Abstract
Numerous studies demonstrate that the Methionine variant of the catechol-O-methyltransferase Val158Met polymorphism, which confers less efficient catabolism of catecholamines, is associated with increased focal activation of prefrontal cortex (PFC) and higher levels of executive function abilities. By and large, however, studies of COMT Val158Met have been conducted with adult samples and do not account for the context in which development is occurring. Effects of early adversity on stress response physiology and the inverted U shape relating catecholamine levels to neural activity in PFC indicate the need to take into account early experience when considering relations between genes such as COMT and executive cognitive ability. Consistent with this neurobiology, we find in a prospective longitudinal sample of children and families (N=1292) that COMT Val158Met interacts with early experience to predict executive function abilities in early childhood. Specifically, the Valine variant of the COMT Val158Met polymorphism, which confers more rather than less efficient catabolism of catecholamines is associated with higher executive function abilities at child ages 48 and 60 months and with faster growth of executive function for children experiencing early adversity, as indexed by cumulative risk factors in the home at child ages 7, 15, 24, and 36 months. Findings indicate the importance of the early environment for the relation between catecholamine genes and developmental outcomes and demonstrate that the genetic moderation of environmental risk is detectable in early childhood.
Keywords: early experience, polymorphism, stress, attention
INTRODUCTION
The relation of catecholamines to cognitive functions associated with prefrontal cortex (PFC) is well established. Numerous studies have demonstrated that the functional form of this relation is that of an inverted U shape curve (Arnsten, 2009). Moderate increases in dopamine and norepinephrine are associated with increased synaptic activity and higher levels of performance on executive function tasks as assessed by measures of working memory and the ability to shift cognitive set. In contrast, very high or very low levels are associated with reduced synaptic activity in PFC and poor executive cognitive performance. This inverted U shape relation between catecholamine levels and performance on tasks dependent on PFC represents a neurobiological manifestation of the now over 100-year-old Yerkes-Dodson law (Diamond, Campbell, Park, Halonen, & Zoladz, 2007).
Given the association between catecholamine levels and cognitive functions associated with PFC, research on the genetic basis for executive functions, particularly working memory, has focused on variants of candidate genes associated with catecholamine activity in the brain. For example, numerous studies have examined variants of the catechol-O-methyltransferase (COMT) gene as well as variants of dopamine receptor and monoamine oxidase genes. COMT is a complex gene with multiple relevant polymorphisms (Nackley et al., 2006); however, the Val158Met polymorphism has been widely studied in adult populations but infrequently examined in children. This variant, a valine to methionine substitution at codon 158, results in less efficient catabolism of catecholamines, particularly, dopamine, leading to the hypothesis that individuals with this substitution will exhibit higher levels of executive function ability in studies conducted in controlled laboratory settings (Tunbridge, Harrison, & Weinberger, 2006).
By and large, examinations of catecholamine genes, including COMT, have tended to demonstrate hypothesized relations between gene variants and neural activity. The findings of this literature generally support the idea that higher catecholamine availability (less efficient catabolism) is associated with more efficient activation of PFC in response to executive function tasks (Mier, Kirsch, & Meyer-Lindberg, 2010). Findings for the relation of the COMT Val158Met polymorphism to measured executive function ability, however, are mixed (Barnett, Heron, Goldman, Jones, & Xu, 2009; Barnett, Scoriels, & Munafò, 2008; Goldman, Weinberger, Malhotra, & Goldberg, 2009). Given the inverted U shaped association between catecholamine levels and executive cognition, the empirical literature is particularly clear on the need to take into account resting or pretask catecholamine levels when examining the relation of COMT genotype to measured executive cognitive ability (Dickinson & Elvevåg, 2009). Specifically, studies demonstrate that increased catecholamine availability, as with the presence of the Met allele in Val158Met, is associated with greater executive cognitive ability when resting catecholamine levels are low, but with reduced executive cognitive ability when pretask levels are high (Mattay et al., 2003; Qin et al., 2012) or when COMT activity is inhibited (Farrell, Tunbridge, Brauetigam, & Harrison, 2012).
