Abstract
Monoclonal antibodies (mAb) targeting immune checkpoint pathways such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1) may confer durable disease control in several malignancies. In some patients, immune checkpoint mAb cause cutaneous immune-related adverse events. Although the most commonly reported cutaneous toxicities are mild, a subset may persist despite therapy and can lead to severe or life-threatening toxicity. Autoimmune blistering disorders are not commonly associated with immune checkpoint mAb therapy. We report a case series of patients who developed bullous pemphigoid (BP), an autoimmune process classically attributed to pathologic autoantibody formation and complement deposition. Three patients were identified. Two patients developed BP while receiving the anti-PD-1 mAb nivolumab, and one while receiving the anti-PD-L1 mAb durvalumab. The clinicopathologic features of each patient and rash, and corresponding radiologic findings at the development of the rash and after its treatment, are described. Patients receiving anti-PD-1/PD-L1 mAb may develop immune-related bullous pemphigoid. This may be related to both T-cell and B-cell mediated responses. Referral to dermatology for accurate diagnosis and management is recommended.
Keywords: anti-PD-1, anti-PD-L1, immune checkpoint blockade, bullous pemphigoid
Background
Immune checkpoint monoclonal antibodies (mAb) that block cytotoxic T-Lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) may generate durable antitumor responses in a number of malignancies(1–3). Side effects of these agents may be attributed to a persistently stimulated immune system, and are thus termed, “immune-related adverse events“ (irAEs). The precise mechanisms underlying the development of irAEs are not fully understood, but are postulated to be largely T cell mediated(4). Examples of such toxicities include: colitis, hepatitis, thyroiditis, pneumonitis, and hypophysitis. Specific target antigens that may underlie the development of these toxicities are not yet known.
The incidence of skin rash as a result of anti–CTLA-4 and anti–PD-1 therapy is over 20%, with a higher reported incidence with anti-CTLA-4 mAb(3, 5). The most common cutaneous irAE is a generalized maculopapular eruption. Pathologic features of this rash include perivascular eosinophilic and leukocytic infiltrates(6), and may be associated with peripheral eosinophilia(7). Although cutaneous irAEs are usually mild to moderate in severity, there are reports of severe reactions including toxic epidermal necrolysis (TEN), Stevens-Johnson-syndrome (SJS), vasculitis or drug reaction with eosinophilia and systemic symptoms (DRESS) as a result of ipilimumab treatment (6, 8, 9). These reactions, although potentially life-threatening, are usually reversible upon discontinuation of the mAb and with systemic treatment. Blistering skin disorders are not commonly associated with immune checkpoint mAb therapy. One case of bullous pemphigoid secondary to the anti–PD-1 agent pembrolizumab has been reported in a patient with metastatic melanoma(10). The underlying mechanisms for the development of this toxicity, and standard approaches for its diagnosis and management in patients receiving immune checkpoint mAb have not been described.
Standard diagnostic work-up for blistering disorders comprises dermatologic referral and a skin biopsy of lesional and perilesional tissue, to initially establish whether the abnormality is intraepidermal or subepidermal by hematoxylin and eosin staining. This is followed by further assessments including direct immunofluorescence (DIF), indirect immunofluorescence (IIF) using monkey esophagus, and serological testing for circulating or tissue-bound autoantibodies by ELISA(11). Bullous pemphigoid (BP) is the most common blistering skin disorder, the pathognomonic features seen with DIF include a subepidermal cleft and linear deposits of IgG and C3 at the blister roof at the dermoepidermal junction, and a band-like pattern at the dermoepidermal junction on IIF using monkey esophagus(11). Other subepidermal blistering disorders are ruled out in order to establish a diagnosis of BP, including epidermolysis bullosa acquisita (EBA), which has positive DIF staining of the blister floor. Patients who develop BP normally present with an initial non-bullous phase of pruritus, followed by development of generalized or localized tense blisters filled with serous or hemorrhagic fluid, and 10–30% of cases show involvement of the oral mucosa (11, 12). Implicated antigenic targets for BP include the hemi-desmosomal structural proteins of the dermoepidermal junction, BP180 (collagen XVII), and BP230(13). Serological testing by ELISA for circulating autoantibodies against BP180 and BP230 may be used to confirm the diagnosis, correlates with disease severity, or monitor response to treatment (13, 14). Whereas classic BP is idiopathic, more than fifty medications are associated with drug-induced BP, including antibiotics, non-steroidal anti-inflammatory drugs, diuretics, oral hypoglycemic agents, anti-hypertensives, and others (15, 16). No specific features differentiate classic BP from drug-induced BP. Drug-induced BP usually resolves after withdrawal of the causative agent, but may follow a chronic course resembling classic BP(15). The underlying mechanisms for drug-induced BP are unclear.
