Abstract
Chlorine is a pulmonary toxicant to which humans can be exposed through accidents or intentional releases. Acute effects of chlorine inhalation in humans and animal models have been well characterized, but less is known about persistent effects of acute, high-level chlorine exposures. In particular, animal models that reproduce the long-term effects suggested to occur in humans are lacking. Here, we report the development of a rabbit model in which both acute and persistent effects of chlorine inhalation can be assessed. Male New Zealand White rabbits were exposed to chlorine while the lungs were mechanically ventilated. After chlorine exposure, the rabbits were extubated and were allowed to survive for up to 24 h after exposure to 800 ppm chlorine for 4 min to study acute effects or up to 7 days after exposure to 400 ppm for 8 min to study longer term effects. Acute effects observed 6 or 24 h after inhalation of 800 ppm chlorine for 4 min included hypoxemia, pulmonary edema, airway epithelial injury, inflammation, altered baseline lung mechanics, and airway hyperreactivity to inhaled methacholine. Seven days after recovery from inhalation of 400 ppm chlorine for 8 min, rabbits exhibited mild hypoxemia, increased area of pressure-volume loops, and airway hyperreactivity. Lung histology 7 days after chlorine exposure revealed abnormalities in the small airways, including inflammation and sporadic bronchiolitis obliterans lesions. Immunostaining showed a paucity of club and ciliated cells in the epithelium at these sites. These results suggest that small airway disease may be an important component of persistent respiratory abnormalities that occur following acute chlorine exposure. This non-rodent chlorine exposure model should prove useful for studying persistent effects of acute chlorine exposure and for assessing efficacy of countermeasures for chlorine-induced lung injury.
Keywords: chlorine, lung injury, rabbit, hypoxemia, bronchiolitis obliterans, airway hyperreactivity
Introduction
Chlorine is a respiratory irritant used in a variety of industrial applications (Das and Blanc, 1993; Evans, 2005). Chlorine has also been used as a chemical weapon and is considered a chemical threat agent (Jones et al., 2010). Exposure to chlorine can occur through household accidents, occupational exposures, and accidental train derailments during chlorine transportation (Evans, 2005; Van Sickle et al., 2009; White and Martin, 2010). When inhaled, chlorine produces adverse acute health effects such as hypoxemia, airway obstruction, and pulmonary edema (Das and Blanc, 1993; Van Sickle et al., 2009; White and Martin, 2010) and long-term effects including respiratory symptoms and altered lung function (Schwartz et al., 1990; Malo et al., 2009; Clark et al., 2016). In a subset of individuals accidentally exposed to high levels of chlorine, such exposures have been associated with a condition termed acute irritant-induced asthma or reactive airways dysfunction syndrome (RADS), which is characterized by airway obstruction and hyperreactivity (Donnelly and FitzGerald, 1990; Malo et al., 2009; Chierakul et al., 2013). Acute high-level chlorine inhalation can lead to chronic airway disease with a host of pathological changes in the airway, such as increased thickness of the basement membrane and increased airway sub-epithelial fibrosis (Gautrin et al., 1994; Takeda et al., 2009).
Chlorine’s status as a chemical threat agent has necessitated the need for countermeasures that can be employed in the wake of an accidental or intentional release of this irritant gas. The goal of the present study was to create an animal model of chlorine inhalation that can be used to study the natural history of chlorine-induced lung disease and to test therapeutic strategies. Most previous studies investigating the effects of chlorine inhalation on the lung have used rodent models (Tian et al., 2008; Tuck et al., 2008; Fanucchi et al., 2012; Musah et al., 2012; Mo et al., 2013; O'Koren et al., 2013). Acute effects of chlorine exposure in small-animal models are pulmonary edema, airway obstruction, airway hyperreactivity, and hypoxemia (Leustik et al., 2008; Tian et al., 2008; Tuck et al., 2008); subacute and chronic effects include airway and interstitial fibrosis, increased mucus production, increased lung resistance, and airway hyperreactivity (Demnati et al., 1998; Yildirim et al., 2004; Fanucchi et al., 2012; Musah et al., 2012; Mo et al., 2013; O'Koren et al., 2013). Studies utilizing rodent models have revealed that chlorine inhalation results in epithelial cell loss and that re-epithelialization following injury to large airways is carried out by basal airway epithelial cells (Tuck et al., 2008; Fanucchi et al., 2012; Musah et al., 2012; Mo et al., 2013; O'Koren et al., 2013). In rodents, pseudostratified airway epithelium containing basal cells is restricted to only the largest airways (Rock and Hogan, 2011; Mo et al., 2013), whereas in humans this type of epithelium is present more distally down to fairly small airways (Boers et al., 1998). Hence larger animal models with more extensive distribution of basal cells may represent important adjuncts for studying persistent effects of chlorine inhalation. Some large animal models to study the effects of chlorine inhalation have been developed (Winternitz et al., 1920; Gunnarsson et al., 1998; Batchinsky et al., 2006). Whole-body exposure to chlorine has been performed in dogs (Winternitz et al., 1920), but more recently developed models have used lung-only exposure based on logistical and safety considerations (Gunnarsson et al., 1998; Batchinsky et al., 2006). In these models, anesthetized, mechanically ventilated pigs or sheep have been exposed to chlorine and then managed on a ventilator for the duration of the post-exposure period. Reported effects of chlorine exposure in these large animal models include epithelial injury, hypoxemia, pneumonitis, pulmonary edema, and pulmonary hypertension (Gunnarsson et al., 1998; Batchinsky et al., 2006). Advantages of such large-animal models are the capacity to perform longitudinal measurements of blood gas analysis and pulmonary function as well as the ability to mimic critical care procedures similar to those instituted in human patients following severe injury from chlorine gas inhalation. However, these published large-animal models of chlorine exposure have limitations, including the inability to characterize the natural history of chlorine-induced lung injury in conscious, spontaneously breathing animals and to examine longer-term sequelae following acute chlorine exposure. Here we report the development of a novel chlorine inhalation model in which rabbits are mechanically ventilated for chlorine exposure, but are then extubated and remain unanesthetized and breathe spontaneously during the post-exposure period. We demonstrate that this model allows investigation of both acute and longer term effects of chlorine exposure on the lungs and should be useful for testing potential treatments for chlorine-induced lung injury.
Materials and Methods
Chlorine exposure
All experiments were performed in accordance with the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the University of Louisville Institutional Animal Care and Use Committee. Male New Zealand White rabbits (2–3 kg) were purchased from Harlan Laboratories, Inc. (Indianapolis, IN) or Robinson Services Inc. (Mocksville, NC). Arterial and iv catheters were placed in ear vessels for collection of blood and administration of drugs. Rabbits were mechanically ventilated and exposed to either chlorine in air or air only via an endotracheal tube. Rabbits were initially anesthetized with propofol (15 mg/kg iv) and intubated with an endotracheal tube. After intubation, propofol was administered by iv constant rate infusion at a rate of 2 mg/kg/h. Rabbits were mechanically ventilated with a tidal volume of 8 ml/kg at 30 breaths/min and positive end-expiratory pressure of 3 cm water. Additional boluses of 3–4 mg/kg propofol were administered until the toe-pinch reflex was absent. Animals were then exposed to either 800 ppm chlorine in air for 4 min, or 400 ppm chlorine in air for 8 min, or to air for either 4 or 8 min. After chlorine or air exposure, propofol administration was discontinued, and the rabbits were given 0.05 mg/kg buprenorphine iv (Reckitt Benckiser Pharmaceuticals, Richmond, VA). When the rabbits regained spontaneous breathing effort (approximately 10 min after the end of exposure), they were removed from the ventilator and extubated, and a transdermal fentanyl patch (25 µg/h for 72 h) was applied to the shaved dorsum. Rabbits were monitored continuously for 24 h after exposure, and blood oxygen saturation (SpO2) was assessed every 10–30 min by pulse oximetry. After 24 h, rabbits were monitored at least daily with measurement of SpO2 and weight. Rabbits were given supplemental oxygen if SpO2 dropped below 90%. Rabbits were euthanized if any of the following humane endpoint criteria were met: SpO2 <80% for >15 min when breathing 100% oxygen, agitation associated with dyspnea, or weight loss of >15% compared with pre-exposure. Fluids were administered iv and sc for the first 24 h after exposure using the formula 70 ml + (body weight in kg × 30 ml). Blood gas measurements were performed 5.5 h, 24 h, and 7 days after exposure using IDEXX VetStat electrolyte and blood gas analyzer (Westbrook, ME). Alveolar-arterial gradient was calculated using the formula [150-(PaCO2/0.8)] – PaO2.
Pulmonary edema
Pulmonary edema was assessed by measuring extravascular lung water in the left lung 6 h after exposure to 800 ppm chlorine or air for 4 min. Extravascular lung water was measured as described previously (Su et al., 2007), except that the density of rabbit blood (1.044 g/ml) (Kenner et al., 1977) was substituted for that of the mouse.
Pulmonary mechanics
Pulmonary mechanics at baseline and in response to inhaled methacholine were measured 24 h or 7 days following chlorine or air exposure. Measurements were performed by forced oscillation technique using a flexiVent system (SCIREQ, Montreal, Quebec, Canada). Rabbits were anesthetized with 4 mg/kg midazolam (West-Ward, Eatontown, NJ) and 10 mg/kg sodium pentobarbital (Sigma-Aldrich, St. Louis, MO) iv and placed on a heating pad. A tracheal cannula connected to a pressure transducer was inserted into the trachea, and the lungs were mechanically ventilated with air at a tidal volume of 10 ml/kg and a frequency of 30 breaths/min. A pressure transducer was connected to a catheter inserted into the auricular or femoral artery to monitor blood pressure during mechanical ventilation. To prevent endogenous breathing effort, rabbits were given cisatracurium besylate (0.12 mg/kg) every 20 min until the completion of airway reactivity measurements. During the experiment, rabbits were given additional sodium pentobarbital to maintain blood pressure at or below the baseline level established before administering the neuromuscular blocking agent. Baseline pulmonary mechanics measurements were collected using 1) a single perturbation at 0.5 Hz to derive resistance and compliance based on the single compartment model, 2) a broadband perturbation from 1 to 20.5 Hz to derive frequency-dependent impedance and parameters based on the constant phase model, and 3) quasi-static pressure-volume curves. Following baseline assessment, measurements were then repeated following administration of increasing doses of aerosolized methacholine generated from solutions of 1.6, 3.1, 6.3, and 12.5 mg/ml.