Although it is well established that background catecholamine levels are relevant to the relation of genes such as COMT to executive cognition, it is notable that few if any studies have examined the relation between COMT and executive cognitive ability using a prospective longitudinal design that takes into account the quality of early life experience. Adverse early experience, such as that associated with early life stress has been shown in both human and animal models to increase catecholamine and glucocorticoid output (Evans, 2003; McEwen and Gianaros, 2011; Meaney, 2001) and to be associated with a pattern of gene expression associated with increased reactivity and decreased regulation of the stress response (Miller et al., 2009). It is therefore likely that early life experience will moderate any relation between variation in the COMT gene and executive function ability. Specifically, among individuals with stressful early life experience, higher catecholamine availability due to less efficient catabolism, as in individuals with a Met allele for COMT Val158Met, would be associated with lower rather than higher executive function ability. In contrast, in keeping with the Yerkes-Dodson inverted U-shape curve, among individuals with greater early life stress, reduced catecholamine availability (more efficient catabolism) associated with the Val-Val genotype would be associated with higher executive function abilities.
Given a theoretical and empirical basis for expected interaction between monoamine gene variants and early experience in the prediction of developmental outcomes, questions about the age in childhood at which associations among gene variants, early experience, and cognitive outcomes might be observed are of interest. Examination of COMT enzyme activity in postmortem human brain indicated a twofold increase between the neonatal period and adulthood (Tunbridge et al., 2006) with levels in childhood at approximately 50% of their adult values. Notably, one prior lab-based study with a small sample of children from middle to upper income homes found, as expected, an association between Met genotype and executive functions in 8 to 14 year olds (Diamond, Briand, Fossella, & Gehlbach, 2004). A second, with a large sequential cohort sample in Sweden found that the Val–Val genotype was associated with working memory ability in young children (age 6 years) but that the Met-Met genotype was associated with better working memory in early adolescence (Dumontheil et al., 2011). No prior studies of which we are aware, however, have examined interaction between COMT and early experience in children with sample sizes large enough to produce reliable estimates of interaction effects. It is not known whether variation in early adversity in the typical range might interact with genetic background to influence trajectories of aspects of development such as executive function abilities in childhood.
Here, we examine expected gene-environment interaction with a diverse prospective longitudinal sample of children and families in predominantly non-urban and low-income communities in two regions of high poverty in the US. It has been shown previously in this sample that early environmental adversity was prospectively associated with elevated cortisol levels in children through age 48 months (Blair et al., 2011a), and that elevated cortisol in the early toddler period partially mediated a relation between early adversity and lower performance on a battery of executive function tasks (Blair et al., 2011b). Here, we build on these prior findings to test the hypothesis that COMT Val158Met genotype will interact with cumulative risk in the family environment in the prediction of executive function development between ages 3 and 5 years. Specifically, the valine variant of COMT Val158Met will be associated with a higher level of executive in the context of high early adversity while the methionine variant will be associated with a higher level of executive function in the context of low early adversity.
METHODS
Participants
The Family Life Project (FLP) was designed to study young children and their families in two of the four major geographical areas of the United States with high poverty rates. Specifically, three counties in eastern North Carolina and three counties in central Pennsylvania were selected to be indicative of the Black South and Appalachia, respectively. The FLP adopted a developmental epidemiological design in which sampling procedures were employed to recruit a representative sample of 1,292 children whose families resided in one of the six counties at the time of the child's birth. Low-income families in both states and African American families in NC were over-sampled. African American families were not over-sampled in PA because African Americans made up <5% of the population of the target communities.
At both sites, recruitment occurred seven days per week over the 12-month recruitment period spanning September 15, 2003 through September 14, 2004. Of those families selected to participate in the study, 1292 (82%) families completed a home visit at 2 months of child age, at which point they were formally enrolled in the study. Detailed descriptions of participating families and communities are available in Vernon-Feagans, Cox, and the FLP Investigators (2013).