Herein, we describe the diagnosis, management and outcomes of three patients who developed BP while receiving anti-PD-1/PD-L1 immune checkpoint mAbs. In addition, we hypothesize that blockade of the PD-1/PD-L1 pathway may increase autoantibody production against the hemidesmosomal protein BP180, through a process that is both T-cell and B-cell mediated. We also hypothesize that this mechanism may contribute to achieving an antitumor response with PD-1/PD-L1 mAbs, as melanoma, non-small cell lung cancers (NSCLC), and the basement membrane of the skin can express BP180 as a common antigen(17, 18). Autoimmune blistering disorders have not been observed with anti CTLA-4 therapy to our knowledge, we thus propose that BP may be a class effect of anti PD-1/PD-L1 therapy.
Case series
Case 1
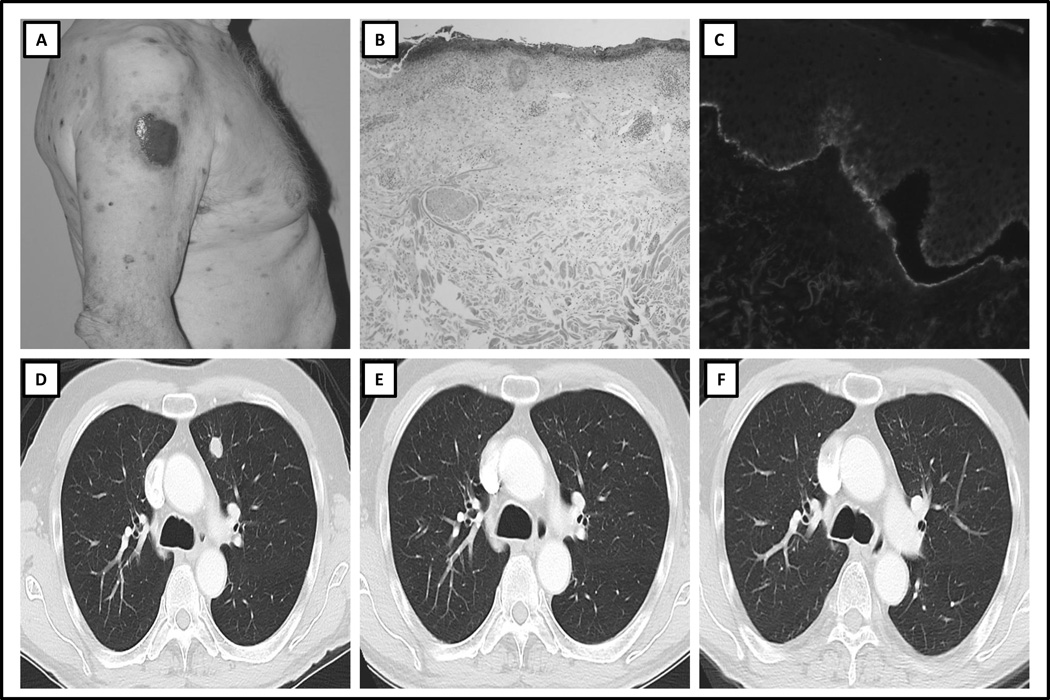
An 80-year-old male with metastatic melanoma, previously treated with ipilimumab (3mg/kg) every three weeks for four cycles of therapy that was complicated by mild pruritus, was treated with second-line nivolumab every 2 weeks. After 10 doses, the patient developed pruritus and a faint maculopapular rash. One month later, he developed tense bullae (Fig. 1A) without mucosal involvement. The patient had no underlying skin or autoimmune disorders, no recent exposure to light or radiation, and no new medications. Comprehensive diagnostic work-up for a blistering disorder was performed including DIF, IIF, BP ELISA and salt split skin analysis to rule out EBA. The histopathology review demonstrated an ulcerated and inflamed subepidermal vesicular dermatitis with eosinophils (Fig. 1B), DIF revealed linear disposition of C3 and IgG at the basal membrane zone (Fig. 1C), IIF on monkey esophagus was positive (IgG 40, IgG4:160), and salt split skin analysis revealed IgG at the epidermal side of the blister, consistent with BP. At the time of the development of the rash, a mildly elevated peripheral eosinophilia of 13.5% was noted on evaluation of the complete blood count, suggestive of BP. From a therapeutic perspective, the patient experienced a near-complete response to nivolumab (Fig. 1D–F), and continued on this therapy with close dermatologic monitoring. He experienced ongoing pruritus and developed urticarial lesions and bullae. His symptoms peaked after each dose of anti–PD-1 therapy, and he was treated with topical steroids, antihistamines, and intermittent oral steroids