Abstract
Physiological responses to threat occur through both the autonomic nervous system (ANS) and the hypothalamic pituitary adrenal (HPA) axis. Activity in these systems can be measured through salivary alpha-amylase (sAA) and salivary cortisol, respectively. Theoretical work and empirical studies have suggested the importance of examining the coordination of these systems in relation to cognitive functioning and behavior problems. Less is known, however, about whether these systems interactively predict more automatic aspects of attention processing such as attention toward emotionally salient threatening stimuli. We used a dot probe task to assess attention bias toward threatening stimuli in 347 kindergarten children. Cortisol and sAA were assayed from saliva samples collected prior to children’s participation in assessments on a subsequent day. Using regression analyses, we examined relations of sAA and cortisol to attention bias. Results indicate that cortisol and sAA interact in predicting attention bias. Higher levels of cortisol predicted greater bias toward threat for children who had high levels of sAA, but predicted greater bias away from threat for children who had low levels of sAA. These results suggest that greater symmetry in HPA and ANS functioning is associated with greater reliance on automatic attention processes in the face of threat.
Keywords: Salivary alpha-amylase, Cortisol, Attention bias, Early childhood
1. Introduction
Physiological responses to threat occur through two main pathways: the limbic hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system (ANS). Through a cascade of processes, the HPA axis controls the release of glucocorticoid hormones including cortisol, which has become a peripheral salivary marker of HPA activity [32]. The ANS pathway regulates the stress response through the release of catecholamines such as norepinephrine. Recently, salivary alpha-amylase (sAA) has been shown to be a reliable peripheral marker of ANS activity [15,26]. Levels of sAA increase during times of acute experiential stress [8,12,14] and are correlated with multiple indicators of ANS activity, including plasma norepinephrine [30], preejection period [7,37], and skin conductance [11]. Higher sAA might indicate higher monoamine response to stress and higher regulation, leading to lower cortisol levels.
The relation of physiological stress processes to cognitive and behavioral outcomes is known to follow an inverted U shape pattern conforming to the Yerkes–Dodson principle [38]. Very high and very low levels of stress hormones are associated with poor performance on complex cognitive tasks whereas moderate levels are associated with more optimal levels of performance. As evidenced on a neurological level, moderate levels of norepinephrine are associated with increased synaptic activity in areas of prefrontal cortex that underlie working memory; at very high levels, however, synaptic activity in prefrontal cortex is suppressed and activity in subcortical areas is increased [29]. Processing in this instance reverts to subcortical brain areas, which underlie more automatic or reactive attentional and motoric responses to stimulation [2]. This shift to subcortical brain areas is also consistent with the less discussed portion of the Yerkes–Dodson Law, which describes a linear relation between arousal and more automatic attentional and fear conditioning processes. Thus, as arousal increases, activation in areas related to processes of focused attention is also increasing, whereas activation in areas related to the modulation of attention follows an inverted-U curve.
Evolutionarily, one important automatic attention process is bias toward threatening stimuli which forces attention to be automatically focused on or held by potential threats when arousal levels, reflective of potential danger, are high [16]. In line with the inverted-U relation of arousal to optimal functioning described above, attention bias toward threat is expected to be greatest under conditions of high or low arousal and lowest under conditions of moderate arousal. Moreover, attention bias can result from either greater facilitation of attention by threatening stimuli or from slower disengagement from threatening stimuli, or from a combination of both processes. More specifically, attention facilitation has to do with the speed at which orienting toward threatening stimuli takes place. Under conditions of higher arousal, individuals would be expected to exhibit greater facilitation because they more quickly orient toward threatening stimuli as these stimuli are more easily captured by attention than are other neutral stimuli. In line with the linear relation of arousal to automatic attention processes, however, we would not expect greater attention facilitation under conditions of low arousal. Attention disengagement on the other hand, has to do with the ability to redirect attention toward an alternate location after attention has already been focused on a threatening stimulus. To the extent that attention disengagement is a somewhat volitional process requiring active manipulation of attention, we may expect to find an inverted-U relation such that both low and high levels of arousal are associated with slower disengagement. This slower disengagement would be expected because, according to the inverted-U, both low and high levels of arousal are associated with difficulty performing more complex attentional tasks.
Considering both facilitation and disengagement further helps to explain the hypothesized inverted-U relation of arousal to attention bias. For those at high levels of arousal, the hypothesis of faster facilitation and slower disengagement would lead us to predict greater attention bias toward threat compared to those with more moderate levels of arousal. For those with low levels of arousal, attention bias might not be augmented since processes of both facilitation and disengagement are expected to be slower. If, however, low levels of arousal are associated with greater slowing of disengagement than slowing of facilitation, as might be expected given that disengagement is typically a slower more effortful process, then individuals with low arousal might also exhibit greater attention biases to threat, but for different reasons than those who have high levels of arousal.