Procedures
Families participating in the study were visited at their homes for data collection at approximately annual intervals beginning at child age 7 months. At each time point, families were visited by highly trained research assistants, and the child's primary caregiver, in almost all cases the mother, was administered questionnaires on household and demographic characteristics and also completed questionnaires on child temperament and behavior. At the home visit for data collection at 36 months, saliva samples were collected using Oragene DNA Self-Collection kits (DNA Genotek, Ottawa, Ontario Canada) in accordance with the manufacturer instructions. At home visits for data collection at 36, 48, and 60 months, children were administered a battery of executive function tasks.
Measures
Genotypes
DNA was extracted according to the manufacturer protocol. COMT genotyping was conducted with the appropriate probes for a Taqman SNP Genotyping Assay using an Allelic Discrimination Assay protocol (Applied Biosystems, Foster City, CA). Forty nanograms of DNA were combined in a volume of 5 μl with 2X Universal PCR Mix (Applied Biosystems) and 1/20 the volume of the Taqman SNP assay in a 384 well plate. A Pre-Read was performed and then PCR as follows: a 10 min hold at 95°C, followed by 40 to 45 cycles of 15 s at 92°C and then 1 min at 60°C in a 7900HT PCR System. After amplification, a Post-Read was performed to analyze. Automatic and manual calls were made (Haberstick and Smolen, 2005).
SNPs were quality controlled using procedures outlined previously (Simmons et al., 2010); briefly, quality control required Hardy-Weinberg equilibrium testing p < .001, missingness by marker <5%, missingness by sample <5%, affirmative relationship checking in PEDCHECK, and Mendelian inconsistency caused genotypes to be dropped at that locus.
Also genotyped was a panel of 48 SNPs that were chosen to include markers that provide information on both sample identification and relatedness to family members (highly polymorphic across the population) and ancestrally informative markers (polymorphic across human populations). This panel was validated for continental populations and also specifically for quantifying admixture in African American samples, as we have described previously (Hou, Phillips, Azaro, Brzustowicz, & Bartlett, 2011). Genotyping was conducted using a custom ligation detection reaction with a probe specifically tagged to identify each allele in a multiplex reaction (Bruse et al., 2008; Simmons et al., 2010). We analyzed population ancestry using principal components analysis (PCA) calculated in EIGENSTRAT that computes PCA scores from SNP data; specifically in this application, 48 ancestrally informative SNPs validated for continental populations and quantifying admixture in African American samples (Hou et al., 2011). We analyzed all samples that passed quality control with all 1000 Genomes samples with >85% of the 48 SNPs in the panel to provide clear reference populations for the three major continental groupings. The first three principal components were visualized graphically for our samples along with the 1000 Genomes samples, all color-coded by ancestry. African American samples displayed variation in the degree of admixture, as expected. As many of these samples did not clearly fall into either continental population according to Price et al. (2008), it is necessary to use the quantitative information on ancestry to control for this potentially confounding variation. Therefore, we included the first 6 principal components to control for the affects of admixture in all analyses.
Executive Functions
The EF battery consisted of six tasks presented in an open spiral bound flipbook with pages that measured 8” × 14”. The experimenter established that the child knew colors, numbers, and animals, and administered training trials and up to three practice trials. If children failed to demonstrate understanding of the goals of the task following practice trials, task administration was discontinued. The battery included three inhibitory control tasks (Simon-like spatial conflict, Stroop-like silly sounds, and farm animal go no-go task), two working memory tasks (a span-like task and a self-ordered pointing task), and one attention shifting task (flexible item selection). Full descriptions of each of the tasks as well as details regarding the administration rules, psychometric properties, and scoring approach for each of the tasks is available in several publications (Willoughby & Blair, 2011; Willoughby, Wirth, & Blair, 2011). A set of item parameters obtained from a calibration sample was applied to all children's EF data across all assessments resulting in a set of item response theory based (i.e., expected a-posteriori [EAP]) scores for each task that were on a common developmental scale.
