Abstract
Quinones represent a class of toxicological intermediates, which can create a variety of hazardous effects in vivo including, acute cytotoxicity, immunotoxicity, and carcinogenesis. In contrast, quinones can induce cytoprotection through the induction of detoxification enzymes, anti-inflammatory activities, and modification of redox status. The mechanisms by which quinones cause these effects can be quite complex. The various biological targets of quinones depend on their rate and site of formation and their reactivity. Quinones are formed through a variety of mechanisms from simple oxidation of catechols/hydroquinones catalyzed by a variety of oxidative enzymes and metal ions to more complex mechanisms involving initial P450-catalyzed hydroxylation reactions followed by two-electron oxidation. Quinones are Michael acceptors, and modification of cellular processes could occur through alkylation of crucial cellular proteins and/or DNA. Alternatively, quinones are highly redox active molecules which can redox cycle with their semiquinone radical anions leading to the formation of reactive oxygen species (ROS) including superoxide, hydrogen peroxide, and ultimately the hydroxyl radical. Production of ROS can alter redox balance within cells through the formation of oxidized cellular macromolecules including lipids, proteins, and DNA. This perspective explores the varied biological targets of quinones including GSH, NADPH, protein sulfhydryls [heat shock proteins, P450s, cyclooxygenase-2 (COX-2), glutathione S-transferase (GST), NAD(P)H:quinone oxidoreductase 1, (NQO1), kelch-like ECH-associated protein 1 (Keap1), IκB kinase (IKK), and arylhydrocarbon receptor (AhR)], and DNA. The evidence strongly suggests that the numerous mechanisms of quinone modulations (i.e., alkylation versus oxidative stress) can be correlated with the known pathology/cytoprotection of the parent compound(s) that is best described by an inverse U-shaped dose–response curve.
1. Introduction
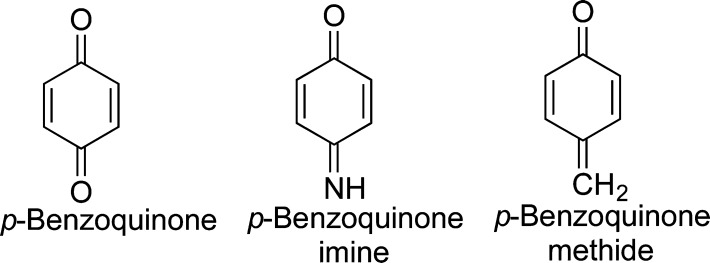
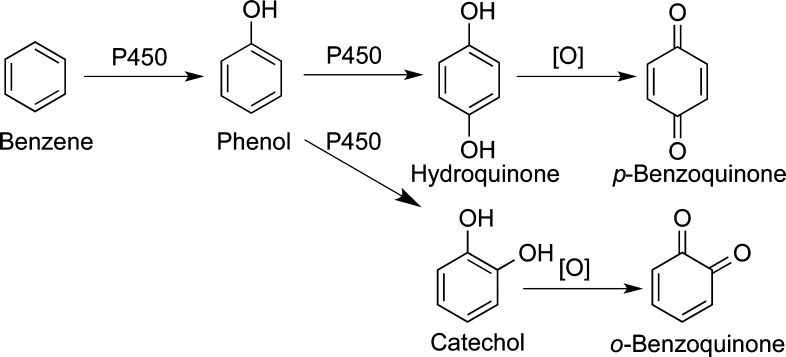
Quinones are a subset of the quinoid family which also contains the quinone imines and the quinone methides (Figure 1).1−3 Quinones, which contain the cyclohexadienedione structure, are the most numerous of the quinoids and are commonly found in several natural products, endogenous biochemicals, drugs, and environmental chemicals and/or are generated through the metabolism of aromatic compounds (Figure 2). In general, the biological properties of quinone precursors (good, bad, or equivocal) are often mediated by their oxidative metabolism to quinones. However, the importance of quinone formation to the off-target effects of drugs, natural products, environmental chemicals, and xenobiotic/endogenous compounds in general is often underestimated. For example, two recent reviews on biological reactive intermediates give varying estimates on the importance of quinone formation. For carcinogenic processes, quinone formation was estimated at 7% of all mutagenic reactive intermediates,4 whereas for all toxic reactions (7% of total metabolism), quinone formation represents closer to 41%.5 Both numbers are likely low since quinones are reactive intermediates and usually cannot be observed directly. In biological systems, as quickly as quinones are formed they react with cellular nucleophiles (GSH, protein sulfhydryls) and/or they redox cycle forming ROS (Figure 3). This perspective will focus on our limited knowledge of the varied biological targets of quinones, resulting in numerous biological effects, which could be beneficial, toxic, equivocal, and/or unknown. Quinone methides and quinone imines have been reviewed recently and are beyond the scope of this perspective.1,2,6−9
Figure 1.
Examples of simple quinoids.
Figure 2.
Quinone formation from aromatic compounds represents a common bioactivation scheme.
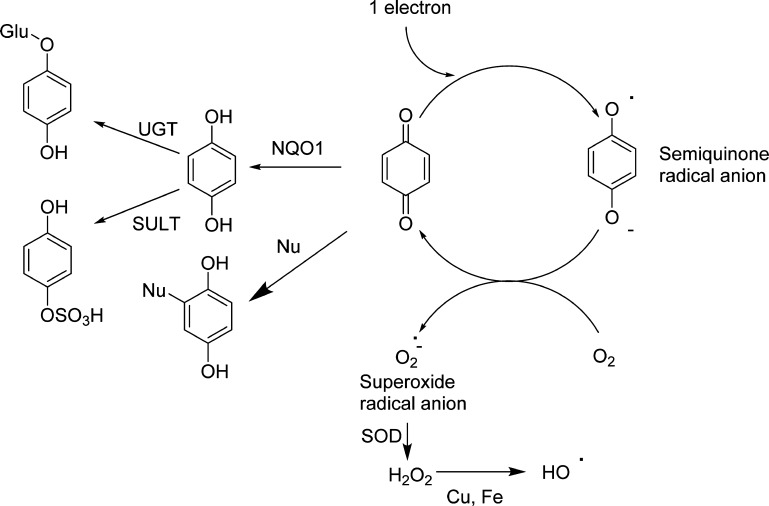
Figure 3.
Mechanisms of quinone toxicity.
2. Mechanism of Quinone Formation
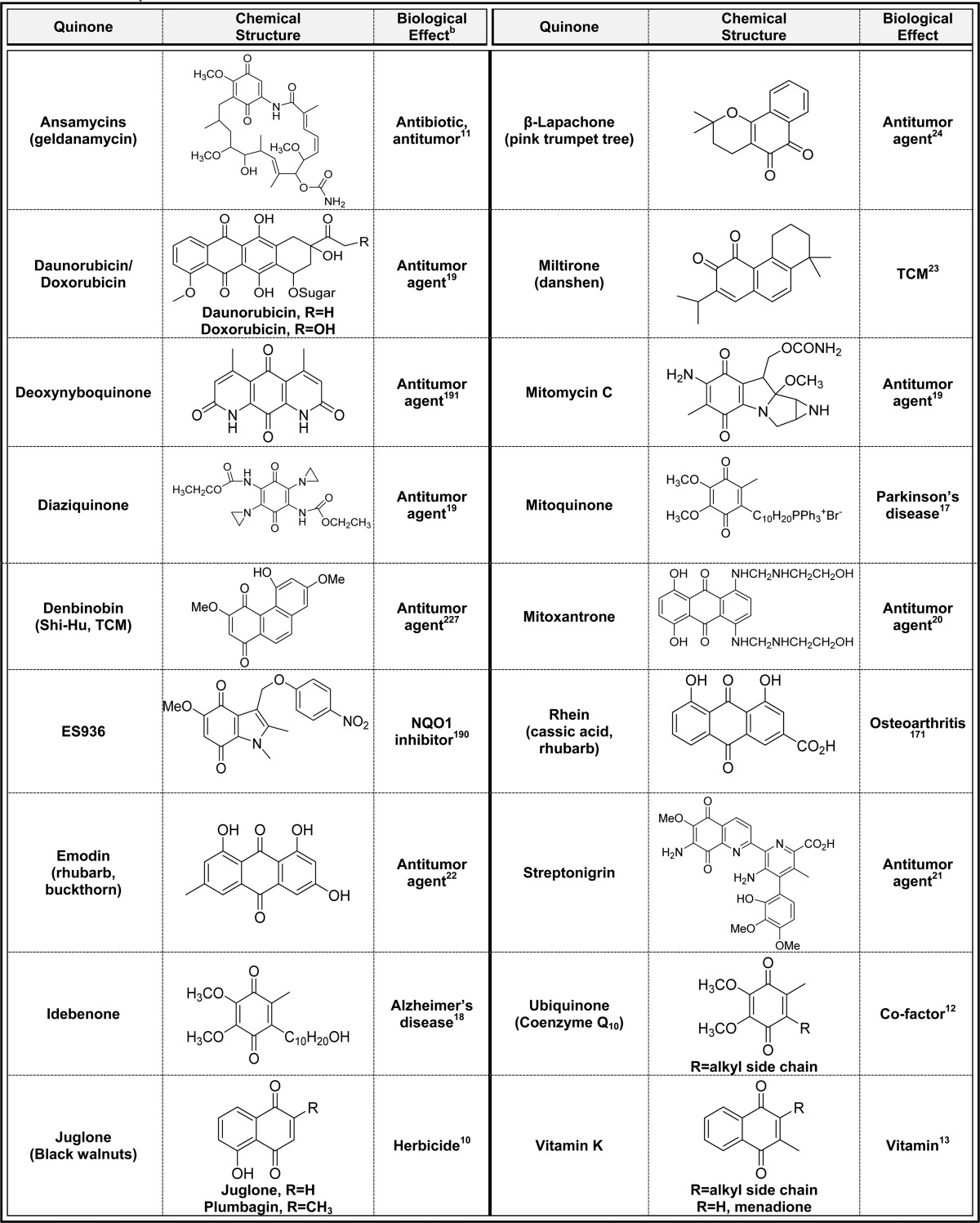
2.1. Stable Quinones (Table 1)
Table 1. Examples of Stable Quinonesa.
Alphabetical list (16 total, partial list).
Only one representative reference is listed for each example.
For the purpose of this perspective, quinones that exist in nature as the quinone form were defined as stable. In general, p-quinones are considerably more stable compared to o-quinones due to the strained 1,2-diketone functionality of the latter. Some p-quinones can be crystallized (t-butylquinone, menadione), and there are examples of natural products with stable p-quinone structures (e.g., juglone, ansamycin antibiotics) as well as endogenous compounds (vitamin K, ubiquinone) (Table 1).10−14 Ubiquinone is an antioxidant in the mitochondria, and its analogues could be useful for the treatment of both Alzheimer’s and Parkinson’s disease since mitochondrial failure, often due to ROS, is involved in the progression of both neurodegenerative diseases.15−17 Mitoquinone is a ubiquinone analogue in clinical trials for the treatment of Parkinson’s disease.15−17 This novel quinone drug is designed to target the mitochondria through its triphenylphosphine cationic moiety that facilitates drug accumulation because of the negative mitochondrial membrane potential. Idebenone is another ubiquinone analogue which may have promise in the treatment of Alzheimer’s disease (Table 1).18 Several potent chemotherapeutic agents [daunorubicin, diaziquone, doxorubicin (adriamycin), emodin, mitomycin C, mitoxantrone, and streptonigrin; Table 1] have highly substituted stable p-quinone functionalities, which likely contribute to their ability to kill cancer cells through redox mechanisms (discussed below) as well as cause reactive oxygen species (ROS)-induced toxicity to normal cells.19−22 The naphthalene o-quinones miltirone isolated from danshen and β-lapachone from the pink trumpet tree are examples of stable o-quinones (Table 1).23,24
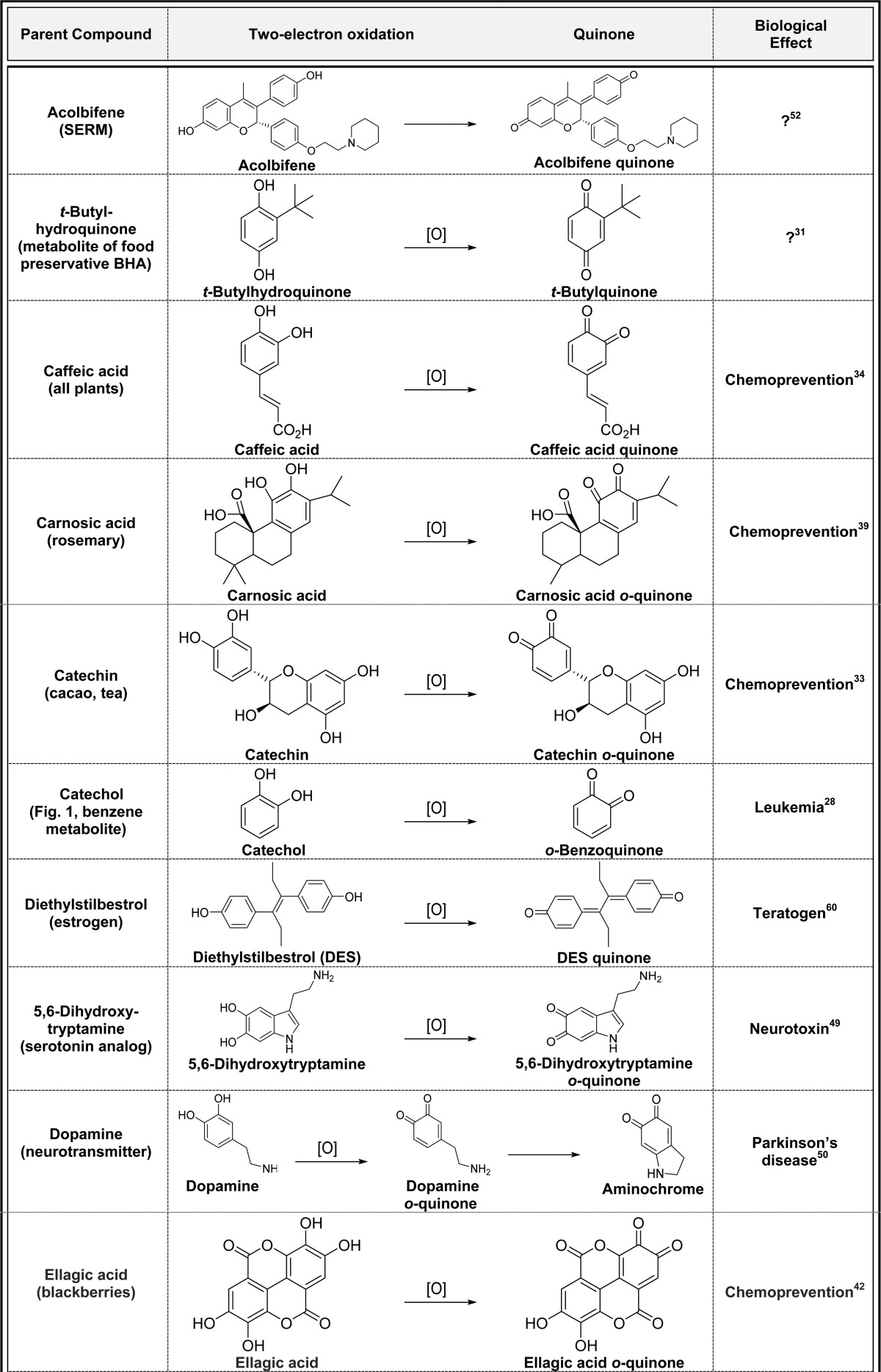
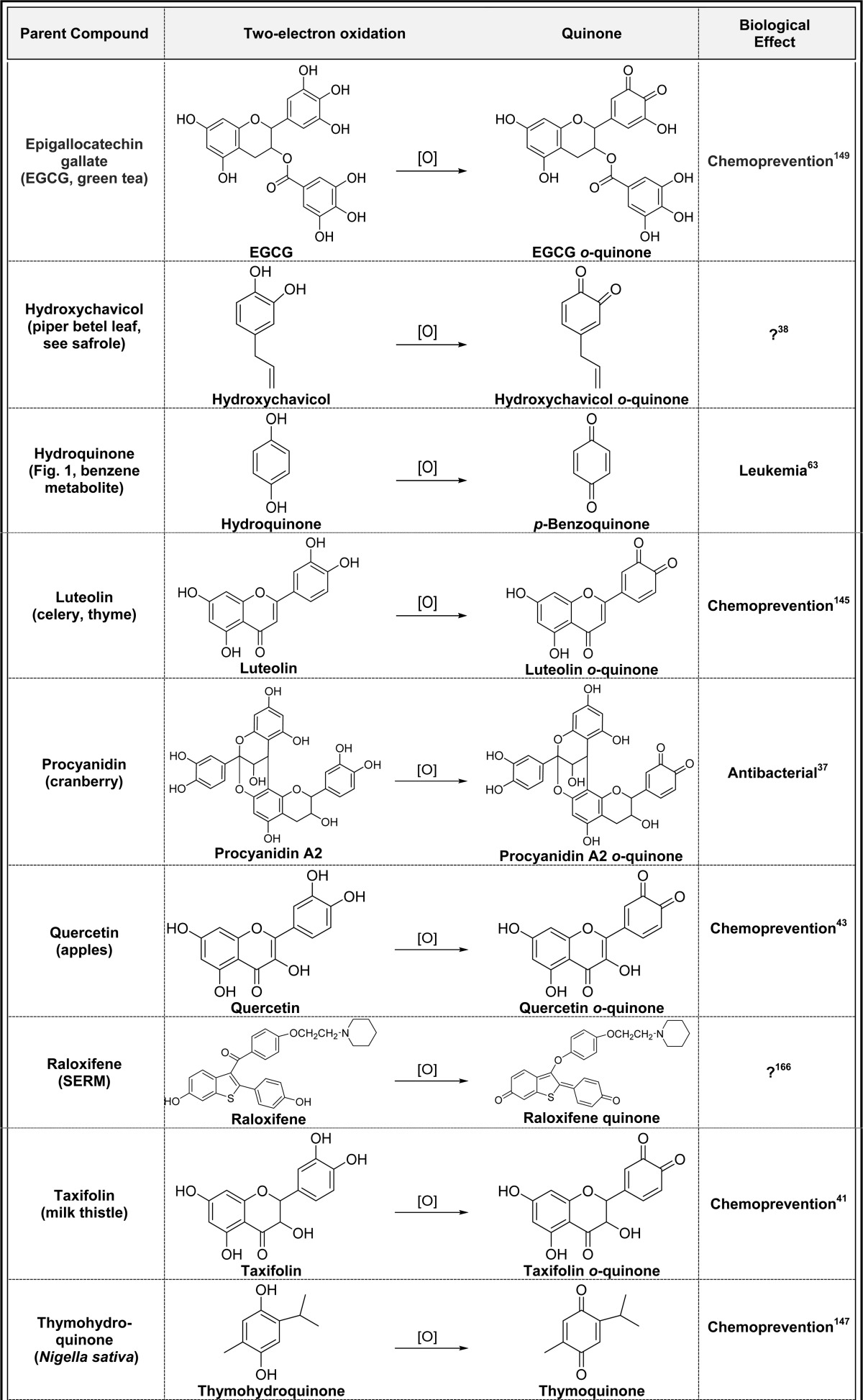
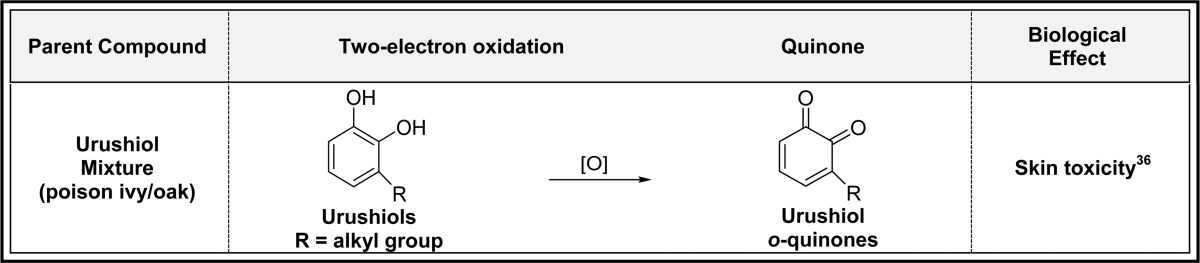
2.2. Two-Electron Oxidation of Hydroquinones/Catechols (Table 2)
Table 2. Two-Electron Oxidation of Hydroquinones/Catechols to Quinonesa.