1.1. Attention bias and stress physiology
There is increasing interest in understanding the physiological processes underlying attention bias because of its robust associations with fearful emotionality as evidenced by measures of anxiety. Extensive work has demonstrated that adults and children with high levels of anxiety show biases toward threatening stimuli (see Ref. [31] for a review). A recent meta-analysis of anxiety related attentional bias, as assessed by a number of different tasks, concluded that anxious individuals robustly exhibit a threat-related bias whereas nonanxious individuals do not [4]. Similarly, attention orienting toward threat has been shown to be related to the stability of anxious behavior across childhood development [31]. For example, behavioral inhibition, or fearful temperament in childhood is a risk factor for social withdrawal in adolescence when children also exhibit greater orienting to threat [27]. Although these studies have demonstrated relations between psychological measures of fearful emotionality and attention bias toward threat, less work has examined relations of physiological markers of arousal and attention bias.
Prior research linking stress physiology to attention biases toward negative stimuli has been largely mixed, and no studies to our knowledge have examined these relations in young children. In a study of 65 healthy young men, participants administered hydrocortisone 60 min prior to an emotional interference task showed greater interference such that they made more errors when naming the colors of aversive words than when naming the colors of neutral words as compared with those who were administered a placebo [17]. Moreover, the authors found evidence that this increased attention to negative stimuli resulted from reduced inhibition of the amygdala. Along similar lines, greater pre- to post-task increases in cortisol were associated with greater attentional bias toward negative stimuli (angry faces) in as sample of 40 male university students (ages 19–26), [34] and shifting attention away from negative words was associated with lower cortisol levels during a recovery period following a stressor in a sample of 135 college student (mean age = 23.8) [10]. In contrast, however, two other studies, one in a mixed gender sample of 28 university students and the other with a sample of only male university students, offered some indication that higher baseline cortisol was related to greater bias away from negative stimuli [33,34]. Furthermore, Ellenbogen et al. [10] also found that neither baseline cortisol levels nor increases in cortisol in response to a stress-inducing task were related to selective attention for emotional words. Thus, it is difficult to draw any firm conclusions from this research linking cortisol to attention bias for negative stimuli.
1.2. A multiple systems approach
Recent work has suggested that examining coordination of the ANS and HPA axis responses to stress may provide important insight into the way these systems are related to cognitive functioning [5]. According to the additive model of Bauer et al. [5], which is actually a multiplicative model, HPA and ANS activity may interact such that having high or low levels of activity in both systems may indicate an overall hyper or hypo responsiveness to stress, respectively. Both the hyper and the hypo responsive patterns are thought to be associated with greater risk because they reflect dysregulation. Having low activity in one system paired with high activity in the other system, however, may suggest overall moderate levels of stress arousal which are associated with peak use of complex cognition and thus lower risk.
Consistent with the idea of coordinated systems, in a sample of 1292 predominantly low-income European-American and African-American children, lower levels of cortisol at 7, 15, and 24 months of age among children with concurrently higher levels of sAA have been predictive of higher executive functioning at 36 months of age and academic skills at pre-kindergarten [6]. Similarly, in a socioeconomically diverse sample of 64 8–9 year old European-American and African-American children, higher basal levels of cortisol among children with higher as compared to lower levels of sAA, were associated with higher levels of externalizing and internalizing behaviors [11]. Similarly, in a sample of maltreated and non-maltreated ethnically diverse adolescents (ages 10–14) from low to middle SES backgrounds, among individuals with low sAA reactivity in response to a stress-inducing task, lower cortisol reactivity was related to higher aggression [14]. In contrast, however, higher cortisol reactivity among those with low sAA reactivity was related to greater parent-reported adjustment problems in a sample of 7–16 year old, primarily Caucasian, children from families with average incomes of $60,000 to $80,000 [1]. Lower resting afternoon levels of cortisol paired with higher levels sAA has also been associated with greater intellectual and reading abilities than lower cortisol paired with lower sAA in a sample of socioeconomically diverse European American and African American 8–9 year old children, although this study differed in that relations were primarily observed for curvilinear and quadratic effects of cortisol and sAA [19]. Although these studies did not necessarily detect the full cross over interaction expected by the multiplicative model, the findings largely support the hypothesis that asymmetries in cortisol and sAA are associated with lower risk for behavioral and cognitive problems whereas symmetries are associated with higher risk.
Few studies have simultaneously examined ANS and HPA in relation to emotional attention biases, and none to our knowledge have examined these relations in young children. One neuroimaging study of 62 healthy young university students found that participants administered high doses of both norepinephrine and cortisol showed increased amygdala activation to faces displaying negative emotions [24]. Similarly, 18–28 year old participants with higher endogenous cortisol levels showed greater amygdala activation to viewing emotional images when noradrenaline was also presumably heightened, but not when noradenaline was presumably low due to administration of a betablocker [35]. Another study examining endogenous cortisol and sAA levels in adult female, primarily White, university students found that when sAA levels were high, higher cortisol was related to greater implicit negative bias on a masked affective priming task [22]. Notably, given an emerging pattern of consistent results across studies, further research is needed to examine relations between sAA and cortisol and, importantly, whether this pattern is present in childhood. Furthermore, research is needed to disentangle the pattern of attention deployment that may underlie the attention bias to negative stimuli. For example, attention bias can be caused either by faster orienting of attention (attention facilitation) or by slower disengagement of attention (attention disengagement) [9], and investigation of these two processes separately may provide further insight into how stress physiology is related to attention bias.