Cumulative Risk
As with prior analyses of FLP data (see Vernon-Feagans et al., 2013), we created a cumulative risk index of 8 variables, including family income-to-need, maternal education, presence of spouse/partner living in the home, hours of employment, occupational prestige, household density, neighborhood noise and safety, and positive parenting that were measured when the target child was 7, 15, 24, and 36 months of age. The index was calculated by averaging scores within a given domain and conducting Principal Components Analysis. This analysis indicated that the data were best represented by a single component with an Eigen value greater than 1 that rose substantially above the scree, accounting for 45% of the variability across the items. The factor loadings were in the expected direction, and ranged from .52 (neighborhood safety) to .83 (family income). Internal-consistency reliability across the items was reasonable (Cronbach's α=.82). We created a continuous cumulative risk index by reverse-scoring the positively framed variables, standardizing each risk measure, and averaging across the risks.
Missing Data
There were N=1123 participating families at the home visit for data collection at child age 36 months, N=1049 at age 48 months, and N=1099 at age 60 months. N=1121 had data for EF at one or more time point. Of the children seen at 36 months, N=992 provided sufficient saliva for genetic analysis, and N=915 had data available for the COMT Val158Met polymorphism. Participants excluded from the analysis due to missing data had marginally lower cumulative risk scores (r=.06, p=.04) and were marginally more likely to be White (r=.06, p=.02). They did not differ in terms of sex and state of residence and were no different from the analysis sample on the outcome variable, executive function ability. Full information maximum likelihood estimation was used to address potential bias associated with missing data.
RESULTS
Descriptive Statistics and Measures of Association
Pearson χ2 statistics were used to analyze the association between COMT genotype and other categorical variables, and multiple regression analyses were used to examine the association between genotype and continuous variables. Frequencies for COMT Val158Met genotype are reported in Table 1 and were as follows: 149 Met–Met (75 female; 33 African American), 333 Val–Met (162 female; 143 African American), 268 Val–Val (130 female; 163 African American). COMT genotype was unrelated to sex, χ2(DF=2)=0.148, p=.929, but was associated with race, χ2(DF=2)=49.053, p<.001. Ordinal logistic regression indicated that the proportional odds assumption was not violated, χ2(DF=1)=1.8755, p=.171, and that African American race was associated with greater odds of having a Val allele, OR=2.96, 95%CI[2.23, 3.91].
Table 1.
Genotype Frequencies
| Genotype Frequencies | |||||
|---|---|---|---|---|---|
| Genotype |
Sex |
Race |
|||
| Overall | Female | Male | African American | Not African American | |
| Val–Val | 268 | 130 | 138 | 163 | 105 |
| Val–Met | 333 | 162 | 171 | 143 | 190 |
| Met–Met | 149 | 75 | 74 | 33 | 116 |
To test for the presence of gene–environment correlation (rGE), we examined COMT as a predictor of cumulative risk scores using multiple regression analysis. There was no overall association between COMT genotype and cumulative risk, F(2, 747)=2.81, p=.061, r2=.0075. Also, to adequately control for potential confounding in our analysis, we included race by risk and race by genotype interactions in the equation predicting executive function growth (Duncan, Pollastri, & Smoller, 2014).
Unconditional Growth Models
We fit unconditional growth models to the executive functioning data at child ages 3, 4, and 5 years using the MIXED procedure in SAS 9.3. Time was centered at the first assessment (3.09 years). Unconditional models indicated significant random effects (i.e., variances) for the intercept, linear slope, and the quadratic slope and significant covariances among the random intercept and linear and quadratic slopes, σ01=−0.084, σ02=0.021, σ12=−0.060, zs=−3.95, 2.47, −2.82. These covariances indicate that high starting values were associated with a more negative than average slope that became more positive (relative to the sample average) over time, and that a more positive initial slope was associated with greater deceleration (relative to the sample average) over time. Executive function increased most rapidly at younger ages and decelerated modestly over time.