Histology and immunofluorescence
Rabbits were euthanized by injection of 4 mg/kg midazolam and 10 mg/kg sodium pentobarbital iv followed by exsanguination. Lungs were inflated with 10% neutral buffered formalin, fixed overnight, processed, paraffin embedded, and sectioned onto slides at a thickness of 5 µm. For histological examination of lungs, hematoxylin and eosin staining was done in addition to staining with Sirius red to visualize collagen. Immunofluorescence was performed using antibodies against airway epithelial cell markers as described (Musah et al., 2012). For the club cell marker club cell secretory protein (CCSP), antibody was generously provided by Dr. Gurmukh Singh (VA Medical Center, Pittsburgh, PA) and used at a dilution of 1: 1000. For the ciliated cell marker acetylated tubulin (AcTub), antibody was purchased from Sigma-Aldrich (St Louis, MO, catalog # T7451) and used at a dilution of 1: 20,000. For the basal cell marker nerve growth factor receptor (NGFR) (Rock et al., 2009), antibody was purchased from Abcam (Cambridge, MA, catalog #ab8877) and used at a dilution of 1:125.
Data analysis
Data was analyzed with GraphPad Prism version 6.0f (GraphPad; La Jolla, CA) and presented as group means ± standard error of the mean (SEM). For most parameters, group means between chorine-exposed and air-exposed rabbits were analyzed by unpaired two-tailed t-test with Welch’s correction. Effects of chlorine exposure on methacholine dose-response curves were analyzed by two-way repeated-measures analysis of variance.
Results
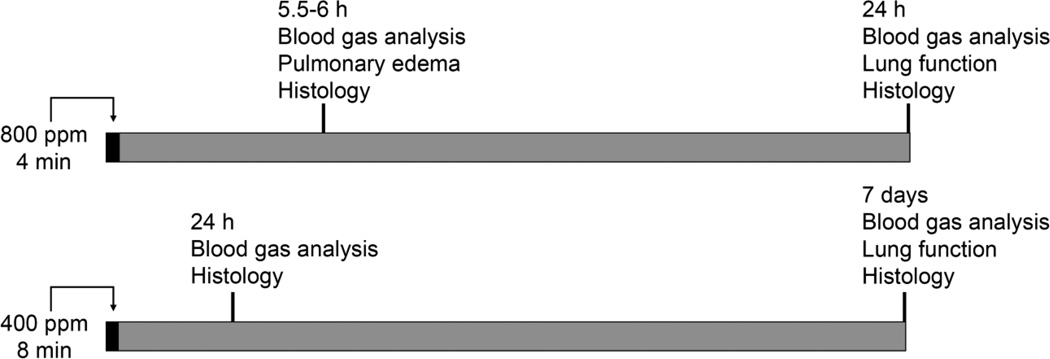
Humans may inhale unhealthy levels of chlorine under a variety of exposure scenarios. Some of these, particularly in large-scale accidents, involve high-intensity, short-term exposures (Jani et al., 2016). Adverse health outcomes from high-level chlorine exposure include both acute lung injury (Van Sickle et al., 2009) and chronic airway disease (Malo et al., 2009; Clark et al., 2013). We therefore wanted to devise exposure protocols that could be used to study both these aspects of chlorine toxicity. We performed dose ranging studies in rabbits using exposure to 400 and 800 ppm chlorine based on our previous experience that short-term exposure of mice to these concentrations resulted in significant pulmonary injury (Hoyle et al., 2010). We sought to develop a non-lethal model in which measurements could be made consistently at defined times after exposure. Preliminary studies indicated that exposure to 800 ppm chlorine for 6 or 8 min resulted in severe lung injury that necessitated euthanasia of some animals prior to 24 h based on predefined humane endpoint criteria. Exposure to 800 ppm for 4 min resulted in moderate lung injury that allowed consistent survival to 24 h, and this exposure protocol was chosen to evaluate acute chlorine injury. However this protocol was inconsistent with longer term survival (described below), and a separate exposure protocol using 400 ppm for 8 min was used to evaluate recovery, repair, and longer term injury (Fig. 1).
