Summary
In an Indian birth cohort, we demonstrate a high and early burden of cryptosporidiosis by polymerase chain reaction and serology. Reinfection was common and infections clustered in a subset of children. Prior infection provided some protection against subsequent infection, but not disease.
Keywords. birth cohort, natural history, cryptosporidiosis, children, diarrhea, India.
Abstract
Background.
Cryptosporidium is a leading cause of moderate to severe childhood diarrhea in resource-poor settings. Understanding the natural history of cryptosporidiosis and the correlates of protection are essential to develop effective and sustainable approaches to disease control and prevention.
Methods.
Children (N = 497) were recruited at birth in semiurban slums in Vellore, India, and followed for 3 years with twice-weekly home visits. Stool samples were collected every 2 weeks and during diarrheal episodes were tested for Cryptosporidium species by polymerase chain reaction (PCR). Serum samples obtained every 6 months were evaluated for seroconversion, defined as a 4-fold increase in immunoglobulin G directed against Cryptosporidium gp15 and/or Cp23 antigens between consecutive sera.
Results.
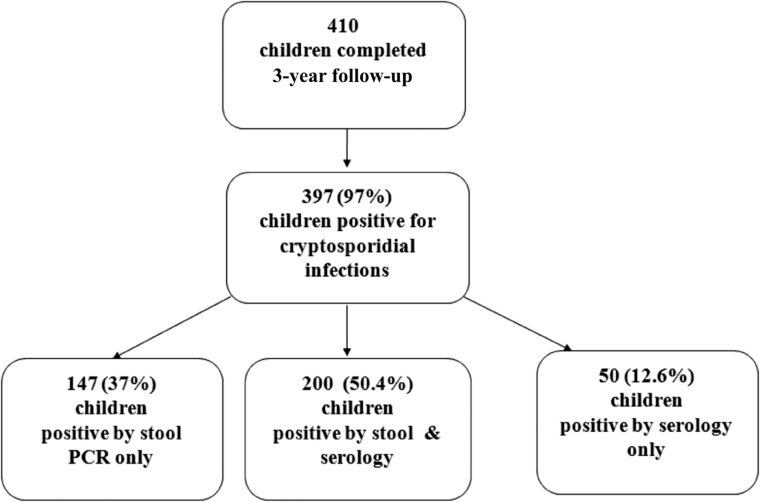
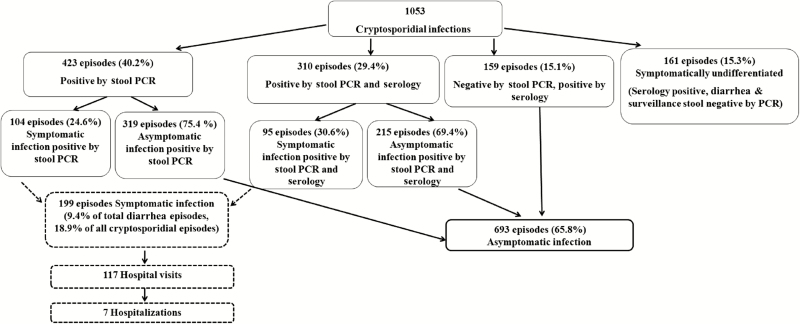
Of 410 children completing follow-up, 397 (97%) acquired cryptosporidiosis by 3 years of age. PCR identified 1053 episodes of cryptosporidiosis, with an overall incidence of 0.86 infections per child-year by stool and serology. The median age for the first infection was 9 (interquartile range, 4–17) months, indicating early exposure. Although infections were mainly asymptomatic (693 [66%]), Cryptosporidium was identified in 9.4% of diarrheal episodes. The proportion of reinfected children was high (81%) and there was clustering of asymptomatic and symptomatic infections (P < .0001 for both). Protection against infection increased with the order of infection but was only 69% after 4 infections. Cryptosporidium hominis (73.3%) was the predominant Cryptosporidium species, and there was no species-specific protection.
Conclusions.
There is a high burden of endemic cryptosporidiosis in southern India. Clustering of infection is suggestive of host susceptibility. Multiple reinfections conferred some protection against subsequent infection.
Cryptosporidium species is a major pathogen causing moderate to severe diarrhea in children [1, 2]. In India alone, cryptosporidiosis causes 3.9–7.1 million diarrheal episodes, 66 400–249 000 hospitalizations, and 5800–14 600 deaths in children aged <2 years [3]. Cryptosporidiosis is associated with long-term sequelae, with significant adverse effects on nutritional status, cognitive development, increased diarrheal burden, and mortality in children [4, 5].
Although studies show high cryptosporidial disease burden in developing countries [3, 5], the epidemiology of human cryptosporidiosis is not clearly understood. There is a dearth of longitudinal data on the course of infection in the absence of overt diarrheal disease. An understanding of the natural history of cryptosporidiosis and correlates of protection are essential in developing effective disease control and preventive measures. We conducted intensive active surveillance of children from birth till 3 years of age in a semiurban area in southern India by harnessing the synergistic benefits of a birth cohort design in a community setting and efficient molecular approaches to detect cryptosporidial infections.