Test of the Hypothesized Interaction Between COMT Genotype and Risk
Covariates included African American race, sex, state (North Carolina vs. Pennsylvania), and mother and child PCA ancestry scores as additional controls for population stratification (Hou et al., 2011) in COMT. Cumulative risk and COMT genotype were included as substantive predictors. Controlling for covariates, cumulative risk was negatively related to the intercept for executive function, whether centered at the first time point, child age 3 years, b=−0.096, se=.019, p<.001, or later time points, ages 4 and 5 years. Risk was unrelated to linear change over time, b=−0.013, se=−.014, p=.311.
COMT genotype, represented by dummy codes with the Val–Val group as the referent uniquely predicted the intercept when placed at age 4 or 5 years but not at age 3 years. The Val–Val group had higher executive function relative to the Val–Met group at age 4, b=−0.058 se=.029, and age 5, b=−0.076, se=.038, both p<.05. Given that the estimate for the comparison of the Met–Met group to the Val–Val group was similar in size and sign to that for the Val-Met group, b=−0.071, se=.038, p=.06 and b=−0.067, se=.048, p=.17, we combined the Met–Met and Val–Met groups and repeated the analysis. The effect of this comparison on the intercept centered at age 5 years is shown in the left-hand column of Table 2,b=−.073, se=.035, p<.05.
Table 2.
Multilevel Model Results
| b | SE | p | b | SE | p | |
|---|---|---|---|---|---|---|
| Intercept | 0.154 | 0.050 | 0.002 | 0.157 | 0.050 | 0.002 |
| State (0=NC, 1=PA) | 0.190 | 0.045 | <0.001 | 0.188 | 0.045 | <0.001 |
| African American | −0.092 | 0.075 | 0.219 | −0.096 | 0.075 | 0.197 |
| Sex (0=Male, 1=Female) | 0.135 | 0.034 | <0.001 | 0.136 | 0.033 | <0.001 |
| Risk | −0.115 | 0.019 | <0.001 | −0.136 | 0.023 | <.001 |
| COMT Val–Met/Met–Met | −0.073 | 0.035 | 0.040 | −0.071 | 0.035 | 0.047 |
| Risk×COMT Val–Met/Met–Met | −0.058 | 0.035 | 0.099 | |||
| Linear Slope | 0.374 | 0.041 | <0.001 | 0.376 | 0.041 | <0.001 |
| State (0=NC, 1=PA) | −0.042 | 0.031 | 0.170 | −0.044 | 0.030 | 0.153 |
| African American | 0.042 | 0.051 | 0.411 | 0.038 | 0.051 | 0.459 |
| Sex (0=Male, 1=Female) | −0.007 | 0.023 | 0.743 | −0.006 | 0.023 | 0.800 |
| Risk | −0.011 | 0.013 | 0.379 | −0.030 | 0.015 | 0.052 |
| COMT Val–Met/Met–Met | −0.011 | 0.024 | 0.647 | −0.009 | 0.024 | 0.702 |
| Risk×COMT Val–Met/Met–Met | −0.052 | 0.024 | 0.028 | |||
| Quadratic Slope | −0.027 | 0.015 | 0.069 | −0.027 | 0.015 | 0.077 |
In addition to this effect on the intercept, we observed an interaction between COMT genotype and the cumulative risk variable in the prediction of the slope. Here, we found as hypothesized that the effect of cumulative risk on the linear slope for executive function in the Val–Val group was distinct from that in the Met-Met/Val-Met group. Results from this model are presented in the right hand column of Table 2 and in Figure 1. As seen in the table, this comparison resulted in a significant interaction, b=−0.052, se=0.024, p<.05, in which the slope of executive function between ages 3 and 5 years became increasingly positive as risk increased in the Val–Val group, meaning that executive function was developing more rapidly for children in this group in high risk homes. In contrast, the slope for executive function became more negative as risk increased in the group with any Met allele, indicating that executive function was developing more slowly in the context of risk for children with a Met allele. That is, as expected the effect of risk on the development of executive functions was moderated by COMT genotype.
FIGURE 1.
