Abstract
Methylglyoxal (MG) is a reactive metabolite that forms adducts on cysteine, lysine and arginine residues of proteins, thereby affecting their function. Methylglyoxal is detoxified by the Glyoxalase system, consisting of two enzymes, Glo1 and Glo2, that act sequentially to convert MG into d-lactate. Recently, the Parkinsonism-associated protein DJ-1 was described in vitro to have glyoxalase activity, thereby detoxifying the MG metabolite, or deglycase activity, thereby removing the adduct formed by MG on proteins. Since Drosophila is an established model system to study signaling, neurodegeneration, and metabolic regulation in vivo, we asked whether DJ-1 contributes to MG detoxification in vivo. Using both DJ-1 knockdown in Drosophila cells in culture, and DJ-1β knock-out flies, we could detect no contribution of DJ-1 to survival to MG challenge or to accumulation of MG protein adducts. Furthermore, we provide data suggesting that the previously reported deglycation activity of DJ-1 can be ascribed to a TRIS buffer artifact.
Keywords: development, Drosophila, glucose metabolism, glycation, metabolism
Introduction
Reactive metabolites have been identified as key players in an increasing number of diseases. While reactive oxygen species (ROS)3 have been linked to cancer and age-related diseases like Parkinsonism (1), reactive carbonyl species (RCS) have been linked to the pathogenesis of diabetic late complications and atherosclerosis (2, 3). One such RCS is methylglyoxal (MG), which forms as a byproduct of flux through various metabolic pathways including glycolysis (4, 5). Methylglyoxal reacts with cysteine, lysine and arginine residues on proteins to form advanced glycation end-products (AGEs) that affect protein function (5, 6). Administration of MG to mice, rats, or cells in culture, results in physiological changes observed in diabetic patients such as collagen accumulation in kidneys, hypercholesterolemia, microvasculature degeneration, and insulin resistance (7–9).
One mechanism that detoxifies MG is the glyoxalase system, composed of two enzymes Glyoxalase 1 (Glo1) and Glyoxalase 2, which act sequentially to convert MG into d-lactate via a mechanism requiring reduced glutathione (2). Recently the Parkinsonism-associated protein PARK7/DJ-1 (10) was reported to be a novel glyoxalase (11). DJ-1 was reported to possess glyoxalase activity in vitro, converting glyoxal or methyglyoxal into glycolic or lactic acid, respectively, in the absence of glutathione, and to protect from methylglyoxal-induced tissue damage in Caenorhabditis elegans (11). Separately, DJ-1 was also reported to have deglycase activity in vitro, enzymatically removing early-stage methylglyoxal and glyoxal adducts from protein side-chains, thereby preventing the formation of irreversible AGEs (12). Both of these findings are intriguing, since they would place DJ-1 at the intersection between detoxification of reactive oxygen species and detoxification of reactive carbonyl species (10).
Drosophila is a well-established model system for studying signaling pathways, lifespan, and neurodegeneration. For instance, mutation of two familial Parkinsonism genes, Pink and Parkin, in Drosophila recapitulates many of the cornerstone molecular and neurological phenotypes of Parkinson disease (13).
To study the role of DJ-1 in MG detoxification in vivo, we study here Drosophila DJ-1 in cell culture and in an animal model system. We find no evidence for a role of Drosophila DJ-1 in protecting cells or flies from methylglyoxal. Instead, we find that the reported in vitro cysteine deglycase activity of DJ-1 is due to a buffer artifact, which can be attributed to TRIS buffer.