Alphabetical list (20 total, partial list). [O] refers to any oxidative enzyme or metal ions and in some cases molecular oxygen.
Only one representative reference is listed for each example.
Hydroquinones and catechols are very easily converted to quinones by oxidation catalyzed by virtually any oxidative enzyme including P450s, COX-2, peroxidase, tyrosinase, xanthine oxidase and monoamine oxidase, and metal ions and in some cases molecular oxygen (Table 2).3,25−28p-Quinones are formed from oxidation of t-butylhydroquinone, hydroquinone, naphthohydroquinone, and thymohydroquinone.25,29−32 Oxidation of catechols in natural products [caffeic acid, carnosic acid, catechin, ellagic acid, epigallocatechin (ECGC), hydroxychavicol, luteolin, procyanidin, quercetin, taxifolin, and urushiols] gives o-quinones with a variety of biological effects (Table 2).33−43 Most of these o-quinones are short-lived, and their contribution to the overall biological profiles of the parent compounds is not clear.44,45 The endogenous catechol amines (5,6-dihydroxytryptamine and dopamine) are also oxidized to o-quinones which may play a role in the etiology of Parkinson’s disease.3,46−50 Dopamine oxidation to dopamine o-quinone as well as other quinones is believed to contribute to neurodegeneration through induction of mitochondria and protein dysfunction and oxidative stress (Table 2).51 The selective estrogen receptor modulators (SERMs) acolbifene and raloxifene form extended quinones in in vitro experiments, although both have very short lifetimes and may not have any beneficial/toxic effects (Table 2).52−57 Extended quinone formation from the estrogen diethylstilbestrol (DES) may contribute to the teratogenic effects of DES in women exposed to this drug to prevent miscarriages and premature births in utero up until its withdrawal in 1971 (Table 2).58−60
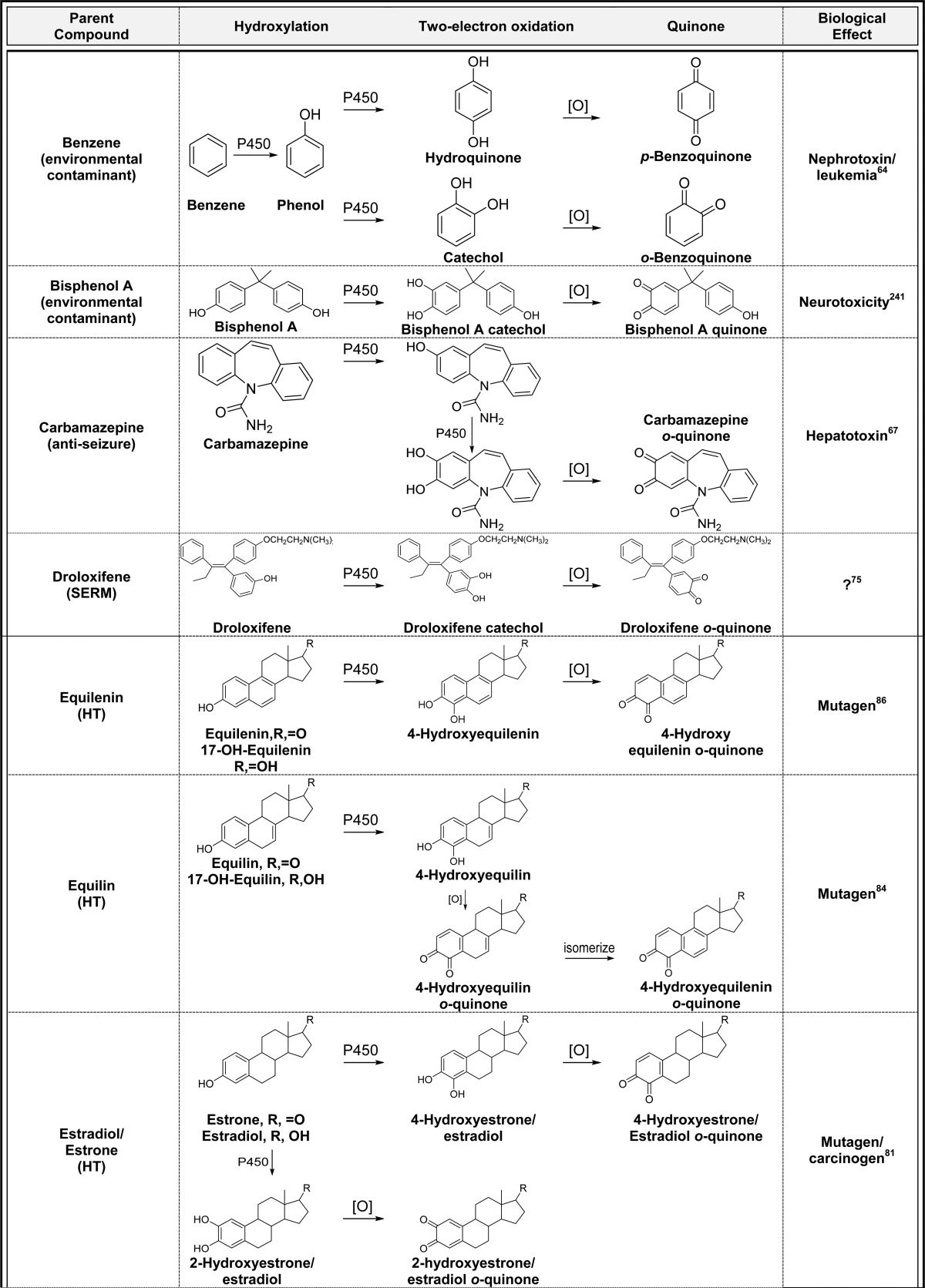
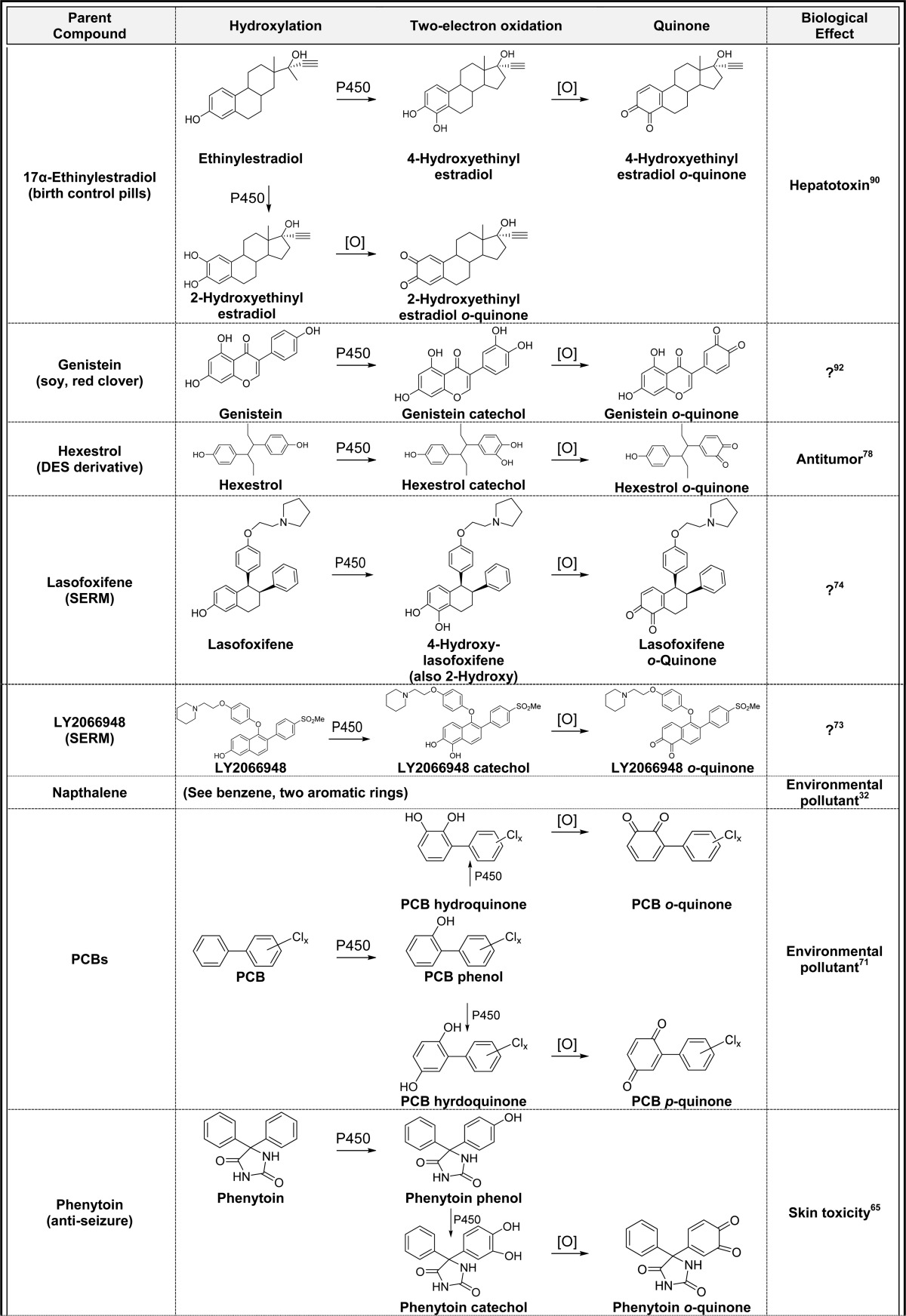
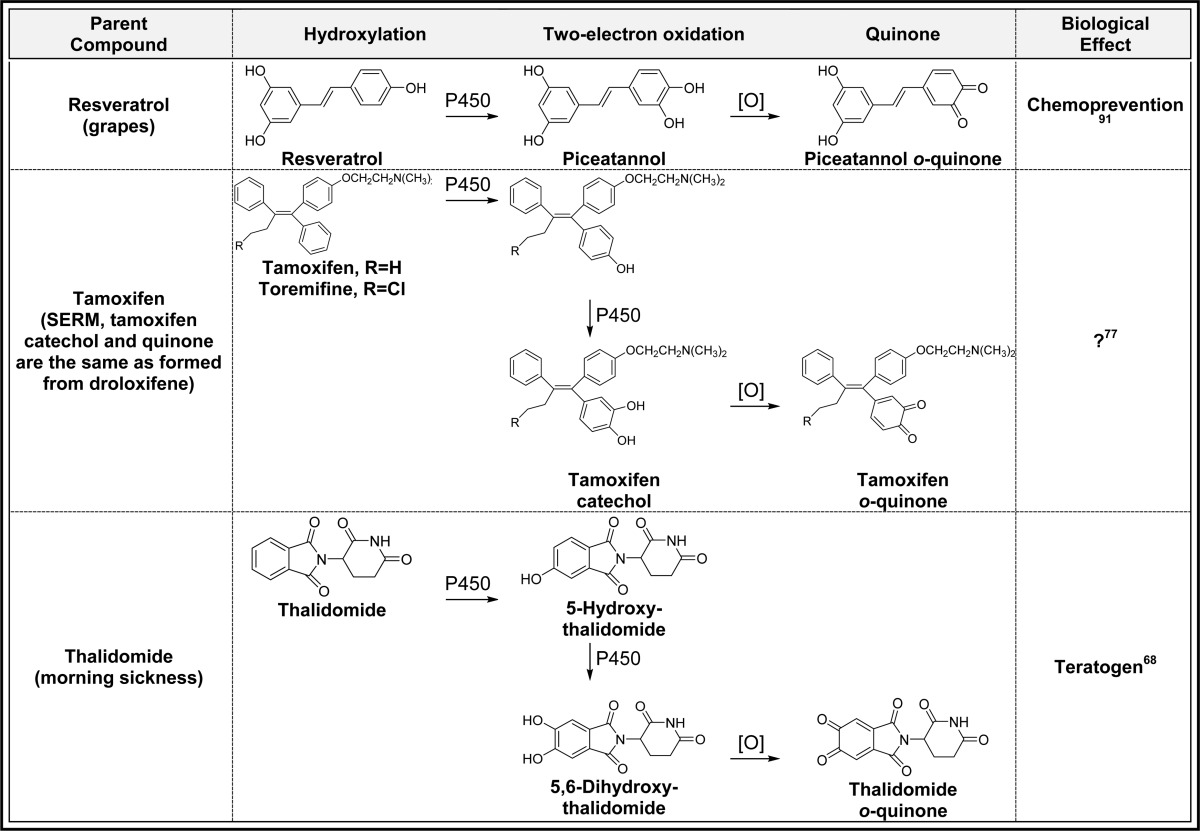
2.3. Aromatic Hydroxylation(s)/Two-Electron Oxidation (Table 3)
Table 3. Aromatic Hydroxylation(s)/Two-Electron Oxidation to Quinonesa.
Alphabetical list (20 total, partial list). [O] refers to any oxidative enzyme or metal ions and in some cases molecular oxygen.
Only one representative reference is listed for each example.
Aromatic rings represent the most common functional group in drugs which suggests that the general bioactivation scheme generating quinones should readily occur as shown for benzene in Figure 2.3,9,61−64 For example, the anticonvulsant phenytoin causes skin toxicity in 5–10% of patients, and P450-catalyzed o-quinone formation has been implicated as the culprit (Table 3).65 The hepatotoxic effects of the epilepsy drug, carbamazepine, has been shown to proceed through a similar mechanism (Table 3).66,67 The teratogen thalidomide is metabolized to a phenol, catechol, and o-quinone catalyzed by P450 which could contribute to the teratogenic mechanism through o-quinone-induced oxidative stress and/or protein binding (Table 3).68 The environmental contaminants naphthalene, bisphenol A, and some polychlorinated biphenyls (PCBs) readily form o- and p-quinones through this mechanism (Table 3).32,69−72 The SERMs (droloxifene, lasofoxifene, LY2066948, raloxifene, tamoxifen, and toremifene) all undergo one or two P-450 catalyzed aromatic hydroxylation reactions followed by two-electron oxidation to o-quinones (Table 3).53,57,73−77 Generally, o-quinone formation from SERMs is a minor bioactivation pathway, and the contribution of quinone metabolites to the overall chemopreventive/toxic properties of the parent SERMs are not known. The synthetic estrogen hexestrol, which is the saturated derivative of DES, is converted to the catechol by P450 followed by two-electron oxidation to the o-quinone (Table 3).59,78 Two o-quinones are formed from the endogenous estrogens (estrone and estradiol, Table 3); the 2-hydroxyestrogen o-quinone pathway is likely a detoxification pathway since the o-quinone formed is very unstable and readily isomerizes to a quinone methide.3,79−81 In contrast, the 4-hydroxyestrogen o-quinone is sufficiently long-lived to produce a variety of mutagenic/carcinogenic effects including DNA oxidation and adduct formation as described below.82,83 Estrogens in hormone therapy (equilin, equilenin, and 8,9-dehydroequilin), all form o-quinones through initial P450-catalyzed aromatic hydroxylation followed by two-electron oxidation (Table 3).79,84−86 Quinone formation from these estrogens could contribute to the increased incidence of hormone-dependent cancer associated with hormone therapy for menopausal symptoms.87−89 17α-Ethinyl estradiol, which is the most common estrogen in birth control pills, is also converted to o-quinones which may contribute to liver toxicity observed at high doses in animal models.90 However, the doses of ethinyl estradiol are significantly lower than HT drugs, and quinone formation is unlikely to contribute to clinical toxicity for birth control pills.90 Finally, the natural products resveratrol and genistein both form catechols and o-quinones by this mechanism which may contribute to their chemopreventive properties in vivo.91,92
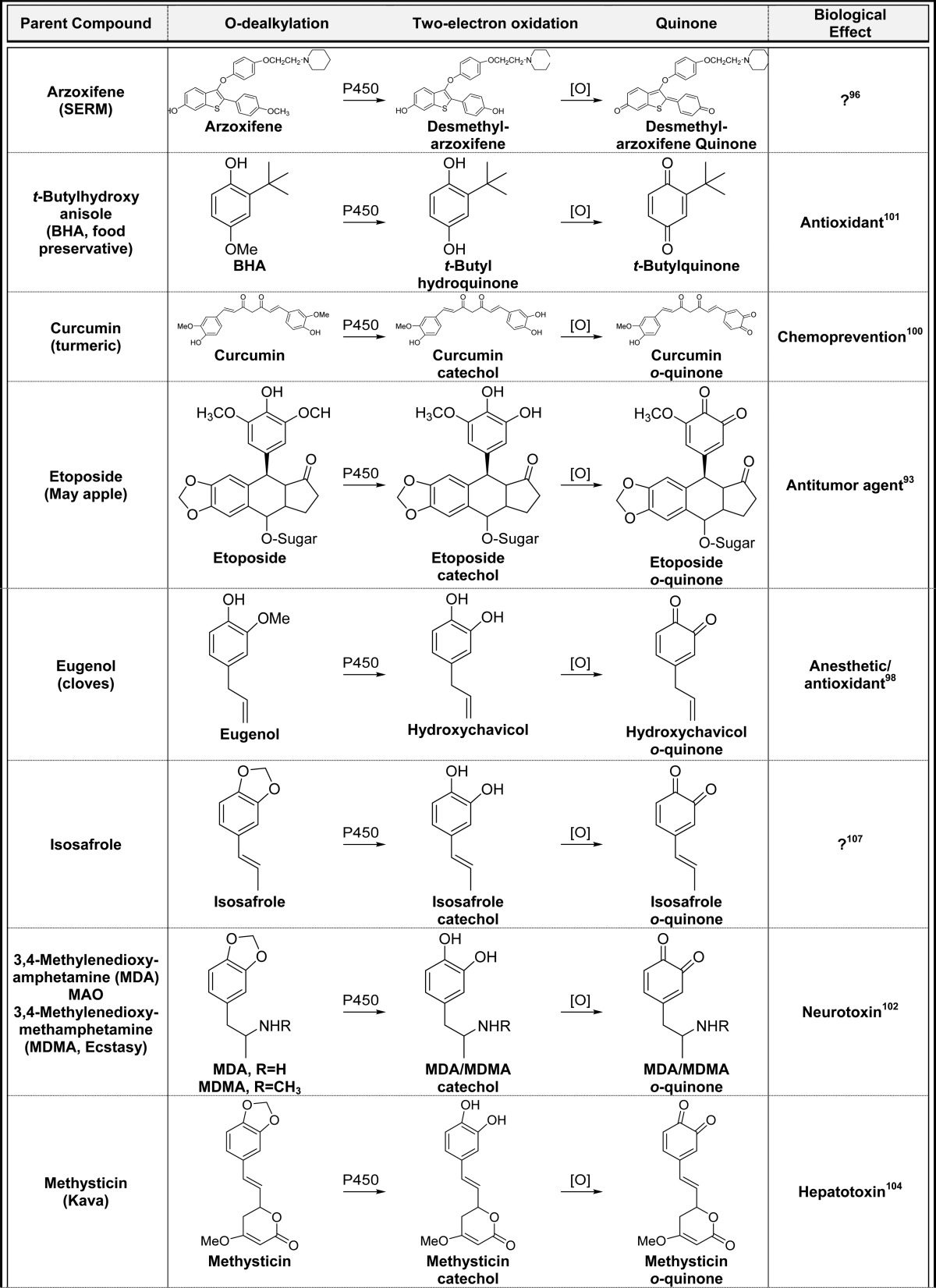
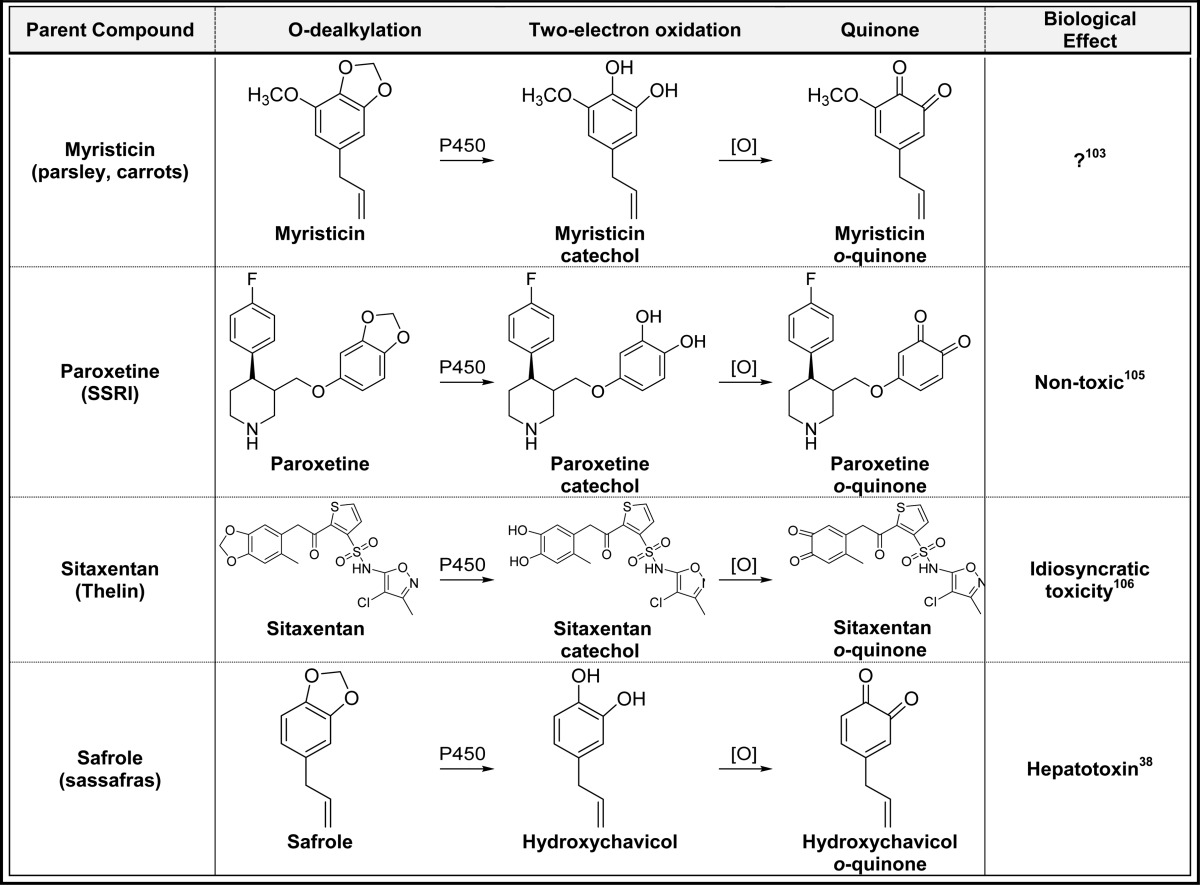
2.4. P450-Catalyzed O-Dealkylation/Two-Electron Oxidation (Table 4)
Table 4. P450-Catalyzed O-Dealkylation/Two-Electron Oxidationa.