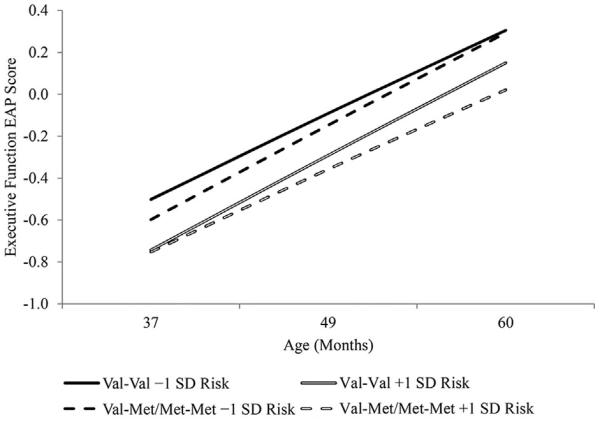
Model-Implied Executive Function EAP Scores as a Function of Cumulative Risk, COMT Val158Met Genotype, and Age.
DISCUSSION
Findings from this predominantly high risk low-income sample indicated that the Val–Val version of the COMT Val158Met gene is associated with higher executive function ability and interacts with adverse early life experience to influence the trajectory of executive function development in early childhood. Children with the Val–Val genotype were found to exhibit a more rapid development of executive function abilities between ages 3 and 5 years in the context of high environmental risk relative to children with the Val–Met or Met–Met genotype. This developmental relation was seen in that the Val–Val genotype was associated with higher executive function at ages 4 and 5 years but not at age 3 years. These findings from a large population-based sample indicate the role of early experience in the relation between genotype and behavior. Prior analyses with adults have indicated substantial main effects of COMT genotype on neural activity in PFC in response to executive function tasks (Mier et al., 2010). In contrast, findings from studies linking COMT genotype with measured executive cognitive ability have been mixed (Barnett et al., 2008). The neurobiology linking catecholamine availability with neural activity in PFC in an inverted U shape curve, however, suggests that any relation of COMT genotype to observed behavior likely depends on background levels of catecholamines. Building on this neurobiology linking neurotransmitter levels, genotype, and behavior, our main finding is that the association of COMT genotype with the development of executive functions is moderated by the quality of early experience. Few prospective longitudinal studies examining gene × early environment interaction have included data on early experience. One, with a sample of preterm infants, found that a relation between the experience of pain in the neonatal period and methylation of the serotonin transporter gene promoter region (SLC6A4), important for mood and behavior regulation, was moderated by COMT Val158Met genotype (Chau et al., 2014). Specifically, SLC64A methylation was reduced for carriers of the Met-Met allele relative to Val-Met and Val-Val carriers.
A second primary finding of this analysis is that relations between genotype and environment are detectable in the preschool period, during a time of rapid development of executive function. No prior studies of which we are aware have shown in a prospective longitudinal sample that genes associated with the metabolism of monoamines interact with the environment to predict the development of executive functions in early childhood. Demonstration of these relations is important given that an increasing number of studies have shown that genetic background interacts with experience in childhood to predict outcomes in adulthood. By and large these studies follow those of two widely cited analyses from a prospective longitudinal study in Dunedin NZ demonstrating that associations between genes related to monoamine (MAOA, 5-HTT) metabolism and later psychopathology depend upon the quality of early experience. Specifically, in the Dunedin sample, individuals carrying the version of the MAOA gene that confers more efficient metabolism of monoamines were at reduced risk for adult antisocial behavior associated with the experience of childhood maltreatment (Caspi et al., 2002). Similarly, for individuals carrying the long allele version of the serotonin transporter gene, conferring more efficient metabolism of serotonin (higher serotonin transporter availability), the effect of stressful life events on depression was reduced (Caspi et al., 2003). Initial attempts at the replication of these gene by environment interactions have been mixed (Munafò, Durrant, Lewis & Flint, 2009), raising questions about the external validity of findings and potential capitalization on chance in relatively small samples with limited power to detect statistical interaction (Duncan & Keller, 2011). More recent meta-analyses, however, have generally supported the original findings (Byrd & Manuck, 2014; Karg, Burmeister, Shedden, & Sen, 2011).
