questions from male sex scale. Third: affirmative answer for two or more of the first four questions plus IMC >35 kg/m2. Fourth: affirmative answer for two or more than the first four questions plus neck circumference (equal to or greater than 43 in men or 41 in women). If three or more answers are affirmative is prudent that a SD specialist will check the patient [6], [45]. There have not been identified studies to assess specifically SDB, with the STOPBang Sleep Apnea Questionnaire, in climacteric women.
Other scale to assessment SDB is The Berlin Questionnaire, specifically proposed for obstructive sleep apnea-hypopnea syndrome (OSAHS). It is the most common type of SDB characterized by airway narrowing during sleep that leads to respiratory disruption, hypoxia and sleep fragmentation. The incidence of OSAHS in the adult female population is 2%. Despite this, the syndrome seems to be underdiagnosed. Pataka [46] evaluated five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. One thousand eighty hundred fifty three (74.4% males) patients (mean age 52±14 years; mean body mass index 32.8±7 kg/m2) of a sleep clinic were studied retrospectively. Berlin questionnaire had the highest sensitivity, OR, and AUC, but rather low specificity. This does not identify studies that involved especially climacteric women.
The evaluation of common causes of SD in middle age women has shown that the primary disorders called apnea syndrome and restless leg syndrome are common in this population [8]. By means of laboratory tests was observed that the 53% of women presented apnea syndrome and restless leg syndrome or both in a study carried out in climacteric women that were experiencing lack of sleep [46], [47]. The best predictors to detect objectively the poor quality of sleep were apnea, restless leg syndrome and awakenings at night. The best predictors to detect subjectively the poor quality of sleep with PSQI were detected as the presence of anxiety and hot flashes at the first half of the night. The adequate management of hot flashes have been proved to improve some manifestations related to lack of sleep; even so, it does not influence in some primary sleep disorders. On the other hand, SD could be associated with higher morbidity and mortality in significant magnitudes. For that reason, is necessary to pay careful attention, as well as to increase the sensibility of the specialist in SD cases and its implications in health women [8].
The Epworth Sleepiness Scale (ESS) allows identifying specifically the daytime somnolence, which also has demonstrated to be a risk factor in the worsening of quality of life during menopause. This scale is easy to apply and allow establishing aspects related to the impact of the sleep disorder in the next day. Additionally, it is very helpful to identify modifications in the quality of life in women as consequence of SD presence; the aforementioned impact has been considered essential and important [9]. The ESS was the only one identified to determine the pathological daytime somnolence; it helps to precise the impact of sleep in daily rest conditions carry out by women [30], [31].
It should always explore aspects related to quality of sleep and the specialist must consider questions that allow identifying SD when climacteric women are evaluated. The identified scales have validity and are good tools to identify subjectively SD and to measure the quality of sleep. It should be noted that sleep absence is not only caused by menopause or hot flashes. The common perception of sleep absence is associated with the ovarian hormonal reduction around the age of menopause, in some women the baseline before menopause has worsened with the elderly [12].
Although, the sleep patterns and SD influences in the personal and social habits, those aspects are not considered in the scales. It was observed that scales do not explore specific habits neither cultural or ethnic conditions. Environmental influences which could generate important modifications.
Through a convenience selection carried out by virtual platform, the interval of time that was considered, the selected languages and databases restrict this study because there are other scales to assess SD in climacteric that have not been identified; but this study has the most widely used scales which have demonstrated the validity to study SD. However, there are other ways to detect SD, for instance, with scales to analyze insomnia, sleep records, consultations and psychological tests. All these scales identify subjectively the SD.
A version of the PSQI includes five questions to be answered by the couple or the carer of the person evaluated. Only laboratory studies such as polysomnography and actigraphy could identify objectively patterns of SD. The initial sleep diagnostic must be carried out with primary scales of health care according to the results. It is possible to get objective evidence and specialized medical care for comorbidities or sleep disturbances. The complexity to advance the studies, laboratory equipment requirements and limitations in the information to make their diagnosis are restricted for specific situations and specialized applications.
5. Conclusion
There are validated and available scales in multiple languages to assess generally or specifically SD, insomnia and excessive daytime sleepiness in climacteric women. They could be used for population studies, to determine its therapeutic efficacy in interventions; even some of them could be applied within health care by doctors with various levels of specialization.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper. The authors are responsible for the content of this paper.
Financing
Vicerrectoría de investigaciones of the Universidad de Cartagena. Colombia. Resolution 03707 of the 2014, process for the obtaining of financial resources in support to the strengthening of research groups. Agreement certificate No. 070-2015.
Acknowledgments
To Teresa Beltrán Barrios for revising this article.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
Appendix A
See
Table A1.