1.3. The current study
The current study examines relations of baseline cortisol and sAA to attention bias to threat using a dot probe task. We hypothesize that greater symmetries as compared to asymmetries in cortisol and sAA levels will be associated with greater attention bias toward threat because these symmetries will be indicative of levels of arousal that promote enhanced attention to threatening stimuli. Moreover, the structure of the attention task allows for investigation of attention facilitation and attention disengagement, two distinct processes that may underlie attention bias to threat. We hypothesize that having higher levels of both sAA and cortisol will be associated with greater attention facilitation. With regard to disengagement, however, we hypothesize that having low levels of both sAA and cortisol may be related to slower disengagement in that this pattern reflects low levels of arousal. It is also possible, however, that disengagement would be slower for individuals with high levels of both sAA and cortisol because disengagement likely requires some volitional modulation of attention.
2. Method
2.1. Participants
Participants were kindergarten children in 28 schools in Massachusetts who were recruited from classrooms participating in a larger curriculum intervention study. Data for the current study come from 411 children who participated in the Dot Probe task during the baseline data collection conducted in the fall of the kindergarten year. Parents provided written consent prior to the assessment and children provided verbal assent at the beginning of the assessment.
2.2. Procedures
Participants were seen individually during two regular school days. On the first day, participants were administered a battery of executive functioning tasks as well as the dot probe task. Here we use data from the dot probe task, which was designed to measure attention bias to threatening stimuli. On the second day of assessment, participants provided saliva samples at three time points during the assessment: immediately after obtaining child assent, 20 min after meeting the child, and 40 min after meeting the child. In between saliva collections, participants completed standardized assessments of reading and literacy skills, math skills, reasoning ability, and processing speed. After collection of the final saliva sample, they also participated in a delay of gratification task. Although we collected 3 cortisol samples, we decided to use the first sample for these analyses because it was the best measure of baseline resting cortisol and sAA levels. Because it was collected immediately following child assent, the first saliva sample captures children’s sAA and cortisol levels when they were going about their normal school day.
2.3. Measures
2.3.1. Dot probe
The dot probe task has been widely used to measure attention biases to emotionally valenced stimuli [13,20,23,36]. Our version of the task begins with 5 neutral control practice trials. Forty test trials are then presented in a semi-random order that is the same across all participants. A trial begins with a fixation cross presented in the center of the screen for 750 ms. Two pictures are then simultaneously presented for 750 ms on the left- and right-hand sides of a computer screen. Picture pairs included images drawn from the International Affective Picture database. For each pair of pictures, a neutral picture (ex. chair, lamp, cup) was paired with either another neutral picture or with a threatening picture (ex. snakes, wolves, car crash). Following the picture presentation, a dot either appears in the same location as the threatening image (congruent trials), in the location opposite the threatening image (incongruent trials), or on either side of the neutral/neutral display (neutral control). The dot remains on the screen for 5000 ms or until the participant responds. Respondents use the keyboard to indicate the location of the dot. The next fixation cross then appears after 2000 ms.
Trials with latencies less than 100 ms or greater than 3 standard deviations above the mean and trials with incorrect responses were excluded from analyses. Mean response latencies were calculated for each trial type if at least 60% of trials were valid. Participants were excluded from analysis if they responded correctly to less than 60% of all trials [39]. We then calculated scores of bias, facilitation, and disengagement. All aggregates were first calculated separately for responses to the left and right sides of the screen and then averaged to create the final aggregate.
Bias was calculated by subtracting the mean latency to respond to congruent trials from the mean latency to respond to incongruent trials. If participants attend to the emotion image, latencies will be shorter for congruent displays compared to incongruent displays and bias scores will be positive. Conversely, if participants avoid the emotion image, latencies will be shorter for incongruent displays compared to congruent displays and bias scores will be negative.
Facilitation was calculated by subtracting mean latency to respond to congruent trials from mean latency to respond to neutral–neutral trials. If participants are vigilant to the emotion image, latencies will be shorter for congruent displays compared to neutral–neutral displays and facilitation scores will be positive.
Disengagement scores were calculated by subtracting mean latency to respond to incongruent trials from mean latency to respond to neutral–neutral trials. If individuals have difficulty disengaging their attention from negative stimuli, response times to incongruent trials should be longer than response times to neutral–neutral trials and disengagement scores would be negative [21].
2.3.2. Salivary cortisol
Saliva samples were collected using Salimetrics Children’s Swabs, were immediately stored on ice, and were then frozen before being shipped to Johns Hopkins University for assay. Samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay US FDA 510 k cleared for use as an in vitro diagnostic measure of adrenal function. The samples were assayed in duplicate. The criterion for repeated testing was variation between duplicates greater than 20%, and the average of the duplicates was used in all analyses. Average intra-and interassay coefficients of variation were less than 10% and 15% respectively. The cortisol distributions were subject to natural log transformation to correct positive skew and values greater than 3 standard deviations above or below the mean were removed prior to analysis.