Results
Loss of DJ-1β Does Not Affect Cellular Response to Methylglyoxal Challenge in Cell Culture
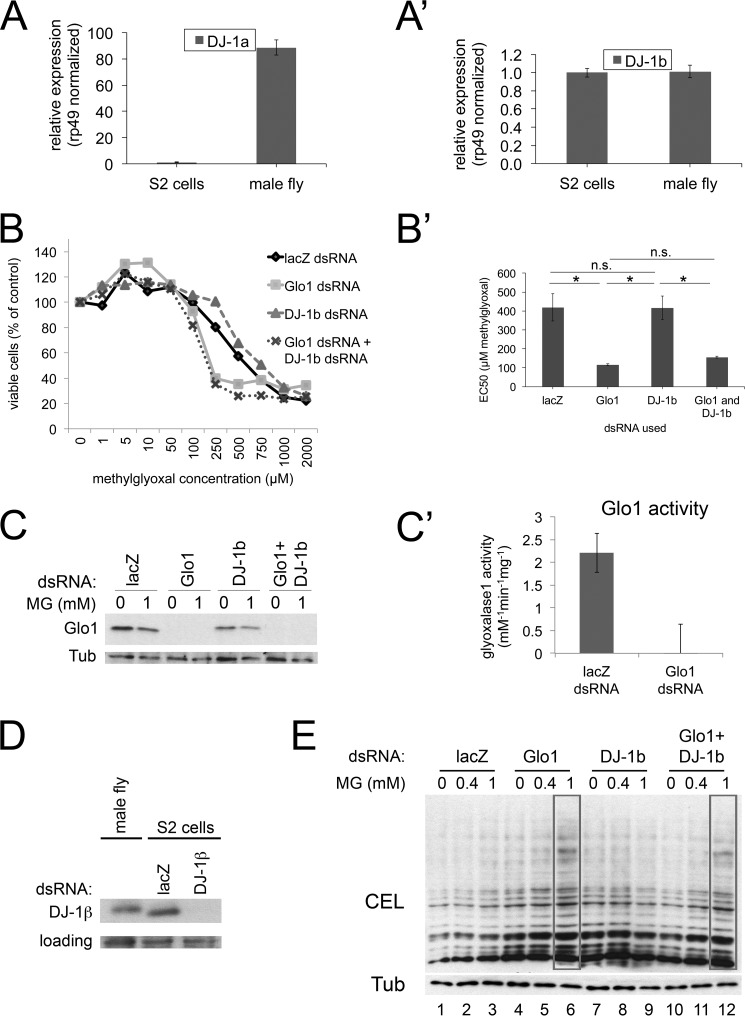
To study the function of DJ-1 in MG detoxification in Drosophila, we first studied Drosophila S2 cells. Drosophila has two DJ-1 homologs, DJ-1α and DJ-1β (14–17). DJ-1α is expressed in male testis whereas DJ-1β is expressed ubiquitously (14–16). Consistent with this, we could detect DJ-1β but not DJ-1α in Drosophila S2 cells (Fig. 1, A–A′), indicating that knocking down DJ-1β is sufficient to remove DJ-1 function in S2 cells. To ask whether DJ-1 contributes meaningfully to MG detoxification in vivo, we performed viability assays on S2 cells treated with MG in the culture medium (Fig. 1, B–B′). S2 cells with a control, non-targeting lacZ dsRNA knockdown display an MG LD50 of ∼400 μm (Fig. 1, B–B′). As a positive control, treatment of S2 cells with Glyoxalase 1 (Glo1) dsRNA, which efficiently knocks down Glo1 protein and activity (Fig. 1, C–C′), sensitizes them to MG treatment, causing the MG LD50 to drop to ∼100 μm (Fig. 1, B–B′). We then optimized the knockdown of DJ-1β so as to lead to an efficient depletion of DJ-1β protein in S2 cells (Fig. 1D). In contrast to Glo1 knockdown, DJ-1β knockdown caused no significant change in the MG LD50 (Fig. 1, B–B′). To test whether DJ-1 and Glo1 might act in a synergistic fashion, or whether a function of DJ-1 might only become visible in the absence of Glo1, we knocked-down DJ-1β and Glo1 simultaneously, but could detect no additional effect of DJ-1β knockdown compared with Glo1 knockdown alone (Fig. 1, B–B′).
FIGURE 1.
DJ-1β knockdown does not affect the response of S2 cells to methylglyoxal challenge. A–A′, S2 cells express detectable DJ-1β but not DJ-1α. mRNA levels detected by quantitative RT-PCR, normalized to rp49, and compared with mRNA from adult males, which express both DJ-1α and DJ-1β. B–D, DJ-1β knockdown does not affect the viability of S2 cells in response to methylglyoxal challenge. S2 cells were treated with a non-targeting negative control dsRNA (lacZ), or dsRNAs targeting glyoxalase Glo1 as a positive control, DJ-1β, or Glo1 and DJ-1β combined. Glo1 dsRNA efficiently knocks down Glo1 protein (C) and activity (C′). DJ-1β knockdown efficiently depletes DJ-1β protein (D). Cells treated with indicated MG concentrations for 48 h were scored for viability via an MTT assay, showing that Glo1 knockdown leads to reduced ability to withstand MG whereas DJ-1β knockdown does not (B–B′). All data points were measured in biological quadruplicates, and the experiment was repeated four times. Error bars: Std. Error (B′), Std. Dev. (C′). E, addition of 1 mm methylglyoxal to S2 cells results in increased levels of the MG adduct CEL only in Glo1 knockdown cells but not in DJ-1β knockdown cells.