Insomnia severity index.
| Insomnia problema | 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|
| 1. | Difficulty falling asleep | None | Mild | Moderate | Severe | Very severe |
| 2. | Difficulty staying asleep | None | Mild | Moderate | Severe | Very severe |
| 3. | Problems waking up too early | None | Mild | Moderate | Severe | Very severe |
| 4. | How satisfied/dissatisfied are you with your current sleep pattern? | Very satisfied | Satisfied | Moderate satisfied | Dissatisfied | Very dissatisfied |
| 5. | How noticeable to others do you think your sleep problem is in terms of impairing the quality of your life? | Not at all noticeable | A Little | Somewhat | Much | Very much noticeable |
| 6. | How worried/distressed are you about your current sleep problem? | Not at all worried | A Little | Somewhat | Much | Very much worried |
| 7. | To what extent do you consider your sleep problem to interfere with your daily functioning (e.g. daytime fatigue, mood, ability to function at work/daily chores, concentration, memory, mood, etc.) currently? | Not at all Interfering | A Little | Somewhat | Much | Very much interfering |
Appendix B
See
Table B1.
Athens insomnia Scale.
| 0 | 1 | 2 | 3 | ||
| 1. | Sleep induction (time it takes you to fall asleep after turning-off the lights) | No problem | Slightly delayed | Markedly delayed | Very delayed or did not sleep at all |
| 2. | Awakenings during the night | No problem | Minor problem | Considerable problem | Serious problem or did not sleep at all |
| 3. | Final awakening earlier than desired | Not earlier | A little earlier | Markedly earlier | Much earlier or did not sleep at all |
| 4. | Total sleep duration | Sufficient | Slightly insufficient | Markedly Insufficient | Very insufficient or did not sleep at all |
| 5. | Overall quality of sleep (no matter how long you slept) | Satisfactory | Slightly unsatisfactory | Markedly Unsatisfactory | Very unsatisfactory or did not sleep at all |
| 6. | Sense of well-being during the day | Normal | Slightly decreased | Markedly decreased | Very decreased |
| 7. | Functioning (physical and mental) during the day | Normal | Slightly decreased | Markedly decreased | Very decreased |
| 8. | Sleepiness during the day | None | Mild | considerable | Intense |
Appendix C
See
Table C1.
Pittsburgh sleep quality index (PSQI).
| The following questions relate to your usual sleep habits during the past month only | |||||
|---|---|---|---|---|---|
| 1. | When have you usually gone to bed? | Bed Time: | |||
| 2. | How long (in minutes) has it taken you to fall asleep each night? | Number of Minutes: | |||
| 3. | What time have you usually gotten up in the morning? | Getting up time: | |||
| 4. | How many hours of actual sleep did you get at night? | Hours of sleep per night: | |||
| 0 | 1 | 2 | 3 | ||
| 5. | During the past month, how often have you had trouble sleeping because you | Not during the past month | Less than once a week | Once or Twice a week | Three or more times a week |
| 5-A. | Cannot get to sleep within 30 min | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-B. | Wake up in the middle of the night or early morning | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-C. | Have to get up to use the bathroom | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-D. | Cannot breathe comfortably | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-E. | Cough or snore loudly | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-F. | Feel too cold | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-G. | Feel too hot | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-H. | Have bad dreams | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-I. | Have pain | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 5-J. | Other reason (s), please describe, including how often you have had trouble sleeping because of this reason (s): | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 6. | During the past month, how often have you taken medicine (prescribed or “over the counter”) to help you sleep? | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 7. | During the past month, how often have you had trouble staying awake while driving, eating meals, or engaging in social activity? | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 8. | During the past month, how much of a problem has it been for you to keep up enthusiasm to get things done? | Not during the past month | Less than once a week | Once or twice a week | Three or more times a week |
| 9. | During the past month, how would you rate your sleep quality overall? | Very good | Fairly good | Fairly bad | Very bad |
Appendix D
See
Table D1.
Epworth sleepiness Scale.
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Sitting and Reading | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
| Watching TV | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
| Sitting, inactive in a public place (e.g. a theater) | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
| Lying down to rest in the afternoon when circumstances permit | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
| Sitting and talking to someone | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
| Sitting quietly after a lunch without alcohol | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
| In a car, whirl stopped for a few minutes in the traffic | Would never doze | Slight chance of dozing | Moderate chance of dozing | High chance of dozing |
Appendix E
See
Table E1.
Jenkins sleep scale (JSS) evaluated in the last month.
| How often in the past month did you: | 0 | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|
| 1. | Have trouble falling asleep | Not at all | 1–3 days | 4–7 days | 8–14 days | 15–21 days | 22–31 days |
| 2. | Wake up several times per night | Not at all | 1–3 days | 4–7 days | 8–14 days | 15–21 days | 22–31 days |
| 3. | Have trouble staying (including waking far too early) | Not at all | 1–3 days | 4–7 days | 8–14 days | 15–21 days | 22–31 days |
| 4. | Wake up after your usual amount of sleep feeling tired worn out | Not at all | 1–3 days | 4–7 days | 8–14 days | 15–21 days | 22–31 days |
Appendix F
See
 |
Appendix G
See
 |
References
- 1.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005.