2.3.3. Salivary alpha-amylase
Samples were assayed for sAA by kinetic reaction assay. The assay employs a chromogenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2 minute period) in absorbance at 405 nm. Average intra- and inter-assay coefficients of variation were less than 10% and 15%. The sAA distributions were subject to square root transformation to correct positive skew and values greater than 3 standard deviations above or below the mean were removed prior to analysis.
2.3.4. Demographic information
Parents completed a questionnaire in which they provided information about their own level of education as well as their child’s race, birth date, and sex. Parental education was categorized on a 4-point scale: 0 = high school education or less, 1 = associate’s degree or some college; 2 = college degree; 3 = graduate degree. Race was categorized as 1 = white; 0 = other.
2.4. Missing data and analytic plan
The sample was limited to the 347 participants for whom we were able to calculate bias scores on the dot probe. Preliminary analyses examined demographic differences between those who did and did not have bias scores on the dot probe task. Participants who did not complete the task tended to be younger (t(385) = −3.14, p = .0002) and were marginally more likely to be male (t(90.40) = 1.93, p = .057). There were no differences in parental education level or child race. Thus, it seems that younger children had difficulty with the task. For analyses predicting facilitation and disengagement on the dot probe, the sample was further limited to the 344 participants who had scores for these measures.
Using regression analyses, we first investigated whether there were any main effects of cortisol or sAA on attention bias to threat and then examined the interaction of cortisol and sAA in predicting attention bias. Analyses controlled for child age, sex, race, parent education, and time of day. To determine whether the associations with attention bias stem from enhanced attention capture or impaired attention disengagement, we conducted the analyses again, this time predicting attention disengagement and attention facilitation. Interaction terms were computed as the product of mean-centered cortisol and sAA. Simple slopes of interactions were probed by recentering the sAA variable at 1 SD below or above the mean, creating a new interaction term, conducting the analysis described above again, and then evaluating the conditional main effect of cortisol [40]. Models were estimated using TYPE = COMPLEX in MPLUS 6.12 to account for clustering of students in schools. Full information maximum likelihood was used to deal with missing values for independent variables.
3. Results
3.1. Descriptive analyses
Participants came from diverse socio-economic backgrounds as indicated by variation in parental education levels. Thirty-five participants’ parents had a high school education or less, seventy-eight had some college or an associate’s degree, seventy-nine had college degrees, and ninety-four had graduate degrees. The majority of participants were White (n = 219), but the other racial backgrounds were somewhat diverse with 1 American Indian/Alaskan Native participant, 17 Hispanic participants, 10 Asian/Pacific Islander participants, 2 South Asian/Indian participants, 7 Black participants, 32 multi-racial participants, and 5 self-identified as ‘other’. Descriptive statistics for the other variables of interest can be found in Table 1. As indicated by the sample sizes for each variable, we did have some missing demographic data because parents either did not return the demographic questionnaire or chose to not respond to certain questions. In regression analyses, missing demographic information was dealt with using full information maximum likelihood as described in Section 2.4.
Table 1.
Descriptive statistics.
| N | Mean | Std. Dev. | |
|---|---|---|---|
| Attention bias (ms) | 347 | 4.77 | 138.63 |
| Attention disengagement (ms) | 344 | −48.73 | 118.42 |
| Attention facilitation (ms) | 344 | −43.82 | 130.80 |
| (ln) Cortisol time 1 (μg/dL) | 295 | −2.26 | 0.50 |
| (ln) Cortisol time 2 (μg/dL) | 292 | −2.34 | 0.50 |
| (ln) Cortisol time 3 (μg/dL) | 291 | −2.39 | 0.52 |
| (sqrt) sAA time 1 (U/mL) | 298 | 7.82 | 3.03 |
| (sqrt) sAA time 2 (U/mL) | 294 | 8.45 | 3.16 |
| (sqrt) sAA time 3 (U/mL) | 292 | 8.07 | 2.88 |
| Time of day | 312 | 10.11 | 1.67 |
| Sex (male = 1) | 347 | 47% | – |
| Age (months) | 327 | 68.52 | 4.18 |
| Race (White = 1) | 293 | 75% | – |
| Parent education | 286 | 1.81 | 1.03 |
3.2. Regression analyses
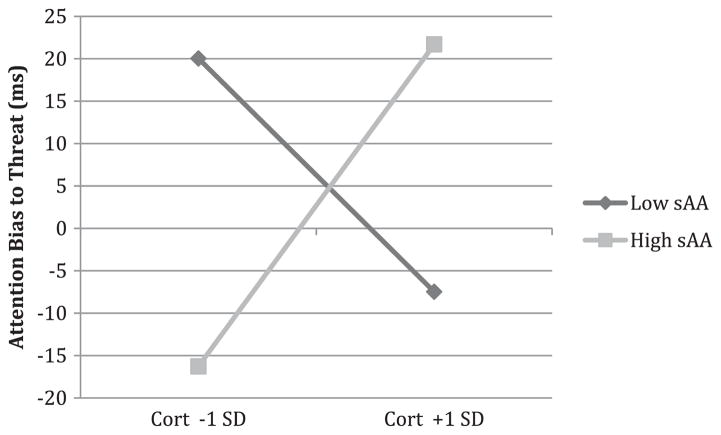
Results indicated that there were no significant main effect associations of cortisol or sAA with attention bias to threat. The interaction of cortisol and sAA, however, was a significant predictor (B = 10.80, S.E. = 4.50, p = .02) of attention bias to threat (Table 2). As shown in Fig. 1, for children with lower sAA, higher cortisol was associated with less attention bias toward threat (simple slope for sAA levels 1 SD below the mean: B = −27.44, S.E. = 16.24, p = .09). Conversely, for children with higher sAA, higher cortisol was associated with greater bias toward threat (simple slope for sAA levels 1 SD above the mean: (B = 37.96, S.E. = 20.97, p = .07). Parental education also predicted bias such that higher parental education was related to less bias toward threat (B = −17.37, S.E. = 9.02, p = .05). Time of day, child sex, child age, and child race were unrelated to attention bias to threat.