Alphabetical list (11 total, partial list). [O] refers to any oxidative enzyme or metal ions and in some cases molecular oxygen.
Only one representative reference is listed for each example.
Another mechanism of quinone formation involves P-450-catalyzed unmasking of phenols/catechols through O-dealkylation reactions followed by two-electron oxidation.9 For example, the methoxy substituent in etoposide is O-dealkylated generating a catechol which is readily oxidized to an o-quinone (Table 4).93o-Quinone formation from etoposide likely contributes to redox toxicity in cells through the generation of ROS contributing to side effects associated with this chemotherapeutic drug.94,95 A similar mechanism is involved in the generation of the extended quinone from arzoxifene; initial O-dealkylation of arzoxifene generates desmethylarzoxifene followed by two-electron oxidation to the extended quinone (Table 4).96 Like the raloxifene extended quinone described above, the arzoxifene quinone has a very short half-life and may not make a significant contribution to the overall biological effects of arzoxifene.57 Eugenol (cloves) can also be metabolized by this pathway initially generating hydroxychavicol followed by two-electron oxidation to the o-quinone (Table 4).9,97,98o-Quinone formation from eugenol represents a minor bioactivation pathway since direct two electron oxidation to the p-quinone methide is a more facile metabolic process.1,99 In an analogous mechanism, curcumin can be O-demethylated giving the catechol, which is further oxidized to an o-quinone which was implicated in curcumin induced oxidative DNA damage (Table 4).100 The synthetic phenolic antioxidant t-butylhydroxyanisole (BHA) also is O-demethylated by P450 to t-butylhydroquinone and further oxidized to the t-butyl-p-benzoquinone (Table 4).101 P450-catalyzed cleavage of the methylenedioxy groups in isosafrole, 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), methysticin, myristicin, paroxetine, safrole, and sitaxentan generate catechols which are readily oxidized to o-quinones by virtually any oxidative enzyme or metal ions (Table 4).102−108 These data suggest that quinone formation from compounds containing a methylenedioxy substituent might be a general mechanism for o-quinone formation.9
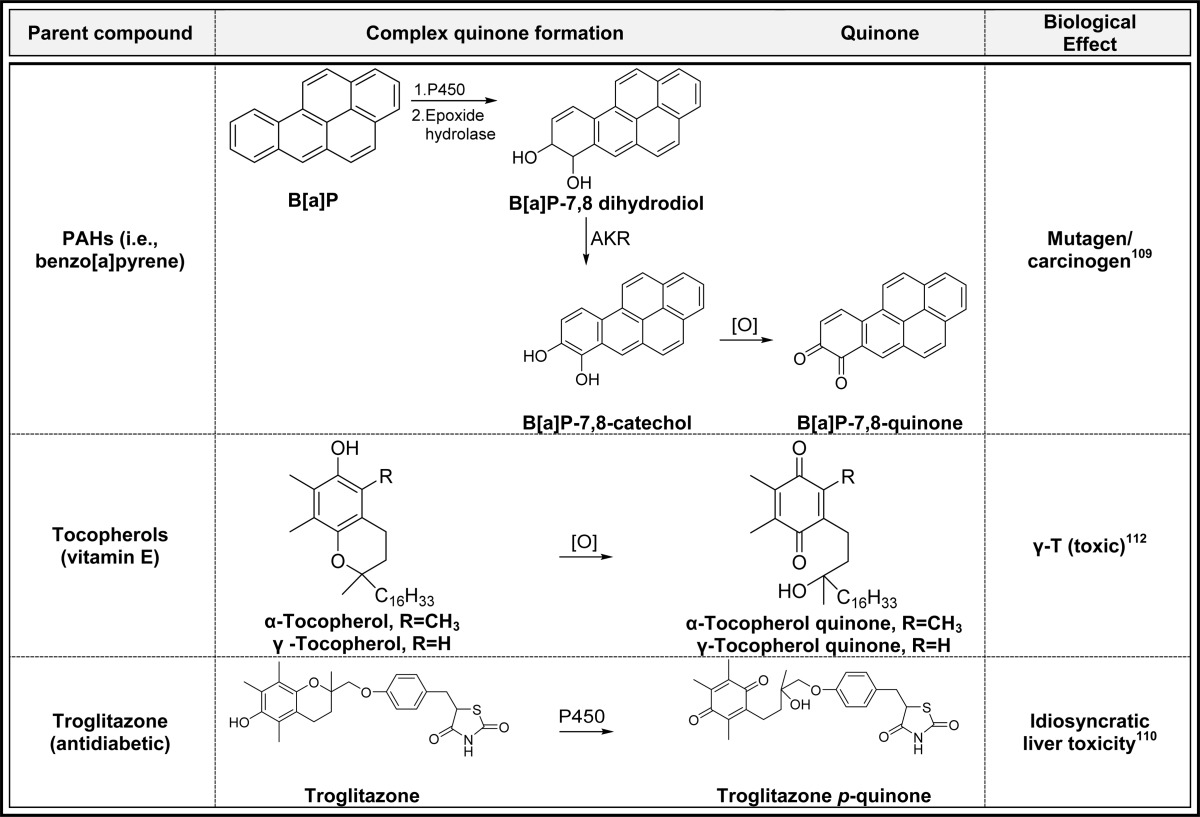
2.5. Complex Quinone Formation (Table 5)
Table 5. Complex Quinone Formationa.
Alphabetical list (3 total, partial list). [O] refers to any oxidative enzyme or metal ions and in some cases molecular oxygen.
Only one representative reference is listed for each example.
Polycyclic aromatic hydrocarbons (PAHs) are metabolized to o-quinones through a complex mechanism involving initial P450-catalyzed arene oxide formation, ring opening catalyzed by epoxide hydrolase, aldo-keto reductase (AKR) mediated formation of the catechol, and two-electron oxidation to o-quinone (Table 5).109 Many AKRs can also catalyze redox cycling (see below) by reducing the PAH o-quinones to the catechol which autoxidize in air back to the o-quinones consuming NADPH.109 This pathway was found to be equally important with the more commonly known diol-epoxide pathway to the activation of benzo[a]pyrene to a human lung carcinogen.109o-Quinone from PAHs present in industrial pollutants and tobacco smoke could make a major contribution to a variety of human cancers.109
A more complex mechanism is also involved in quinone formation from diabetic/anti-inflammatory drug troglitazone and the tocopherols (vitamin E) (Table 5).110−112 The troglitazone quinone likely contributes to the idiosyncratic liver toxicity which led to the withdrawal of troglitazone from the U.S. market in 2000.113 The highly substituted tocopherol p-quinone likely contributes to the antioxidant effects and signaling mechanisms attributed to the parent vitamin (Table 5).114,115 The quinone formed from γ-tocopherol has one less methyl group compared to that from α-tocopherol which could influence its biological effects in vivo. Even though humans consume considerably more γ-tocopherol in the diet compared to α-tocopherol, α-tocopherol is selectively retained likely due to the enhanced reactivity/toxicity of the less substituted γ-tocopherol quinone.114,116
3. Chemistry of Quinones
3.1. Covalent Modification
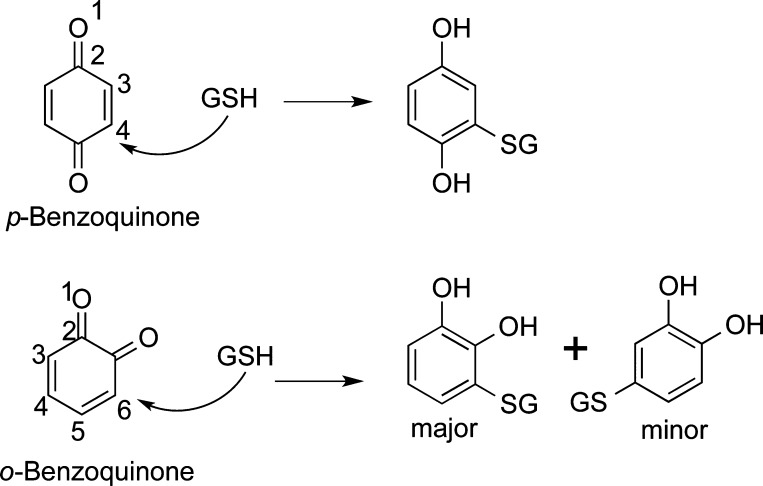
Quinones are Michael acceptors and readily react with soft nucleophiles like sulfhydryl residues on cysteine in GSH and proteins (Figure 4). p-Quinones react via 1,4-reductive addition reactions regenerating the hydroquinone with covalent attachment to the cysteine residue. Unsubstituted o-quinones generally undergo 1,6-reductive addition reactions with thiol nucleophiles due to extended conjugation, although 1,4-reductive addition is often observed as a minor product (Figure 4).38,117 There is some evidence to suggest that the conjugation reaction with cysteine residues is reversible, which could regenerate the quinone and free cysteine and could play a role in quinone signaling mechanisms.118−121 The hydroquinone/catechol thioethers are more readily oxidized compared to unsubstituted parent compounds, and quinone thioethers with multiple cysteine substituents are often reported.122,123 Reaction with nitrogen nucleophiles such as lysine, histidine, N-terminal amino acids, and purine and pyrimidine bases on DNA are much slower compared to that of sulfur nucleophilic additions, although they do occur.71,124 Generally, reductive 1,4-Michael additions predominate over Schiff base formation.36 Very unstable o-quinones (t1/2 < 1 s) like those formed from genistein (Table 3), luteolin, and quercetin o-quinones (Table 2) which have a C2–C3 double bond in the C ring are rapidly hydrated and unlikely to damage important biological molecules.45,92,125 Instead, quinone formation from these natural products might be a chemopreventive mechanism through the Keap1/Nrf2 pathway as discussed below.125
Figure 4.
Michael addition of GSH to quinones.
3.2. Redox Chemistry
Quinones are also potent redox active compounds.3,126 They are readily reduced by one electron catalyzed by P450/NADPH oxidoreductase generating semiquinone radical anions (Figure 3). These intermediates are very unstable and are easily oxidized back up to quinones by molecular oxygen. The reduction of molecular oxygen generates the first of the ROS, the superoxide anion radical. The superoxide anion radical is dismutated by superoxide dismutase generating hydrogen peroxide. Hydrogen peroxide can be reduced by Fenton chemistry through oxidation of iron and/or copper generating the highly reactive hydroxyl radical. This futile redox cycling is responsible for oxidative stress within cells and is a major contributor to overall quinone toxicity.3 Most quinones can also be reduced by two electrons back to the hydroquinone or catechol catalyzed by NADPH/quinone oxidoreductase (NQO1), which represents a major detoxification mechanism.127,128 In some cases as with the PAH o-quinones, AKRs can catalyze the two-electron reduction reaction.109 The relative ability of quinones to redox cycle is highly dependent on the ring substituents. Electron rich substituents, extended ring systems, and extensive substitution promote redox cycling since they stabilize the semiquinone radical anion.126 This often leads to a paradox where GSH conjugation does not result in detoxification since the quinone-thioether quinone is more redox active than the unsubstituted quinone due to the electron donating sulfur substituent.2
In normal cells, a strict balance is maintained between oxidation and reduction, and anything that changes this delicate redox balance is thought to contribute to a number of diseases.129 Quinones cause oxidative stress through the consumption of reducing equivalents including GSH (alkylation and oxidation) and NAD(P)H (oxidation).130 Production of ROS can alter redox balance within cells through the formation of oxidized cellular macromolecules including lipids, proteins, and DNA. Formation of oxidatively damaged bases such as 8-oxodeoxyguanosine (8-oxo-dG) has been associated with aging and carcinogenesis.131,132 Furthermore, ROS can activate a number of signaling pathways including protein kinase C and RAS.133 The redox potential between the quinone and hydroquinone/catechol pair has a major effect on their prooxidant and/or antioxidant effects and ultimately their cytotoxic versus cytoprotective biological properties.134 For example, it has recently been shown that the more easily p-hydroquinones are oxidized, the more potent their antiproliferative effects are in MC38 cells.135 It is likely that the equine catechol estrogens (4-hydroxyequilin, 4-hydroxyequilenin) and PAH quinones, which only require oxygen to autoxidize to their respective highly redox active o-quinones, cause considerably more oxidative stress as compared to those formed from the endogenous catechol estrogens from estradiol and estrone.79,109,136 These differences in redox activities may explain variations in epidemiology studies on the link between different types of hormone therapies and risk of breast cancer.137,138
4. Quinone Targets (Figures 5 and 6)
Figure 5.
Quinone toxicity targets.
Figure 6.
Quinone signaling targets.
4.1. GSH (Figure 5)
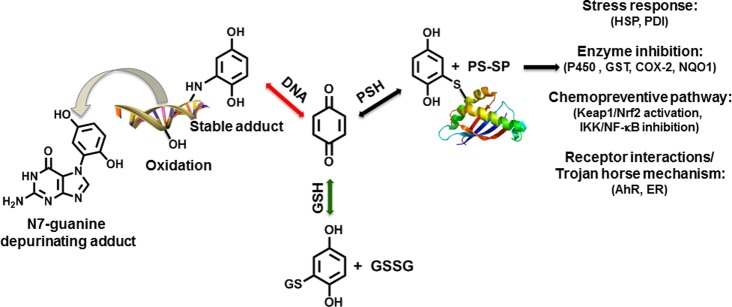
The most important nonprotein sulfhydryl in cells is glutathione (GSH) whose major purpose is to protect cells from reactive electrophiles and free radicals.139 As soft electrophiles, quinones are particularly susceptible to GSH conjugation reactions, and usually, the reaction is so facile that enzymatic catalysis by glutathione S-transferase (GSTs) is not required. Model oxidation reactions with microsomes, peroxidase/hydrogen peroxide, and tyrosinase, in the presence of GSH, are all effective methods for generating and trapping quinones.9,108,122,123,140 Under circumstances where quinone formation is low and GSH levels are high (i.e., healthy liver cells, 5–10 mM GSH), nonenzymatic reaction with GSH generates reduced GSH hydroquinone/catechol conjugates, which are readily excreted through the mercapturic acid pathway (Figures 3 and 4). Along with two-electron reduction catalyzed by NQO1 and or AKR, GSH conjugation represents a common detoxification pathway for most quinones. GSH can also be oxidized to GSSG by hydroxyl radicals generated during redox cycling of quinones.130 Several drugs, environmental chemicals, natural products, and endogenous compounds form quinone GSH conjugates and/or cause GSH depletion.3,10,70,79,92,103,105,106,109,121,141−149
4.2. Protein Modification
4.2.1. General Protein Modification (Figure 5)
As GSH concentrations are depleted, other sulfhydryl nucleophiles such as cysteine residues on proteins become quinone biological targets. For highly reactive quinones, the major proteins alkylated are those present in the highest concentration containing the largest number of cysteine residues. For example, heat shock proteins [Hsp60, Hsp70, Hsp90, and protein disulfide isomerases (PDIs)] are produced in cells in response to stressful conditions including the generation of electrophilic/redox active quinones.114,150 These stress proteins are cysteine-rich and thus ideal protein targets of quinones both for alkylation and oxidation (Figure 5).151−156 For example, using covert oxidatively activated tag (COATag) methodology the extended quinone formed from two-electron oxidation of raloxifene was shown to mainly modify the stress protein GrP78/BiP, three PDIs, and a microsomal GST in rat liver microsomes.152 PCB p-quinones also modify GrP78/BiP and induce the unfolded protein response.157 The arylating quinone from γ-tocopherol but not the more substituted α-tocopherol quinone induced endoplasmic reticulum stress.114 The ansamycin quinones have potent antitumor activity through mechanisms involving inactivation, destabilization, and degrading Hsp90 proteins.11,158 Some dopamine o-quinones cause mitochondrial protein damage which has been implicated in Parkinson’s disease.159 The dopamine quinones were found to inhibit the 20/26S proteasome, which is a cytosol protein with numerous cysteines.156 There are a variety of protein targets of quinones, and identifying reversible unstable protein adducts is challenging for proteomic research, as well as determining the relative importance of the identified biological targets to the overall cytotoxic/cytoprotective effects.160 Some specific quinone protein targets are enzymes that catalyze their formation and detoxification as well as proteins involved in cell signaling (GST/MAPK, Keap1/Nrf2, IKK/NF-κB) and nuclear receptor proteins [arylhydrocarbon receptor (AhR), estrogen receptor (ER)] as outlined below.