- 2.American Psychiatric Association . American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and statistical manual of mental disorders. DSM-V-TR. [Google Scholar]
- 3.World Health Organization. Available from: 〈http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf〉 [accessed 24.07.16].
- 4.National Center on Sleep Disorders Research. National sleep disorders research plan. Available from: 〈http://www.nhlbi.nih.gov/files/docs/resources/sleep/201101011NationalSleepDisordersResearchPlanDHHSPublication11-7820.pdf〉 [last accessed 24.07.16].
- 5.Monterrosa-Castro A., Marrugo-Flórez M., Romero- Pérez I., Fernández-Alonso A., Chedraui P., Pérez-López F.R. Assessment of sleep quality and correlates in a large cohort of Colombian women around menopause. Menopause. 2013;20(4):464–469.. doi: 10.1097/gme.0b013e31826e7649. [DOI] [PubMed] [Google Scholar]
- 6.Lee K.A., Anderson D.J. Screening midlife women for sleep problems: why, how, and who should get a referral? Menopause. 2015;22(7):783–785.. doi: 10.1097/GME.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 7.Escobar-Córdoba F., Chica-Urzola H., Cuevas-Cendales F. Sleep disorders related to the female menopause and their treatment. Rev Col Obstet Ginecol. 2008;59(2):131–139. [Google Scholar]
- 8.Shaver J.L., Woods N.F. Sleep and menopause: a narrative review. Menopause. 2015;22(8):899–915.. doi: 10.1097/GME.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 9.Blümel J.E., Cano A., Mezones-Holguín E., Barón G., Bencosme A., Benítez Z. A multinational study of sleep disorders during female mid-life. Maturitas. 2012;72(4):359–366.. doi: 10.1016/j.maturitas.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Arakane M., Castillo C., Rosero M.F., Peñafiel R., Pérez-López F.R., Chedraui P. Factors relating to insomnia during the menopausal transition as evaluated by the insomnia Severity Index. Maturitas. 2011;69:157–161.. doi: 10.1016/j.maturitas.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Lampio L., Saaresranta T., Polo O., Polo-Kantola P. Päivi. subjective sleep in premenopausal and postmenopausal women during workdays and leisure days: a sleep diary study. Menopause. 2013;20(6):655660.. doi: 10.1097/gme.0b013e31827ae954. [DOI] [PubMed] [Google Scholar]
- 12.Freeman E.W., Sammel M.D., Gross S.A., Pien G.W. Poor sleep in relation to natural menopause: a population-based 14-year follow-up of midlife women. Menopause. 2014;22(7):719–726.. doi: 10.1097/GME.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joffe H., Massler A., Sharkey K.M. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28:404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Frías C., Figueroa-Vega N., Malacara J.M. Relationship of sleep alterations with perimenopausal and postmenopausal symptoms. Menopause. 2014;21:1017–1022.. doi: 10.1097/GME.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 15.Akashiba T., Kawahara S., Akahoshi T., Omori C., Saito O., Saito O., Majima T., Horie T. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122(3):861–865.. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 16.Xu M., Bélanger L., Ivers H., Guay B., Zhang J., Morin C.M. Comparison of subjective and objective sleep quality in menopausal and non-menopausal women with insomnia. Sleep Med. 2011;12(1):65–69.. doi: 10.1016/j.sleep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 18.Monterrosa-Castro A., Marrugo-Flórez M., Romero-Pérez I., Chedraui P., Fernández-Alonso A.M., Pérez-López F.R. Prevalence of insomnia and related factors in a large mid-aged female Colombian sample. Maturitas. 2014;74(4):346–351. doi: 10.1016/j.maturitas.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Mendoza J., Rodríguez, Vela-Bueno A., Olavarrieta-Bernardino S., Calhoun S.L., Bixler E.O., Vgontzas A.N. The Spanish version of the Insomnia severity Index: a confirmatory factor analysis. Sleep Med. 2012;13(2):207–210. doi: 10.1016/j.sleep.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Morin C.M., Belleville G., Bélanger L., Ivers H. The Insomnia severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608.. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Cuadros J.L., Fernández-Alonso A.M., Cuadros-Celorrio A.M., Fernández-Luzón N., Guadix-Peinado M.J., del Cid-Martín N., Chedraui P., Pérez-López F.R. Perceived stress, insomnia and related factors in women around the menopause. Maturitas. 2012;72(4):367–372. doi: 10.1016/j.maturitas.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Benito J., Ruiz C., Guilera G. A Spanish version of the Athens Insomnia scale. Qual Life Res. 2011;20(6):931–937. doi: 10.1007/s11136-010-9827-x. [DOI] [PubMed] [Google Scholar]