Table 2.
Regression analyses.
| Attention bias
|
Attention facilitation
|
|||||
|---|---|---|---|---|---|---|
| Estimate | S.E. | p-Value | Estimate | S.E. | p-Value | |
| Cortisol | 5.23 | 12.90 | 0.69 | 14.30 | 14.57 | 0.33 |
| sAA | −0.59 | 3.13 | 0.85 | 1.02 | 2.64 | 0.70 |
| Cortisol * sAA | 10.80 | 4.50 | 0.02 | 10.60 | 5.28 | 0.05 |
| Time of day | 5.01 | 6.34 | 0.43 | 4.38 | 6.79 | 0.52 |
| Sex | −0.99 | 14.11 | 0.94 | 16.24 | 13.16 | 0.22 |
| Parent education | −17.37 | 9.02 | 0.05 | −14.20 | 7.79 | 0.07 |
| Age | −0.30 | 1.56 | 0.85 | −0.69 | 1.47 | 0.64 |
| Race | −0.19 | 24.56 | 0.99 | 13.31 | 21.39 | 0.53 |
| Intercept | 4.49 | 8.16 | 0.58 | −43.80 | 5.94 | < .001 |
Fig. 1.
Fig. 1 depicts the relation of cortisol to attention bias to threat for children who have low sAA levels (1 SD below the mean) and for children who have high sAA levels (1 SD above the mean). All other predictors are mean centered.
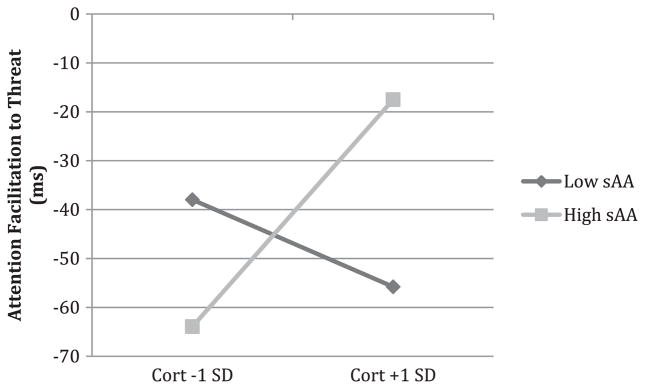
We then examined whether the relations with bias might be a result of slower disengagement from threat or faster orientation to threat. Neither the main effects nor the interaction of cortisol and sAA were associated with disengagement of attention from threatening stimuli. There were also no main effects of cortisol or sAA on facilitation of attention. Cortisol and sAA did interact, however, to predict attention facilitation in a way that was largely consistent with findings for attention bias (B = 10.60, S.E. = 5.28, p = .05). As shown in Fig. 2, for children with higher levels of sAA, higher cortisol was associated with greater facilitation to threat (simple slope for sAA levels 1 SD above the mean: B = 46.40, S.E. = 21.17, p = .03). Conversely, for children with lower sAA, higher cortisol was descriptively associated with less facilitation to threat, but the simple slope of cortisol for children with low sAA (1 SD below the mean) was not significant. Parental education also marginally predicted attention facilitation (B = −14.20, S.E. = 7.79, p = .07) such that children whose parents had higher education showed less attention facilitation to threat. No other covariates were significantly related to attention facilitation.
Fig. 2.
Fig. 2 depicts the relation of cortisol to attention facilitation to threat for children who have low sAA levels (1 SD below the mean) and for children who have high sAA levels (1 SD above the mean). All other predictors are mean centered.