4.2.2. P450 Inhibition (Figure 5)
Since P450 is the most likely enzyme catalyzing quinone formation, it is to be expected that particularly reactive quinone metabolites have been reported to be inhibitors of P450s.103,161−163 For example, 4-hydroxytamoxifen and raloxifene were found to be potent mechanism-based inactivators of P450 as a result of quinone-mediated alkylation of the apoprotein.164−166 With raloxifene, analysis of the P450 3A4 peptide showed that Cys239 was the site of the raloxifene extended quinone reaction.167 Sitaxentan contains a methylenedioxy ring, which is metabolized by P450 to the catechol and oxidized to the o-quinone, which could be responsible for the reported inhibition of P450 3A4 (Table 4).106o-Quinone formation by sitaxentan could be responsible for the reports of idiosyncratic drug toxicity, which led to its worldwide withdrawal in December, 2010. Similarly, the methyenedioxy group in myristicin is oxidized to an o-quinone leading to mechanism-based inhibition of P4501A2.103 GSH was shown to block the inhibition which suggests that the o-quinone was the inhibitor and not the carbene formed from the methylenedioxy moiety.103 The SSRI paroxetine was shown to be an inhibitor of P450 2D6, and the o-quinone metabolite was implicated as the reactive intermediate responsible for the inhibitory effect which ultimately leads to clinical drug–drug interactions with other P450 2D6 substrates.168,169 Finally, the methoxyestrogens are feedback inhibitors of P450 1A1 and P450 1B1 likely through the formation of the catechol estrogen o-quinones.170
4.2.3. COX-2 Inhibition (Figure 5)
Several synthetic and natural stable quinones have been reported to have anti-inflammatory activity through a COX-2 inhibitory mechanism with a potency similar to indomethacin.171 For example, nanomolar IC50s have been reported for jugalone and thymoquinone against human recombinant COX-2 activity (Table 1).171,172 The catechol metabolite of resveratrol, piceatannol (Table 3), was found to be a selective COX-2 inhibitor with potency similar to that of celecoxib which may contribute to the chemopreventive properties of both compounds.91,173,174 The diethylester prodrug, diacerein (rhubarb), which is hydrolyzed to the stable p-quinone rhein (Table 1), has been shown to be effective for treatment of osteoarthritis.175,176 Like P450, the peroxidase activity of COX-2 catalyzes the oxidation of catechols and hydroquinones during prostaglandin biosynthesis, and these quinones can inhibit the enzyme.27 For example, the peroxidase activity of COX-2 catalyzes the formation of the dopamine quinone, which is implicated in the pathogenesis of Parkinson’s disease (Table 2).177 It has been suggested that COX-2 inhibitors may be valuable therapies aimed at slowing the progression of this neurodegenerative disease.178 Similarly, COX-2 catalyzed formation of the methamphetamine o-quinone as well as endogenous neurotransmitter o-quinones causing oxidative stress and mitochondrial protein damage has been implicated in neurotoxicity and aging.179,180 Finally, COX-2 has been shown to oxidize diethylstilbestrol and catechol estrogens to the extended quinone and o-quinones, respectively (Table 3).181−183
4.2.4. COMT Inhibition (Figure 5)
In addition to inhibiting enzymes which catalyze their formation, quinones can also inhibit enzymes which detoxify them or their hydroquinone/catechol precursors. For example, catechol O-methyl transferase (COMT) catalyzes the conversion of catechols to methoxyethers which cannot be further oxidized to quinones and therefore represents a catechol detoxification mechanism. The equine estrogen metabolites 4-hydroxyequilenin and 4-hydroxyequilin have been shown to be potent inhibitors of COMT likely because they can autoxidize to o-quinones (Table 3).184,185 Inhibition of COMT-mediated catechol estrogen clearance may play a role in the toxicity of endogenous estrogens and estrogens in HT.186 The catecholamine quinones are also potent irreversible inhibitors of COMT which could contribute to the neurotoxicity mechanism (Table 3).187,188
4.2.5. NQO1 Inhibition (Figure 5)
Two-electron reduction of quinones to catechols and hydroquinones is a major detoxification mechanism for most quinones.128 However, some quinones have been shown to be mechanism-based inhibitors of NQO1. For example, the p-quinone ES936 (Table 1) is a nanomolar inhibitor of NQO1 in cell-based assays.189 Similarly, the antitumor agent mitomycin C (Table 1) undergoes bioreductive activation catalyzed by NQO1, but it is also a mechanism-based inhibitor of the enzyme both in vitro and in vivo.190 Since NQO1 activity is much higher in cancer cells compared to that in normal cells, quinone-mediated inhibition of NQO1 could be an effective anticancer strategy.191 For example, the natural product deoxynyboquinone is a potent NQO1 substrate and inhibitor through an NQO1-catalyzed redox cycling mechanism and deoxynyboquinone could have considerable potential as a chemotherapeutic agent (Table 2).191
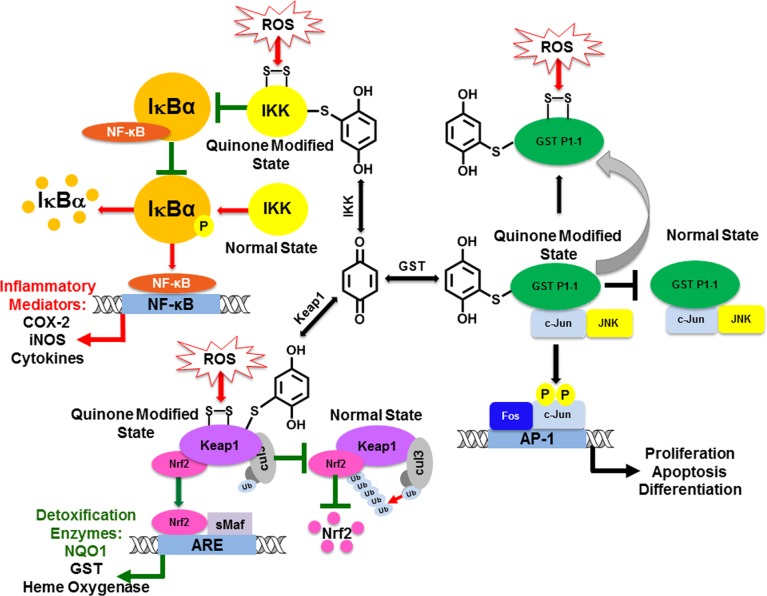
4.2.6. GST Inhibition (Figure 6)
GSTs are often covalently modified and/or oxidized by quinones likely in an attempt by GSTs to catalyze detoxification of quinones.192−198 This usually results in the inhibition of GSTs, and the effect on GST P1–1 in particular could play a role in regulating the c-Jun N-terminal kinase (JNK) cell signaling pathway. JNK is a MAPK which effects cell growth, differentiation, stress response, and cellular transformation.192,199−201 GST P1–1 forms an inhibitory complex with JNK–c-Jun that inhibits the phosphorylation of c-Jun by JNK. Quinone mediated oxidative or covalent modification of GST P1–1 leads to activation of JNK phosphorylation of c-Jun resulting in the induction of stress response, differentiation, and proliferation. Some specific examples include metabolites of the equine estrogens 4-hydroxyequilenin o-quinone and 4-hydroxyequilin o-quinone, which were found to be potent irreversible inhibitors of GST P1–1 (Table 3).79,192,198 In contrast, the o-quinones of the endogenous catechol estrogen 4-hydroxyestrone o-quinone did not significantly inhibit GST P1–1 activity in human breast cancer cells perhaps due to differences in the reactivity of these estrogen quinones.193 Plant polyphenols including quercetin, ellagic acid, juglone, luteolin, and caffeic acid have been reported as GST inhibitors likely through quinone formation.196,202,203
4.2.7. Keap1/Nrf2/ARE Pathway (Figure 6)
para and o-quinones were among the first characterized small molecule inducers of the Keap1/Nrf2/ARE pathway.204 This pathway controls gene expression of an elaborate network of protective proteins including heme oxygenase, NQO1, AKT, and GST that defend cells from electrophiles and free radicals.205,206 Under normal conditions, the levels of these protective enzymes is low due to the repressor function of Keap1, which sets up Nrf2 for ubiquitination and proteosomal degradation (Figure 6). Modification of Keap1 by either alkylation or oxidation of crucial cysteine residues (CYS23, 151, 273, and 288) leads to the loss of the repressor function of Keap1, increased stability of Nrf2, translocation of Nrf2 to the nucleus, and activation of ARE-mediated target genes.204,207,208 For example, BHA and its metabolite t-butylhydroquinone are classical activators of Nrf2 genes resulting in potent induction of heme oxygenase and NQO1 (Table 4).209 It has been shown that catechol estrogen o-quinones (Table 3) can covalently modify Keap1 leading to Nrf2 activation and induction of heme oxygenase.210 Similarly, catechols from green tea including EGCG and its epimer gallocatechin gallate and flavonoids such as quercetin form o-quinones can activate the Keap1/Nrf2/ARE pathway leading to cytoprotection (Table 2).125,211−214 In addition, the quercetin quinone methide formed by isomerization of the quercetin o-quinone could also contribute to Keap1/Nrf2/ARE activation.43 Interestingly, the level of induction could be enhanced by pretreatment with the GSH synthesis inhibitor buthionine sulfoximine and diminished in the presence of the electrophile scavenger N-acetylcysteine.125,214 Other natural product catechols including carnosic acid and caffeic acid had analogous Keap1 modulatory effects through their corresponding o-quinone mediated mechanisms (Table 2).129,215,216
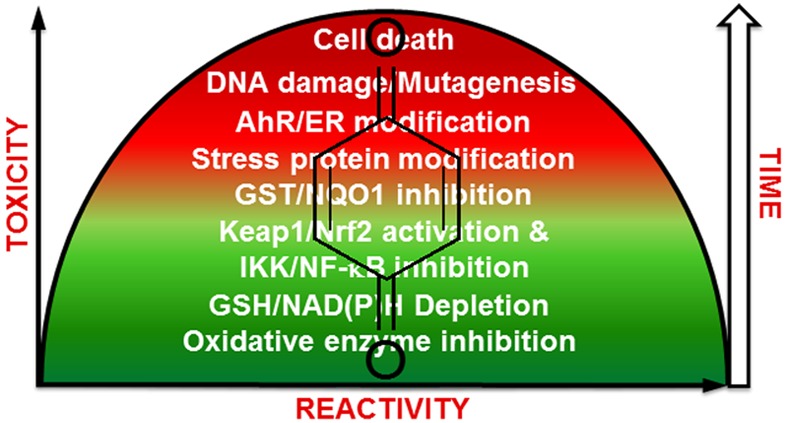
Activators of the Keap1-Nrf2 pathway are generally considered as chemopreventive agents since they enhance the levels of detoxification enzymes. However, it is important to realize that Nrf2 is very much involved in the initiation, promotion, and metastasis of cancer.207,208,217−219 In fact, cancer cells hijack Nrf2 to support their malignant growth, and activators of Nrf2 could have unwanted effects including the development of resistance to chemotherapeutic agents. For example, t-butylhydroquinone upregulated Nrf2 and enhanced the resistance of cancer cells to cisplatin, doxorubicin, and etoposide in neuroblastoma cells.217 Similarly, the electrophilic and prooxidant signals produced from PAH o-quinones can lead to the induction of ARE-regulated genes which could influence the initiation and promotion stages of PAH carcinogenesis (Table 4).206,220 Quinone Nrf2 activators could be an effective strategy for cancer chemoprevention; however, the effect of quinone exposure on long-term Nrf2 levels are not known, and they may not be safe for cancer patients. Finally, based on the crucial role of Nrf2 in redox homeostasis in cells, it is likely that the dose–response curve for quinone Nrf2 activators is U-shaped and that cytoprotection/chemoresistance depends on the amount of quinone formed as well as its electrophilic/redox activity (Figure 7).206
Figure 7.
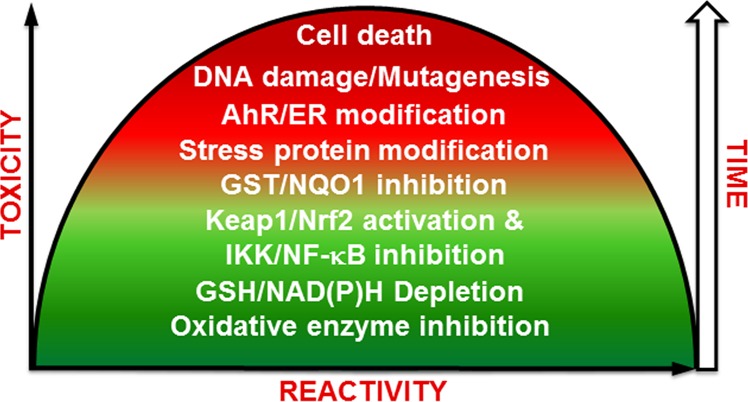
Inverse U-shaped modulation of toxicity as a function of dose and reactivity of quinone. Reactivity is defined as d[P]/dt = k [quinone]. The timeline refers to the likely order of events occurring within the cell.
4.2.8. NF-κB Pathway (Figure 6)
NF-κB proteins are a family of transcription factors that play a major role in inflammation and carcinogenesis.221,222 NF-κB activity is regulated by the IκB proteins, which are controlled through phosphorylation by upstream IκB kinases (IKK). These kinases are attractive targets for electrophilic quinones, which can covalently modify IKK leading to the inhibition of IKK and NF-κB-mediated gene transcription resulting in anti-inflammatory activities. For example, it has been shown that 1,2-naphthoquinone and β-lapachone (Table 1) inhibited phosphorylation of IκB by IKK and disrupted NF-κB signaling.223,224 Similarly, the catechol metabolite of resveratrol, piceatannol (Table 3), is oxidized to an o-quinone, which targets Cys179 of IKK leading to inhibition of phorbol ester induced NF-κB activation.225,226 The antitumor anthroquinone denbinobin also inhibits NF-κB through the modulation of IKK in human leukemic cells, and the inhibitory effects could be prevented by N-acetylcysteine.227 The ansamycin antibiotic herbimycin A (Table 1) preferentially inhibits IKK likely through interaction with Cys59, resulting in the prevention of expression of NF-κB-dependent genes and anti-inflammatory activity.228 In contrast, the carcinogenic metabolite of estradiol, 4-hydroxyestradiol (Table 3), is oxidized to an o-quinone which generates ROS causing the activation of IKK and NF-κB leading to the transformation of MCF-10A breast cells into a malignant phenotype.229
4.2.9. Nuclear Receptor Modification (Figure 5)
It has been shown that PAH o-quinones and 1,2-naphthoquinone can covalently modify AhR leading to the activation of the AhR/XRE pathway and induction of P450 1A1.230,231 Similar results have been reported for 1,4-benzoquinone, t-butyl-1,4-benzoquinone, and PCB quinones suggesting that some quinones could be bifunctional inducers of both phase I and phase II enzymes.230−232 Interaction of these genotoxic quinones with AhR targets them into the nucleus where they can redox cycle generating ROS and cause oxidative damage to DNA in a manner similar to the Trojan horse estrogen receptor mechanism and 4-hydroxyequilenin (see below).136 In support of this mechanism, the levels of DNA strand breaks and 8-oxo-dG levels were lower in AhR-deficient Hepa and AhR knockdown H358 cells treated with the PAH o-quinone, benzo[a]pyrene-7,8-dione.233
A similar Trojan horse DNA damage mechanism was observed with the estrogen receptor (ER) and the equine catechol estrogen, 4-OHEN.136 The rate of 4-OHEN-induced oxidative DNA damage was significantly enhanced in ERα positive cells. The mechanism likely involves ERα acting as a Trojan horse, which is covalently modified by 4-OHEN o-quinone, concentrating the ER-quinone complex in the nucleus, accelerating the rate of ROS formation, and enhancing DNA oxidation.79 The Trojan horse mechanism of estrogen carcinogenesis could explain why there is a positive association between 8-oxo-dG excretion levels and risk for ER positive breast cancer.234
4.3. DNA
Several quinones are known to cause a variety of DNA lesions, including depurinating bases resulting from N7-guanine or N3-adenine adduction, stable adducts at the exocyclic amino groups of guanine and adenine, as well as oxidized bases (Figure 5).3 Depurinating N7-guanine and N3-adenine adducts have been reported from dopamine o-quinones as well as from 4-hydroxyestrone/estradiol o-quinones.82,235,236 The estrogen depurinating DNA adducts have been detected in much higher levels in prostate and breast cancer patients compared to that in samples from disease-free individuals.237−239 There is speculation and some in vitro studies suggesting that similar depurinating adducts could be formed by a number of quinones including those from diethylstilbestrol, bisphenol A, PAHs, and benzene.69,240−242 In contrast to these unstable depurinating adducts which would be repaired by base excision repair enzymes, the equine estrogens 4-hydroxyequilin and 4-hydroxyequilenin o-quinones also form stable cyclic adducts similar to those reported for the PAH o-quinones.3,243−251 Highly mutagenic cyclic DNA adducts have also been reported for p-benzoquinone which could contribute to the carcinogenic effects of benzene.252−254 DNA adducts have been reported from quercetin, although these adducts likely result from covalent modification by the quinone methide tautomer of the quercetin o-quinone.255 In addition, these quercetin DNA adducts were found to be transient and are unlikely to contribute to mutagenicity in vivo. Although quercetin has tested positive in the Ames test in the presence of S9 activation,256 the transient nature of the DNA adducts likely explains why no genotoxic effects have been observed in animal models.257
Oxidative damage to DNA is also a common cytotoxic mechanism for quinones. Biomarkers for oxidative damage to DNA include the formation of 8-oxo-dG which is considered to be an important biomarker in carcinogenesis.258,259 PAH o-quinones and PCB quinones have been reported to cause oxidative DNA damage.109,260,261 PAH o-quinones generated oxidized pyrimidines and 8-oxo-dG through redox cycling resulting in G → T transversions which may contribute to lung cancer risk.109,262,263 8-oxo-dG formation has been correlated with p53 mutations observed in human lung cancer which strongly implicates the PAH o-quinones as ultimate carcinogens.264,265
The excessive production of ROS in breast cancer tissue has been linked to metastasis of tumors in women with breast cancer.266 The source of ROS has been suggested to be the result of redox cycling between the estrogen o-quinones and their semiquinone radicals generating superoxide, hydrogen peroxide, and ultimately reactive hydroxyl radicals (Figure 3) which cause oxidative cleavage of the phosphate-sugar backbone as well as oxidation of the purine/pyrimidine residues of DNA.267 In support of this mechanism, a variety of ROS mediated damage has been reported in hamsters treated with 17β-estradiol including DNA single-strand breaks, 8-oxo-dG formation, and chromosomal abnormalities.267−271 The equine catechol estrogens, 4-hydroxyequilin, and 4-hydroxyequilenin are much more redox active since they autoxidize to o-quinones generating extensive oxidative DNA damage.79,272,273 For example, 4-OHEN induced DNA single-strand breaks as well as the formation of 8-oxo-dA and 8-oxo-dG.274−276 These and other data are evidence for a mechanism of estrogen-induced tumor initiation by redox cycling of estrogen quinones generating ROS which damage DNA. Finally, a variety of redox active halogenated quinones have been shown to induce epigenetic modulation of DNA demethylation affecting the expression of thousands of genes involved in a broad range of cellular processes.277
5. Toxicity versus Cytoprotection. Importance of Dose, Time, and Reactivity
It is now becoming generally accepted that quinones and compounds that form them likely have inverse U-shaped dose–response curves (Figure 7).129,206,208,278,279 This is because they have opposing effects in biological systems which depending on the dose, cellular targets, and time of exposure could result in toxicity or cytoprotection. At low doses and for relatively stable/selective quinones, cytoprotection comes from the electrophilic counterattack resulting in the induction of detoxification enzymes through the Keap1/Nrf2 pathway. At higher doses and for less selective/more reactive quinones, GSH depletion, NAD(P)H oxidation, and protein degradation led to the endoplasmic reticulum stress response and altered signal transduction. DNA adducts especially from estrogen and PAH quinones which are not repaired efficiently over long-term exposure can initiate and promote the carcinogenic process. Very reactive quinones likely only target the enzymes that sythesize them (P450, COX-2, etc.) or react with water and have little toxic/cytoprotective effects.
In general, the higher the potency of the drug, the lower is the dose and the decreased risk of toxicity.9 For example, paroxetine does form an o-quinone which can decrease GSH and inhibit P450 2D6; however, the dose of the drug is low, and o-quinone formation is unlikely to contribute to significant clinical problems.168 Similarly, o-quinones are the major metabolites of endogenous estrogens and estrogens in estrogen replacement formulations. As with paroxetine, the dose is very low for these drugs especially for oral contraceptives, and o-quinone formation may not be an issue. However, in addition to the generally accepted hormonal carcinogenesis mechanism, long-term exposure to low doses of estrogen o-quinones in estrogen replacement formulations could contribute to enhanced risk of breast cancer especially in older women.