according to severity. Bullous pemphigoid ELISA was analyzed on two separate occasions during treatment with systemic corticosteroids and was positive (Table 1). After 26 doses of nivolumab, the patient began to develop erosions and vesicles on the buccal mucosa. This was treated with oral tacrolimus ointment and dexamethasone swish/spit. The patient’s nivolumab was then withheld. Over the next 4 months and at the time of this report, the patient had been treated with nicotinamide and a short course of antibiotic therapy for superimposed skin infection. The severity of the rash ranged from grade 1–2 during the patient‘s clinical course. The patient’s melanoma remains in complete remission now five months since the administration of the last dose of nivolumab.
Figure 1. Clinicopathologic Features and Serial Radiologic Imaging of Case 1.
A) Clinical presentation: multiple excoriated blisters on the trunk, B) Hematoxylin and eosin histopathologic slide, demonstrating subepidermal cleft and eosinophilic infiltrate, C) Direct immunofluorescence demonstrating IgG positivity at the dermo-epidermal junction, D) Baseline computed tomography (CT) scan image before treatment with immune checkpoint therapy, demonstrating lung metastasis, E) CT scan image at time of development of bullous pemphigoid, demonstrating partial radiologic response, F) CT scan image 6 months after diagnosis of bullous pemphigoid, demonstrating ongoing partial response.
Table 1.
Patient Demographics and Diagnostic Workup
| Age (yrs) |
Sex | Tumor Type |
Current Therapy |
Prior Therapies |
DIF | IIF | Salt Split Skin Analysis |
Time of Rash Onset (from start of Therapy) |
BP Elisa Titers (from time taken since start of therapy) |
|---|---|---|---|---|---|---|---|---|---|
| 80 | Male | Melanoma | Anti- PD-1 mAb |
Ipilimumab | + | + | + | 24 weeks |
At 33.9 weeks: BP180: 13.8* BP230: 10.0* At 47.0 weeks BP180: 25.6* BP230: 12.2* |
| 78 | Female | Melanoma | Anti- PD-1 mAb |
Ipilimumab | + | + | + | 17.9 weeks |
At 44.1 weeks: BP180: 72* BP230: 21.7* |
| 85 | Male | NSCLC | Anti- PD-L1 mAb |
Carboplatin + Gemcitabine |
+ | NA | NA | 6.1 weeks |
At 55.1 weeks: BP180: 1.1* BP230: 0.9* |
NSCLC= Non-small cell lung cancer, mAb= monoclonal antibody, NA= not applicable (not completed), DIF= direct immunofluroescence, IIF= immunofixation, BP ELISA= bullous pemphigoid enzyme-linked immunosorbent assay,
BP ELISA taken during steroid therapy.