4. Discussion
Our main results indicated that cortisol and sAA interacted to predict attention bias to threat. For children with higher levels of sAA, higher cortisol was associated with greater attention bias to threat. For children with lower levels of sAA, however, lower cortisol was associated with greater attention bias to threat. These results add to a growing literature demonstrating that cortisol and sAA interact to predict a variety of outcomes including executive functioning and academic skills [6] as well as internalizing and externalizing behaviors [11]. Broadly, this literature demonstrates that using information about both cortisol and sAA to predict outcomes is better than using information about only one of them. Generally, having high levels of either cortisol or sAA in combination with low levels of the other has been described as reflecting an asymmetrical physiological profile and is thought to reflect moderate levels of arousal. High levels of both cortisol and sAA or low levels of both cortisol and sAA, however, are described as symmetrical profiles which likely reflect hyper- and hypo-arousal, respectively. As such, our results are consistent with the findings for other developmental outcomes in that greater asymmetry has been generally associated with greater use of cognitive and behavioral self-regulation, whereas greater symmetry has been generally associated with more reactive behaviors that are often associated with developmental risk. Moreover these results are consistent with one previous study which specifically found that symmetries in cortisol and sAA are associated with greater attention bias to threat [22]. The current study extends this past work by demonstrating that the same pattern of results holds for a sample of young children that includes both boys and girls. Interestingly, our results appear to demonstrate the full cross over interaction whereas the previous study did not find any relation of cortisol to attention bias for those with low sAA. It is important to note, however, that the attention bias simple slopes for both low and high levels of sAA were only marginally significant, and thus the characterization of the interaction as a full cross-over should be interpreted with caution.
The full crossover interaction would be highly consistent with the multiplicative model [5] of coordination between the HPA and ANS systems. In the multiplicative model, symmetrically high or low activation in both systems is thought to be a marker of physiological dysregulation and thus a marker for risk whereas asymmetric combinations of high activity in one system paired with low activity in the other is thought to be indicative of well-regulated stress physiology and thus be associated with lower risk. This pattern of results is also consistent with what is known about the time course of activation in the HPA and sympathetic adrenal systems, including opposite patterns of diurnal change in which cortisol decreases and sAA increases throughout the day [25], and patterns of response to stress in which increases and decreases in sAA precede those in cortisol, leading to higher cortisol but lower sAA 10+ minutes post-stressor [12,14,28]. Furthermore, temporally linked patterns of activation in the sympathetic adrenal and HPA systems are central to understanding effects of stress on learning [18].
The results indicating that higher levels of cortisol are associated with higher attention bias when sAA is also high are similarly consistent with the inverted-U model. This model describes high levels of arousal as being associated with less effortful processing and greater automatic, or reactive, processing of stimuli such as threatening pictures, which are evolutionarily salient. More specifically, the results for attention facilitation demonstrate that the higher bias may result from enhanced automatic attention capture by threatening stimuli when levels of sAA and cortisol are both high, rather than from impaired disengagement from threatening stimuli. This enhanced attention facilitation is consistent with the inverted U model and with the linear relation between arousal and automatic attention.
The results demonstrating that symmetrically low levels of sAA and cortisol were associated with higher attention bias, however, are slightly more complicated to explain. As symmetrically low-levels indicate hypo-activation of these threat response systems, it is unclear what attentional processes might lead to an overall bias. One possibility is that this hypo-responsiveness is a result of high allostatic load resulting from high levels of psychosocial stress. In this scenario, it is possible that greater attention bias to threat may have been tuned while arousal levels were high and may have then remained as a more permanent psychological phenomenon even after children developed a physiological profile of hypo-responsivity. Some research with high-risk children has suggested that hypocortisolism could be a marker of allostatic load in prepubertal children [3]. Moreover, in a diverse community-based sample, family adversity was found to be curvilinearly to kindergarten children’s cortisol levels such that both high and low levels of family adversity were associated with lower cortisol levels [41]. Thus, it is possible that a profile of hypo-responsivity could be emerging even in early childhood as a result of allostatic load.
An alternative possibility is that because the profile of low sAA and low cortisol reflects lower arousal, these children would be generally slower to process and respond to the threatening stimuli. Consistent with our hypothesis that children with a profile of low cortisol and low sAA would not be expected to show faster deployment of attention, we found no relation of cortisol to attention facilitation for children with low sAA. Rather, we hypothesized that these children may be slower to disengage their attention either because their automatic attention processes are slower, making them less alert to the appearance of the dot on the screen or because their low levels of arousal make it more difficult to volitionally shift attention to the location of the dot. Unexpectedly, however, we did not find any evidence for an interaction of sAA and cortisol in relation to attention disengagement. Given the preliminary nature and somewhat inconclusive nature of these results, future work is needed to replicate and further examine the relation of this pattern of low cortisol and low sAA levels to attention bias.
Furthermore, an extensive literature demonstrates that cognitive bias toward threat is associated with mental health issues such as anxiety (see Bar-Haim et al. [4] for a review). Based on that literature, our results may suggest that having physiological profiles of high cortisol and high sAA or low cortisol and low sAA may be indicative of risk for developing mental health problems. Importantly, given that we find that the coordination of these threat response systems is already related to attention bias in early childhood, understanding the long-term developmental outcomes associated with these patterns of stress physiology will be important.