Figure 1. Chlorine exposure and analysis.
The diagram shows the overall experimental design, including the two chlorine exposure protocols and the types of analyses performed at different times after exposure.
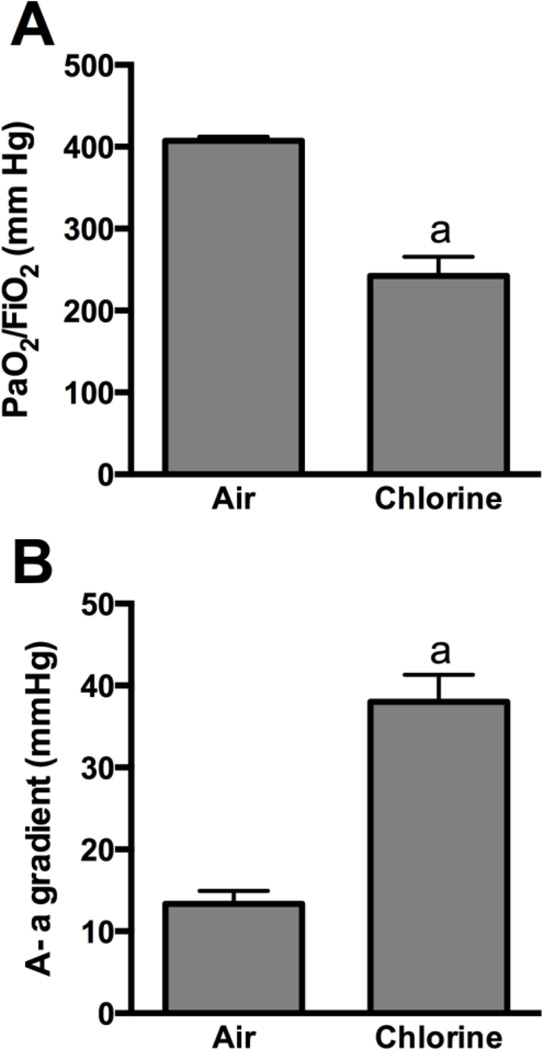
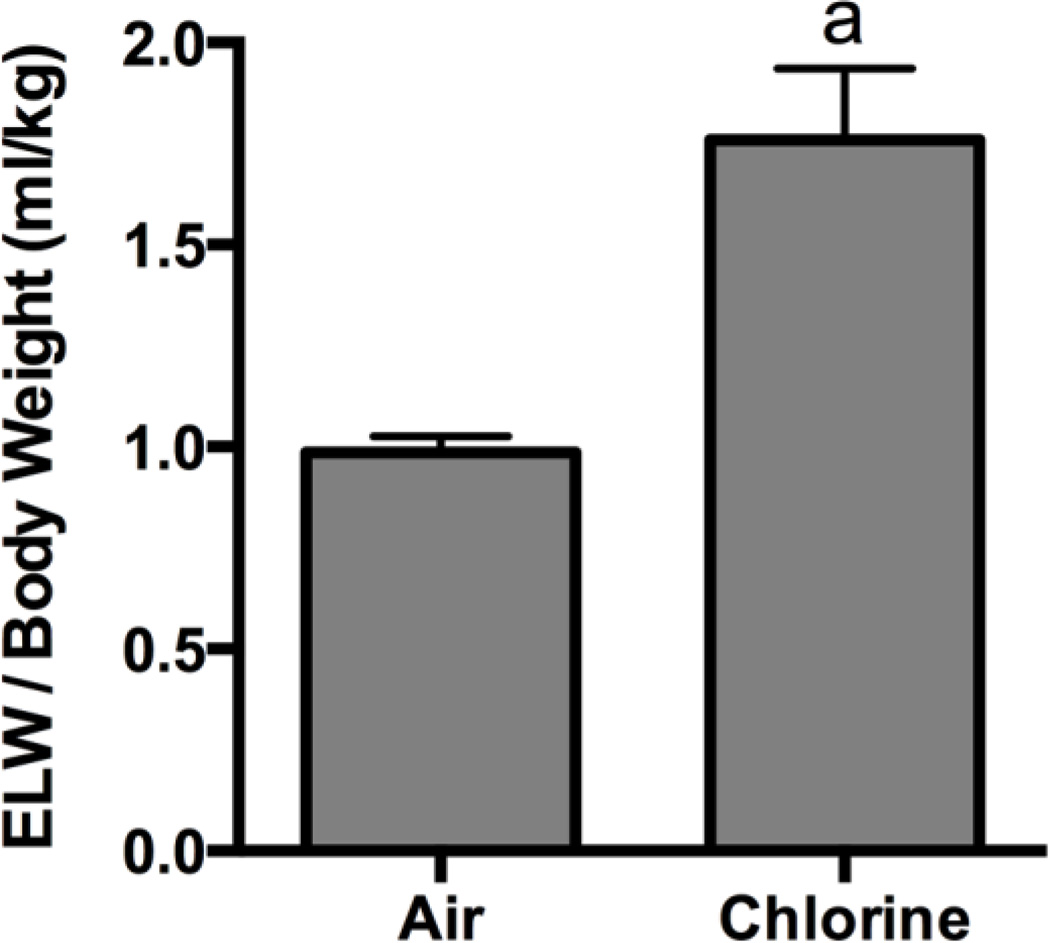
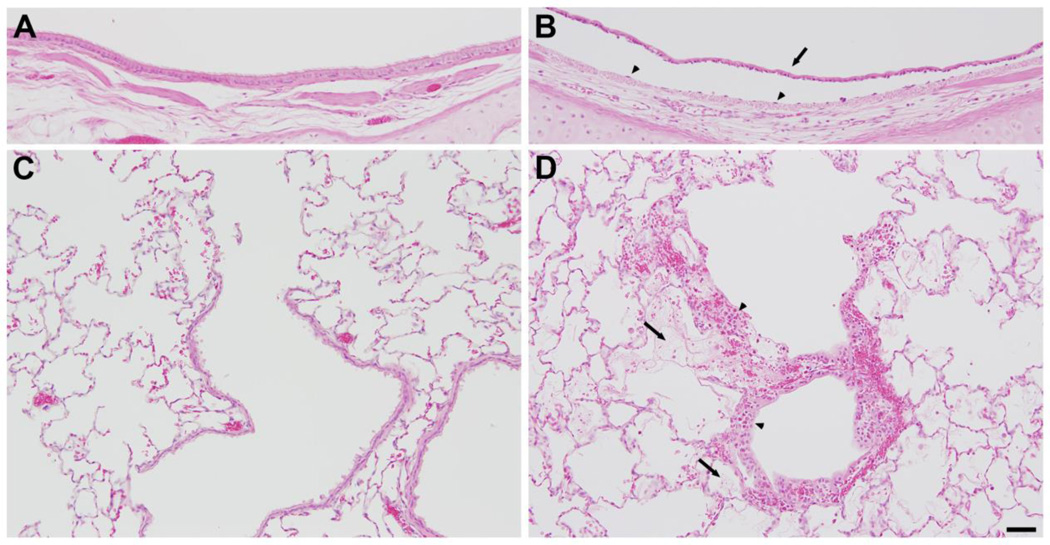
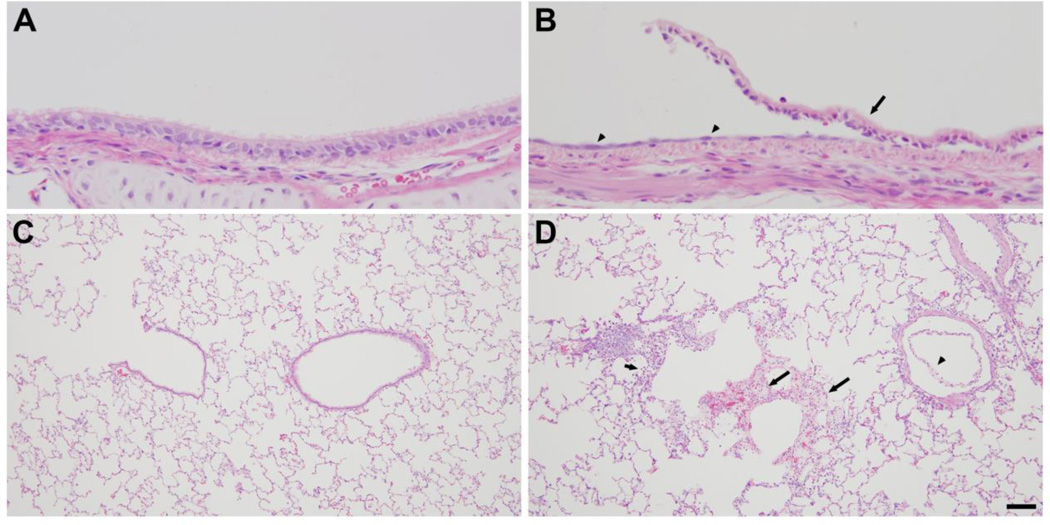
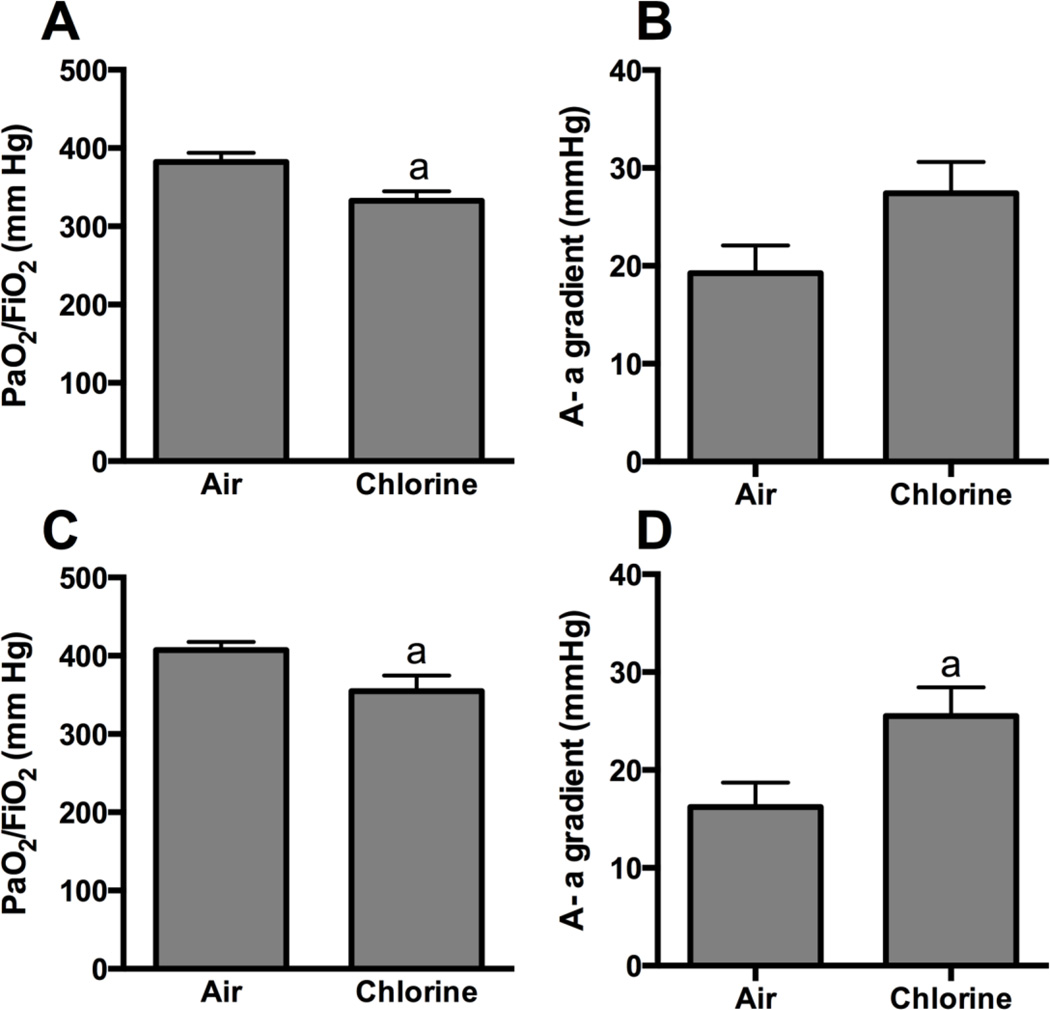
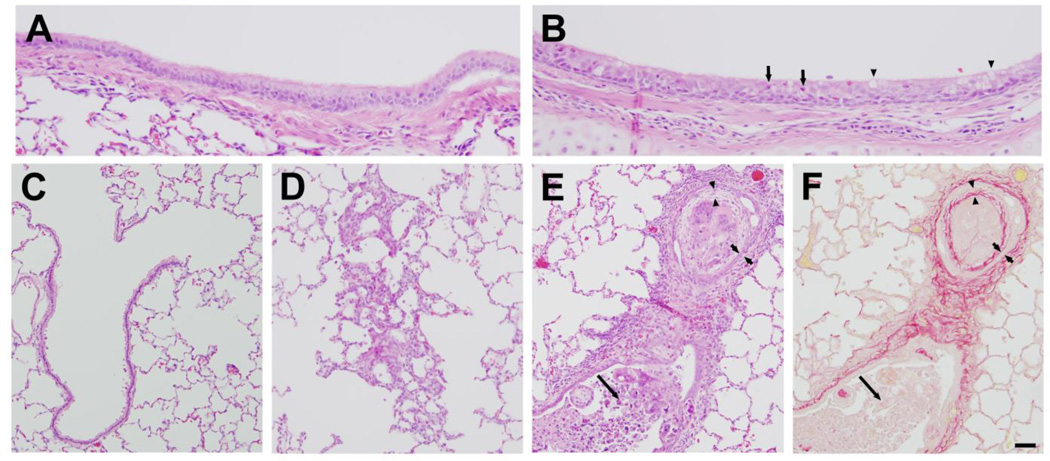
Rabbits were exposed to 800 ppm chlorine for 4 min and were evaluated for pulmonary toxicity at 6 and 24 h after exposure. These time points were based on the kinetics of chlorine-induced acute lung injury in humans (Van Sickle et al., 2009; White and Martin, 2010) and in animal models (Gunnarsson et al., 1998; Batchinsky et al., 2006; Leustik et al., 2008), in particular our previous studies in mice (Tian et al., 2008; Chang et al., 2012). Exposure of rabbits to 800 ppm chlorine for 4 min produced hypoxemia as evidenced by a decreased ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2; p<0.05) (Fig. 2A) and increased alveolar-arterial gradient (Fig. 2B) compared with air-exposed animals (p<0.05). Measurement of extravascular lung water to assess pulmonary edema 6 h after chlorine or air exposure showed significantly increased extravascular lung water in chlorine-exposed rabbits compared with their air-exposed counterparts (p<0.05) (Fig. 3). Histological analysis of lungs collected 6 h after exposure revealed that inhalation of 800 ppm chlorine for 4 min produced injury throughout the pulmonary airway tree (Fig. 4). Large cartilaginous airways in air-exposed rabbits contained normal pseudostratified epithelium (Fig. 4A). Six h after chlorine exposure, damaged epithelial cells had sloughed from larger airways, but thinly spread basal cells could be observed still attached to the basement membrane (Fig. 4B). Air-exposed rabbits showed normal structure of alveoli and small airways, including terminal bronchioles (Fig. 4C). Chlorine-exposed rabbits had damaged bronchioles characterized by injured and sloughed epithelial cells, hemorrhage, and inflammation (Fig. 4D). Alveolar injury that included proteinaceous material and fibrin deposition within air spaces was also observed. Alveolar injury was patchy and tended to cluster around injured bronchioles. Patchy air space enlargement was also observed adjacent to injured bronchioles.