6. Conclusions and Future Directions
The above are several examples of both structurally simple and complex aromatic compounds for which data strongly implicate quinone intermediates as mediators of toxicity and/or cytoprotection for a variety of drugs, natural products, environmental contaminants, and endogenous chemicals. These reactive metabolites could be considerably more important to the metabolism and biological properties of synthetic and naturally occurring aromatic compounds than is currently recognized. Quinones are formed both enzymatically and nonenzymatically, but the details of these processes and relationships to the structures of aromatic compounds are just beginning to emerge. Variations in the contributions of electrophilicity/redox activity modulate quinone reactivity over a wide range, suggesting substantial differences in the biological targets and intracellular effects of quinones. It is clear that covalent modification of both proteins and DNA competes with detoxification mechanisms such as reaction with GSH and that quinones are capable of inducing cytotoxic and cytoprotective responses. Future studies will seek to clarify relationships between reactivities and biological actions of these electrophiles and to gain insight into the mechanisms involved in cellular damage/cytoprotection. These data obtained will assist in clarifying the complex biological properties of a number of aromatic drugs and natural products and provide new information on intracellular targets as a function of electrophile/redox reactivity, which may be applicable to other types of electrophilic intermediates. Developing a better understanding of factors affecting phase II conjugation and rapid elimination of the parent aromatic compounds versus formation of quinones, their reactivities, and biological targets will allow advances in the drug discovery process to either enhance or prevent this pathway in vivo. Given the high cost of drug withdrawal especially for idiosyncratic drug toxicity, it is important to screen for potential quinone formation early in the drug discovery process.
Acknowledgments
We thank Birgit Dietz and Atieh Hajirahimkhan for helpful discussions.
Glossary
Abbreviations
- AKR
aldo-keto reductase
- ARE
antioxidant response element
- BHA
t-butylhydroxyanisole
- COX-2
cyclooxygenase-2
- dA
2′-deoxyadenosine
- dG
2′-deoxyguanosine
- DT
diaphorase
- ER
estrogen receptor
- GST
glutathione S-transferase
- γ-GT
γ-glutamyl transpeptidase
- 5-HT
5-hydroxy-tryptamine
- HT
hormone therapy
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- KEAP1
kelch-like ECH-associated protein 1
- MAPK
mitogen-activated protein kinase
- MDA
3,4-(+)-methylenedioxyamphetamine
- MDMA
3,4-(+)-methylenedioxymethamphetamine
- α-MeDA
α-methyldopamine
- NF-κB
nuclear factor kappa B
- Nrf2
NF-E2-related factor 2
- NQO1
NAD(P)H:quinone oxidoreductase
- 2-OHE
2-hydroxyestrone
- 4-OHE
4-hydroxyestrone
- 4-OHEN
4-hydroxyequilenin
- 8-oxo-dA
8-oxodeoxyadenosine
- 8-oxo-dG
8-oxodeoxyguanosine
- P450
cytochrome P450
- PAHs
polycyclic aromatic hydrocarbons
- PDIs
protein disulfide isomerases
- ROS
reactive oxygen species
- XRE
xenobiotic response element
Biographies
Judy L. Bolton, Ph.D. I am currently a full professor and Head of the Department of Medicinal Chemistry and Pharmacognosy at the University of Illinois at Chicago. I am an associate editor for Chemical Research in Toxicology, and I have served as chair of the Cancer Etiology Study Section, NIH. My research is focused on bioactivation of estrogens and antiestrogens and mechanisms of botanical dietary supplements. I currently have over 150 publications and have already mentored 22 Ph.D. students, 8 masters students, and 27 postdoctoral fellows. Finally, I was recognized with Fellow of the American Chemical Society, 2011.
Tareisha L. Dunlap, Ph.D. I am currently a senior research specialist in the Department of Medicinal Chemistry and Pharmacognosy at the University of Illinois at Chicago. I am a former American Chemical Society scholar, and I more recently received a NIH National Center for Complementary and Alternative Medicine Research Training Fellowship (2013–2015). My research is focused on studying the effects of botanical dietary supplements on cross-talk between biomolecules in the inflammatory and estrogen chemical carcinogenesis pathways. I have several manuscripts (9 total) on cancer prevention, both published and in preparation.
This work was supported in part by the Office of Dietary Supplements (ODS) and National Center for Complementary and Integrated Health (NCCIH) through the NIH grant P50 AT000155 and T32 AT007533.
The authors declare no competing financial interest.
References
- Bolton J. L. (2014) Quinone methide bioactivation pathway: contribution to toxicity and/or cytoprotection?. Curr. Org. Chem. 18, 61–69. 10.2174/138527281801140121123046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks T. J.; Jones D. C. (2002) The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug Metab. 3, 425–438. 10.2174/1389200023337388. [DOI] [PubMed] [Google Scholar]
- Bolton J. L.; Trush M. A.; Penning T. M.; Dryhurst G.; Monks T. J. (2000) Role of quinones in toxicology. Chem. Res. Toxicol. 13, 135–160. 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- Rendic S.; Guengerich F. P. (2012) Contributions of human enzymes in carcinogen metabolism. Chem. Res. Toxicol. 25, 1316–1383. 10.1021/tx300132k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa B.; Pedretti A.; Vistoli G. (2012) Reactions and enzymes in the metabolism of drugs and other xenobiotics. Drug Discovery Today 17, 549–560. 10.1016/j.drudis.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Baillie T. A.; Rettie A. E. (2011) Role of biotransformation in drug-induced toxicity: influence of intra- and inter-species differences in drug metabolism. Drug Metab. Pharmacokinet. 26, 15–29. 10.2133/dmpk.DMPK-10-RV-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. A.; Isin E. M.; Ogese M. O.; Mettetal J. T.; Williams D. P. (2016) Reactive Metabolites: Current and Emerging Risk and Hazard Assessments. Chem. Res. Toxicol. 29, 505–533. 10.1021/acs.chemrestox.5b00410. [DOI] [PubMed] [Google Scholar]
- Leung L.; Kalgutkar A. S.; Obach R. S. (2012) Metabolic activation in drug-induced liver injury. Drug Metab. Rev. 44, 18–33. 10.3109/03602532.2011.605791. [DOI] [PubMed] [Google Scholar]
- Kalgutkar A. S.; Gardner I.; Obach R. S.; Shaffer C. L.; Callegari E.; Henne K. R.; Mutlib A. E.; Dalvie D. K.; Lee J. S.; Nakai Y.; O’Donnell J. P.; Boer J.; Harriman S. P. (2005) A comprehensive listing of bioactivation pathways of organic functional groups. Curr. Drug Metab. 6, 161–225. 10.2174/1389200054021799. [DOI] [PubMed] [Google Scholar]
- Inbaraj J. J.; Chignell C. F. (2004) Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 17, 55–62. 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- Miyata Y. (2005) Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 11, 1131–1138. 10.2174/1381612053507585. [DOI] [PubMed] [Google Scholar]
- Lenaz G.; Genova M. L. (2009) Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim. Biophys. Acta, Bioenerg. 1787, 563–573. 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Mukai K.; Morimoto H.; Kikuchi S.; Nagaoka S. (1993) Kinetic study of free-radical-scavenging action of biological hydroquinones (reduced forms of ubiquinone, vitamin K and tocopherol quinone) in solution. Biochim. Biophys. Acta, Gen. Subj. 1157, 313–317. 10.1016/0304-4165(93)90115-O. [DOI] [PubMed] [Google Scholar]
- Acosta M. J.; Vazquez Fonseca L.; Desbats M. A.; Cerqua C.; Zordan R.; Trevisson E.; Salviati L. (2016) Coenzyme Q biosynthesis in health and disease. Biochim. Biophys. Acta, Bioenerg. 1857, 1079–1085. 10.1016/j.bbabio.2016.03.036. [DOI] [PubMed] [Google Scholar]
- Tauskela J. S. (2007) MitoQ--a mitochondria-targeted antioxidant. IDrugs 10, 399–412. [PubMed] [Google Scholar]
- Smith R. A.; Murphy M. P. (2010) Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 1201, 96–103. 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- Doughan A. K.; Dikalov S. I. (2007) Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid. Redox Signaling 9, 1825–1836. 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Singh A. (2015) A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 6, 206. 10.3389/fphar.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G. (1987) Metabolism and reactions of quinoid anticancer agents. Pharmacol. Ther. 35, 57–162. 10.1016/0163-7258(87)90105-7. [DOI] [PubMed] [Google Scholar]
- Faulds D.; Balfour J. A.; Chrisp P.; Langtry H. D. (1991) Mitoxantrone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs 41, 400–449. 10.2165/00003495-199141030-00007. [DOI] [PubMed] [Google Scholar]
- Bolzan A. D.; Bianchi M. S. (2001) Genotoxicity of streptonigrin: a review. Mutat. Res., Rev. Mutat. Res. 488, 25–37. 10.1016/S1383-5742(00)00062-4. [DOI] [PubMed] [Google Scholar]
- Srinivas G.; Babykutty S.; Sathiadevan P. P.; Srinivas P. (2007) Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 27, 591–608. 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- Chang H. M.; Chui K. Y.; Tan F. W.; Yang Y.; Zhong Z. P.; Lee C. M.; Sham H. L.; Wong H. N. (1991) Structure-activity relationship of miltirone, an active central benzodiazepine receptor ligand isolated from Salvia miltiorrhiza Bunge (danshen). J. Med. Chem. 34, 1675–1692. 10.1021/jm00109a022. [DOI] [PubMed] [Google Scholar]
- Pardee A. B.; Li Y. Z.; Li C. J. (2002) Cancer therapy with beta-lapachone. Curr. Cancer Drug Targets 2, 227–242. 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- O’Brien P. J. (1991) Molecular mechanisms of quinone cytotoxicity. Chem.-Biol. Interact. 80, 1–14. 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- Monks T. J.; Hanzlik R. P.; Cohen G. M.; Ross D.; Graham D. G. (1992) Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 112, 2–16. 10.1016/0041-008X(92)90273-U. [DOI] [PubMed] [Google Scholar]
- Wangpradit O.; Moman E.; Nolan K. B.; Buettner G. R.; Robertson L. W.; Luthe G. (2010) Observation of an unusual electronically distorted semiquinone radical of PCB metabolites in the active site of prostaglandin H synthase-2. Chemosphere 81, 1501–1508. 10.1016/j.chemosphere.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmin T. R.; Swerdloff M. D.; Rogie M. M. (1981) Copper(II)-Induced oxidations of aromatic substrates: catalytic conversion of catechols to o-benzoquinones. copper phenoxides as intermediates in the oxidation of phenol and a single-step conversion of phenol, ammonia, and oxygen into muconic acid mononitrile. J. Am. Chem. Soc. 103, 5795–5804. 10.1021/ja00409a030. [DOI] [Google Scholar]
- Yuan X.; Miller C. J.; Pham A. N.; Waite T. D. (2014) Kinetics and mechanism of auto- and copper-catalyzed oxidation of 1,4-naphthohydroquinone. Free Radical Biol. Med. 71, 291–302. 10.1016/j.freeradbiomed.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Marsik P.; Kokoska L.; Landa P.; Nepovim A.; Soudek P.; Vanek T. (2005) In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and −2-catalyzed prostaglandin E2. Planta Med. 71, 739–742. 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- Gharavi N.; Haggarty S.; El-Kadi A. O. (2007) Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr. Drug Metab. 8, 1–7. 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- Kumagai Y.; Shinkai Y.; Miura T.; Cho A. K. (2012) The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 52, 221–247. 10.1146/annurev-pharmtox-010611-134517. [DOI] [PubMed] [Google Scholar]
- Moridani M. Y.; Scobie H.; Salehi P.; O’Brien P. J. (2001) Catechin metabolism: glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome p450. Chem. Res. Toxicol. 14, 841–848. 10.1021/tx000235o. [DOI] [PubMed] [Google Scholar]
- Moridani M. Y.; Scobie H.; Jamshidzadeh A.; Salehi P.; O’Brien P. J. (2001) Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: glutathione conjugate formation. Drug Metab. Dispos. 29, 1432–1439. [PubMed] [Google Scholar]
- Galati G.; Sabzevari O.; Wilson J. X.; O’Brien P. J. (2002) Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 177, 91–104. 10.1016/S0300-483X(02)00198-1. [DOI] [PubMed] [Google Scholar]
- Liberato D. J.; Byers V. S.; Dennick R. G.; Castagnoli N. (1981) Regiospecific attack of nitrogen and sulfur nucleophiles on quinones derived from poison oak/ivy catechols (urushiols) and analogues as models for urushiol-protein conjugate formation. J. Med. Chem. 24, 28–33. 10.1021/jm00133a007. [DOI] [PubMed] [Google Scholar]
- Beart J. E.; Lilley T. H.; Haslam E. (1985) Polyphenol interactions. part 2. covalent binding of procyanidins to proteins during acid-catalysed decomposition; observation of some polymeric proanthocyanidins. J. Chem. Soc., Perkin Trans. 2 1439–1443. 10.1039/p29850001439. [DOI] [Google Scholar]
- Bolton J. L.; Acay N. M.; Vukomanovic V. (1994) Evidence that 4-allyl-o-quinones spontaneously rearrange to their more electrophilic quinone methides: potential bioactivation mechanism for the hepatocarcinogen safrole. Chem. Res. Toxicol. 7, 443–450. 10.1021/tx00039a024. [DOI] [PubMed] [Google Scholar]
- Masuda T.; Inaba Y.; Takeda Y. (2001) Antioxidant mechanism of carnosic acid: structural identification of two oxidation products. J. Agric. Food Chem. 49, 5560–5565. 10.1021/jf010693i. [DOI] [PubMed] [Google Scholar]
- Galati G.; Moridani M. Y.; Chan T. S.; O’Brien P. J. (2001) Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: glutathione oxidation and conjugation. Free Radical Biol. Med. 30, 370–382. 10.1016/S0891-5849(00)00481-0. [DOI] [PubMed] [Google Scholar]
- Awad H. M.; Boersma M. G.; Boeren S.; van Bladeren P. J.; Vervoort J.; Rietjens I. M. (2002) The regioselectivity of glutathione adduct formation with flavonoid quinone/quinone methides Is pH-dependent. Chem. Res. Toxicol. 15, 343–351. 10.1021/tx010132l. [DOI] [PubMed] [Google Scholar]
- Munoz-Munoz J. L.; Garcia-Molina F.; Garcia-Molina M.; Tudela J.; Garcia-Canovas F.; Rodriguez-Lopez J. N. (2009) Ellagic acid: characterization as substrate of polyphenol oxidase. IUBMB Life 61, 171–177. 10.1002/iub.143. [DOI] [PubMed] [Google Scholar]
- Boersma M. G.; Vervoort J.; Szymusiak H.; Lemanska K.; Tyrakowska B.; Cenas N.; Segura-Aguilar J.; Rietjens I. M. (2000) Regioselectivity and reversibility of the glutathione conjugation of quercetin quinone methide. Chem. Res. Toxicol. 13, 185–191. 10.1021/tx990161k. [DOI] [PubMed] [Google Scholar]
- Rietjens I. M.; Boersma M. G.; van der Woude H.; Jeurissen S. M.; Schutte M. E.; Alink G. M. (2005) Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat. Res., Fundam. Mol. Mech. Mutagen. 574, 124–138. 10.1016/j.mrfmmm.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Tu T. T.; Giblin D.; Gross M. L. (2011) Structural determinant of chemical reactivity and potential health effects of quinones from natural products. Chem. Res. Toxicol. 24, 1527–1539. 10.1021/tx200140s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings T. G.; Lewis D. A.; Zigmond M. J. (1996) Reactive dopamine metabolites and neurotoxicity: implications for Parkinson’s disease. Adv. Exp. Med. Biol. 387, 97–106. 10.1007/978-1-4757-9480-9_13. [DOI] [PubMed] [Google Scholar]
- Shen X. M.; Dryhurst G. (1996) Further insights into the influence of L-cysteine on the oxidation chemistry of dopamine: reaction pathways of potential relevance to Parkinson’s disease. Chem. Res. Toxicol. 9, 751–763. 10.1021/tx960008f. [DOI] [PubMed] [Google Scholar]
- Napolitano A.; Manini P.; d’Ischia M. (2011) Oxidation chemistry of catecholamines and neuronal degeneration: an update. Curr. Med. Chem. 18, 1832–1845. 10.2174/092986711795496863. [DOI] [PubMed] [Google Scholar]
- Singh S.; Dryhurst G. (1991) Reactions of the serotonergic neurotoxin 5,6-dihydroxytryptamine with glutathione. J. Org. Chem. 56, 1767–1773. 10.1021/jo00005a021. [DOI] [Google Scholar]
- Shen X.; Xia B.; Wrona M. Z.; Dryhurst G. (1996) Synthesis, redox properties, in vivo formation, and neurobehavioral effects of N-acetylcysteinyl conjugates of dopamine: possible metabolites of relevance to Parkinson’s disease. Chem. Res. Toxicol. 9, 1117–1126. 10.1021/tx960052v. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J.; Paris I.; Munoz P.; Ferrari E.; Zecca L.; Zucca F. A. (2014) Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 129, 898–915. 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- Liu J.; Liu H.; van Breemen R. B.; Thatcher G. R.; Bolton J. L. (2005) Bioactivation of the selective estrogen receptor modulator acolbifene to quinone methides. Chem. Res. Toxicol. 18, 174–182. 10.1021/tx0497752. [DOI] [PubMed] [Google Scholar]
- Yu L.; Liu H.; Li W.; Zhang F.; Luckie C.; van Breemen R. B.; Thatcher G. R.; Bolton J. L. (2004) Oxidation of raloxifene to quinoids: potential toxic pathways via a diquinone methide and o-quinones. Chem. Res. Toxicol. 17, 879–888. 10.1021/tx0342722. [DOI] [PubMed] [Google Scholar]
- Yu L., Zhang F., Nikolic D., Li W., van Breemen R. B., and Bolton J. L. (2002) Formation and Reactivity of a Raloxifene Quinone Methide, Aug 18–23, American Chemical Society, Washington, DC. [Google Scholar]
- Liu H.; Qin Z.; Thatcher G. R.; Bolton J. L. (2007) Uterine peroxidase-catalyzed formation of diquinone methides from the selective estrogen receptor modulators raloxifene and desmethylated arzoxifene. Chem. Res. Toxicol. 20, 1676–1684. 10.1021/tx7001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. D.; Reilly C. A.; Yost G. S. (2010) CYP3A4-Mediated oxygenation versus dehydrogenation of raloxifene. Biochemistry 49, 4466–4475. 10.1021/bi902213r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowers T. S.; Qin Z. H.; Thatcher G. R.; Bolton J. L. (2006) Bioactivation of selective estrogen receptor modulators (SERMs). Chem. Res. Toxicol. 19, 1125–1137. 10.1021/tx060126v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders E. J.; Saunders J. A. (1990) Drug therapy in pregnancy: the lessons of diethylstilbestrol, thalidomide, and Bendectin. Health Care Women Int. 11, 423–432. 10.1080/07399339009515912. [DOI] [PubMed] [Google Scholar]