- 24.Chedraui P., San Miguel G., Villacreses D., Dominguez A., Jaramillo W., Escobar G.S. Assessment of insomnia and related risk factors in postmenopausal women screened for the metabolic syndrome. Maturitas. 2013;74:154–159. doi: 10.1016/j.maturitas.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Macías Fernández J.A., Royuela Rico A. La versión española del índice de Calidad de sueño de Pittsburgh. Inf Psiquiátricas. 1996;146:465–472. [Google Scholar]
- 27.Escobar-Córdoba F., Eslava-Schmalbach J. Validación colombiana del índice de calidad de sueño de Pittsburgh. Rev Neurol. 2005;40:150–155.. [PubMed] [Google Scholar]
- 28.Taavoni S., Ekbatani N.N., Haghani H. Postmenopausal women's quality of sleep and its related factors. J Midlife Health. 2015;6(1):21–25. doi: 10.4103/0976-7800.153611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johns M.W. A new method for measuring daytime sleepiness: the Epworth Sleepiness scale. Sleep. 1991;14:540–545.. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Chedraui P., Pérez-López F., Mendoza M., Leimberg M.L., Martínez M.A., Vallarinob V., Hidalgo L. Factors related to increased daytime sleepiness during the menopausal transition as evaluated by the Epworth sleepiness Scale. Maturitas. 2010;65(1):75–80.. doi: 10.1016/j.maturitas.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Yazdi Z., Sadeghniiat-Haghighi K., Ziaee A., Elmizadeh K., Ziaeeha M. Influence of sleep disturbances on quality of life of iranian menopausal. Women J Psychol. 2013;1:1–5. doi: 10.1155/2013/907068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins C.D., Stanton B.A., Niemcryk S.J., Rose R.M. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41(4):313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 33.Ornat L., Martínez-Dearth R., Chedraui P., Pérez-López F.R. Assessment of subjective sleep disturbance and related factors during female mid-life with the Jenkins sleep Scale. Maturitas. 2014;77(4):344–350. doi: 10.1016/j.maturitas.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Taillard J., Philip P., Bioulac B. Morningness/eveningness and the need for sleep. J Sleep Res. 1999;8(4):291–295. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 35.Partinem M., Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjetive sleep complaints. J Sleep Res. 1995;4(1):150–155. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 36.Sarti C.D., Chiantera A., Graziottin A., Ognisanti F., Sidoli C., Mincigrucci M., Parazzini F. Hormone therapy and sleep quality in women around menopause. Menopause. 2005;12(5):545–551. doi: 10.1097/01.gme.0000172270.70690.5e. [DOI] [PubMed] [Google Scholar]
- 37.Ellis B.W., Johns M.W., Lancaster R., Raptopoulos P., Angelopoulos N., Priest R.G. The St. Mary's Hospital sleep Questionnaire: a study of reliability. Sleep. 1981;4(1):93–97. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 38.Pien G.W., Sammel M.D., Freeman E.W., Lin H., Deblasis T.L. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31(7):991–999. [PMC free article] [PubMed] [Google Scholar]
- 39.Kravitz H.M., Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38:567–586. doi: 10.1016/j.ogc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartz A., Ross J.J., Noyes R., Williams P. Somatic symptoms and psycho- logical characteristics associated with insomnia in postmenopausal women. Sleep Med. 2013;14:71–78. doi: 10.1016/j.sleep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007;(433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 42.Mirer A.G., Peppard P.E., Palta M., Benca R.M., Rasmuson A., Young T. Menopausal hormone therapy and sleep-disordered breathing evidence for a healthy user bias. Ann Epidemiol. 2015;25(1):779–784. doi: 10.1016/j.annepidem.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahe C., Czira M.E., Teismann H., Berger K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015;16(10):1225–1228.. doi: 10.1016/j.sleep.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Ameratunga D., Goldin J., Hickey M. Sleep disturbance in menopause. Intern Med J. 2012;42:742–747. doi: 10.1111/j.1445-5994.2012.02723.x. [DOI] [PubMed] [Google Scholar]
- 45.Toronto Western. Hospital, University Health Network. University of Toronto. Available from: 〈http://www.stopbang.ca/translation/translation.php〉 [accesed 24.07.16]; 2016.
- 46.Pataka A., Daskalopoulou E., Kalamaras G., Passa K.F., Argyropoulou P. Evaluation of five different questionnaires for assessing sleep apnea syndrome in sleep clinic. Sleep Med. 2014;15(7):776–781.. doi: 10.1016/j.sleep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 47.State-of-the-Science NIH. Conference Statement on management of menopause-related symptoms. NIH Consent State Sci Statements; 22: pp. 1–38; 2005. [PubMed]