Importantly, validation or refutation of putative gene by environment interactions must proceed on grounds of biological plausibility as well as statistical significance. Across studies, there exists variation in measures and populations that might explain heterogeneity in findings (Goldman et al., 2009). Perhaps most importantly in the current context, findings of monoamine gene by environment interaction, like those reported here, are predicated on the idea that early adverse experience elevates catecholamine levels and/or alters neural circuitry and sensitivity in monoamine systems. No studies have contained direct measures of monoamine levels or of relevant neural circuitry that could be used to directly test hypotheses about the way in which early adverse experience and genetic background combine to influence behavior. Findings here are supported by the prior literature (e.g., Evans, 2003; Miller et al., 2009), including analyses from this sample (Blair et al., 2011a), demonstrating that early adversity is associated with elevated levels of indicators of stress physiology in children.
Study Limitations and Conclusions
Study limitations include the correlational nature of the associations among variables and the fact that the sample is predominantly high-risk and low-income. To the extent to which the range of environmental risk is attenuated in the sample, coefficients might underestimate the magnitude of effects. As well, executive functions can be difficult to measure, particularly in young children, and this likely places a further limit on the precision of the estimates. This concern is obviated to some extent by the use of a battery of tasks with known psychometric properties and established reliability. Further, although the COMT Val158Met polymorphism is widely studied, it is only one relevant variant of a complex gene that has also been related to pain perception and emotionality in addition to executive function. In addition, although the interaction of COMT Val158Met with early experience is statistically significant, the extent to which it is practically meaningful must be raised. The interaction effect reported here accounts for a relatively small amount of incremental variance and as such requires a large sample to detect it.
Although limited in certain respects, the finding of the interaction provides evidence of an expected relation between a genetic mechanism and environmental context that likely illustrates a more general developmental principle and sheds light on a potential early pathway through which genotype and environment combine to increase risk for later psychopathology. Findings from prior longitudinal studies indicate reduced risk for psychopathology associated with adverse early experience among individuals with a genotype that confers more efficient metabolism of monoamines. The finding here that the more catabolically efficient version of COMT is associated with more rapid development of executive functions in early childhood in the context of high environmental adversity recommends executive function development as one potential mechanism through which risk for later psychopathology is reduced. Executive function abilities are associated with the effective regulation of emotion and attention, higher levels of school achievement, and lower levels of behavior problems and substance use. As such, a focus on the promotion of executive function development in early childhood for children facing adversity is likely to prove an effective strategy for supporting healthy development into young adulthood, particularly for children at increased genetic risk.
Acknowledgments
We would like to thank the many families and research assistants that made this study possible. Support for this research was provided by the National Institute of Child Health and Human Development grants R01 HD51502 and P01 HD39667 with co-funding from the National Institute on Drug Abuse. The Family Life Project Key Investigators include Lynne Vernon-Feagans, The University of North Carolina, Mark Greenberg, The Pennsylvania State University, Martha Cox, The University of North Carolina, Clancy Blair, New York University, Peg Burchinal, The University of North Carolina, Michael Willoughby, The University of North Carolina, Patricia Garrett-Peters, The University of North Carolina, Roger Mills-Koonce, The University of North Carolina, Maureen Ittig, The Pennsylvania State University.