- Cavalieri E.; Rogan E. (2014) The molecular etiology and prevention of estrogen-initiated cancers: Ockham’s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol. Aspects Med. 36, 1–55. 10.1016/j.mam.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehr J. G.; DaGue B. B.; Ballatore A. M.; Henkin J. (1983) Diethylstilbestrol (DES) quinone: A reactive intermediate in DES metabolism. Biochem. Pharmacol. 32, 3711–3718. 10.1016/0006-2952(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Mao F.; Ni W.; Xu X.; Wang H.; Wang J.; Ji M.; Li J. (2016) Chemical structure-related drug-like criteria of global approved drugs. Molecules 21, 75. 10.3390/molecules21010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E. L.; Rogan E. G. (2004) A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Ann. N. Y. Acad. Sci. 1028, 247–257. 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- Schwartz C. S.; Snyder R.; Kalf G. F. (1985) The inhibition of mitochondrial DNA replication in vitro by the metabolites of benzene, hydroquinone and p-benzoquinone. Chem.-Biol. Interact. 53, 327–350. 10.1016/S0009-2797(85)80108-3. [DOI] [PubMed] [Google Scholar]
- Seaton M. J.; Schlosser P. M.; Bond J. A.; Medinsky M. A. (1994) Benzene metabolism by human liver microsomes in relation to cytochrome P450 2E1 activity. Carcinogenesis 15, 1799–1806. 10.1093/carcin/15.9.1799. [DOI] [PubMed] [Google Scholar]
- Cuttle L.; Munns A. J.; Hogg N. A.; Scott J. R.; Hooper W. D.; Dickinson R. G.; Gillam E. M. (2000) Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation. Drug Metab. Dispos. 28, 945–950. [PubMed] [Google Scholar]
- Pearce R. E.; Lu W.; Wang Y.; Uetrecht J. P.; Correia M. A.; Leeder J. S. (2008) Pathways of carbamazepine bioactivation in vitro. III. The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab. Dispos. 36, 1637–1649. 10.1124/dmd.107.019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C.; Uetrecht J. P. (1999) Detection of 2-hydroxyiminostilbene in the urine of patients taking carbamazepine and its oxidation to a reactive iminoquinone intermediate. J. Pharmacol. Exp. Ther. 288, 51–56. [PubMed] [Google Scholar]
- Chowdhury G.; Shibata N.; Yamazaki H.; Guengerich F. P. (2014) Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analogue of thalidomide. Chem. Res. Toxicol. 27, 147–156. 10.1021/tx4004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E. L.; Rogan E. G. (2010) Is bisphenol A a weak carcinogen like the natural estrogens and diethylstilbestrol?. IUBMB Life 62, 746–751. 10.1002/iub.376. [DOI] [PubMed] [Google Scholar]
- Qiu S. X.; Yang R. Z.; Gross M. L. (2004) Synthesis and liquid chromatography/tandem mass spectrometric characterization of the adducts of bisphenol A o-quinone with glutathione and nucleotide monophosphates. Chem. Res. Toxicol. 17, 1038–1046. 10.1021/tx049953r. [DOI] [PubMed] [Google Scholar]
- Amaro A. R.; Oakley G. G.; Bauer U.; Spielmann H. P.; Robertson L. W. (1996) Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem. Res. Toxicol. 9, 623–629. 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- McLean M. R.; Bauer U.; Amaro A. R.; Robertson L. W. (1996) Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 9, 158–164. 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Gherezghiher T. B.; Michalsen B.; Chandrasena R. E.; Qin Z.; Sohn J.; Thatcher G. R.; Bolton J. L. (2012) The naphthol selective estrogen receptor modulator (SERM), LY2066948, is oxidized to an o-quinone analogous to the naphthol equine estrogen, equilenin. Chem.-Biol. Interact. 196, 1–10. 10.1016/j.cbi.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalsen B. T.; Gherezghiher T. B.; Choi J.; Chandrasena R. E.; Qin Z.; Thatcher G. R.; Bolton J. L. (2012) Selective estrogen receptor modulator (SERM) lasofoxifene forms reactive quinones similar to estradiol. Chem. Res. Toxicol. 25, 1472–1483. 10.1021/tx300142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D.; Zhang F.; Yu L.; Yang Y.; van Breemen R. B.; Bolton J. L. (2001) Synthesis and reactivity of potential toxic metabolites of tamoxifen analogues: droloxifene and toremifene o-quinones. Chem. Res. Toxicol. 14, 1643–1653. 10.1021/tx010137i. [DOI] [PubMed] [Google Scholar]
- Liu X.; Pisha E.; Tonetti D. A.; Yao D.; Li Y.; Yao J.; Burdette J. E.; Bolton J. L. (2003) Antiestrogenic and DNA damaging effects induced by tamoxifen and toremifene metabolites. Chem. Res. Toxicol. 16, 832–837. 10.1021/tx030004s. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Fan P. W.; Liu X.; Shen L.; van Breeman R. B.; Bolton J. L. (2000) Synthesis and reactivity of a potential carcinogenic metabolite of tamoxifen: 3,4-dihydroxytamoxifen-o-quinone. Chem. Res. Toxicol. 13, 53–62. 10.1021/tx990145n. [DOI] [PubMed] [Google Scholar]
- Liehr J. G.; Ballatore A. M.; Dague B. B.; Ulubelen A. A. (1985) Carcinogenicity and metabolic activation of hexestrol. Chem.-Biol. Interact. 55, 157–176. 10.1016/S0009-2797(85)80125-3. [DOI] [PubMed] [Google Scholar]
- Bolton J. L.; Thatcher G. R. (2008) Potential mechanisms of estrogen quinone carcinogenesis. Chem. Res. Toxicol. 21, 93–101. 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J. L.; Shen L. (1996) p-Quinone methides are the major decomposition products of catechol estrogen o-quinones. Carcinogenesis 17, 925–929. 10.1093/carcin/17.5.925. [DOI] [PubMed] [Google Scholar]
- Cao K.; Stack D. E.; Ramanathan R.; Gross M. L.; Rogan E. G.; Cavalieri E. L. (1998) Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine, and glutathione. Chem. Res. Toxicol. 11, 909–916. 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- Cavalieri E. L.; Rogan E. G. (2016) Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention. Clin. Transl. Med. 5, 12. 10.1186/s40169-016-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager J. D. (2015) Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention - A review. Steroids 99, 56–60. 10.1016/j.steroids.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Chen Y.; Pisha E.; Shen L.; Xiong Y.; van Breemen R. B.; Bolton J. L. (1999) The major metabolite of equilin, 4-hydroxyequilin, autoxidizes to an o-quinone which isomerizes to the potent cytotoxin 4-hydroxyequilenin-o-quinone. Chem. Res. Toxicol. 12, 204–213. 10.1021/tx980217v. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Yao D.; Hua Y.; van Breemen R. B.; Bolton J. L. (2001) Synthesis and reactivity of the catechol metabolites from the equine estrogen, 8,9-dehydroestrone. Chem. Res. Toxicol. 14, 754–763. 10.1021/tx010049y. [DOI] [PubMed] [Google Scholar]
- Shen L.; Pisha E.; Huang Z.; Pezzuto J. M.; Krol E.; Alam Z.; van Breemen R. B.; Bolton J. L. (1997) Bioreductive activation of catechol estrogen-ortho-quinones: Aromatization of the B ring in 4-hydroxyequilenin markedly alters quinoid formation and reactivity. Carcinogenesis 18, 1093–1101. 10.1093/carcin/18.5.1093. [DOI] [PubMed] [Google Scholar]
- Brass L. M. (2004) Hormone replacement therapy and stroke: clinical trials review. Stroke 35, 2644–2647. 10.1161/01.STR.0000143218.20061.ac. [DOI] [PubMed] [Google Scholar]
- Chlebowski R. T.; Manson J. E.; Anderson G. L.; Cauley J. A.; Aragaki A. K.; Stefanick M. L.; Lane D. S.; Johnson K. C.; Wactawski-Wende J.; Chen C.; Qi L.; Yasmeen S.; Newcomb P. A.; Prentice R. L. (2013) Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J. Natl. Cancer Inst. 105, 526–535. 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson J. (2014) The Women’s Health Initiative: the latest findings from long-term follow-up. Women's Health 10, 125–128. 10.2217/whe.14.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L.; O’Brien P. (2014) Molecular mechanism of 17alpha-ethinylestradiol cytotoxicity in isolated rat hepatocytes. Can. J. Physiol. Pharmacol. 92, 21–26. 10.1139/cjpp-2013-0267. [DOI] [PubMed] [Google Scholar]
- Piotrowska H.; Kucinska M.; Murias M. (2012) Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat. Res., Rev. Mutat. Res. 750, 60–82. 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Tu T.; d’Avignon D. A.; Gross M. L. (2009) Balance of beneficial and deleterious health effects of quinones: a case study of the chemical properties of genistein and estrone quinones. J. Am. Chem. Soc. 131, 1067–1076. 10.1021/ja806478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen J. M. S.; de Vries J.; Pappie D.; van den Akker E.; Lafleur M. V. M; Retel J.; van der Greef J.; Pinedo H. M. (1987) Cytochrome P450-mediated O-demethylation: A route in the metabolic activation of etoposide (VP-16–213). Cancer Res. 47, 4658–4662. [PubMed] [Google Scholar]
- Zheng N.; Pang S.; Oe T.; Felix C. A.; Wehrli S.; Blair I. A. (2006) Characterization of an etoposide-glutathione conjugate derived from metabolic activation by human cytochrome P450. Curr. Drug Metab. 7, 897–911. 10.2174/138920006779010638. [DOI] [PubMed] [Google Scholar]
- Barrera G. (2012) Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 1. 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Liu J.; van Breemen R. B.; Thatcher G. R.; Bolton J. L. (2005) Bioactivation of the selective estrogen receptor modulator desmethylated arzoxifene to quinoids: 4′-fluoro substitution prevents quinoid formation. Chem. Res. Toxicol. 18, 162–173. 10.1021/tx049776u. [DOI] [PubMed] [Google Scholar]
- Bertrand F.; Basketter D. A.; Roberts D. W.; Lepoittevin J. P. (1997) Skin sensitization to eugenol and isoeugenol in mice: Possible metabolic pathways involving ortho-quinone and quinone methide intermediates. Chem. Res. Toxicol. 10, 335–343. 10.1021/tx960087v. [DOI] [PubMed] [Google Scholar]
- Sakano K.; Inagaki Y.; Oikawa S.; Hiraku Y.; Kawanishi S. (2004) Copper-mediated oxidative DNA damage induced by eugenol: possible involvement of O-demethylation. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 565, 35–44. 10.1016/j.mrgentox.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Thompson D. C.; Thompson J. A.; Sugumaran M.; Moldeus P. (1993) Biological and toxicological consequences of quinone methide formation. Chem.-Biol. Interact. 86, 129–162. 10.1016/0009-2797(93)90117-H. [DOI] [PubMed] [Google Scholar]
- Sakano K.; Kawanishi S. (2002) Metal-mediated DNA damage induced by curcumin in the presence of human cytochrome P450 isozymes. Arch. Biochem. Biophys. 405, 223–230. 10.1016/S0003-9861(02)00302-8. [DOI] [PubMed] [Google Scholar]
- Schilderman P. A.; Rhijnsburger E.; Zwingmann I.; Kleinjans J. C. (1995) Induction of oxidative DNA damages and enhancement of cell proliferation in human lymphocytes in vitro by butylated hydroxyanisole. Carcinogenesis 16, 507–512. 10.1093/carcin/16.3.507. [DOI] [PubMed] [Google Scholar]
- Monks T. J.; Jones D. C.; Bai F.; Lau S. S. (2004) The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther. Drug Monit. 26, 132–136. 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Yang A. H.; He X.; Chen J. X.; He L. N.; Jin C. H.; Wang L. L.; Zhang F. L.; An L. J. (2015) Identification and characterization of reactive metabolites in myristicin-mediated mechanism-based inhibition of CYP1A2. Chem.-Biol. Interact. 237, 133–140. 10.1016/j.cbi.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Johnson B. M.; Qiu S. X.; Zhang S.; Zhang F.; Burdette J. E.; Yu L.; Bolton J. L.; van Breemen R. B. (2003) Identification of novel electrophilic metabolites of piper methysticum Forst (Kava). Chem. Res. Toxicol. 16, 733–740. 10.1021/tx020113r. [DOI] [PubMed] [Google Scholar]
- Zhao S. X.; Dalvie D. K.; Kelly J. M.; Soglia J. R.; Frederick K. S.; Smith E. B.; Obach R. S.; Kalgutkar A. S. (2007) NADPH-dependent covalent binding of [3H]paroxetine to human liver microsomes and S-9 fractions: identification of an electrophilic quinone metabolite of paroxetine. Chem. Res. Toxicol. 20, 1649–1657. 10.1021/tx700132x. [DOI] [PubMed] [Google Scholar]
- Erve J. C.; Gauby S.; Maynard J. W. Jr.; Svensson M. A.; Tonn G.; Quinn K. P. (2013) Bioactivation of sitaxentan in liver microsomes, hepatocytes, and expressed human P450s with characterization of the glutathione conjugate by liquid chromatography tandem mass spectrometry. Chem. Res. Toxicol. 26, 926–936. 10.1021/tx4001144. [DOI] [PubMed] [Google Scholar]
- Klungsoyr J.; Scheline R. R. (1982) Metabolism of isosafrole and dihydrosafrole in the rat. Biomed. Mass Spectrom. 9, 323–329. 10.1002/bms.1200090803. [DOI] [PubMed] [Google Scholar]
- Bolton J. L.; Pisha E.; Shen L.; Krol E. S.; Iverson S. L.; Huang Z.; van Breemen R. B.; Pezzuto J. M. (1997) The reactivity of o-quinones which do not isomerize to quinone methides correlates with alkylcatechol-induced toxicity in human melanoma cells. Chem.-Biol. Interact. 106, 133–148. 10.1016/S0009-2797(97)00066-5. [DOI] [PubMed] [Google Scholar]
- Penning T. M. (2014) Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 27, 1901–1917. 10.1021/tx500298n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H.; Shibata A.; Suzuki M.; Nakajima M.; Shimada N.; Guengerich F. P.; Yokoi T. (1999) Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-450 2C8 and P-450 3A4 in human liver microsomes. Drug Metab. Dispos. 27, 1260–1266. [PubMed] [Google Scholar]
- Wu J. H.; Croft K. D. (2007) Vitamin E metabolism. Mol. Aspects Med. 28, 437–452. 10.1016/j.mam.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Cornwell D. G.; Ma J. (2007) Studies in vitamin E: biochemistry and molecular biology of tocopherol quinones. Vitam. Horm. 76, 99–134. 10.1016/S0083-6729(07)76005-3. [DOI] [PubMed] [Google Scholar]
- Tafazoli S.; Spehar D. D.; O’Brien P. J. (2005) Oxidative stress mediated idiosyncratic drug toxicity. Drug Metab. Rev. 37, 311–325. 10.1081/DMR-200055227. [DOI] [PubMed] [Google Scholar]
- Wang X.; Thomas B.; Sachdeva R.; Arterburn L.; Frye L.; Hatcher P. G.; Cornwell D. G.; Ma J. (2006) Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc. Natl. Acad. Sci. U. S. A. 103, 3604–3609. 10.1073/pnas.0510962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I.; Biswas S. K.; Kirkham P. A. (2006) Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 72, 1439–1452. 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Cornwell D. G.; Williams M. V.; Wani A. A.; Wani G.; Shen E.; Jones K. H. (2002) Mutagenicity of tocopheryl quinones: evolutionary advantage of selective accumulation of dietary alpha-tocopherol. Nutr. Cancer 43, 111–118. 10.1207/S15327914NC431_13. [DOI] [PubMed] [Google Scholar]
- Iverson S. L.; Hu L. Q.; Vukomanovic V.; Bolton J. L. (1995) The influence of the para-alkyl substituent on the isomerization of o-quinones to p-quinone methides: Potential bioactivation mechanism for catechols. Chem. Res. Toxicol. 8, 537–544. 10.1021/tx00046a007. [DOI] [PubMed] [Google Scholar]
- Chandrasena R. E.; Edirisinghe P. D.; Bolton J. L.; Thatcher G. R. (2008) Problematic detoxification of estrogen quinones by NAD(P)H-dependent quinone oxidoreductase and glutathione-S-transferase. Chem. Res. Toxicol. 21, 1324–1329. 10.1021/tx8000797. [DOI] [PubMed] [Google Scholar]
- Abiko Y.; Kumagai Y. (2013) Interaction of Keap1 modified by 2-tert-butyl-1,4-benzoquinone with GSH: evidence for S-transarylation. Chem. Res. Toxicol. 26, 1080–1087. 10.1021/tx400085h. [DOI] [PubMed] [Google Scholar]
- Peng K. W.; Chang M.; Wang Y. T.; Wang Z.; Qin Z.; Bolton J. L.; Thatcher G. R. (2010) Unexpected hormonal activity of a catechol equine estrogen metabolite reveals reversible glutathione conjugation. Chem. Res. Toxicol. 23, 1374–1383. 10.1021/tx100129h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H. M.; Boersma M. G.; Boeren S.; Van Bladeren P. J.; Vervoort J.; Rietjens I. M. (2003) Quenching of quercetin quinone/quinone methides by different thiolate scavengers: stability and reversibility of conjugate formation. Chem. Res. Toxicol. 16, 822–831. 10.1021/tx020079g. [DOI] [PubMed] [Google Scholar]
- Monks T. J.; Lau S. S. (1998) The pharmacology and toxicology of polyphenolic-glutathione conjugates. Annu. Rev. Pharmacol. Toxicol. 38, 229–255. 10.1146/annurev.pharmtox.38.1.229. [DOI] [PubMed] [Google Scholar]
- Monks T. J.; Lau S. S. (1992) Toxicology of quinone-thioethers. Crit. Rev. Toxicol. 22, 243–270. 10.3109/10408449209146309. [DOI] [PubMed] [Google Scholar]
- Bittner S. (2006) When quinones meet amino acids: chemical, physical and biological consequences. Amino Acids 30, 205–224. 10.1007/s00726-005-0298-2. [DOI] [PubMed] [Google Scholar]
- Rietjens I. M.; Al Huseiny W.; Boersma M. G. (2011) Flavonoids and alkenylbenzenes: New concepts in bioactivation studies. Chem.-Biol. Interact. 192, 87–95. 10.1016/j.cbi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Brunmark A.; Cadenas E. (1989) Redox and addition chemistry of quinoid compounds and its biological implications. Free Radical Biol. Med. 7, 435–477. 10.1016/0891-5849(89)90126-3. [DOI] [PubMed] [Google Scholar]
- Ross D.; Beall H.; Traver R. D.; Siegel D.; Phillips R. M.; Gibson N. W. (1994) Bioactivation of quinones by DT-diaphorase, molecular, biochemical, and chemical studies. Oncol. Res. 6, 493–500. [PubMed] [Google Scholar]
- Colucci M. A.; Moody C. J.; Couch G. D. (2008) Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1: from synthetic organic chemistry to compounds with anticancer potential. Org. Biomol. Chem. 6, 637–656. 10.1039/B715270A. [DOI] [PubMed] [Google Scholar]
- Satoh T.; McKercher S. R.; Lipton S. A. (2013) Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radical Biol. Med. 65, 645–657. 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant T. W.; Rao D. N.; Mason R. P.; Cohen G. M. (1988) Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem.-Biol. Interact. 65, 157–173. 10.1016/0009-2797(88)90052-X. [DOI] [PubMed] [Google Scholar]
- Freitas A. A.; de Magalhaes J. P. (2011) A review and appraisal of the DNA damage theory of ageing. Mutat. Res., Rev. Mutat. Res. 728, 12–22. 10.1016/j.mrrev.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Roszkowski K.; Jozwicki W.; Blaszczyk P.; Mucha-Malecka A.; Siomek A. (2011) Oxidative damage DNA: 8-oxoGua and 8-oxodG as molecular markers of cancer. Med. Sci. Monit. 17, CR329–CR333. 10.12659/MSM.881805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V.; Yin Z.; Tew K. D.; Ronai Z. (1999) Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18, 6104–6111. 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Satoh T., Stalder R., McKercher S. R., Williamson R. E., Roth G. P., and Lipton S. A. (2015) Nrf2 and HSF-1 pathway activation via hydroquinone-based proelectrophilic small molecules is regulated by electrochemical oxidation potential. ASN Neuro. 7, DOI: 10.1177/1759091415593294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanidad K. Z.; Sukamtoh E.; Wang W.; Du Z.; Florio E.; He L.; Xiao H.; Decker E. A.; Zhang G. (2016) Oxidative conversion mediates antiproliferative effects of tert-butylhydroquinone: structure and activity relationship study. J. Agric. Food Chem. 64, 3743–3748. 10.1021/acs.jafc.6b00711. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wijewickrama G. T.; Peng K. W.; Dietz B. M.; Yuan L.; van Breemen R. B.; Bolton J. L.; Thatcher G. R. (2009) Estrogen Receptor {alpha} Enhances the Rate of Oxidative DNA Damage by Targeting an Equine Estrogen Catechol Metabolite to the Nucleus. J. Biol. Chem. 284, 8633–8642. 10.1074/jbc.M807860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen R. J.; Allred D. C.; Ardoin S. P.; Archer D. F.; Boyd N.; Braunstein G. D.; Burger H. G.; Colditz G. A.; Davis S. R.; Gambacciani M.; Gower B. A.; Henderson V. W.; Jarjour W. N.; Karas R. H.; Kleerekoper M.; Lobo R. A.; Manson J. E.; Marsden J.; Martin K. A.; Martin L.; Pinkerton J. V.; Rubinow D. R.; Teede H.; Thiboutot D. M.; Utian W. H.; Endocrine S. (2010) Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 95, s1–s66. 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opatrny L.; Dell’Aniello S.; Assouline S.; Suissa S. (2008) Hormone replacement therapy use and variations in the risk of breast cancer. BJOG 115, 169–175. 10.1111/j.1471-0528.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- Forman H. J.; Zhang H.; Rinna A. (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 30, 1–12. 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton R. C.; Garle M. J.; Fry J. R. (2000) Glutathione depletion in a liver microsomal assay as an in vitro biomarker for reactive metabolite formation. Biomarkers 5, 285–294. 10.1080/135475000413836. [DOI] [PubMed] [Google Scholar]