Case 2
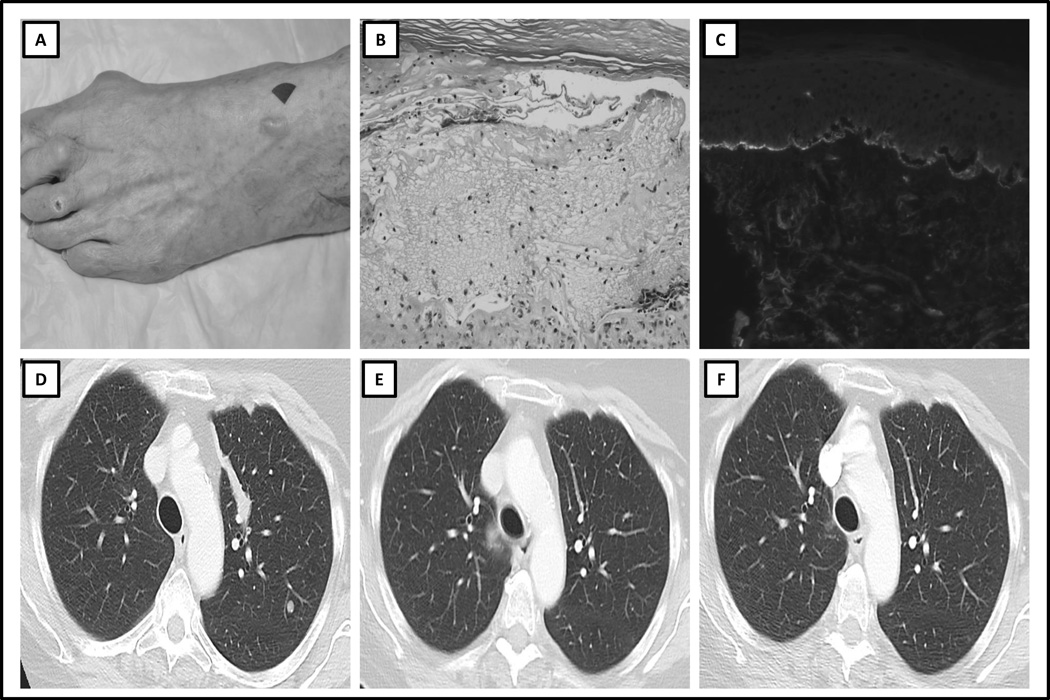
A 78-year-old female with metastatic melanoma was treated with durvalumab as second-line therapy, after first-line ipilimumab (3mg/kg) every three weeks for four cycles of therapy without associated irAEs. The patient had no relevant history of skin or autoimmune disorders, recent exposure to light or radiation, and no new medications. After 8 doses of durvalumab, the patient developed a maculopapular rash on her back, which was managed successfully with topical steroids. After an additional 4 doses of anti–PD-L1 therapy, a biopsy of the rash demonstrated pauci-inflammatory lichenoid dermatitis, suggesting a drug reaction. Subsequently, after almost one year of durvalumab therapy, she developed two fluid-filled, pruritic, tense blisters on the dorsum of her foot (Fig. 2A), accompanied by a new, intensely pruritic rash involving her torso and extremities. In light of these findings, subsequent dosing was withheld. A skin biopsy of the new blistering rash revealed a subepidermal cleft (Fig. 2B) with deposition of IgG and C3 on DIF (Fig. 2C), positive IIF on monkey esophagus (IgG 320, IgG4:160), negative salt-split skin analysis, and elevated BP180 (72.0) and BP230 (21.7) titers on serum ELISA while receiving topical steroid therapy (Table 1). The patient then developed buccal mucosal involvement and her rash evolved from erythematous patches into tense discrete bullae. At the time of initial development of the rash, her eosinophil count increased compared to pre-treatment levels but remained within normal range. Her skin condition improved slightly with topical steroids alone. The severity of the patient’s rash ranged from grade 1–2. The patient demonstrated a partial response to therapy by radiologic assessment at the time of development of BP, which is ongoing, one year post discontinuation of therapy (Fig. 2D–F). She continues to develop intermittent isolated pruritic lesions on her trunk, which are treated effectively with topical steroids.
Figure 2. Clinicopathologic Features and Serial Radiologic Imaging of Case 2.
A) Clinical presentation: tense blister on the dorsum of the left foot, B) Hematoxylin and eosin histopathologic slide, demonstrating subepidermal cleft and eosinophilic infiltrate C) Direct immunofluorescence demonstrating subepidermal cleft and C3 deposition at the dermo-epidermal junction, D) Baseline CT scan image prior to treatment with immune checkpoint therapy, demonstrating lung metastasis, E) CT scan image at the time of diagnosis of bullous pemphigoid, demonstrating partial response, F) CT scan image 12 months after discontinuation of treatment, demonstrating no evidence of disease.