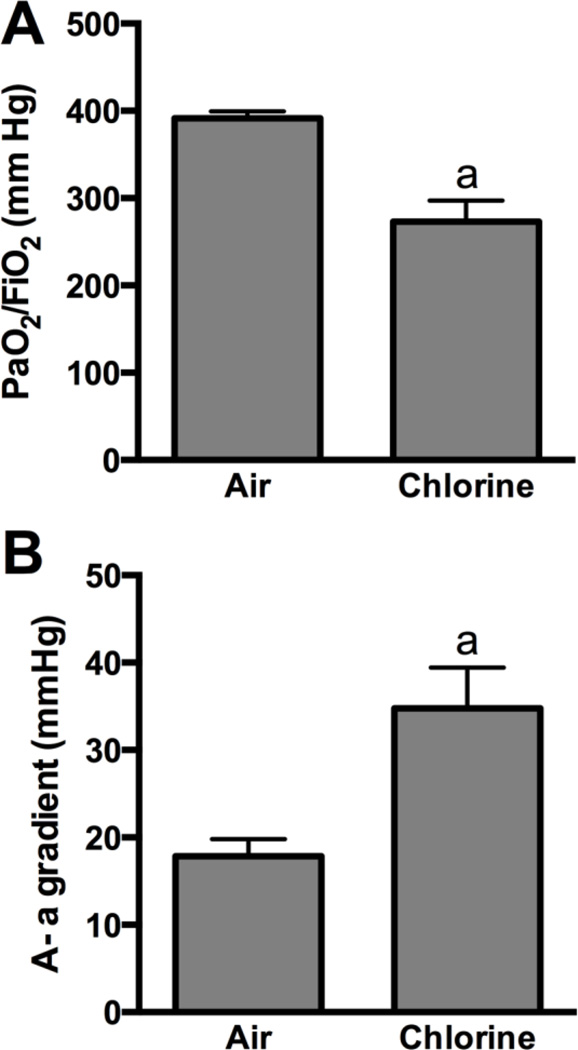
Figure 2. Arterial blood oxygenation 5.5 h after exposure to 800 ppm chlorine for 4 min.
Arterial blood gas analysis was performed in rabbits 5.5 h after exposure to 800 ppm chlorine or air for 4 min. A) Ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2). B) Alveolar-arterial (A-a) gradient. a, p<0.05 vs. air. n=9–10 rabbits/group.
Figure 3. Pulmonary edema 6 h after exposure to 800 ppm chlorine for 4 min.
Pulmonary edema was assessed in rabbits 6 h after exposure to 800 ppm chlorine or air for 4 min by measurement of extravascular lung water (ELW) in the left lung. a, p<0.05 vs. air. n=8 rabbits/group.
Figure 4. Lung histology 6 h after exposure to 800 ppm chlorine for 4 min.
All panels show hematoxylin and eosin staining of lung sections collected 6 h after exposure to 800 ppm chlorine for 4 min. A) Lobar bronchus from air-exposed rabbit showing normal airway structure. B) Lobar bronchus from chlorine-exposed rabbit showing sloughed epithelium (arrow) and residual, thinly spread basal cells (arrowheads). C) Section from air-exposed rabbit showing normal structure of alveoli and terminal bronchiole. D) Section from chlorine-exposed rabbit showing injured terminal bronchiole, including damaged epithelium and inflammation (arrowheads). Alveolar injury with fibrin deposition is indicated by arrows. Scale bar in D represents 50 µm for all panels. Results are representative of 8 chlorine-exposed and 8 sham-exposed rabbits.
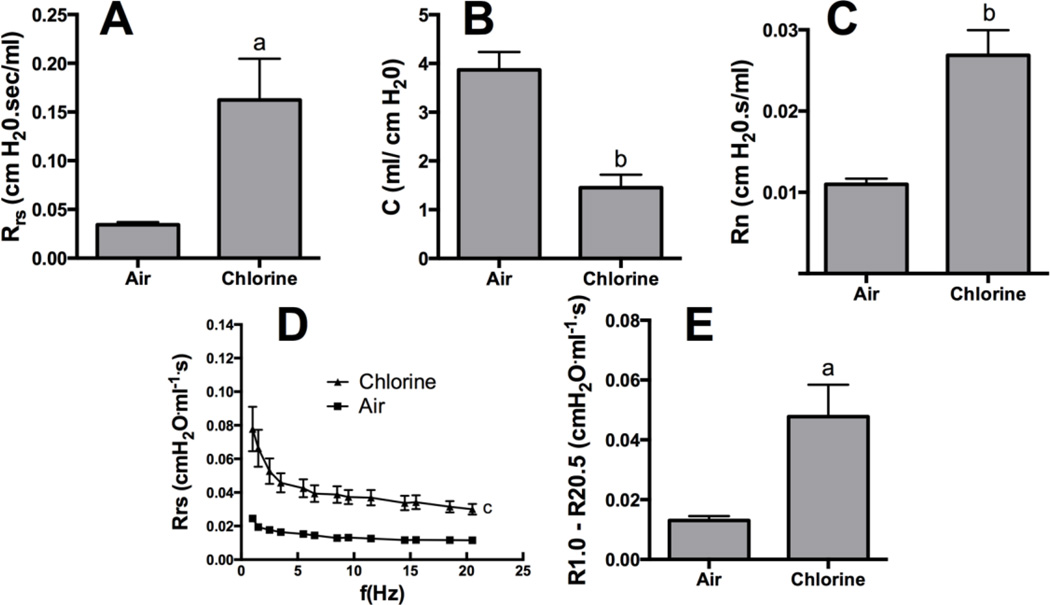
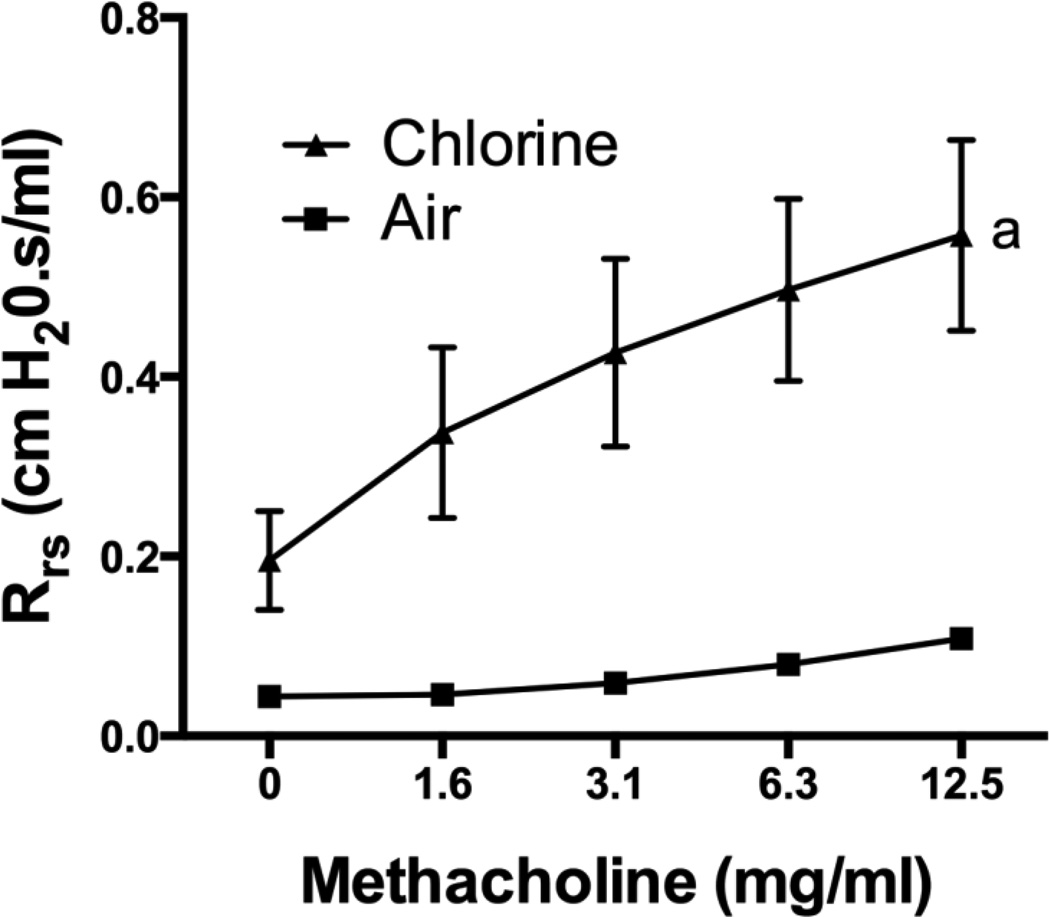
Assessment 24 h after exposure to air or 800 ppm chlorine revealed a significantly decreased PaO2/FiO2 in chlorine-exposed rabbits compared with air-exposed rabbits (p<0.05) (Fig 5A), as well as significantly increased alveolar-arterial gradient (p<0.05) (Fig 5B). Pulmonary mechanics measured at baseline in rabbits 24 h after air or chlorine exposure revealed altered lung function in chlorine-exposed rabbits, including increased resistance (p<0.05) (Fig 6A), decreased compliance (p<0.01) (Fig 6B), and increased Newtonian resistance (p<0.01) (Fig 6C), which is related to resistance in the central or conducting airways. Respiratory resistance (i.e. the real part of respiratory system impedance) was elevated at high frequencies in chlorine-exposed rabbits compared with air-exposed (p<0.05), indicative of central airway obstruction (Fig 6D). Chlorine-exposed rabbits also exhibited exaggerated frequency-dependent changes in resistance (assessed as R1.0-R20.5) (p<0.05) indicative of effects in the distal lung (Fig.6D and 6E). Chlorine-exposed rabbits also exhibited airway hyperreactivity in response to methacholine challenge (p<0.05) (Fig 7). Histological analysis showed that 24 h after air exposure, lungs continued to show normal airway structure (Fig.8A and 8C). In larger airways of chlorine-exposed animals, sloughed epithelium could still be observed; epithelial sheets tended to remain loosely attached to denuded basement membrane, but not to areas containing repairing epithelial cells (Fig. 8B). Repairing epithelium tended to be thicker at 24 h than at 6 h, suggesting some proliferative repair had taken place. In lung parenchyma, similar features to those at 6 h were observed, including sloughed epithelium in bronchioles, inflammation, alveolar flooding adjacent to injured bronchioles, and enlarged airspaces near injured airways (Fig. 8D).