- Munns A. J.; De Voss J. J.; Hooper W. D.; Dickinson R. G.; Gillam E. M. (1997) Bioactivation of phenytoin by human cytochrome P450: characterization of the mechanism and targets of covalent adduct formation. Chem. Res. Toxicol. 10, 1049–1058. 10.1021/tx9700836. [DOI] [PubMed] [Google Scholar]
- Iverson S. L.; Shen L.; Anlar N.; Bolton J. L. (1996) Bioactivation of estrone and its catechol metabolites to quinoid-glutathione conjugates in rat liver microsomes. Chem. Res. Toxicol. 9, 492–499. 10.1021/tx950178c. [DOI] [PubMed] [Google Scholar]
- Flowers-Geary L.; Harvey R. G.; Penning T. M. (1993) Cytotoxicity of polycyclic aromatic hydrocarbon o-quinones in rat and human hepatoma cells. Chem. Res. Toxicol. 6, 252–260. 10.1021/tx00033a002. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Schreiber E. M.; Giorgianni A.; Yalowich J. C.; Day B. W. (2006) Myeloperoxidase-catalyzed metabolism of etoposide to its quinone and glutathione adduct forms in HL60 cells. Chem. Res. Toxicol. 19, 937–943. 10.1021/tx0600595. [DOI] [PubMed] [Google Scholar]
- Shi F.; Zhao P.; Li X.; Pan H.; Ma S.; Ding L. (2015) Cytotoxicity of luteolin in primary rat hepatocytes: the role of CYP3A-mediated ortho-benzoquinone metabolite formation and glutathione depletion. J. Appl. Toxicol. 35, 1372–1380. 10.1002/jat.3106. [DOI] [PubMed] [Google Scholar]
- Ludewig G.; Lehmann L.; Esch H.; Robertson L. W. (2008) Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ. Toxicol. Pharmacol. 25, 241–246. 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalife K. H.; Lupidi G. (2007) Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radical Res. 41, 153–161. 10.1080/10715760600978815. [DOI] [PubMed] [Google Scholar]
- Sang S.; Yang I.; Buckley B.; Ho C. T.; Yang C. S. (2007) Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (−)-epigallocatechin-3-gallate: studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radical Biol. Med. 43, 362–371. 10.1016/j.freeradbiomed.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang S.; Lambert J. D.; Hong J.; Tian S.; Lee M. J.; Stark R. E.; Ho C. T.; Yang C. S. (2005) Synthesis and structure identification of thiol conjugates of (−)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 18, 1762–1769. 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- Kensler T. W.; Wakabayashi N.; Biswal S. (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89–116. 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Lame M. W.; Jones A. D.; Wilson D. W.; Segall H. J. (2003) Protein targets of 1,4-benzoquinone and 1,4-naphthoquinone in human bronchial epithelial cells. Proteomics 3, 479–495. 10.1002/pmic.200390062. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li Q.; Yang X.; van Breemen R. B.; Bolton J. L.; Thatcher G. R. (2005) Analysis of protein covalent modification by xenobiotics using a covert oxidatively activated tag: raloxifene proof-of-principle study. Chem. Res. Toxicol. 18, 1485–1496. 10.1021/tx0501738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. W.; Sok D. E. (2003) Identification of alkylation-sensitive target chaperone proteins and their reactivity with natural products containing Michael acceptor. Arch. Pharmacal Res. 26, 1047–1054. 10.1007/BF02994757. [DOI] [PubMed] [Google Scholar]
- Nardai G.; Sass B.; Eber J.; Orosz G.; Csermely P. (2000) Reactive cysteines of the 90-kDa heat shock protein, Hsp90. Arch. Biochem. Biophys. 384, 59–67. 10.1006/abbi.2000.2075. [DOI] [PubMed] [Google Scholar]
- Jia Z.; Person M. D.; Dong J.; Shen J.; Hensley S. C.; Stevens J. L.; Monks T. J.; Lau S. S. (2004) Grp78 is essential for 11-deoxy-16,16-dimethyl PGE2-mediated cytoprotection in renal epithelial cells. Am. J. Physiol Renal Physiol 287, F1113–1122. 10.1152/ajprenal.00138.2004. [DOI] [PubMed] [Google Scholar]
- Xiong R.; Siegel D.; Ross D. (2014) Quinone-induced protein handling changes: implications for major protein handling systems in quinone-mediated toxicity. Toxicol. Appl. Pharmacol. 280, 285–295. 10.1016/j.taap.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.; Su C.; Song X.; Shi Q.; Fu J.; Hu L.; Xia X.; Song E.; Song Y. (2015) Polychlorinated biphenyl quinone induces endoplasmic reticulum stress, unfolded protein response, and calcium release. Chem. Res. Toxicol. 28, 1326–1337. 10.1021/acs.chemrestox.5b00124. [DOI] [PubMed] [Google Scholar]
- Reigan P.; Siegel D.; Guo W.; Ross D. (2011) A mechanistic and structural analysis of the inhibition of the 90-kDa heat shock protein by the benzoquinone and hydroquinone ansamycins. Mol. Pharmacol. 79, 823–832. 10.1124/mol.110.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S. B.; Hastings T. G. (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J. Neurochem. 73, 1127–1137. 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Pierce E. N.; Piyankarage S. C.; Dunlap T.; Litosh V.; Siklos M. I.; Wang Y. T.; Thatcher G. R. (2016) Prodrugs bioactivated to quinones target NF-kappaB and multiple protein networks: identification of the quinonome. Chem. Res. Toxicol. 29, 1151. 10.1021/acs.chemrestox.6b00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar J.; Liu J.; Foroozesh M.; Klein Stevens C. L. (2012) Inhibition of cytochrome p450 enzymes by quinones and anthraquinones. Chem. Res. Toxicol. 25, 357–365. 10.1021/tx2004163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg P. F.; Kent U. M.; Bumpus N. N. (2008) Mechanism-based inactivation of human cytochromes P450s: experimental characterization, reactive intermediates, and clinical implications. Chem. Res. Toxicol. 21, 189–205. 10.1021/tx7002504. [DOI] [PubMed] [Google Scholar]
- Korobkova E. A. (2015) Effect of Natural Polyphenols on CYP Metabolism: Implications for Diseases. Chem. Res. Toxicol. 28, 1359–1390. 10.1021/acs.chemrestox.5b00121. [DOI] [PubMed] [Google Scholar]
- Sridar C.; Kent U. M.; Notley L. M.; Gillam E. M.; Hollenberg P. F. (2002) Effect of tamoxifen on the enzymatic activity of human cytochrome CYP2B6. J. Pharmacol. Exp. Ther. 301, 945–952. 10.1124/jpet.301.3.945. [DOI] [PubMed] [Google Scholar]
- Notley L. M.; Crewe K. H.; Taylor P. J.; Lennard M. S.; Gillam E. M. (2005) Characterization of the human cytochrome P450 forms involved in metabolism of tamoxifen to its alpha-hydroxy and alpha,4-dihydroxy derivatives. Chem. Res. Toxicol. 18, 1611–1618. 10.1021/tx050140s. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Ngui J. S.; Doss G. A.; Wang R. W.; Cai X.; DiNinno F. P.; Blizzard T. A.; Hammond M. L.; Stearns R. A.; Evans D. C.; Baillie T. A.; Tang W. (2002) Cytochrome P450 3A4-mediated bioactivation of raloxifene: irreversible enzyme inhibition and thiol adduct formation. Chem. Res. Toxicol. 15, 907–914. 10.1021/tx0200109. [DOI] [PubMed] [Google Scholar]
- Baer B. R.; Wienkers L. C.; Rock D. A. (2007) Time-dependent inactivation of P450 3A4 by raloxifene: identification of Cys239 as the site of apoprotein alkylation. Chem. Res. Toxicol. 20, 954–964. 10.1021/tx700037e. [DOI] [PubMed] [Google Scholar]
- Bertelsen K. M.; Venkatakrishnan K.; Von Moltke L. L.; Obach R. S.; Greenblatt D. J. (2003) Apparent mechanism-based inhibition of human CYP2D6 in vitro by paroxetine: comparison with fluoxetine and quinidine. Drug Metab. Dispos. 31, 289–293. 10.1124/dmd.31.3.289. [DOI] [PubMed] [Google Scholar]
- Preskorn S. H.; Shah R.; Neff M.; Golbeck A. L.; Choi J. (2007) The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J. Psychiatr. Pract. 13, 5–12. 10.1097/00131746-200701000-00002. [DOI] [PubMed] [Google Scholar]
- Dawling S.; Roodi N.; Parl F. F. (2003) Methoxyestrogens exert feedback inhibition on cytochrome P450 1A1 and 1B1. Cancer Res. 63, 3127–3132. [PubMed] [Google Scholar]
- Landa P.; Kutil Z.; Temml V.; Vuorinen A.; Malik J.; Dvorakova M.; Marsik P.; Kokoska L.; Pribylova M.; Schuster D.; Vanek T. (2012) Redox and non-redox mechanism of in vitro cyclooxygenase inhibition by natural quinones. Planta Med. 78, 326–333. 10.1055/s-0031-1280430. [DOI] [PubMed] [Google Scholar]
- Marsik P.; Kokoska L.; Landa P.; Nepovim A.; Soudek P.; Vanek T. (2005) In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and −2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 71, 739–742. 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- Murias M.; Handler N.; Erker T.; Pleban K.; Ecker G.; Saiko P.; Szekeres T.; Jager W. (2004) Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure-activity relationship. Bioorg. Med. Chem. 12, 5571–5578. 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Seyed M. A.; Jantan I.; Bukhari S. N.; Vijayaraghavan K. (2016) A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J. Agric. Food Chem. 64, 725–737. 10.1021/acs.jafc.5b05993. [DOI] [PubMed] [Google Scholar]
- Dougados M.; Nguyen M.; Berdah L.; Mazieres B.; Vignon E.; Lequesne M.; Group E. I. S. (2001) Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum. 44, 2539–2547. . [DOI] [PubMed] [Google Scholar]
- Rintelen B.; Neumann K.; Leeb B. F. (2006) A meta-analysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch. Intern. Med. 166, 1899–1906. 10.1001/archinte.166.17.1899. [DOI] [PubMed] [Google Scholar]
- Teismann P.; Tieu K.; Choi D. K.; Wu D. C.; Naini A.; Hunot S.; Vila M.; Jackson-Lewis V.; Przedborski S. (2003) Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 100, 5473–5478. 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P. (2012) COX-2 in the neurodegenerative process of Parkinson’s disease. Biofactors 38, 395–397. 10.1002/biof.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. M.; Kuhn D. M. (2005) Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. J. Pharmacol. Exp. Ther. 313, 870–876. 10.1124/jpet.104.080242. [DOI] [PubMed] [Google Scholar]
- Goncalves L. L.; Ramkissoon A.; Wells P. G. (2009) Prostaglandin H synthase-1-catalyzed bioactivation of neurotransmitters, their precursors, and metabolites: oxidative DNA damage and electron spin resonance spectroscopy studies. Chem. Res. Toxicol. 22, 842–852. 10.1021/tx800423s. [DOI] [PubMed] [Google Scholar]
- Degen G. H.; Metzler M.; Sivarajah K. S. (1986) Co-oxidation of diethylstilbestrol and structural analogs by prostaglandin synthase. Carcinogenesis 7, 137–142. 10.1093/carcin/7.1.137. [DOI] [PubMed] [Google Scholar]
- Ross D.; Mehlhorn R. J.; Moldeus P.; Smith M. T. (1985) Metabolism of diethylstilbestrol by horseradish peroxidase and prostaglandin-H synthase. Generation of a free radical intermediate and its interaction with glutathione. J. Biol. Chem. 260, 16210–16214. [PubMed] [Google Scholar]
- Zahid M.; Kohli E.; Saeed M.; Rogan E.; Cavalieri E. (2006) The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: Implications for tumor-initiating activity. Chem. Res. Toxicol. 19, 164–172. 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- Li Y.; Yao J.; Chang M.; Nikolic D.; Yu L.; Yager J. D.; Mesecar A. D.; van Breemen R. B.; Bolton J. L. (2004) Equine catechol estrogen 4-hydroxyequilenin is a more potent inhibitor of the variant form of catechol-O-methyltransferase. Chem. Res. Toxicol. 17, 512–520. 10.1021/tx0342464. [DOI] [PubMed] [Google Scholar]
- Yao J.; Li Y.; Chang M.; Wu H.; Yang X.; Goodman J. E.; Liu X.; Liu H.; Mesecar A. D.; Van Breemen R. B.; Yager J. D.; Bolton J. L. (2003) Catechol estrogen 4-hydroxyequilenin is a substrate and an inhibitor of catechol-O-methyltransferase. Chem. Res. Toxicol. 16, 668–675. 10.1021/tx0340549. [DOI] [PubMed] [Google Scholar]
- Yager J. D.; Davidson N. E. (2006) Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 354, 270–282. 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T.; Smissman E. E.; Nerland D.; Reid J. R. (1976) Catechol O-methyltransferase. 7. Affinity labeling with the oxidation products of 6-aminodopamine. J. Med. Chem. 19, 30–37. 10.1021/jm00223a007. [DOI] [PubMed] [Google Scholar]
- Smythies J.; Galzigna L. (1998) The oxidative metabolism of catecholamines in the brain: a review. Biochim. Biophys. Acta, Gen. Subj. 1380, 159–162. 10.1016/S0304-4165(97)00131-1. [DOI] [PubMed] [Google Scholar]
- Dehn D. L.; Siegel D.; Swann E.; Moody C. J.; Ross D. (2003) Biochemical, cytotoxic, and genotoxic effects of ES936, a mechanism-based inhibitor of NAD(P)H:quinone oxidoreductase 1, in cellular systems. Mol. Pharmacol. 64, 714–720. 10.1124/mol.64.3.714. [DOI] [PubMed] [Google Scholar]
- Gustafson D. L.; Siegel D.; Rastatter J. C.; Merz A. L.; Parpal J. C.; Kepa J. K.; Ross D.; Long M. E. (2003) Kinetics of NAD(P)H:quinone oxidoreductase I (NQO1) inhibition by mitomycin C in vitro and in vivo. J. Pharmacol. Exp. Ther. 305, 1079–1086. 10.1124/jpet.103.050070. [DOI] [PubMed] [Google Scholar]
- Parkinson E. I.; Hergenrother P. J. (2015) Deoxynyboquinones as NQO1-activated cancer therapeutics. Acc. Chem. Res. 48, 2715–2733. 10.1021/acs.accounts.5b00365. [DOI] [PubMed] [Google Scholar]
- Chang M.; Shin Y. G.; van Breemen R. B.; Blond S. Y.; Bolton J. L. (2001) Structural and functional consequences of inactivation of human glutathione S-transferase P1–1 mediated by the catechol metabolite of equine estrogens, 4-hydroxyequilenin. Biochemistry 40, 4811–4820. 10.1021/bi002513o. [DOI] [PubMed] [Google Scholar]
- Abel E. L.; Lyon R. P.; Bammler T. K.; Verlinde C. L.; Lau S. S.; Monks T. J.; Eaton D. L. (2004) Estradiol metabolites as isoform-specific inhibitors of human glutathione S-transferases. Chem.-Biol. Interact. 151, 21–32. 10.1016/j.cbi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- van Ommen B.; Ploemen J. H. T. M.; Bogaards J. J. P.; Monks T. J.; Lau S. S.; van Bladeren P. J. (1991) Irreversible inhibition of rat glutathione S-transferase 1–1 by quinones and their glutathione conjugates. Biochem. J. 276, 661–666. 10.1042/bj2760661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen B.; Koster A.; Verhagen H.; van Bladeren P. J. (1992) The glutathione conjugates of tert-butyl hydroquinone as potent redox cycling agents and possible reactive agents underlying the toxicity of butylated hydroxyanisole. Biochem. Biophys. Res. Commun. 189, 309–314. 10.1016/0006-291X(92)91559-9. [DOI] [PubMed] [Google Scholar]
- Balyan R.; Kudugunti S. K.; Hamad H. A.; Yousef M. S.; Moridani M. Y. (2015) Bioactivation of luteolin by tyrosinase selectively inhibits glutathione S-transferase. Chem.-Biol. Interact. 240, 208–218. 10.1016/j.cbi.2015.08.011. [DOI] [PubMed] [Google Scholar]
- van Zanden J. J.; Ben Hamman O.; van Iersel M. L.; Boeren S.; Cnubben N. H.; Lo Bello M.; Vervoort J.; van Bladeren P. J.; Rietjens I. M. (2003) Inhibition of human glutathione S-transferase P1–1 by the flavonoid quercetin. Chem.-Biol. Interact. 145, 139–148. 10.1016/S0009-2797(02)00250-8. [DOI] [PubMed] [Google Scholar]
- Yao J.; Chang M.; Li Y.; Pisha E.; Liu X.; Yao D.; Elguindi E. C.; Blond S. Y.; Bolton J. L. (2002) Inhibition of cellular enzymes by equine catechol estrogens in human breast cancer cells: specificity for glutathione S-transferase P1–1. Chem. Res. Toxicol. 15, 935–942. 10.1021/tx020018i. [DOI] [PubMed] [Google Scholar]
- Tew K. D.; Townsend D. M. (2011) Regulatory functions of glutathione S-transferase P1–1 unrelated to detoxification. Drug Metab. Rev. 43, 179–193. 10.3109/03602532.2011.552912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V.; Yin Z.; Fuchs S. Y.; Benezra M.; Rosario L.; Tew K. D.; Pincus M. R.; Sardana M.; Henderson C. J.; Wolf C. R.; Davis R. J.; Ronai Z. (1999) Regulation of JNK signaling by GSTp. EMBO J. 18, 1321–1334. 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde E. (2010) Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 17, 1373–1380. 10.1038/cdd.2010.80. [DOI] [PubMed] [Google Scholar]
- Lee K. W. (1991) Glutathione S-transferase activities in phytophagous insects - induction and inhibition by plant phototoxins and phenols. Insect Biochem. 21, 353–361. 10.1016/0020-1790(91)90001-U. [DOI] [Google Scholar]
- Kudugunti S. K.; Thorsheim H.; Yousef M. S.; Guan L.; Moridani M. Y. (2011) The metabolic bioactivation of caffeic acid phenethyl ester (CAPE) mediated by tyrosinase selectivity inhibits glutathione S-transferase. Chem.-Biol. Interact. 192, 243–256. 10.1016/j.cbi.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T.; Wang X. J. (2011) Induction of the Keap1/Nrf2/ARE pathway by oxidizable diphenols. Chem.-Biol. Interact. 192, 101–106. 10.1016/j.cbi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Surh Y. J.; Kundu J. K.; Na H. K. (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 74, 1526–1539. 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- Tebay L. E.; Robertson H.; Durant S. T.; Vitale S. R.; Penning T. M.; Dinkova-Kostova A. T.; Hayes J. D. (2015) Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radical Biol. Med. 88, 108–146. 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T.; Yamamoto M. (2015) Molecular basis of the Keap1-Nrf2 system. Free Radical Biol. Med. 88, 93–100. 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Kensler T. W.; Wakabayashi N. (2010) Nrf2: friend or foe for chemoprevention?. Carcinogenesis 31, 90–99. 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum Y. S.; Han Y. H.; Liew C.; Kim J. H.; Xu C.; Yuan X.; Shakarjian M. P.; Chong S.; Kong A. N. (2006) Induction of Heme Oxygenase-1 (HO-1) and NAD[P]H: Quinone Oxidoreductase 1 (NQO1) by a Phenolic Antioxidant, Butylated Hydroxyanisole (BHA) and Its Metabolite, tert-Butylhydroquinone (tBHQ) in Primary-Cultured Human and Rat Hepatocytes. Pharm. Res. 23, 2586–2594. 10.1007/s11095-006-9094-2. [DOI] [PubMed] [Google Scholar]