Case 3
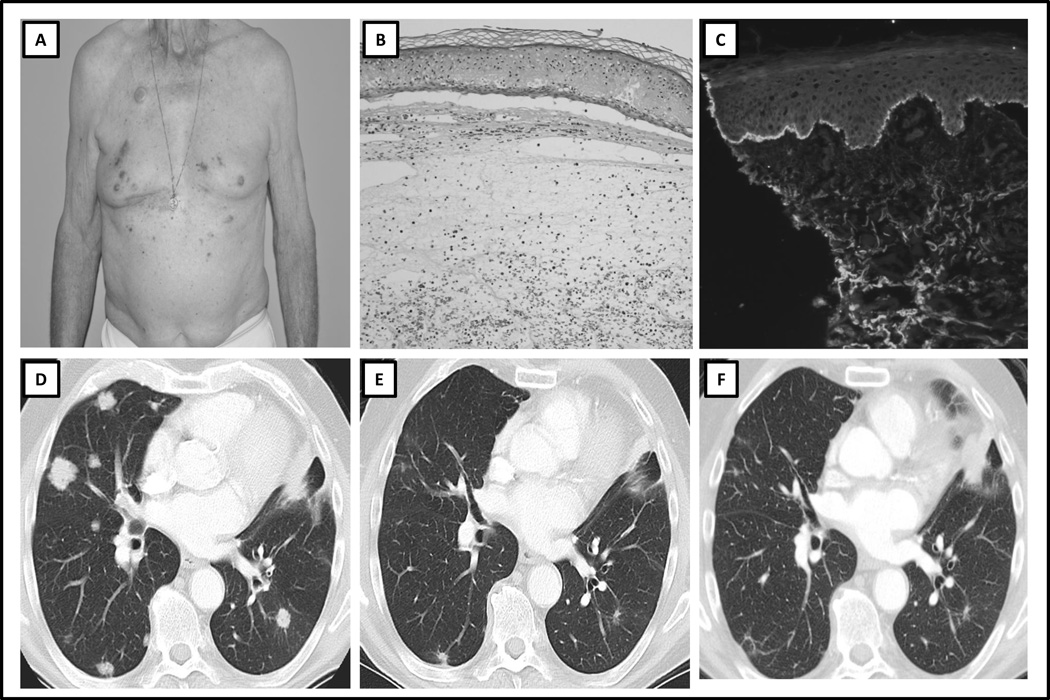
An 85 year old male with metastatic squamous cell carcinoma of the lung with progressive disease following six cycles of first-line platinum doublet chemotherapy, was treated with nivolumab every 2 weeks. After 3 months of therapy, the patient developed a pruritic, maculopapular, and erythematous rash. The patient had no history of underlying skin or autoimmune disorders, recent exposure to light or radiation, and no new medications. Histopathologic evaluation of the rash demonstrated a spongiotic, vesicular, and superficial perivascular dermatitis, thought to represent drug-induced hypersensitivity. The rash improved with topical high-potency steroids. However, after the patient’s ninth dose of nivolumab, he developed small cutaneous vesicles and bullae, which progressed to involve more than 30% of his body surface, consistent with a grade 3 rash (Fig. 3A). His nivolumab therapy was subsequently discontinued. A repeat skin biopsy demonstrated a subepidermal bullous dermatitis with eosinophils (Fig. 3B). Blood eosinophils increased compared to pretreatment and peaked at the time of diagnosis of BP, but remained within normal limits. Direct immunofluorescence revealed linear deposition of IgG and C3 at the basement membrane zone of the dermoepidermal junction, consistent with BP (Fig. 3C). BP180 and BP230 autoantibodies were negative, however these were tested after initiation and while the patient was receiving oral steroids. The cutaneous eruption remained stable with oral steroid therapy, dosed according to severity. The patient continued to develop new BP lesions after discontinuation of nivolumab for over 10 months. He was subsequently tapered off oral prednisone and treated with intermittent topical steroids. The patient’s squamous cell lung cancer has been stable on restaging CT scans since commencement of therapy and remains stable 15 months after the patient’s last dose was administered (Fig. 3D–F).
Figure 3. Clinicopathologic Features and Serial Radiologic Imaging of Case 3.
A) Clinical presentation: multiple papules and blisters on the trunk, B) Hematoxylin and eosin histopathologic slide, demonstrating subepidermal cleft and eosinophilic infiltrate, C) Direct immunofluorescence demonstrating subepidermal cleft and C3 deposition at the dermo-epidermal junction, D) Baseline CT scan image prior to treatment with immune checkpoint therapy, demonstrating lung metastases, E) CT scan image at time of diagnosis of bullous pemphigoid, demonstrating partial response, F) CT scan image 12 months after discontinuation of immune checkpoint therapy, demonstrating ongoing partial response.