MG causes formation of advanced glycation end-products on proteins, one of which is Nϵ-carboxymethyllysine (CEL), which can be detected by immunoblotting whole cell lysates. To test a readout of MG that is more direct than cell viability, we detected CEL adducts in lysates of S2 cells treated with MG (Fig. 1E). Control cells show little to no increase in CEL adducts when treated with 1 mm MG (Fig. 1E, lanes 1–3), indicating they are capable of efficiently detoxifying MG. Knockdown of Glo1 causes a detectable impairment of MG detoxification activity in vivo, since CEL adducts are clearly increased upon treatment with 1 mm MG (Fig. 1E, lane 6). In contrast, DJ-1β knockdown caused no detectable increase in CEL adducts compared with control cells (lane 9), and combined knockdown of DJ-1β and Glo1 showed no additional phenotype compared with Glo1 knockdown alone (Fig. 1E, lanes 12 versus 6). Together, these results indicate that we can detect no contribution of DJ-1 to methylglyoxal tolerance in Drosophila cell culture.
Loss of DJ-1β Does Not Lead to Elevated AGE Formation in Vivo in Flies
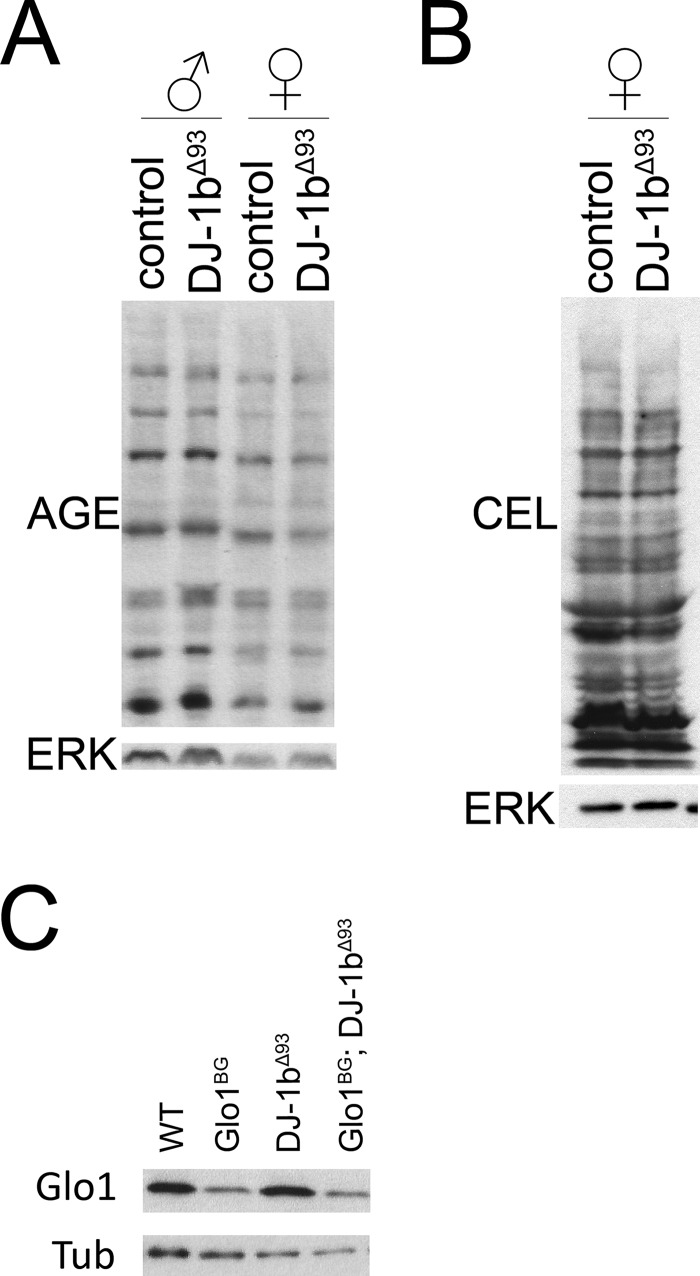
We next studied the role of DJ-1 in vivo in the fly, using previously described DJ-1β[Δ93]-null flies which completely lack the DJ-1β gene (15). As we observed in Drosophila S2 cells, knock-out of DJ-1β did not lead to elevated levels of MG adducts in vivo, assayed either with an antibody that detects a range of MG adducts such as CML or imidazolone (AGE, Fig. 2A) or an antibody that specifically detects CEL (Fig. 2B). In particular, elevated MG adducts could not be detected in females that lack expression of the other DJ-1 homolog DJ-1α (Fig. 2, A–B). Furthermore, we crossed the DJ-1β[Δ93] mutation into a genetic background with strongly reduced Glo1 protein levels (Glo1[BG02656] mutants, Fig. 2C), to test whether the two genes interact genetically. However, DJ-1β[Δ93], Glo1[BG02656] double mutants had no obvious phenotypes or impaired viability.