- Sumi D.; Numasawa Y.; Endo A.; Iwamoto N.; Kumagai Y. (2009) Catechol estrogens mediated activation of Nrf2 through covalent modification of its quinone metabolite to Keap1. J. Toxicol. Sci. 34, 627–635. 10.2131/jts.34.627. [DOI] [PubMed] [Google Scholar]
- Magesh S.; Chen Y.; Hu L. (2012) Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 32, 687–726. 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa S.; Fujii M.; Hou D. X. (2007) Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radical Biol. Med. 42, 1690–1703. 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Lee-Hilz Y. Y.; Boerboom A. M.; Westphal A. H.; Berkel W. J.; Aarts J. M.; Rietjens I. M. (2006) Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 19, 1499–1505. 10.1021/tx060157q. [DOI] [PubMed] [Google Scholar]
- Muzolf-Panek M.; Gliszczynska-Swiglo A.; de Haan L.; Aarts J. M.; Szymusiak H.; Vervoort J. M.; Tyrakowska B.; Rietjens I. M. (2008) Role of catechin quinones in the induction of EpRE-mediated gene expression. Chem. Res. Toxicol. 21, 2352–2360. 10.1021/tx8001498. [DOI] [PubMed] [Google Scholar]
- Satoh T.; Kosaka K.; Itoh K.; Kobayashi A.; Yamamoto M.; Shimojo Y.; Kitajima C.; Cui J.; Kamins J.; Okamoto S.; Izumi M.; Shirasawa T.; Lipton S. A. (2008) Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J. Neurochem. 104, 1116–1131. 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.; Shin D. H.; Kim J. H.; Hong S.; Choi D.; Kim Y. J.; Kwak M. K.; Jung Y. (2010) Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: structural analysis for NFkappaB inhibition. Eur. J. Pharmacol. 643, 21–28. 10.1016/j.ejphar.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Wang X. J.; Sun Z.; Villeneuve N. F.; Zhang S.; Zhao F.; Li Y.; Chen W.; Yi X.; Zheng W.; Wondrak G. T.; Wong P. K.; Zhang D. D. (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29, 1235–1243. 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B.; Liby K. T. (2012) NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer 12, 564–571. 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. (2013) Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426. 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.; Penning T. M. (2007) Aldo-keto reductases and bioactivation/detoxication. Annu. Rev. Pharmacol. Toxicol. 47, 263–292. 10.1146/annurev.pharmtox.47.120505.105337. [DOI] [PubMed] [Google Scholar]
- Morgan M. J.; Liu Z. G. (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 21, 103–115. 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi M. M.; Sung B.; Yadav V. R.; Kannappan R.; Aggarwal B. B. (2011) NF-kappaB addiction and its role in cancer: ’one size does not fit all’. Oncogene 30, 1615–1630. 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi D.; Akimori M.; Inoue K.; Takano H.; Kumagai Y. (2010) 1,2-Naphthoquinone suppresses lipopolysaccharide-dependent activation of IKKbeta/NF-kappaB/NO signaling: an alternative mechanism for the disturbance of inducible NO synthase-catalyzed NO formation. J. Toxicol. Sci. 35, 891–898. 10.2131/jts.35.891. [DOI] [PubMed] [Google Scholar]
- Dong G. Z.; Oh E. T.; Lee H.; Park M. T.; Song C. W.; Park H. J. (2010) Beta-lapachone suppresses radiation-induced activation of nuclear factor-kappaB. Exp. Mol. Med. 42, 327–334. 10.3858/emm.2010.42.5.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son P. S.; Park S. A.; Na H. K.; Jue D. M.; Kim S.; Surh Y. J. (2010) Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-{kappa}B activation and cyclooxygenase-2 expression in human breast epithelial cells: cysteine 179 of IKK{beta} as a potential target. Carcinogenesis 31, 1442–1449. 10.1093/carcin/bgq099. [DOI] [PubMed] [Google Scholar]
- Liu L.; Li J.; Kundu J. K.; Surh Y. J. (2014) Piceatannol inhibits phorbol ester-induced expression of COX-2 and iNOS in HR-1 hairless mouse skin by blocking the activation of NF-kappaB and AP-1. Inflammation Res. 63, 1013–1021. 10.1007/s00011-014-0777-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Duffhues G.; Calzado M. A.; de Vinuesa A. G.; Appendino G.; Fiebich B. L.; Loock U.; Lefarth-Risse A.; Krohn K.; Munoz E. (2009) Denbinobin inhibits nuclear factor-kappaB and induces apoptosis via reactive oxygen species generation in human leukemic cells. Biochem. Pharmacol. 77, 1401–1409. 10.1016/j.bcp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Ogino S.; Tsuruma K.; Uehara T.; Nomura Y. (2004) Herbimycin A abrogates nuclear factor-kappaB activation by interacting preferentially with the IkappaB kinase beta subunit. Mol. Pharmacol. 65, 1344–1351. 10.1124/mol.65.6.1344. [DOI] [PubMed] [Google Scholar]
- Park S. A.; Na H. K.; Kim E. H.; Cha Y. N.; Surh Y. J. (2009) 4-hydroxyestradiol induces anchorage-independent growth of human mammary epithelial cells via activation of IkappaB kinase: potential role of reactive oxygen species. Cancer Res. 69, 2416–2424. 10.1158/0008-5472.CAN-08-2177. [DOI] [PubMed] [Google Scholar]
- Burczynski M. E.; Penning T. M. (2000) Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 60, 908–915. [PubMed] [Google Scholar]
- Abiko Y.; Puga A.; Kumagai Y. (2015) Covalent binding of quinones activates the Ah receptor in Hepa1c1c7 cells. J. Toxicol. Sci. 40, 873–886. 10.2131/jts.40.873. [DOI] [PubMed] [Google Scholar]
- Xiao W.; Son J.; Vorrink S. U.; Domann F. E.; Goswami P. C. (2015) Ligand-independent activation of aryl hydrocarbon receptor signaling in PCB3-quinone treated HaCaT human keratinocytes. Toxicol. Lett. 233, 258–266. 10.1016/j.toxlet.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H.; Mangal D.; Frey A. J.; Harvey R. G.; Blair I. A.; Penning T. M. (2009) Aryl hydrocarbon receptor facilitates DNA strand breaks and 8-oxo-2′-deoxyguanosine formation by the aldo-keto reductase product benzo[a]pyrene-7,8-dione. J. Biol. Chem. 284, 29725–29734. 10.1074/jbc.M109.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S.; Olsen A.; Moller P.; Poulsen H. E.; Tjonneland A. (2013) Association between 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case-control study. Cancer Epidemiol., Biomarkers Prev. 22, 1289–1296. 10.1158/1055-9965.EPI-13-0229. [DOI] [PubMed] [Google Scholar]
- Zahid M.; Saeed M.; Yang L.; Beseler C.; Rogan E.; Cavalieri E. L. (2011) Formation of dopamine quinone-DNA adducts and their potential role in the etiology of Parkinson’s disease. IUBMB Life 63, 1087–1093. 10.1002/iub.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E. L.; Rogan E. G. (2010) Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 6, 75–91. 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Gaikwad N. W.; Meza J.; Cavalieri E. L.; Muti P.; Trock B.; Rogan E. G. (2009) Novel biomarkers for risk of prostate cancer: results from a case-control study. Prostate 69, 41–48. 10.1002/pros.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markushin Y.; Gaikwad N.; Zhang H.; Kapke P.; Rogan E. G.; Cavalieri E. L.; Trock B. J.; Pavlovich C.; Jankowiak R. (2006) Potential biomarker for early risk assessment of prostate cancer. Prostate 66, 1565–1571. 10.1002/pros.20484. [DOI] [PubMed] [Google Scholar]
- Markushin Y.; Zhong W.; Cavalieri E. L.; Rogan E. G.; Small G. J.; Yeung E. S.; Jankowiak R. (2003) Spectral characterization of catechol estrogen quinone (CEQ)-derived DNA adducts and their identification in human breast tissue extract. Chem. Res. Toxicol. 16, 1107–1117. 10.1021/tx0340854. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Fang J.; Li S.; Wei J.; Yang Z.; Zhao H.; Zhao C.; Cai Z. (2016) Interaction of bisphenol A 3,4-quinone metabolite with glutathione and ribonucleosides/deoxyribonucleosides in vitro. J. Hazard. Mater. 10.1016/j.jhazmat.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Edmonds J. S.; Nomachi M.; Terasaki M.; Morita M.; Skelton B. W.; White A. H. (2004) The reaction of bisphenol A 3,4-quinone with DNA. Biochem. Biophys. Res. Commun. 319, 556–561. 10.1016/j.bbrc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- McCoull K. D.; Rindgen D.; Blair I. A.; Penning T. M. (1999) Synthesis and characterization of polycyclic aromatic hydrocarbon o-quinone depurinating N7-guanine adducts. Chem. Res. Toxicol. 12, 237–246. 10.1021/tx980182z. [DOI] [PubMed] [Google Scholar]
- Shen L.; Qiu S.; Chen Y.; Zhang F.; van Breemen R. B.; Nikolic D.; Bolton J. L. (1998) Alkylation of 2′-deoxynucleosides and DNA by the Premarin metabolite 4-hydroxyequilenin semiquinone radical. Chem. Res. Toxicol. 11, 94–101. 10.1021/tx970181r. [DOI] [PubMed] [Google Scholar]
- Shen L.; Qiu S.; van Breemen R. B.; Zhang F.; Chen Y.; Bolton J. L. (1997) Reaction of the Premarin® metabolite 4-hydroxyequilenin semiquinone radical with 2′-deoxyguanosine: Formation of unusual cyclic adducts. J. Am. Chem. Soc. 119, 11126–11127. 10.1021/ja971396q. [DOI] [Google Scholar]
- Balu N.; Padgett W. T.; Lambert G. R.; Swank A. E.; Richard A. M.; Nesnow S. (2004) Identification and characterization of novel stable deoxyguanosine and deoxyadenosine adducts of benzo[a]pyrene-7,8-quinone from reactions at physiological pH. Chem. Res. Toxicol. 17, 827–838. 10.1021/tx034207s. [DOI] [PubMed] [Google Scholar]
- Balu N.; Padgett W. T.; Nelson G. B.; Lambert G. R.; Ross J. A.; Nesnow S. (2006) Benzo[a]pyrene-7,8-quinone-3′-mononucleotide adduct standards for 32P postlabeling analyses: detection of benzo[a]pyrene-7,8-quinone-calf thymus DNA adducts. Anal. Biochem. 355, 213–223. 10.1016/j.ab.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Edirisinghe P.; Sohn J.; Qin Z.; Geacintov N. E.; Thatcher G. R.; Bolton J. L. (2009) Development of a liquid chromatography electrospray ionization tandem mass spectrometry method for analysis of stable 4-hydroxyequilenin-DNA adducts in human breast cancer cells. Chem. Res. Toxicol. 22, 1129–1136. 10.1021/tx900063g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.; Wang Y.; Kolbanovskiy A.; Durandin A.; Bolton J. L.; van Breemen R. B.; Broyde S.; Geacintov N. E. (2008) Determination of absolute configurations of 4-hydroxyequilenin-cytosine and -adenine adducts by optical rotatory dispersion, electronic circular dichroism, density functional theory calculations, and mass spectrometry. Chem. Res. Toxicol. 21, 1739–1748. 10.1021/tx800095f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbanovskiy A.; Kuzmin V.; Shastry A.; Kolbanovskaya M.; Chen D.; Chang M.; Bolton J. L.; Geacintov N. E. (2005) Base selectivity and effects of sequence and DNA secondary structure on the formation of covalent adducts derived from the equine estrogen metabolite 4-hydroxyequilenin. Chem. Res. Toxicol. 18, 1737–1747. 10.1021/tx050190x. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Swanson S. M.; van Breemen R. B.; Liu X.; Yang Y.; Gu C.; Bolton J. L. (2001) Equine estrogen metabolite 4-hydroxyequilenin induces DNA damage in the rat mammary tissues: formation of single-strand breaks, apurinic sites, stable adducts, and oxidized bases. Chem. Res. Toxicol. 14, 1654–1659. 10.1021/tx010158c. [DOI] [PubMed] [Google Scholar]
- Huang M.; Blair I. A.; Penning T. M. (2013) Identification of stable benzo[a]pyrene-7,8-dione-DNA adducts in human lung cells. Chem. Res. Toxicol. 26, 685–692. 10.1021/tx300476m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracz K.; Bodell W. J. (1991) Detection of 3′-hydroxy-1,N6-benzetheno-2′-deoxyadenosine 3′-phosphate by 32P postlabeling of DNA reacted with p-benzoquinone. Chem. Res. Toxicol. 4, 199–202. 10.1021/tx00020a012. [DOI] [PubMed] [Google Scholar]
- Wang P.; Gao J.; Li G.; Shimelis O.; Giese R. W. (2012) Nontargeted analysis of DNA adducts by mass-tag MS: reaction of p-benzoquinone with DNA. Chem. Res. Toxicol. 25, 2737–2743. 10.1021/tx300363a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.; Zhang Y.; Guliaev A. B.; Shen H.; Hang B.; Singer B.; Wang Z. (2005) The p-benzoquinone DNA adducts derived from benzene are highly mutagenic. DNA Repair 4, 1399–1409. 10.1016/j.dnarep.2005.08.012. [DOI] [PubMed] [Google Scholar]
- van der Woude H.; Alink G. M.; van Rossum B. E.; Walle K.; van Steeg H.; Walle T.; Rietjens I. M. (2005) Formation of transient covalent protein and DNA adducts by quercetin in cells with and without oxidative enzyme activity. Chem. Res. Toxicol. 18, 1907–1916. 10.1021/tx050201m. [DOI] [PubMed] [Google Scholar]
- Resende F. A.; Vilegas W.; Dos Santos L. C.; Varanda E. A. (2012) Mutagenicity of flavonoids assayed by bacterial reverse mutation (Ames) test. Molecules 17, 5255–5268. 10.3390/molecules17055255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek-van den Hil E. F.; van Schothorst E. M.; van der Stelt I.; Hollman P. C.; Keijer J.; Rietjens I. M. (2015) Quercetin tests negative for genotoxicity in transcriptome analyses of liver and small intestine of mice. Food Chem. Toxicol. 81, 34–39. 10.1016/j.fct.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K.; Ames B. N. (1991) Assays for 8-hydroxy-2′-deoxyguanosine: A biomarker of in vivo oxidative DNA damage. Free Radical Biol. Med. 10, 211–216. 10.1016/0891-5849(91)90078-H. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. (1990) The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis 11, 1447–1450. 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Dong H.; Shi Q.; Song X.; Fu J.; Hu L.; Xu D.; Su C.; Xia X.; Song E.; Song Y. (2015) Polychlorinated biphenyl quinone induces oxidative DNA damage and repair responses: The activations of NHEJ, BER and NER via ATM-p53 signaling axis. Toxicol. Appl. Pharmacol. 286, 10–16. 10.1016/j.taap.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Seike K.; Murata M.; Oikawa S.; Hiraku Y.; Hirakawa K.; Kawanishi S. (2003) Oxidative DNA damage induced by benz[a]anthracene metabolites via redox cycles of quinone and unique non-quinone. Chem. Res. Toxicol. 16, 1470–1476. 10.1021/tx034103h. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Troxel A. B.; Harvey R. G.; Penning T. M. (2006) Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the aldo-keto-reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-oxo-dGuo via reactive oxygen species. Chem. Res. Toxicol. 19, 719–728. 10.1021/tx0600245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H.; Mangal D.; Tacka K. A.; Quinn A. M.; Harvey R. G.; Blair I. A.; Penning T. M. (2008) Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc. Natl. Acad. Sci. U. S. A. 105, 6846–6851. 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P.; Pfeifer G. P. (2001) Patterns of p53 G-- > T transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis 22, 367–374. 10.1093/carcin/22.3.367. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Gelhaus S.; Vedantam S.; Oliva A. L.; Batra A.; Blair I. A.; Troxel A. B.; Field J.; Penning T. M. (2008) The pattern of p53 mutations caused by PAH o-quinones is driven by 8-oxo-dGuo formation while the spectrum of mutations is determined by biological selection for dominance. Chem. Res. Toxicol. 21, 1039–1049. 10.1021/tx700404a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. S.; Bicknell R. (2001) Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3, 323–327. 10.1186/bcr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Liehr J. G. (1994) 8-Hydroxylation of guanine bases in kidney and liver DNA of hamsters treated with estradiol. Role of free radicals in estrogen-induced carcinogenesis. Cancer Res. 54, 5515–5517. [PubMed] [Google Scholar]
- Nutter L. M.; Ngo E. O.; Abul-Hajj Y. J. (1991) Characterization of DNA damage induced by 3,4-estrone-o-quinone in human cells. J. Biol. Chem. 266, 16380–16386. [PubMed] [Google Scholar]
- Nutter L. M.; Wu Y.; Ngo E. O.; Sierra E. E.; Gutierrez P. L.; Abul-Hajj Y. J. (1994) An o-quinone form of estrogen produces free radicals in human breast cancer cells: Correlation with DNA damage. Chem. Res. Toxicol. 7, 23–28. 10.1021/tx00037a004. [DOI] [PubMed] [Google Scholar]
- Li J. J.; Gonzalez A.; Banerjee S.; Banerjee S. K.; Li S. A. (1993) Estrogen carcinogenesis in the hamster kidney: role of cytotoxicity and cell proliferation. Environ. Health Perspect. 101, 259–264. 10.1289/ehp.93101s5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K.; Banerjee S.; Li S. A.; Li J. J. (1994) Induction of chromosome aberrations in Syrian hamster renal cortical cells by various estrogens. Mutat. Res., Fundam. Mol. Mech. Mutagen. 311, 191–197. 10.1016/0027-5107(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Spencer W. A.; Vadhanam M. V.; Jeyabalan J.; Gupta R. C. (2012) Oxidative DNA damage following microsome/Cu(II)-mediated activation of the estrogens, 17beta-estradiol, equilenin, and equilin: role of reactive oxygen species. Chem. Res. Toxicol. 25, 305–314. 10.1021/tx200356v. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Chandrasena E. R.; Yuan Y.; Peng K. W.; van Breemen R. B.; Thatcher G. R.; Bolton J. L. (2010) Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens. Chem. Res. Toxicol. 23, 1365–1373. 10.1021/tx1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Shen L.; Zhang F.; Lau S. S.; van Breemen R. B.; Nikolic D.; Bolton J. L. (1998) The equine estrogen metabolite 4-hydroxyequilenin causes DNA single strand breaks and oxidation of DNA bases in vitro. Chem. Res. Toxicol. 11, 1105–1111. 10.1021/tx980083l. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Liu X.; Pisha E.; Constantinou A. I.; Hua Y.; Shen L.; van Breemen R. B.; Elguindi E. C.; Blond S. Y.; Zhang F.; Bolton J. L. (2000) A metabolite of equine estrogens, 4-hydroxyequilenin, induces DNA damage and apoptosis in breast cancer cell lines. Chem. Res. Toxicol. 13, 342–350. 10.1021/tx990186j. [DOI] [PubMed] [Google Scholar]
- Han X.; Liehr J. G. (1995) Microsome-mediated 8-hydroxylation of guanine bases of DNA by steroid estrogens: correlation of DNA damage by free radicals with metabolic activation to quinones. Carcinogenesis 16, 2571–2574. 10.1093/carcin/16.10.2571. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Yang Y.; Wang X.; Chong Z.; Yin R.; Song S. H.; Zhao C.; Li C.; Huang H.; Sun B. F.; Wu D.; Jin K. X.; Song M.; Zhu B. Z.; Jiang G.; Rendtlew Danielsen J. M.; Xu G. L.; Yang Y. G.; Wang H. (2014) Redox-active quinones induces genome-wide DNA methylation changes by an iron-mediated and Tet-dependent mechanism. Nucleic Acids Res. 42, 1593–1605. 10.1093/nar/gkt1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietjens I. M. C. M.; Alink G. M. (2006) Future of toxicology - Low-dose toxicology and risk- benefit analysis. Chem. Res. Toxicol. 19, 977–981. 10.1021/tx0601051. [DOI] [PubMed] [Google Scholar]
- Dunlap T.; Piyankarage S. C.; Wijewickrama G. T.; Abdul-Hay S.; Vanni M.; Litosh V.; Luo J.; Thatcher G. R. (2012) Quinone-induced activation of Keap1/Nrf2 signaling by aspirin prodrugs masquerading as nitric oxide. Chem. Res. Toxicol. 25, 2725–2736. 10.1021/tx3003609. [DOI] [PMC free article] [PubMed] [Google Scholar]