4.1. Limitations and conclusion
The correlational design of this study does not allow us to determine whether differences in the levels of cortisol and sAA caused differences in attention bias to threat or whether differences in attention bias to threat instead caused differences in the levels of these stress hormones. Experimental manipulation of these stress hormones may help to clarify the directionality of these processes. Similarly, this study does not explain the physiological mechanisms by which these two systems are related to each other or by which they are interactively related to developmental outcomes, and much future work is needed in this area. Moreover, because saliva samples were collected during the regular school day and as such, we did not know the date or time of each assessment prior to its occurrence, it was not possible to control for factors such as medications or foods the child may have consumed prior to collection of the sample. As the collection of saliva samples outside of laboratory or home settings continues to become more widespread, investigating ways to control for potential confounds such as food or medication in these settings will be a productive avenue for future research. Similarly, because the collection of saliva samples took place in schools, it was not practical to collect repeated samples over a period of several days. Although multiple samples can help in attaining more precise measurements of baseline cortisol and sAA, past studies have successfully used a single cortisol sample [6]. Finally, given the limited number of studies examining relations of cortisol and sAA to developmental outcomes, future work should also be done to replicate these findings.
Despite these limitations, this study extends prior research to demonstrate that the interaction of sAA and cortisol is related to attention bias to threat in children. In doing so, it advances a growing body of literature indicating that taking a multiple systems approach to stress physiology is essential for understanding the relation of stress physiology to important developmental outcomes.
HIGHLIGHTS.
We assess children’s attention bias to threat and markers of stress physiology.
Salivary alpha-amylase (sAA) and cortisol interacted to predict attention bias.
Higher cortisol predicted greater bias to threat when sAA was high.
Higher cortisol predicted greater bias away from threat when sAA was low.
Acknowledgments
The research reported in this publication was supported by the Institute of Education Sciences Grant R305A1000058. The first author’s role in this research was also supported by the Institute of Education Sciences, U.S. Department of Education, through Grant R305B080019 to New York University. The opinions expressed are those of the authors and do not represent views of the Institute or the U.S. Department of Education.
References
- 1.Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biol Psychol. 2011;88(1):57–64. doi: 10.1016/j.biopsycho.2011.06.008. http://dx.doi.org/10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnsten AFT. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7(1–2):133–46. doi: 10.1155/NP.2000.133. http://dx.doi.org/10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23(3):881–96. doi: 10.1017/S095457941100037X. http://dx.doi.org/10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. http://dx.doi.org/10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. J Dev Behav Pediatr. 2002;23(2):102–13. doi: 10.1097/00004703-200204000-00007. http://dx.doi.org/10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Berry D, Blair C, Willoughby M, Granger DA the FLP Key Investigators. Salivary alpha-amylase and cortisol in infancy and toddlerhood: direct and indirect relations with executive functioning and academic ability in childhood. Psychoneuroendocrinology. 2012;37(10):1700–11. doi: 10.1016/j.psyneuen.2012.03.002. http://dx.doi.org/10.1016/j.psyneuen.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Bosch JA, de Geus EJC, Veerman ECI, Hoogstraten J, Nieuw Amerongen AV. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med. 2003;65(2):245–58. doi: 10.1097/01.psy.0000058376.50240.2d. http://dx.doi.org/10.1097/01.PSY.0000058376.50240.2D. [DOI] [PubMed] [Google Scholar]
- 8.Chatterton RT, Vogelsong KM, Lu Y, Ellman AB, Hudgens GA. Salivary a-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16(4):433–48. doi: 10.1111/j.1475-097x.1996.tb00731.x. http://dx.doi.org/10.1111/j.1475-097X.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 9.Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: a critical review. Cognit Therapy Res. 2009;33(2):221–34. doi: 10.1007/s10608-007-9161-y. http://dx.doi.org/10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellenbogen MA, Schwartzman AE, Stewart J, Walker C. Stress and selective attention: the interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39(6):723–32. doi: 10.1111/1469-8986.3960723. http://dx.doi.org/10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- 11.El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: the moderating role of sympathetic nervous system activity. J Abnorm Child Psychol. 2008;36(4):601–11. doi: 10.1007/s10802-007-9204-6. http://dx.doi.org/10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 12.Engert V, Vogel S, Efanov SI, Duchesne A, Corbo V, Ali N, et al. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology. 2011;36(9):1294–302. doi: 10.1016/j.psyneuen.2011.02.018. http://dx.doi.org/10.1016/j.psyneuen.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. http://dx.doi.org/10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 14.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–87. doi: 10.1016/j.psyneuen.2006.05.010. http://dx.doi.org/10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-Amylase in biobehavioral research. Ann N Y Acad Sci. 2007;1098:122–44. doi: 10.1196/annals.1384.008. http://dx.doi.org/10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- 16.Hanoch Y, Vitouch O. When less is more: information, emotional arousal and the ecological reframing of the Yerkes–Dodson law. Theor Psychol. 2004;14(4):427–52. http://dx.doi.org/10.1177/0959354304044918. [Google Scholar]