FIGURE 2.
DJ-1β knock-out flies do not have elevated levels of AGEs. A–B, DJ-1β knock-out flies do not have elevated levels of AGEs. Flies were grown under density controlled conditions and aged 6 weeks to allow accumulation of MG adducts. MG-adducts were detected either with an antibody that recognizes a variety of MG adducts (AGE, panel A), or specifically CEL (panel B). C, DJ-1β knock-out mutation was crossed into Glo1[BG02656] hypomorphic mutants, which have strongly reduced levels of Glo1 protein, detected by immunoblotting.
In Vitro Cysteine Deglycase Activity of DJ-1 May Be a Buffer Artifact Attributable to TRIS
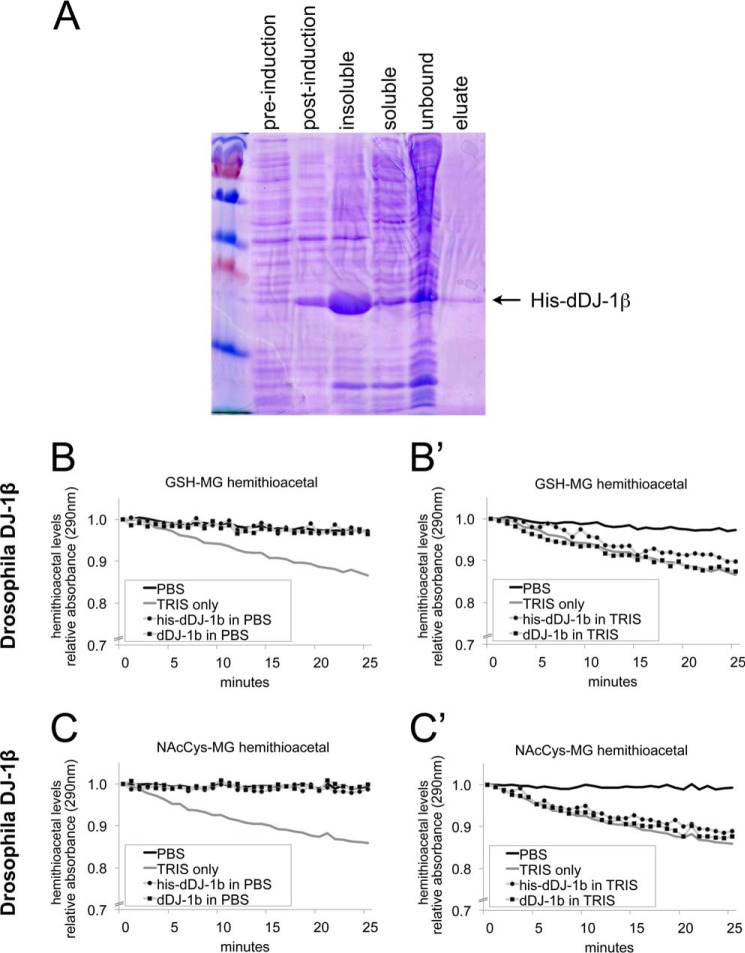
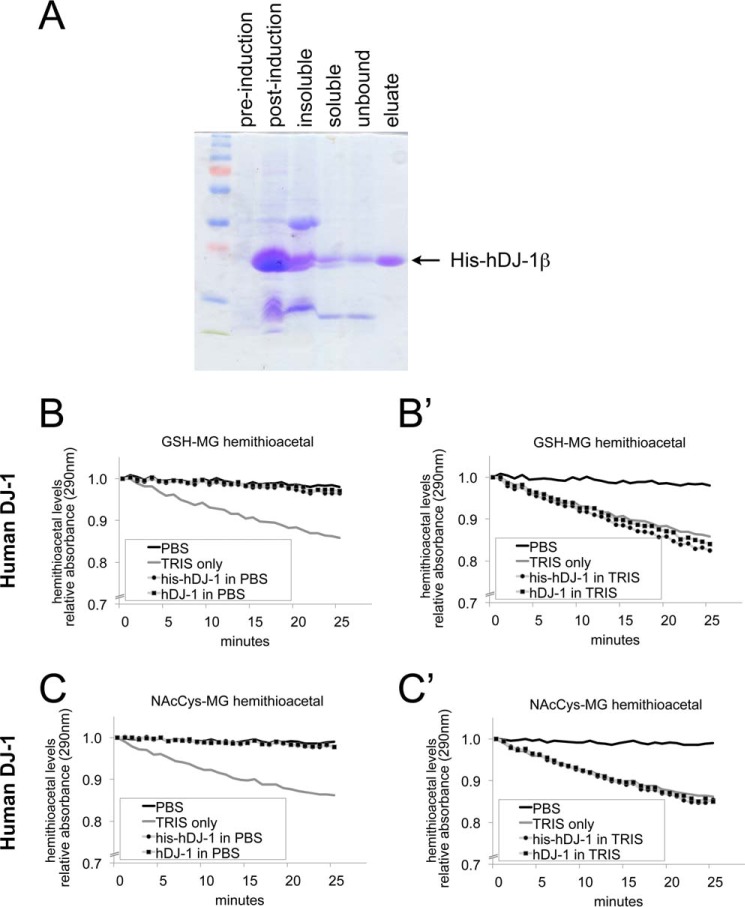
When methylglyoxal is combined with either GSH or N-acetylcysteine in vitro, this leads to formation of a hemithioacetal between MG and the respective cysteines, which can be detected by absorption at 290 nm (12). Recombinant DJ-1 was reported to reverse formation of these hemithioacetals, as it causes a progressive drop in A290 when added in vitro to these hemithioacetals (12). To test whether Drosophila DJ-1 also has this activity, we expressed and purified recombinant His-tagged Drosophila DJ-1β (his-dDJ-1β) (Fig. 3A). To perform the deglycase assay, we first dialyzed his-dDJ-1β protein into a TRIS-containing buffer, as was previously described (12). We subsequently realized when performing control experiments; however, that TRIS buffer alone is sufficient to cause a drop in A290 (Fig. 3, B–C′). In TRIS buffer, his-dDJ-1β shows a similar drop in A290 as TRIS buffer alone (Fig. 3, B′ and C′). When dialyzed into PBS, however, his-dDJ-1β protein shows no deglycase activity (Fig. 3, B and C). In case this was due to the presence of the His tag, we cleaved off the His tag with TEV protease, and tested non-tagged dDJ-1β. Just as for the tagged protein, the un-tagged protein also showed no deglycase activity in PBS (Fig. 