Figure 5. Arterial blood oxygenation 24 h after exposure to 800 ppm chlorine for 4 min.
Arterial blood gas analysis was performed in rabbits 24 h after exposure to 800 ppm chlorine or air for 4 min. A) Ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2). B) Alveolar-arterial (A-a) gradient. a, p<0.05 vs. air; n=9–10 rabbits/group.
Figure 6. Baseline lung mechanics 24 h after exposure to 800 ppm chlorine for 4 min.
Pulmonary mechanics were assessed in rabbits 24 h after exposure to 800 ppm chlorine or air for 4 min by forced oscillation technique using a single frequency perturbation at 0.5 Hz (A and B) or a broadband perturbation from 1 to 20.5 Hz (C, D, and E). A) Resistance. B) Compliance. C) Newtonian resistance. D) Respiratory system resistance (the real part of respiratory system impedance) as a function of frequency. E) Frequency-dependent resistance calculated as R1.0-R20.5. a, p<0.05 vs. air; b, p<0.01 vs. air; c, p<0.001 chlorine vs. air curves; n=5–6 rabbits/group.
Figure 7. Airway reactivity 24 h after exposure to 800 ppm chlorine for 4 min.
Respiratory system resistance (assessed at 0.5 Hz) following increasing doses of inhaled methacholine was measured in rabbits 24 h after exposure to 800 ppm chlorine or air for 4 min. a, p<0.05 vs. air. n=5–6 rabbits/group.
Figure 8. Lung histology 24 h after exposure to 800 ppm chlorine for 4 min.
A) Lobar bronchus from air-exposed rabbit showing normal airway structure. B) Lobar bronchus from chlorine-exposed rabbit showing sloughed epithelium (arrow) and repairing epithelium that appears thicker than at 6 h (arrowheads). C) Section from air-exposed rabbit showing normal structure of small bronchiole, terminal bronchiole, and alveoli. D) Section from chlorine-exposed rabbit showing sloughed epithelium in small bronchiole (arrowhead), inflammation (short arrow), and alveolar flooding adjacent to terminal bronchiole (long arrows). Scale bar in panel D represents 25 µm for A and B, 100 µm for C and D. Results are representative of 10 chlorine-exposed and 5 sham-exposed rabbits.
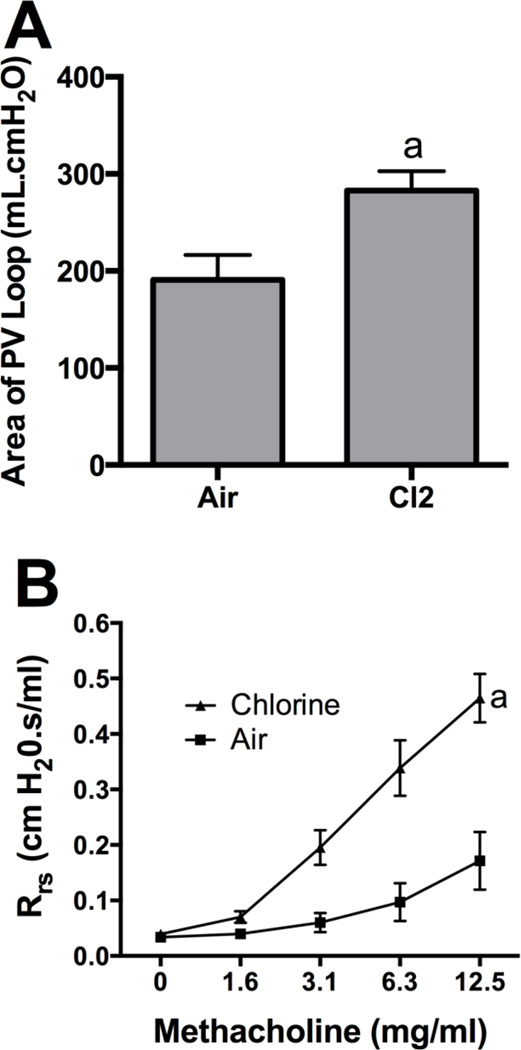
We sought to assess repair of lung injury and to examine longer term consequences of acute chlorine inhalation by allowing chlorine-exposed rabbits to survive for 7 days after exposure. Two rabbits were allowed to survive beyond 24 h after inhalation of 800 ppm chlorine for 4 min. Both these animals developed severe and worsening hypoxia and were euthanized 27 h and 3 days after exposure. These results precluded the use of this exposure regimen to examine longer term effects of chlorine exposure. To develop a model in which recovery from injury could be followed and lung repair could be investigated, rabbits were exposed to 400 ppm chlorine for 8 min. Although this provides the same nominal dose as 800 ppm for 4 min (53 ppm-h), our previous results in mice indicated that chlorine inhalation does not obey Haber’s law such that the lower concentration exposure will produce a lesser toxic effect (Hoyle et al., 2010). Exposure of rabbits to 400 ppm chlorine for 8 min produced modest hypoxemia when assessed 24 h after exposure (p<0.05 for PaO2/FiO2 ratio) (Fig.9A and 9B), and impaired oxygenation was still apparent 7 days after exposure (p<0.05 for PaO2/FiO2 ratio and A-a gradient) (Fig.9C and 9D). Most baseline lung mechanics parameters, including respiratory system resistance, compliance, and Newtonian resistance, were not significantly different between chlorine- and air-exposed animals 7 days after exposure (not shown). Chlorine-exposed rabbits had abnormal pressure-volume loops with increased area [i.e. increased hysteresis (p<0.05) (Fig. 10A)]. Chlorine-exposed rabbits also exhibited airway hyperreactivity to inhaled methacholine 7 days after exposure (p<0.01) (Fig 10B). Seven days after air exposure, lungs had histologically normal structure (Fig. 11A and 11C). Exposure of rabbits to 400 ppm chlorine for 8 min resulted in injury and sloughing of epithelial cells throughout the airway tree from bronchi to terminal bronchioles observed 24 h after exposure, but alveolar injury was less pronounced than for exposure to 800 ppm for 4 min (not shown). Large airways from chlorine-exposed rabbits were repopulated with a pseudostratified epithelium (Fig. 11B); focal areas contained granulocytes within the epithelium and increased numbers of goblet cells. Within the lung parenchyma, some areas of hyperplasia with abnormal alveolar structure were observed (Fig. 11D). Many small bronchioles showed a robust influx of inflammatory cells; in sporadic bronchioles the airway lumen was nearly occluded by bronchiolitis obliterans lesions (Fig. 11E and 11F). These lesions were composed of an abnormal epithelial layer, a collagen-containing fibrotic layer, and inflammatory cells and debris filling the central lumen of the airway.
Figure 9. Arterial blood oxygenation after exposure to 400 ppm chlorine for 8 min.
Arterial blood gas analysis was performed in rabbits 1 (A and B) and 7 days (C and D) after exposure to 400 ppm chlorine or air for 8 min. A) and C) Ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2). B) and D) Alveolar-arterial (A-a) gradient. a, p<0.05 vs. air. n=9–10 rabbits/group.
Figure 10. Pressure-volume curve area and airway reactivity 7 d after exposure to 400 ppm chlorine for 8 min.
Rabbits were exposed to 400 ppm chlorine or air for 8 min and subjected to lung function measurements 7 days after exposure. A) Area of quasi-static pressure-volume curves. a, p<0.05 vs. air; n=5–6 rabbits/group. B) Airway reactivity measured as respiratory system resistance in response to increasing doses of inhaled methacholine. a, dose-response curves different at p<0.01 vs. air; n=5–6 rabbits/group.
Figure 11. Lung histology 7 days after exposure.
A) Lobar bronchus from air-exposed rabbit showing normal airway structure. B) Lobar bronchus from chlorine-exposed rabbit showing repaired pseudostratified epithelium containing granulocytes (arrows) and increased goblet cells (arrowheads). C) Section from air-exposed rabbit showing normal structure of terminal bronchiole and alveoli. D) Hyperplasia and abnormal alveolar structure in chlorine-exposed rabbit. E) Terminal bronchiole from chlorine-exposed rabbit containing inflammation and bronchiolitis obliterans lesion. Part of the airway contains an identifiable lumen with considerable inflammatory cells and cell debris (long arrow). Part of the airway cut in cross-section contains concentric layers of abnormal epithelium (bracketed by short arrows), fibrosis (bracketed by arrowheads), and inflammatory cells and cell debris filling the lumen. F) Adjacent section to panel E stained with Sirius red. Red staining shows normal collagen in airway wall and abnormal fibrotic layer (bracketed by arrowheads) inside the epithelium (bracketed by short arrows). Long arrow indicates inflammation and cell debris inside airway lumen. Scale bar represents 25 µm for panels A and B, 50 µm for panels C–F. Results are representative of 9 chlorine-exposed and 7 sham-exposed rabbits.