- 17.Henckens MJ, van Wingen GA, Joëls M, Fernández G. Time-dependent effects of cortisol on selective attention and emotional interference: a functional MRI study. Front Integr Neurosci. 2012;6(66) doi: 10.3389/fnint.2012.00066. http://dx.doi.org/10.3389/fnint.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10(4):152–8. doi: 10.1016/j.tics.2006.02.002. http://dx.doi.org/10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Keller PS, El-Sheikh M, Granger DA, Buckhalt JA. Interactions between salivary cortisol and alpha-amylase as predictors of children’s cognitive functioning and academic performance. Physiol Behav. 2012;105(4):987–95. doi: 10.1016/j.physbeh.2011.11.005. http://dx.doi.org/10.1016/j.physbeh.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression, and the processing of emotional stimuli in non-referred girls and boys. Behav Sci Law. 2006;24(1):21–37. doi: 10.1002/bsl.668. http://dx.doi.org/10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- 21.Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. 2004;42(10):1183–92. doi: 10.1016/j.brat.2003.08.001. http://dx.doi.org/10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Kreher DA, Powers SI, Granger DA. The relationship between cortisol, salivary alpha-amylase, and cognitive bias in young women. Behav Neurosci. 2012;126(1):157–66. doi: 10.1037/a0026654. http://dx.doi.org/10.1037/a0026654. [DOI] [PubMed] [Google Scholar]
- 23.Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, et al. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. J Abnorm Child Psychol. 2011;39(1):125–35. doi: 10.1007/s10802-010-9438-6. http://dx.doi.org/10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kukolja J, Schläpfer TE, Keysers C, Klingmüller D, Maier W, Fink GR, et al. Modeling a negative response bias in the human amygdala by noradrenergic–glucocorticoid interactions. J Neurosci. 2008;28(48):12868–76. doi: 10.1523/JNEUROSCI.3592-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32(4):392–401. doi: 10.1016/j.psyneuen.2007.02.007. http://dx.doi.org/10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Nater U, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34(4):486–96. doi: 10.1016/j.psyneuen.2009.01.014. http://dx.doi.org/10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349–57. doi: 10.1037/a0018486. http://dx.doi.org/10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quesada AA, Wiemers US, Schoofs D, Wolf OT. Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology. 2012;37(1):125–36. doi: 10.1016/j.psyneuen.2011.05.013. http://dx.doi.org/10.1016/j.psyneuen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113(3):523–36. doi: 10.1016/j.pharmthera.2006.11.006. http://dx.doi.org/10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: an indicator of sympathetic activity? Ann N Y Acad Sci. 2004;1032:258–63. doi: 10.1196/annals.1314.033. http://dx.doi.org/10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- 31.Shechner T, Britton JC, Perez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, et al. Attention biases, anxiety, and development: toward or away from threats or rewards? Depress Anxiety. 2012;29(4):282–94. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69(6):1503–13. http://dx.doi.org/10.1111/j.1467-8624.1998.tb06173.x. [PubMed] [Google Scholar]
- 33.van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, et al. Baseline salivary cortisol levels and preconscious selective attention for threat: a pilot study. Psychoneuroendocrinology. 1998;23(7):741–7. doi: 10.1016/s0306-4530(98)00047-x. http://dx.doi.org/10.1016/S0306-4530(98)00047-X. [DOI] [PubMed] [Google Scholar]
- 34.van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, et al. Conscious and preconscious selective attention to social threat: different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25(6):577–91. doi: 10.1016/s0306-4530(00)00011-1. http://dx.doi.org/10.1016/S0306-4530(00)00011-1. [DOI] [PubMed] [Google Scholar]
- 35.van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SARB. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87(1):57–66. doi: 10.1016/j.nlm.2006.05.008. http://dx.doi.org/10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Waters AM, Nitz AB, Craske MG, Johnson C. The effects of anxiety upon attention allocation to affective stimuli. Behav Res Ther. 2007;45(4):763–74. doi: 10.1016/j.brat.2006.07.002. http://dx.doi.org/10.1016/j.brat.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 37.West SG, Granger DA, Kivlighan KT, Psota TL, Hurston KL. Salivary alpha-amylase response to the cold pressor is correlated with cardiac markers of sympathetic activation. Presented at the Annual Meeting of the American Psychosomatic Society; Denver, CO. 2006. [Google Scholar]
- 38.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18(5):459–82. http://dx.doi.org/10.1002/cne.920180503. [Google Scholar]
- 39.Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. J Abnorm Child Psychol. 2011;39(1):125–35. doi: 10.1007/s10802-010-9438-6. http://dx.doi.org/10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 41.Bush N, Obradović J, Adler N, Boyce WT. Developmental markers of allostatic load: Examining the evidence in a longitudinal sample of kindergarten children. Dev Psychopathol. 2011;23:1089–106. doi: 10.1017/S0954579411000514. [DOI] [PubMed] [Google Scholar]