3, B and C). To test whether there is a difference between Drosophila and human DJ-1, we also expressed and purified recombinant His-tagged human DJ-1 (Fig. 4A), and subsequently removed the His tag by TEV protease cleavage. As for the Drosophila protein, neither his-hDJ-1, nor untagged hDJ-1 showed any deglycase activity on cysteine in vitro, in addition to the TRIS-mediated effect (Fig. 4, B–C′). In sum, this raises the possibility that the reported deglycase effects on cysteine may be due to the TRIS buffer.
FIGURE 3.
Drosophila DJ-1β does not have detectable deglycase activity, whereas TRIS does. A, Coomassie-stained SDS-PAGE gel showing successive steps in the purification process of His-tagged Drosophila DJ-1β. BL21 Escherichia coli cells were transformed with vectors containing N-terminal His-tagged DJ-1 full-length open reading frames. Single colonies were grown in LB medium to OD 0.6 (pre-induction), then IPTG was added to 0.5 mm final. After incubation at 18 °C overnight (post-induction), the bacteria were centrifuged and lysed, yielding an insoluble pellet (insoluble) and a supernatant with soluble protein (soluble). His-DJ-1β was purified on Ni-NTA-agarose beads and eluted in a TRIS buffer containing imidazole (eluate). B–C, deglycation assayed as in Ref. 12, as the drop in absorption at 290 nm, which detects the hemithioacetal formed by MG reacting with either glutathione (B–B′) or N-acetylcysteine (C–C′). Addition of TRIS buffer (20 mm, pH 8.0) leads to a drop in A290 whereas addition of PBS buffer does not. Drosophila DJ-1β does not show any deglycase activity when dialyzed in PBS (panels B and C), whereas in TRIS buffer, it causes a similar drop in A290 as TRIS buffer alone (panels B′ and C′). Both His-tagged proteins, as well as proteins with the His tag cleaved off, were tested. A290 is shown relative to A290 at time point 0, when the indicated proteins and/or buffers were added to the reaction.