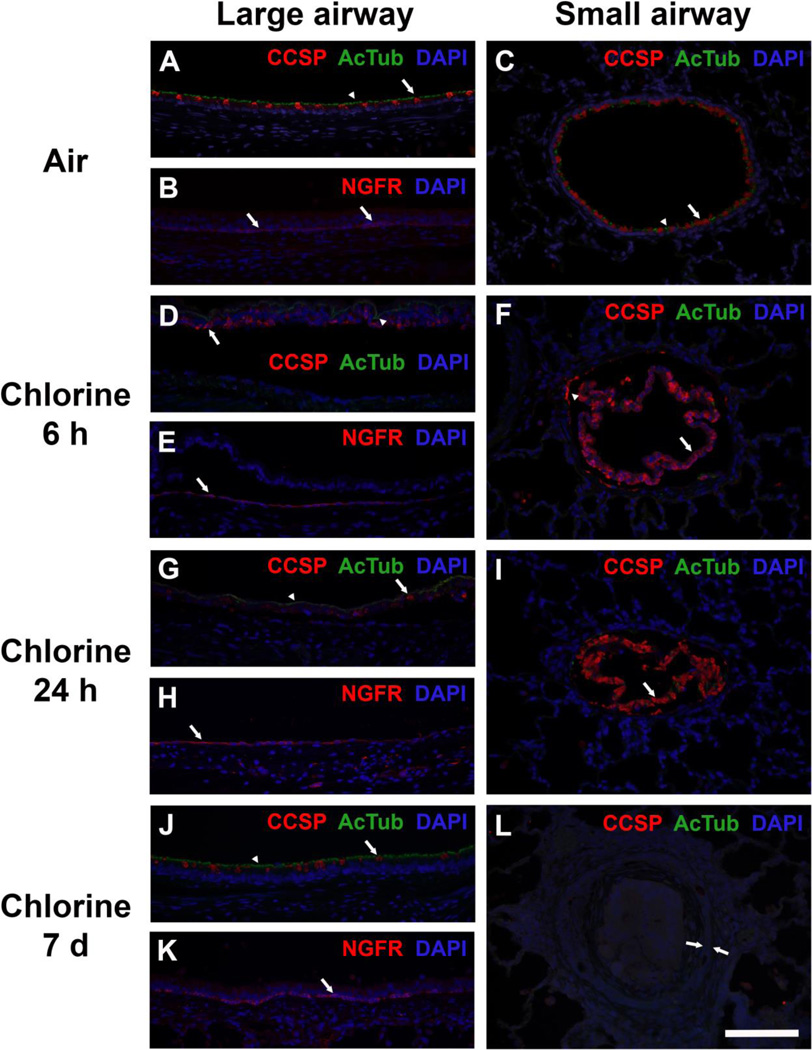
Immunostaining for epithelial cell markers was done to investigate repair of injured airway epithelium after chlorine inhalation (Fig. 12). Staining for club cell secretory protein (CCSP; club cell marker) and acetylated tubulin (AcTub; ciliated cell marker) revealed abundant club and ciliated cells in large airways, with ciliated cells predominating (Fig. 12A). Large airways also contained basal cells as part of the pseudostratified epithelium, and these cells were identified by staining for the low-affinity nerve growth factor receptor (NGFR; Fig. 12B). Small airways were lined with a simple epithelium containing club and ciliated cells, with club cells predominating (Fig. 12C), but lacking basal cells (not shown). Six h after exposure to 800 ppm chlorine for 4 min, injured epithelial cells had sloughed from large airways, and CCSP and AcTub staining was observed in sloughed epithelium within the airway lumen (Fig. 12D). Numerous surviving basal cells could be observed still attached to the inner surface of the airway (Fig. 12E). In areas where basal cells were sparse, the cells were thin and appeared to be spreading to cover the injured airway. In small airways 6 h after exposure to 800 ppm chlorine for 4 min, the sloughed epithelium containing abundant CCSP staining could be seen filling the airway lumen (Fig. 12F). Occasionally CCSP staining could also be observed in a few surviving club cells that remained attached to the airway; however, these were absent from many profiles of small airways. AcTub staining was sparse or absent from small airways at this time after chlorine exposure. Epithelial staining 24 h after exposure to 800 ppm chlorine for 4 min was generally similar to that observed at 6 h. Residual CCSP and AcTub could still be detected in epithelium that had sloughed from large airways (Fig. 12G). Basal cells generally appeared to be spread thinner than at 6 h (Fig. 12H). Sloughed epithelium in small airways still showed CCSP staining, but these cells no longer stained for DAPI, indicating that they were in the process of degrading (Fig. 12I). Effects on airway epithelium in rabbits exposed to 400 ppm chlorine for 8 min were assessed 24 h and 7 days after exposure. At 24 h, immunostaining for airway epithelial cell markers was similar to that in rabbits exposed to 800 ppm for 4 min (not shown). After 7 days of recovery following exposure to 400 ppm chlorine for 8 min, large airways again contained an intact pseudostratified epithelium lined with club and ciliated cells (Fig. 12J). However, the distribution of these cells appeared different from that in air-exposed animals in that there appeared to be more ciliated and fewer club cells in the regenerated epithelium. Basal cells appeared to have returned to their normal distribution and shape at this time (Fig. 12K). In bronchioles with obliterative lesions, airway epithelial cells lacked staining for CCSP, AcTub (Fig. 12L), or NGFR (not shown), indicating the presence of an abnormal, undifferentiated epithelium within these lesions.
Figure 12. Immunohistochemical staining for airway epithelial cell markers.
A) Large airway from air-exposed rabbit showing normal distribution of ciliated cells (green AcTub staining indicated by arrowhead) and club cells (red CCSP staining indicated by arrow). B) Large airway from air-exposed rabbit showing normal distribution of basal cells (red NGFR staining indicated by arrows). C) Small airway from air-exposed rabbit showing normal distribution of ciliated cells (green AcTub staining indicated by arrowhead) and club cells (red CCSP staining indicated by arrow). D) Large airway from rabbit 6 h after exposure to 800 ppm chlorine for 4 min. AcTub (green, arrowhead) and CCSP (red, arrow) staining is confined to epithelial cell debris that has sloughed from the airway. E) Large airway from rabbit 6 h after exposure to 800 ppm chlorine for 4 min. Surviving basal cells staining for NGFR remain attached to the airway wall and adopt a more spread morphology (arrow). F) Small airway from rabbit 6 h after exposure to 800 ppm chlorine for 4 min. Most CCSP staining is observed in injured epithelium that has sloughed from the airway (arrow). In some airways, a few residual club cells remain attached (arrowhead). AcTub staining is virtually absent from most small airways. G) Large airway from rabbit 24 h after exposure to 800 ppm chlorine for 4 min. Similar to what was observed at 6 h, AcTub (arrowhead) and CCSP (arrow) staining is confined to sloughed epithelial debris in the airway lumen and is not present in the airway proper. H) Large airway from rabbit 24 h after exposure to 800 ppm chlorine for 4 min. NGFR-stained basal cells with squamous morphology are observed in the airway. I) Small airway from rabbit 24 h after exposure to 800 ppm chlorine for 4 min. Most CCSP staining is observed in injured epithelium that has sloughed from the airway (arrow). Lack of DAPI staining suggests that this cellular debris has continued to break down between 6 and 24 h. J) Large airway from rabbit 7 days after exposure to 400 ppm chlorine for 8 min. A regenerated airway epithelium containing AcTub-stained ciliated cells (arrowhead) and CCSP-stained club cells (arrow) is present, but it appears to have a lower density of club cells compared with air-exposed mice. K) Large airway from rabbit 7 days after exposure to 400 pm chlorine for 8 min showing normal morphology of NGFR-stained basal cells (arrow). L) Small airway with bronchiolitis obliterans lesion from rabbit 7 days after exposure to 400 ppm chlorine for 8 min. The epithelial layer lining this airway (bracketed by arrows) is devoid of staining for AcTub and CCSP. Scale bar represents 100 µm for all panels. Results are representative of 7 rabbits/group for A–C, 6 rabbits/group for D–F, 9 rabbits/group for G–I, and 9 rabbits/group for J–L.
Discussion
We have developed a model that allows the study of acute and long-term effects of chlorine inhalation in rabbits. We showed that chlorine inhalation results in acute epithelial injury to the pulmonary tree, pulmonary edema, and hypoxemia, as well as more persistent effects, including airway hyperreactivity, aberrant repair in the large airways, and inflammation and bronchiolitis obliterans in the small airways. The rabbit model described here overcomes some limitations of previously developed non-rodent models. Studies utilizing rabbit models to investigate chlorine inhalation have either used one rabbit per time point per dose with the evaluation of only limited endpoints (Barrow and Smith, 1975), or used isolated rabbit lungs, which cannot be used to study the long-term effects of chlorine (Menaouar et al., 1997). In most other non-rodent models of chlorine inhalation, only acute effects could be evaluated, as animals were not removed from mechanical ventilation following chlorine exposure (Gunnarsson et al., 1998; Batchinsky et al., 2006). One study in dogs utilized whole-body exposure to chlorine so that long-term effects of acute chlorine inhalation could be examined. Assessment of histology was the main endpoint, and airway inflammatory and fibrotic lesions including bronchiolitis obliterans were the main abnormalities detected (Winternitz et al., 1920). Our results agree with this previous study in that we have detected bronchiolitis obliterans as a type of lung pathology that develops after recovery from chlorine-induced acute lung injury, but we have also expanded upon the endpoints measured to include physiological analyses.
Exposure to chlorine in this study was performed in mechanically ventilated animals, which has advantages as well as disadvantages. The main disadvantage of this procedure is that it does not represent the manner in which humans are expected to be exposed to chlorine, which would be through spontaneous breathing. However, because of the different structures of the respiratory tract and physiology of breathing, exposing rabbits via the ventilated method may better replicate some aspects of injury in humans. Rabbits are obligate nose breathers with a relatively large olfactory surface area compared to humans. Injury to the nasal passages from irritant gas exposure in obligate nose breathing animals can result in forced oral breathing. As forced oral breathing can by itself produce respiratory distress, hypoxemia, lung injury, and even death (Ramadan, 1983; Kalogjera et al., 1991), it can represent a confounding effect that would not be observed in humans. In addition, the large surface area of nasal epithelium in obligate nose breathing animals produces effective scrubbing of inhaled reactive chemicals, thereby lowering the effective dose to which the lung is exposed. Bypassing the nasal passages by intubation may result in a distribution of lung injury more representative of that in humans. This has been shown previously with other reactive chemical threat agents, such as sulfur mustard (Veress et al., 2015a) and nerve agents (Nambiar et al., 2006; Che et al., 2009). An additional advantage of this approach is the ability to deliver a defined dose of chlorine to the lungs through mechanical ventilation. Stimulation of sensory nerves by irritant gases triggers protective changes in ventilation patterns that are highly variable even within inbred mouse strains (our unpublished observations); bypassing these by mechanical ventilation has the potential to generate a more reproducible injury model.