Discussion
These cases illustrate the diagnosis, management, and anti-tumor response seen in three patients who developed BP while receiving nivolumab and durvalumab, respectively. In the context of one previous case report describing this phenomenon with pembrolizumab (10), we hypothesize that this clinical manifestation is likely to represent a class effect of these agents.
These cases display characteristics of both classic and drug-induced BP. Diagnostic tests for BP were positive in all three cases discussed above, after early referral to a dermatologist and diagnostic workup. Their clinical courses were distinct from traditional drug-induced BP, which usually resolves abruptly upon withdrawal of the causative agent(16). BP associated with anti–PD-1/PD-L1 mAbs may persist for several months after discontinuation of the agent. This could be explained by the continued in vivo effect of immune checkpoint mAb, which persists regardless of discontinued dosing, due to continued immune activation(3). This is also supported by the fact that all three patients had continued antitumor response or stable disease, in addition to continued blistering. These observations will need additional investigation to confirm a relationship between both findings. In case 1, cutaneous symptoms peaked after each dose of treatment, suggesting BP flare due to repeated dosing. In cases 2 and 3, patients exhibited ongoing cutaneous eruptions, despite cessation of the offending agent. Thus, although continued dosing may worsen BP, discontinuing the offending agent may not lead to complete resolution, and these patients may require intermittent or ongoing BP treatment.
BP with anti–PD-1/PD-L1 mAbs may occur within several months after initiation of the offending agent, and may be accompanied or preceded by pruritus. In the patients described, the onset of pruritus occurred within the first 3–4 months of initiation of anti–PD-1/PD-L1 therapy. Pruritus in the setting of mAb therapy is a common clinical finding; however, patients who manifest this symptom could represent a subset of patients with a non-bullous form of BP. Circulating autoantibodies against BP180 may be present prior to the development of blisters, or during the pruritic phase. Thus, in patients with persistent or unusual pruritus, dermatologic evaluation for potential subclinical BP may be considered.
In terms of treatment, topical and systemic steroids were administered according to severity. In a comparison of oral and topical steroid therapy in patients with moderate to severe BP of classic-type, topical steroid treatment with clobetasol (40 g/day) was superior to systemic treatment with prednisone (1 mg/kg/day), with regard to BP control, occurrence of side effects, and duration of hospitalization(19). In the above reported cases, patients were successfully managed mainly with topical steroids.
Why some patients develop immune-related BP and others do not, is currently unclear, however this could be related to the possession of a common target antigen located both at the dermoepidermal junction and on tumor cells. BP180 is an antigen that can be expressed on the surface of malignant melanocytic tumor cells, NSCLC cells, and the basement membrane of the skin (17, 18). Thus, it is possible that this phenomenon is a demonstration of T-cells targeting BP180 on tumor cells, as well as the basement membrane of the skin. Since BP180 is also expressed in other tissues, it is possible that other irAEs may develop as a result of a similar underlying mechanism involving autoantibody production(20). In our series, two of three cases demonstrated elevated serum BP180 and BP230 autoantibodies, however one of these patients had testing while receiving systemic steroids, and no patient had autoantibody titers assessed both before and after therapy, which limits our ability to make strong conclusions from this observation. From here, we plan to assess the primary and metastatic tumor tissue of the three patients in this case series for expression of BP180 using immunohistochemistry, in an attempt to demonstrate a potential on-target effect. Future studies may be done to assess BP180 and BP230 levels in before and after steroid therapy in patients who develop BP in the context of anti–PD-1/PD-L1 therapy.