FIGURE 4.
Human DJ-1 does not have detectable deglycase activity, whereas TRIS does. A, Coomassie-stained SDS-PAGE gel showing successive steps in the purification process of His-tagged human DJ-1. BL21 E. coli cells were transformed with vectors containing N-terminal His-tagged DJ-1 full-length open reading frames. Single colonies were grown in LB medium to OD 0.6 (pre-induction), then IPTG was added to 0.5 mm final. After incubation at 18 °C overnight (post-induction), the bacteria were centrifuged and lysed, yielding an insoluble pellet (insoluble) and a supernatant with soluble protein (soluble). His-DJ-1 was purified on Ni-NTA-agarose beads and eluted in a TRIS buffer containing imidazole (eluate). B–C, deglycation assayed as in Ref. 12, as the drop in absorption at 290 nm, which detects the hemithioacetal formed by MG reacting with either glutathione (B–B′) or N-acetylcysteine (C–C′). Addition of TRIS buffer (20 mm, pH 8.0) leads to a drop in A290 whereas addition of PBS buffer does not. Human DJ-1 does not show any deglycase activity when dialyzed in PBS (panels B and C), whereas in TRIS buffer, it causes a similar drop in A290 as TRIS buffer alone (panels B′ and C′). Both His-tagged proteins, as well as proteins with the His tag cleaved off, were tested. A290 is shown relative to A290 at time point 0, when the indicated proteins and/or buffers were added to the reaction.
Discussion
The connection between Parkinsonism and Diabetes mellitus has been subject to discussion for a long time, in particular because of the role of reactive metabolites in the pathogenesis of both diseases. The reported enzymatic activities of DJ-1 as a glyoxalase or a deglycase (11, 12) would create a link between these two diseases since DJ-1 has been linked to Parkinsonism and MG detoxification has been linked to diabetes (6, 10). We therefore examined, using Drosophila as a model system, whether DJ-1 contributes toward MG detoxification in vivo, but could find no evidence to support this. Knockdown of DJ-1β causes no change in the ability of S2 cells to survive an MG challenge, and no detectable change in the amount of MG adducts formed in S2 cells upon MG treatment. Likewise, in vivo in the fly, we saw no increased formation of MG adducts in DJ-1β mutant flies compared with controls. When replicating the in vitro deglycase experiments described in Ref. 12, we realized the cysteine deglycase activity can be attributed to the TRIS buffer. We could detect no cysteine deglycase activity of either Drosophila or human DJ-1, either His-tagged or untagged, after dialyzing away the TRIS buffer. In principle, the difference in activity observed in this report versus (12) could be due to a difference in purification techniques. In Ref. 12, DJ-1 protein was purified by two-step chromatography using first a DEAE-Sephacel anion exchanger and then a hydroxylapatite column, whereas we used affinity chromatography. Our purification technique, however, yielded fairly pure protein (Figs. 3A and 4A). Furthermore, the TRIS buffer effect is present regardless of the purification technique, and as far as we can tell, in Ref. 12, TRIS buffer alone was not used as a negative control. This caveat might also need to be considered when interpreting data relating to the deglycase activity of other proteins such as Hsp31 (18). In addition, DJ-1 has also been reported to act as a glutathione-independent glyoxalase (11). In our in vitro assays, we have looked specifically at the deglycase activity, and not the glyoxalase activity of DJ-1. That said, we see no effects on MG adducts in vivo in DJ-1 knock-out flies. In sum, although there may be differences between flies and worms, we find no evidence in Drosophila supporting the notion that DJ-1 helps detoxify MG in vivo.
Materials and Methods
Cell Culture and Treatments
Drosophila S2 cells were grown in Gibco Serum-Free Medium (SFM) supplemented with 20 mm l-glutamine (Gibco), penicillin (50 units/ml), and streptomycin (50 μg/ml) from PAA at 25 °C. For dsRNA treatments, dsRNA was synthesized by PCR amplifying the region of the transcript to be targeted with primers containing a T7 promoter, and the PCR product was used as the template for in vitro transcription reaction with T7 transcriptase (Fermentas). dsRNA knockdown was performed by treating cells with 6 μg/ml dsRNA for 5 days and then diluting them to 1 million cells/ml and reseeding 1 ml per well of a 6-well plate. For control wells 1 ml of medium was added, for methylglyoxal treatment 1 ml of medium containing twice the final methylglyoxal concentration (Sigma M0252) was added. Cells were then lysed 24 h after treatment, either in Laemmli buffer for Western blot analysis or in Trizol for RNA extraction.