Acute lung injury due to chlorine inhalation is characterized by key aspects such as epithelial cell death, inflammation, pulmonary edema, hypoxemia, and pulmonary function abnormalities in animal models as well in human clinical studies (Gunnarsson et al., 1998; Martin et al., 2003; Batchinsky et al., 2006; Leustik et al., 2008; Tian et al., 2008; Van Sickle et al., 2009; Mohan et al., 2010; Chang et al., 2012; Jonasson et al., 2013). In our chlorine-exposed rabbit model, we observed damaged epithelial cells in the large airways, damaged bronchioles, alveolar injury, and inflammation. Epithelial damage and inflammation have been reported in studies of chlorine exposure in several animal models, including mice (Tian et al., 2008; Tuck et al., 2008; Jonasson et al., 2013; Mo et al., 2013; O'Koren et al., 2013), rats (Leustik et al., 2008), and pigs (Gunnarsson et al., 1998). We also observed pulmonary edema 6 and 24 h after chlorine exposure. This agrees with previous studies of chlorine exposure in isolated rabbit lungs (Menaouar et al., 1997), in mechanically ventilated pigs (Gunnarsson et al., 1998), and in spontaneously breathing mice (Tian et al., 2008; Chang et al., 2012; Jonasson et al., 2013). In human clinical cases, increased pulmonary edema was also reported in patients accidentally exposed to chlorine gas. The time course of the development of pulmonary edema within 6–8 h after exposure is consistent with what is observed in animal models (Mohan et al., 2010). Hypoxemia is an important clinical sign indicative of chlorine-induced acute lung injury (White and Martin, 2010). Exposure of rabbits to 800 ppm chlorine for 4 min resulted in a PO2/FiO2 ratio in the range of 200–300, which clinically would be considered mild acute respiratory distress syndrome (ARDS) (ARDS Definition Task Force, 2012). Following the Graniteville, SC chlorine disaster, 22% of hospitalized patients who had blood gas analysis fell into this category, with 26% >300 (non-ARDS) and 53% <200 (moderate/severe ARDS) (Van Sickle et al., 2009). Thus the acute affects from exposure to this chlorine dose in rabbits were representative of a significant fraction of human chlorine exposure victims in this exposure incident. The model could be modified to produce more severe injury by increasing the chlorine dose, but this would likely require continued mechanical ventilation after exposure to support animals with respiratory failure, as has been reported previously using pig and sheep models (Gunnarsson et al., 1998; Batchinsky et al., 2006).
Chlorine exposure produced acute alterations in pulmonary mechanics that correlated with lung pathology observed histologically. Acute effects of chlorine inhalation on pulmonary mechanics measured 24 h after exposure included increased resistance, decreased compliance, and airway hyperreactivity to inhaled methacholine. Both Newtonian resistance (indicative of central airway resistance) and total resistance were increased in chlorine-exposed compared with air-exposed mice. Analysis of resistance measured using a broadband forced oscillation perturbation as a function of frequency showed increased resistance at high frequencies, again consistent with acute effects of chlorine in the central airways (Bates et al., 2011). Chlorine-exposed rabbits also exhibited exaggerated increases in resistance at lower frequencies, which has been termed frequency-dependent resistance and is indicative of changes in the peripheral lung (Smith et al., 2005). Analysis of data in this manner is useful in that it could be compared to impulse oscillometry measurements that can be collected noninvasively in humans. Overall, these observations supported the general conclusion that acute chlorine injury impaired function of both large airways and distal lung and were consistent with the histological findings of damage to large airways, small airways, and alveoli within 24 h of exposure. Pulmonary function changes, including increased resistance and decreased compliance, have been consistently observed in rodents in the acute period after chlorine exposure (Demnati et al., 1998; Martin et al., 2003; Hoyle et al., 2010; Song et al., 2011; Fanucchi et al., 2012; Jonasson et al., 2013), and decreased compliance was observed in chlorine-exposed pigs (Gunnarsson et al., 1998). In studies of humans accidentally exposed to high levels of chlorine, both obstructive and restrictive abnormalities have been identified shortly after exposure (Kaufman and Burkons, 1971; Ploysongsang et al., 1982; Das and Blanc, 1993; Balte et al., 2013). Obstructive changes in the acute period after chlorine exposure have been attributed to airway injury and bronchospasm, whereas restrictive deficits have been attributed to pulmonary edema (Ploysongsang et al., 1982). These overall conclusions from human studies are consistent with our observations of both central airways and distal lung contributing to abnormal pulmonary physiology acutely after chlorine exposure.
We previously reported that in mice epithelial repair in the large airways is carried out by basal cells after chlorine inhalation (Musah et al., 2012; Mo et al., 2013). In our current study, large airways were repopulated with a pseudostratified epithelium lined with club and ciliated cells 7 days after chlorine inhalation. Repopulation of the pseudostratified epithelium was apparently carried out by basal cells, as numerous surviving basal cells were observed still attached to the inner surface of the airway when most other cells had been sloughed off into the airway lumen following chlorine inhalation. Although the pseudostratified epithelium in the large airways seemed intact after recovery for 7 days, there were areas containing an abnormally high proportion of ciliated cells compared with club cells in the regenerated epithelium. We have also observed abnormal distributions of airway epithelial cells in our previous study in mice after chlorine inhalation (Mo et al., 2013), which suggests that this phenomenon may be a common manifestation of airway injury after irritant gas exposure. In the smaller airways, we observed an ongoing process of inflammation and injury 7 days after chlorine exposure. Bronchiolitis obliterans developed in some airways, and this was associated with a lack of differentiated epithelial cell markers indicative of aberrant repair. These findings were consistent with observations in dogs that chlorine inhalation led to progressive bronchiolitis obliterans, which led to the death of the animals between 15 and 193 days after exposure (Winternitz et al., 1920). Sulfur mustard, another pulmonary toxicant that produces airway injury, also produces long-term effects of bronchiolitis obliterans in humans (Beheshti et al., 2006; Ghanei et al., 2008; Saber et al., 2012) and rats (Veress et al., 2015b). It is unclear why the persistent abnormalities in rabbits were limited to small airways, as the entire airway tree was injured by chlorine. The inflammatory and bronchiolitis obliterans lesions were restricted to areas of the lung that normally lack pseudostratified epithelium and basal cells. In chlorine-exposed mice, injured pseudostratified epithelium is repaired by surviving basal cells, and our histology and immunostaining results are consistent with a similar mechanism operating in injured rabbit lung. Thus we postulate that repair processes in distal airways without basal cells are less efficient at restoring normal airway structure and that an abnormal epithelium triggers the downstream events, including inflammation, that lead to persistent lung pathology at these sites.
At 7 days after chlorine exposure, most pulmonary mechanics parameters, including resistance and compliance, were not different between chlorine- and air-exposed rabbits. This result was consistent with the resolution of central airway injury and pulmonary edema. Although small airway pathology was noted at this time, apparently the sporadic nature of the bronchiolitis obliterans lesions did not produce an increase in total lung resistance. However, increased hysteresis of lung pressure-volume curves was observed; this phenomenon can be associated with small airway disease when occluded airways open during the lung inflation maneuver (Sorkness and Tuffaha, 2004). In rabbits examined 7 days after exposure in the present study, chlorine exposure resulted in airway hyperreactivity, which appears to be a common consequence of irritant gas exposure. In mice and rats examined a week or more after exposure, chlorine inhalation has been shown to cause airway hyperreactivity associated with histological changes including airway remodeling and fibrosis (Demnati et al., 1998; Tuck et al., 2008; Fanucchi et al., 2012; Mo et al., 2013). In humans, exposure to pulmonary irritants can produce acute irritant-induced asthma, also known as reactive airway dysfunction syndrome (RADS) (Brooks et al., 1985; Malo et al., 2009). Methacholine hyperreactivity and airway structural changes have been commonly observed in individuals with acute irritant-induced asthma (Boulet et al., 1997; Takeda et al., 2009).
In summary, we have characterized the acute effects of chlorine on lung injury and have examined the initial repair after injury in a novel model of chlorine exposure in rabbits. The studies provide a non-rodent model in which the animals can be monitored after exposure to examine long-term consequences of acute, high-level chlorine inhalation and to test potential therapeutic interventions for chlorine-induced lung disease.
Highlights.
A novel rabbit model of chlorine-induced lung disease was developed.
Acute effects of chlorine were pulmonary edema, hypoxemia and impaired lung function.
Persistent small airway disease developed following recovery from acute injury.
Small airway disease included inflammation and bronchiolitis obliterans lesions.
The model should be useful for studying chlorine lung injury and testing treatments.
Acknowledgments
The authors thank Ashley McDonal for excellent technical assistance.