A number of theories have been proposed to explain the immunologic mechanism of BP. In a mouse model, antibodies to BP180 were pathogenic, and depletion of complement, neutrophils, or mast cells abrogated the pathogenic effect of the BP180 antibodies, thus highlighting the role of the innate immune system in BP(21). The role of T cells in the pathogenesis of BP remains unclear. In drug-induced BP, it has been postulated that exposure to certain drugs may lead to depletion of CD4+CD25+Foxp3+ regulatory T cells, which can in turn lead to proliferation of autoantibody-secreting B-cell clones(22). In the context of PD-1/PD-L1 blockade, the principal effect of blocking this axis is the reinvigoration of exhausted T cells (23). It has also been postulated that anti–PD-1/PD-L1 therapy leads to an interaction between PD-1/PD-L1 expressing B cells and PD-1+ follicular helper T cells, thus facilitating a B-cell germinal center response, that favors a humoral rather than a cellular response (24, 25). Although a similar interaction and clinical cases of BP have not been described to our knowledge in the context of CTLA-4 blockade, further studies are required to determine whether BP may develop with mAbs to CTLA-4.
Conclusion
Clinicians prescribing anti–PD-1/PD-L1 therapy should be aware of the clinical manifestations of BP and recognize that this may be a class effect of these agents. We recommend that if BP is suspected, clinicians refer patients to a dermatologist for early evaluation and institute topical or systemic immunosuppressive therapy when necessary. Discontinuation of anti-PD-1/PD-1 therapy may be required. Dermatologic evaluation should also be considered in patients experiencing persistent pruritus to evaluate for the non-bullous variant of BP. Mechanisms underlying the development of irAEs with immune checkpoint mAbs warrant further investigation. Although autoimmune phenomena caused by immune checkpoint mAbs are assumed to be T cell mediated, B cells and the innate immune system may be closely interlinked and could play a crucial role in the development of irAEs. Dermatologic manifestations like BP may be instructive, in that they may identify potential target antigens and stimulate further study of the mechanisms underlying irAEs.
Acknowledgments
This work was not supported by a specific grant or source of funding.
KTC: Consultant: EAISI Other: AMGEN, Physician Education Resource, Dematology Nurses Association, Oncology Nursing Society, Clinical Assistance Programs LLC
MAP: Research funding: Bristol-Myers Squibb, Consulting: Bristol-Myers Squibb
AML: Advisory Board and Research funding: Bristol-Myers Squibb
JEC: Advisory Board: Genentech
NHS: Research funding: Genentech, Merck, MedImmune, Bristol-Myers Squibb, Consulting: Bristol-Myers Squibb, Roche, MedImmune, Pfizer
JDW: Research funding and Consulting: Genentech, Merck, MedImmune, Bristol-Myers Squibb
MEL: Commercial Research Grant: Berg, Bristol-Myers Squibb, Consulting: Roche, Genentech, Bristol-Myers Squibb, Merck, Novartis
Footnotes
Financial Disclosures:
No relevant financial disclosures.
Conflicts of Interest:
JN, KS, CAQ, KB, JC, DBP, AW, ASL, EAQ, PKP, MD, SPA : No relevant disclosures
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. PubMed PMID: 20525992. Pubmed Central PMCID: 3549297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011 Jun 30;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. PubMed PMID: 21639810. Epub 2011/06/07. eng. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012 Jun 28;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. PubMed PMID: 22658127. Pubmed Central PMCID: 3544539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Apr 27; doi: 10.1200/JCO.2014.60.0379. PubMed PMID: 25918278. Epub 2015/04/29. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minkis K, Garden BC, Wu S, Pulitzer MP, Lacouture ME. The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. Journal of the American Academy of Dermatology. 2013 Sep;69(3):e121–e128. doi: 10.1016/j.jaad.2012.12.963. PubMed PMID: 23357570. Epub 2013/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 6.Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. Journal of the American Academy of Dermatology. 2014 Apr 23; doi: 10.1016/j.jaad.2014.02.035. PubMed PMID: 24767731. Epub 2014/04/29. Eng. [DOI] [PubMed] [Google Scholar]
- 7.Jaber SH, Cowen EW, Haworth LR, Booher SL, Berman DM, Rosenberg SA, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Archives of dermatology. 2006 Feb;142(2):166–172. doi: 10.1001/archderm.142.2.166. PubMed PMID: 16490844. Epub 2006/02/24. eng. [DOI] [PubMed] [Google Scholar]
- 8.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PloS one. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. PubMed PMID: 23341990. Pubmed Central PMCID: Pmc3544906. Epub 2013/01/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yervoy [package insert] Princeton NB-MS. 2011 http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125377s0000lbl.pdf.