Contract grant sponsor: National Institute of Child Health and Human Development
Contract grant numbers: R01 HD51502, P01 HD39667
Contract grant sponsor: National Institute on Drug Abuse
REFERENCES
- Arnsten AFT. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol- o-methyltransferase gene val158/108met polymorphism. Biological Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Heron J, Goldman D, Jones PB, Xu K. Effects of catechol-o-methyltransferase on normal variation in the cognitive function of children. American Journal of Psychiatry. 2009;166:909–916. doi: 10.1176/appi.ajp.2009.08081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L, the Family Life Project Investigators Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011a;44:1095–1109. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Mills- Koonce R, Cox M, Greenberg MT, the Family Life Project Investigators Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011b;82:1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruse S, Moreau M, Azaro M, Zimmerman R, Brzustowicz L. Improvements to bead-based oligonucleotide ligation SNP genotyping assays. Biological Techniques. 2008;45:559–571. doi: 10.2144/000112960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: Meta-analysis of a gene-environment interaction. Biological Psychiatry. 2014;75:9–17. doi: 10.1016/j.biopsych.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Poulon R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulon R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chau CMY, Ranger M, Sulistyoningrum D, Devlin AM, Oberlander TF, Grunau RE. Neonatal pain and COMTVal158Met genotype in relation to serotonin transporter (SLC6A4) promoter methylation in very preterm children at school age. Frontiers in Behavioral Neuroscience. 2014;8:409. doi: 10.3389/fnbeh.2014.00409. DOI: 10.3389/fnbeh.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Elvevåg B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Influence of the COMT genotype on working memory and brain activity changes during development. Biological Psychiatry. 2011;70:222–229. doi: 10.1016/j.biopsych.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Pollastri AR, Smoller JW. Mind the gap: Why many geneticists and psychological scientists have discrepant views about gene? environment interaction (G× E) research. American Psychologist. 2014;69:249–268. doi: 10.1037/a0036320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. Comt val158met genotype determines the direction of cognitive effects produced by catechol-o-methyltransferase inhibition. Biological Psychiatry. 2012;71:538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: Evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behavior Genetics. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Goldman D, Weinberger DR, Malhotra AK, Goldberg TE. The role of COMT Val158Met in cognition. Biological Psychiatry. 2009;65:e1. doi: 10.1016/j.biopsych.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick B, Smolen A. Genotyping of three single nucleotide polymorphisms following whole genome preamplification of DNA collected from buccal cells. Behavior Genetics. 2005;34:541–547. doi: 10.1023/B:BEGE.0000038492.50446.25. [DOI] [PubMed] [Google Scholar]
- Hou LC, Phillips M, Azaro M, Brzustowicz LM, Bartlett CW. Validation of a cost-efficient multi-purpose SNP panel for disease based research. PLOS ONE. 2011;6:e19699. doi: 10.1371/journal.pone.0019699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5- httlpr), stress, and depression meta-analysis revisited: Evidence of genetic moderation. JAMA Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M. Gene × environment interaction studies have not properly controlled for potential confounders: The problem and the (simple) solution. Biological Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Weinberger DR. Catechol -O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress-and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Kobor MS. 2009. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences USA. 106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene × environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Price AL, Butler J, Patterson N, Capelli C, Pascali VL, Scarnicci F, Hirschhorn JN. Discerning the ancestry of European Americans in genetic association studies. PLoS Genetics. 2008;4:e236. doi: 10.1371/journal.pgen.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Cousijn H, Rijpkema M, Luo J, Franke B, Hermans EJ, Fernandez G. The effect of moderate acute psychological stress on working memory-related neural activity is modulated by a genetic variation in catecholaminergic function in humans. Frontiers in Integrative Neuroscience. 2012;6:16. doi: 10.3389/fnint.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons TR, Flax JF, Azaro MA, Hayter JE, Justice LM, Petrill SA, Bartlett CW. Increasing genotype-phenotype model determinism: application to bivariate reading/language traits and epistatic interactions in language-impaired families. Human Heredity. 2010;70:232–244. doi: 10.1159/000320367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val 158met and beyond. Biological Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox M, the Family Life Project Investigators The Family Life Project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development. 2013;78:310. doi: 10.1111/mono.12046. [DOI] [PubMed] [Google Scholar]
- Willoughby M, Blair C. Test-retest reliability of a new executive function battery for use in early childhood. Child Neuropsychology. 2011;17:564–579. doi: 10.1080/09297049.2011.554390. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Wirth RJ, Blair CB. Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment. 2012;24:418–431. doi: 10.1037/a0025779. [DOI] [PMC free article] [PubMed] [Google Scholar]