MTT Cell Viability Assay
For the MTT assay, dsRNA-treated cells were diluted to 1 million cells/ml, and then 100 μl were seeded onto 96-well plates. Then 100 μl of methylglyoxal diluted in medium (2× of final concentration) was added to each well. After 48 h of incubation, 50 μl of MTT solution (2 mg/ml in PBS, Sigma M5655) was added and incubated for 4 h. Then the medium was discarded, and 200 μl of DMSO were added. After 1 h of incubation, absorbance was measured at 590 nm with a reference filter of 620 nm. Cell viability (%) was plotted versus concentration, and the EC50 was determined by non-linear regression using the program PRISM.
Glyoxalase Assay
Ten male flies (2 weeks of age) were homogenized in 1 ml of homogenization buffer (10 mm sodium phosphate buffer, pH 7.0, 0.02% Triton X-100) and then sonicated at 30% power for 15 s. After clarification by centrifugation at 14,000 rpm for 30 min at 4 °C, 10 μl of the supernatant were added to the reaction mix in a 96-well glass-bottom UV plate (per well: 100 μl of 100 mm sodium phosphate buffer pH 6.6, 60 μl of H2O, 20 μl of 20 mm methylglyoxal, and 20 μl of 20 mm reduced glutathione). Absorption was continuously measured at 235 nm for 20 min, then the average slopes of all replicates were used to calculate enzymatic activity. The values obtained were normalized to the protein content measured by Qubit Fluorometer. For glyoxalase assay on cells, 1 million S2 cells were lysed in homogenization buffer, and then processed as described above.
Recombinant DJ-1β
Recombinant DJ-1 proteins were generated by transforming BL21 RP competent cells with vectors containing N-terminally His-tagged Drosophila DJ-1β or Human DJ-1 full-length open reading frames. In each case, a single colony was grown in LB medium to OD 0.6, then IPTG was added to 0.5 mm final. After incubation at 18 °C overnight, the bacteria were centrifuged and lysed, and the protein was purified on Ni-NTA-agarose beads and eluted in 20 mm TRIS buffer, pH 8.0, 150 mm NaCl, and 333 mm imidazole. For the deglycase assay the eluted protein was dialyzed three times into PBS to remove all TRIS buffer. For removing the His tag, recombinant proteins were incubated with His-tagged TEV protease (Sigma T4455) for 12 h at 4 °C. Both HIS-TEV and non-cleaved his-DJ1 proteins were subsequently removed by incubating with Ni-NTA-agarose beads. The resulting untagged DJ-1 proteins were dialyzed two more times into PBS to remove the TRIS buffer present in the TEV protease solution.
Deglycase Assay
Experiments were performed at 25 °C in 50 mm sodium phosphate buffer, pH 7.0. Methylglyoxal and reduced glutathione (or N-acetylcysteine) were premixed to a final concentration of 20 mm. After reaching steady-state absorption levels at 290 nm (∼10 min) recombinant DJ-1 was added to a final concentration of 4 μm in treatment wells, or an identical volume of just PBS or just TRIS were added as controls. A290 was then measured for 30 min.
Fly Stocks
The DJ-1βΔ93 line was supplied by the Bloomington Drosophila Stock Center (33601). Flies were grown in controlled conditions by placing newly-hatch first instar larvae into vials at defined density (60/vial), allowing them to grow to adulthood, and then collecting animals that eclose within 1 day and aging them for 2 weeks.
Antibodies
Rabbit anti-GloI (Santa Cruz Biotechnology sc-67351), anti-tubulin AA4.3-s (DS Hybridoma Bank), mouse anti-CEL (Biologo CEL025), rabbit anti-AGE (abcam ab23720), rabbit anti-ERK (Cell Signaling 4695). The DJ-1β antibody was made by immunizing rabbits with recombinant Drosophila DJ-1β. For Western blotting, antibodies were used at 1:1000 dilution in 5% skim milk/PBS-T except for the CEL and AGE antibodies, which were used at 1:500 dilution in 5% BSA/PBS-T.