- 10.Carlos G, Anforth R, Chou S, Clements A, Fernandez-Penas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma research. 2015 Jun;25(3):265–268. doi: 10.1097/CMR.0000000000000155. PubMed PMID: 25831416. [DOI] [PubMed] [Google Scholar]
- 11.Baum S, Sakka N, Artsi O, Trau H, Barzilai A. Diagnosis and classification of autoimmune blistering diseases. Autoimmunity reviews. 2014 Apr-May;13(4–5):482–489. doi: 10.1016/j.autrev.2014.01.047. PubMed PMID: 24434358. Epub 2014/01/18. eng. [DOI] [PubMed] [Google Scholar]
- 12.Sticherling M, Erfurt-Berge C. Autoimmune blistering diseases of the skin. Autoimmunity reviews. 2012 Jan;11(3):226–230. doi: 10.1016/j.autrev.2011.05.017. PubMed PMID: 21640850. Epub 2011/06/07. eng. [DOI] [PubMed] [Google Scholar]
- 13.Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clinical reviews in allergy & immunology. 2007 Oct;33(1–2):67–77. doi: 10.1007/s12016-007-0030-y. PubMed PMID: 18094948. Epub 2007/12/21. eng. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Archives of dermatology. 2000 Feb;136(2):174–178. doi: 10.1001/archderm.136.2.174. PubMed PMID: 10677092. Epub 2000/02/17. eng. [DOI] [PubMed] [Google Scholar]
- 15.Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014 Jan 10; doi: 10.1111/jdv.12366. PubMed PMID: 24404939. Epub 2014/01/11. Eng. [DOI] [PubMed] [Google Scholar]
- 16.Vassileva S. Drug-induced pemphigoid: bullous and cicatricial. Clin Dermatol. 1998 May-Jun;16(3):379–387. doi: 10.1016/s0738-081x(98)00008-x. PubMed PMID: 9642531. Epub 1998/06/27. eng. [DOI] [PubMed] [Google Scholar]
- 17.Krenacs T, Kiszner G, Stelkovics E, Balla P, Teleki I, Nemeth I, et al. Collagen XVII is expressed in malignant but not in benign melanocytic tumors and it can mediate antibody induced melanoma apoptosis. Histochemistry and cell biology. 2012 Oct;138(4):653–667. doi: 10.1007/s00418-012-0981-9. PubMed PMID: 22688676. [DOI] [PubMed] [Google Scholar]
- 18.Papay J, Krenacs T, Moldvay J, Stelkovics E, Furak J, Molnar B, et al. Immunophenotypic profiling of nonsmall cell lung cancer progression using the tissue microarray approach. Applied immunohistochemistry & molecular morphology : AIMM/official publication of the Society for Applied Immunohistochemistry. 2007 Mar;15(1):19–30. doi: 10.1097/01.pai.0000213143.32030.f5. PubMed PMID: 17536303. Epub 2007/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. The New England journal of medicine. 2002 Jan 31;346(5):321–327. doi: 10.1056/NEJMoa011592. PubMed PMID: 11821508. Epub 2002/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 20.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Science translational medicine. 2014 Apr 2;6(230):230ra45. doi: 10.1126/scitranslmed.3008002. PubMed PMID: 24695685. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Sui W, Zhao M, Li Z, Li N, Thresher R, et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. Journal of autoimmunity. 2008 Dec;31(4):331–338. doi: 10.1016/j.jaut.2008.08.00. Pubmed Central PMCID: Pmc2642586. Epub 2008/10/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowman C, Delrieu O. Immunogenetics of drug-induced skin blistering disorders. Part I: Perspective. Pharmacogenomics. 2009 Apr;10(4):601–621. doi: 10.2217/pgs.09.11. PubMed PMID: 19374517. Epub 2009/04/21. eng. [DOI] [PubMed] [Google Scholar]
- 23.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002 Sep 17;99(19):12293–12297. doi: 10.1073/pnas.192461099. PubMed PMID: 12218188. Pubmed Central PMCID: Pmc129438. Epub 2002/09/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012 Nov 1;1(8):1223–1225. doi: 10.4161/onci.21335. PubMed PMID: 23243584. Pubmed Central PMCID: Pmc3518493. Epub 2012/12/18. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature immunology. 2010 Jun;11(6):535–542. doi: 10.1038/ni.1877. PubMed PMID: 20453843. Pubmed Central PMCID: Pmc2874069. Epub 2010/05/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]