Funding Source
This work was supported by the National Institutes of Health CounterACT Program through the Office of the Director and the National Institute of Environmental Health Sciences (Award U01 ES015673 to G.W.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Balte PP, Clark KA, Mohr LC, Karmaus WJ, Van Sickle D, Svendsen ER. The Immediate Pulmonary Disease Pattern following Exposure to High Concentrations of Chlorine Gas. Pulmonary Medicine. 2013;2013:325869. doi: 10.1155/2013/325869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow RE, Smith RG. Chlorine-induced pulmonary function changes in rabbits. American Industrial Hygiene Association Journal. 1975;36:398–403. [Google Scholar]
- Batchinsky AI, Martini DK, Jordan BS, Dick EJ, Fudge J, Baird CA, Hardin DE, Cancio LC. Acute respiratory distress syndrome secondary to inhalation of chlorine gas in sheep. Journal of Trauma. 2006;60:944–956. doi: 10.1097/01.ta.0000205862.57701.48. [DOI] [PubMed] [Google Scholar]
- Bates JH, Irvin CG, Farre R, Hantos Z. Oscillation mechanics of the respiratory system. Comprehensive Pysiology. 2011;1:1233–1272. doi: 10.1002/cphy.c100058. [DOI] [PubMed] [Google Scholar]
- Beheshti J, Mark EJ, Akbaei HM, Aslani J, Ghanei M. Mustard lung secrets: long term clinicopathological study following mustard gas exposure. Pathology Research and Practice. 2006;202:739–744. doi: 10.1016/j.prp.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. American Journal of Respiratory and Critical Care Medicine. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Boulet LP, Laviolette M, Turcotte H, Cartier A, Dugas M, Malo JL, Boutet M. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest. 1997;112:45–52. doi: 10.1378/chest.112.1.45. [DOI] [PubMed] [Google Scholar]
- Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest. 1985;88:376–384. doi: 10.1378/chest.88.3.376. [DOI] [PubMed] [Google Scholar]
- Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicology and Applied Pharmacology. 2012;263:251–258. doi: 10.1016/j.taap.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che MM, Conti M, Chanda S, Boylan M, Sabnekar P, Rezk P, Amari E, Sciuto AM, Gordon RK, Doctor BP, Nambiar MP. Post-exposure treatment with nasal atropine methyl bromide protects against microinstillation inhalation exposure to sarin in guinea pigs. Toxicology and Applied Pharmacology. 2009;239:251–257. doi: 10.1016/j.taap.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Chierakul N, Rittayamai N, Passaranon P, Chamchod C, Suntiwuth B. Respiratory health effect of persons accidentally expose to high concentration of chlorine gas. Journal of the Medical Association of Thailand. 2013;96(Suppl 2):S17–S21. [PubMed] [Google Scholar]
- Clark KA, Chanda D, Balte P, Karmaus WJ, Cai B, Vena J, Lawson AB, Mohr LC, Gibson JJ, Svendsen ER. Respiratory symptoms and lung function 8–10 months after community exposure to chlorine gas: a public health intervention and cross-sectional analysis. BMC Public Health. 2013;13:945. doi: 10.1186/1471-2458-13-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Karmaus WJ, Mohr LC, Cai B, Balte P, Gibson JJ, Ownby D, Lawson AB, Vena JE, Svendsen ER. Lung Function before and after a Large Chlorine Gas Release in Graniteville, South Carolina, USA. Annals of the American Thoracic Society. 2016;13:356–363. doi: 10.1513/AnnalsATS.201508-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Blanc PD. Chlorine gas exposure and the lung: a review. Toxicology and Industrial Health. 1993;9:439–455. doi: 10.1177/074823379300900304. [DOI] [PubMed] [Google Scholar]
- Demnati R, Fraser R, Martin JG, Plaa G, Malo JL. Effects of dexamethasone on functional and pathological changes in rat bronchi caused by high acute exposure to chlorine. Toxicological Sciences. 1998;45:242–246. doi: 10.1006/toxs.1998.2532. [DOI] [PubMed] [Google Scholar]
- Donnelly SC, FitzGerald MX. Reactive airways dysfunction syndrome (RADS) due to chlorine gas exposure. Irish journal of medical science. 1990;159:275–276. doi: 10.1007/BF02993611. discussion 276–277. [DOI] [PubMed] [Google Scholar]
- Evans RB. Chlorine: state of the art. Lung. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. American Journal of Respiratory Cell and Molecular Biology. 2012;46:599–606. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautrin D, Boulet LP, Boutet M, Dugas M, Bherer L, L'Archeveque J, Laviolette M, Cote J, Malo JL. Is reactive airways dysfunction syndrome a variant of occupational asthma? Journal of Allergy and Clinical Immunology. 1994;93:12–22. doi: 10.1016/0091-6749(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Tazelaar HD, Chilosi M, Harandi AA, Peyman M, Akbari HM, Shamsaei H, Bahadori M, Aslani J, Mohammadi A. An international collaborative pathologic study of surgical lung biopsies from mustard gas-exposed patients. Respiratory Medicine. 2008;102:825–830. doi: 10.1016/j.rmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. Journal of Applied Toxicology. 1998;18:249–255. doi: 10.1002/(sici)1099-1263(199807/08)18:4<249::aid-jat507>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Chang W, Chen J, Schlueter CF, Rando RJ. Deviations from Haber's law for multiple measures of acute lung injury in chlorine-exposed mice. Toxicological Sciences. 2010;118:696–703. doi: 10.1093/toxsci/kfq264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. International Journal of Environmental Health Research. 2016;26:58–74. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson S, Koch B, Bucht A. Inhalation of chlorine causes long-standing lung inflammation and airway hyperresponsiveness in a murine model of chemical-induced lung injury. Toxicology. 2013;303:34–42. doi: 10.1016/j.tox.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Jones R, Wills B, Kang C. Chlorine gas: an evolving hazardous material threat and unconventional weapon. Western Journal of Emergency Medicine. 2010;11:151–156. [PMC free article] [PubMed] [Google Scholar]
- Kalogjera L, Pegan B, Petric V. Compensatory mechanisms induced by high oropharyngeal airway resistance in rats. Acta Otolaryngologica. 1991;111:384–388. doi: 10.3109/00016489109137406. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Burkons D. Clinical, roentgenologic, and physiologic effects of acute chlorine exposure. Archives of Environmental Health. 1971;23:29–34. doi: 10.1080/00039896.1971.10665950. [DOI] [PubMed] [Google Scholar]
- Kenner T, Leopold H, Hinghofer-Szalkay H. The continuous high-precision measurement of the density of flowing blood. Pflugers Archiv. 1977;370:25–29. doi: 10.1007/BF00707941. [DOI] [PubMed] [Google Scholar]
- Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008;295:L733–L743. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo JL, L'Archeveque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. American Journal of Respiratory and Critical Care Medicine. 2009;179:923–928. doi: 10.1164/rccm.200810-1550OC. [DOI] [PubMed] [Google Scholar]
- Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. American Journal of Respiratory and Critical Care Medicine. 2003;168:568–574. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- Menaouar A, Anglade D, Baussand P, Pelloux A, Corboz M, Lantuejoul S, Benchetrit G, Grimbert FA. Chlorine gas induced acute lung injury in isolated rabbit lung. European Respiratory Journal. 1997;10:1100–1107. doi: 10.1183/09031936.97.10051100. [DOI] [PubMed] [Google Scholar]
- Mo Y, Chen J, Schlueter CF, Hoyle GW. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. American Journal of Physiology Lung Cell and Molecular Physiology. 2013;304:L92–L102. doi: 10.1152/ajplung.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Kumar SN, Rao MH, Bollineni S, Manohar IC. Acute accidental exposure to chlorine gas: clinical presentation, pulmonary functions and outcomes. Indian Journal of Chest Diseases and Allied Sciences. 2010;52:149–152. [PubMed] [Google Scholar]
- Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respiratory Research. 2012;13:107. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar MP, Wright BS, Rezk PE, Smith KB, Gordon RK, Moran TS, Richards SM, Sciuto AM. Development of a microinstillation model of inhalation exposure to assess lung injury following exposure to toxic chemicals and nerve agents in Guinea pigs. Toxicology Mechanisms and Methods. 2006;16:295–306. doi: 10.1080/15376510600748760. [DOI] [PubMed] [Google Scholar]
- O'Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. American Journal of Respiratory Cell and Molecular Biology. 2013;49:788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploysongsang Y, Beach BC, DiLisio RE. Pulmonary function changes after acute inhalation of chlorine gas. Southern Medical Journal. 1982;75:23–26. doi: 10.1097/00007611-198201000-00007. [DOI] [PubMed] [Google Scholar]
- Ramadan MF. Experimental nasal obstruction and changes in the arterial blood gases. Clinical Otolaryngology & Allied Sciences. 1983;8:245–250. doi: 10.1111/j.1365-2273.1983.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annual Review of Cell and Developmental Biology. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences (USA) 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber H, Saburi A, Ghanei M. Clinical and paraclinical guidelines for management of sulfur mustard induced bronchiolitis obliterans; from bench to bedside. Inhalation Toxicology. 2012;24:900–906. doi: 10.3109/08958378.2012.725783. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Smith DD, Lakshminarayan S. The pulmonary sequelae associated with accidental inhalation of chlorine gas. Chest. 1990;97:820–825. doi: 10.1378/chest.97.4.820. [DOI] [PubMed] [Google Scholar]
- Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impluse oscillometry. European Respiratory Society Monographs. 2005;31:72–105. [Google Scholar]
- Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure Administration of a {beta}2-Agonist Decreases Chlorine-Induced Airway Hyperreactivity in Mice. American Journal of Respiratory Cell and Molecular Biology. 2011;45:88–94. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkness RL, Tuffaha A. Contribution of airway closure to chronic postbronchiolitis airway dysfunction in rats. Journal of Applied Physiology. 2004;96:904–910. doi: 10.1152/japplphysiol.00674.2003. [DOI] [PubMed] [Google Scholar]
- Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. American Journal of Respiratory Cell and Molecular Biology. 2007;37:186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Maghni K, Daigle S, L'Archeveque J, Castellanos L, Al-Ramli W, Malo JL, Hamid Q. Long-term pathologic consequences of acute irritant-induced asthma. Journal of Allergy and Clinical Immunology. 2009;124:975–981. doi: 10.1016/j.jaci.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhalation Toxicology. 2008;20:783–793. doi: 10.1080/08958370802007841. [DOI] [PubMed] [Google Scholar]
- Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respiratory Research. 2008;9:61. doi: 10.1186/1465-9921-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. American Journal of Emergency Medicine. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress LA, Anderson DR, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, Loader JE, Paradiso DC, Smith RW, Rancourt RC, Holmes WW, White CW. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicological Sciences. 2015a;143:178–184. doi: 10.1093/toxsci/kfu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress LA, Osborne C, Houin W, Anderson D, Hendry-Hofer TB, Loader J, Garlick R, Rioux J, Smith R, Burns C, White CW. Animal Model of Bronchiolitis Obliterans and Interstitial Fibrosis after Sulfur Mustard Inhalation. American Journal of Respiratory and Critical Care Medicine. 2015b;191:A3455. [Google Scholar]
- White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proceedings of the American Thoracic Society. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winternitz MC, Lambert RA, Jackson L, Smith GH. The Pathology of Chlorine Poisoning. In: Winternitz MC, editor. Collected Studies on the Pathology of War Gas Poisoning. New Haven: Yale University School of Medicine; 1920. pp. 1–32. [Google Scholar]
- Yildirim C, Kocoglu H, Goksu S, Cengiz B, Sari I, Bagci C. Long-term pulmonary histopathologic changes in rats following acute experimental exposure to chlorine gas. Inhalation Toxicology. 2004;16:911–915. doi: 10.1080/08958370490520749. [DOI] [PubMed] [Google Scholar]