Author Contributions
D. P. and T. F. performed experiments. All authors designed experiments, evaluated data, and wrote the manuscript.
Acknowledgments
Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
This work was supported by the DFG SFB1118 and by the Helmholtz Portfolio Topic “Metabolic Dysfunction.” The authors declare that they have no conflicts of interest with the contents of this article.
- ROS
- reactive oxygen species
- RCS
- reactive carbonyl species
- MG
- methylglyoxal
- AGE
- advanced glycation endproducts
- Glo
- glyoxalase
- CEL
- Nϵ-carboxymethyllysine.
References
- 1. Kim G. H., Kim J. E., Rhie S. J., and Yoon S. (2015) The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thornalley P. J. (2003) Glyoxalase, Structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 31, 1343–1348 [DOI] [PubMed] [Google Scholar]
- 3. Bierhaus A., Fleming T., Stoyanov S., Leffler A., Babes A., Neacsu C., Sauer S. K., Eberhardt M., Schnölzer M., Lasitschka F., Lasischka F., Neuhuber W. L., Kichko T. I., Konrade I., Elvert R. et al. (2012) Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 18, 926–933 [DOI] [PubMed] [Google Scholar]
- 4. Thornalley P. J. (1996) Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification–a role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. 27, 565–573 [DOI] [PubMed] [Google Scholar]
- 5. Ramasamy R., Yan S. F., and Schmidt A. M. (2006) Methylglyoxal comes of AGE. Cell 124, 258–260 [DOI] [PubMed] [Google Scholar]
- 6. Thornalley P. J. (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metabol. Drug Interact. 23, 125–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golej J., Hoeger H., Radner W., Unfried G., and Lubec G. (1998) Oral administration of methylglyoxal leads to kidney collagen accumulation in the mouse. Life Sci. 63, 801–807 [DOI] [PubMed] [Google Scholar]
- 8. Berlanga J., Cibrian D., Guillén I., Freyre F., Alba J. S, Lopez-Saura P., Merino N., Aldama A., Quintela A. M., Triana M. E, Montequin J. F., et al. (2005) Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin. Sci. 109, 83–95 [DOI] [PubMed] [Google Scholar]
- 9. Riboulet-Chavey A., Pierron A., Durand I., Murdaca J., Giudicelli J., and Van Obberghen E. (2006) Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 55, 1289–1299 [DOI] [PubMed] [Google Scholar]
- 10. Raninga P. V., Trapani G. D., and Tonissen K. F. (2014) Cross talk between two antioxidant systems, thioredoxin and DJ-1: Consequences for Cancer. Oncoscience 1, 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J. Y., Song J., Kwon K., Jang S., Kim C., Baek K., Kim J., and Park C. (2012) Human and its homologs are novel glyoxalases. Hum. Mol. Genet. 21, 3215–3225 [DOI] [PubMed] [Google Scholar]
- 12. Richarme G., Mihoub M., Dairou J., Bui L. C., Leger T., and Lamouri A. (2015) Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 290, 1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imai Y. (2012) Mitochondrial Regulation by PINK1-Parkin Signaling. ISRN Cell Biol. 15, doi: 10.5402/2012/926160. [DOI] [Google Scholar]
- 14. Menzies F. M., Yenisetti S. C., and Min K. T. (2005) Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr. Biol. 15, 1578–1582 [DOI] [PubMed] [Google Scholar]
- 15. Meulener M., Whitworth A. J., Armstrong-Gold C. E., Rizzu P., Heutink P., Wes P. D., Pallanck L. J., and Bonini N. M. (2005) Drosophila mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr. Biol. 15, 1572–1577 [DOI] [PubMed] [Google Scholar]
- 16. Park J., Kim S. Y., Cha G. H., Lee S. B., Kim S., and Chung J. (2005) Drosophila mutants show oxidative stress-sensitive locomotive dysfunction. Gene 361, 133–139 [DOI] [PubMed] [Google Scholar]
- 17. Lavara-Culebras E., and Paricio N. (2007) Drosophila DJ-1 mutants are sensitive to oxidative stress and show reduced lifespan and motor deficits. Gene 400, 158–165 [DOI] [PubMed] [Google Scholar]
- 18. Mihoub M., Abdallah J., Gontero B., Dairou J., and Richarme G. (2015) The DJ-1 superfamily member Hsp31 repairs proteins from glycation by methylglyoxal and glyoxal. Biochem. Biophys. Res. Commun. 463, 1305–1310 [DOI] [PubMed] [Google Scholar]