MATERIALS AND METHODS
Site Recruitment, Follow-up, and Definitions
A birth cohort of 497 newborns was recruited between March 2009 and May 2010 in periurban Vellore, Tamil Nadu, India. In this population, human immunodeficiency virus (HIV) prevalence in antenatal women is <0.3% and there is an effective prevention of transmission program; hence, we did not screen for HIV. Children with very low birth weight (<1500 g) or congenital malformations were excluded. Enrollment, twice-weekly follow-up, and a description of illness in the cohort have been published [6]. Surveillance stool samples were collected every 2 weeks. For diarrhea, 3 stool samples were collected on separate days from day 1 to day 7 from the start of the episode. Severity of diarrheal episodes was assessed using the Vesikari scoring system [7]. Cord blood, where possible, or peripheral blood within 45 days after birth was collected from the infant. Every 6 months, 3- to 5-mL blood samples were collected from the study children.
Diarrhea was defined as ≥3 loose, watery stools in a 24-hour period [8], with an episode defined as at least 1 day of diarrhea, preceded and followed by at least 2 days without diarrhea. Cryptosporidiosis/cryptosporidial infection was identified by polymerase chain reaction (PCR) detection of Cryptosporidium species in stool or a 4-fold increase in immunoglobulin G (IgG) levels to gp15 and/or Cp23 between 2 serum samples. A diarrheal episode was termed symptomatic cryptosporidiosis if a stool sample collected within ±7 days was PCR positive. Cryptosporidiosis identified by stool PCR was asymptomatic if the stool was PCR positive but there was no diarrhea ±14 days. A new asymptomatic episode was considered when there was at least a 2-week gap with an intervening stool sample negative for Cryptosporidium species. Asymptomatic cryptosporidiosis identified by serology was a 4-fold increase in IgG to gp15 and/or Cp23 between 2 sera with no diarrhea or stool PCR positivity during that period. When a child was positive for asymptomatic infection by both stool PCR and serology during a defined interval, only stool PCR was considered and the child was not double-counted. Symptomatically undifferentiated cryptosporidiosis occurred when there was a 4-fold increase in IgG levels between 2 sera with a history of diarrhea during the period, but all stools were PCR negative. Cryptosporidial infection includes asymptomatic and symptomatic infections, while disease refers to symptomatic infection.
Testing for Cryptosporidium in Stool
DNA extracted from stool using a QIAamp DNA stool mini kit (Qiagen, Valencia, California) was screened by conventional 18S ribosomal RNA (rRNA) nested PCR for Cryptosporidium species using published protocols [9, 10]. Appropriate negative (no DNA template) and positive (known C. hominis or C. parvum PCR-positive stool) controls were included in every extraction and PCR run. For PCR-positive samples, species was determined by restriction fragment-length polymorphism and confirmed by 18S rRNA amplicon sequencing [11].
Identification of Enteric Pathogens in Diarrhea
Other than testing for Cryptosporidium species, which was by PCR, all diarrheal stool samples were tested for the presence of bacterial, viral, and parasitic pathogens using the Interactions of Malnutrition and Enteric Infections (MAL-ED) study protocols [12], which tested for multiple bacterial, viral, and parasitic pathogens.
ELISA for IgG to Gp15 and Cp23 in Serum
Serum IgG was measured by enzyme-linked immunosorbent assay (ELISA) using recombinant gp15 and Cp23 [13–15] as antigens, with results expressed as arbitrary ELISA units. Samples with positive ELISA unit values were considered seropositive.
MHC Class II Typing
Major histocompatibility complex (MHC) class II typing was performed in a subset of 74 children, of whom 41 had only asymptomatic infection and 33 had ≥2 infections with at least 1 symptomatic infection. We chose children from whom sufficient genomic DNA was available, so a power calculation was not performed to determine the ideal sample size. There is no information of human leukocyte antigen (HLA) alleles in this population, which consists of a mix of religions and ethnic backgrounds in southern India. DNA was extracted from blood using a DNeasy Blood and Tissue kit (Qiagen). HLA class II (DR and DQ) alleles expressed by the MHC genes were identified by PCR with sequence-specific oligonucleotide probes using a Luminex-based method with a Rapid Lifecodes kit (Tepnel Lifecodes Corporation, Stamford, Connecticut).
Statistical Analysis
Data were analyzed using Stata software, version 12.1 for Windows (StataCorp, College Station, Texas) and R version 2.12.1 (http://www.r-project.org/), with analyses confined to the 410 children who completed 3 years of follow-up. The baseline demographic characteristics of 87 children who did not complete the study did not differ significantly from those included in the analysis (Supplementary Table 1). Comparisons used the χ2 or Fisher exact test for categorical variables and 2-tailed t test or Wilcoxon rank-sum test for continuous variables. The cumulative incidence of cryptosporidial infections was calculated using survival analysis, adjusted for the duration of follow-up of each child and expressed as number of episodes per child-year. Based on the assumption that cryptosporidiosis would follow a Poisson distribution, the number of children expected to have 0, 1, 2, 3, 4, 5, and 6 episodes of cryptosporidiosis was calculated and compared with the observed frequency to verify if there was clustering of cryptosporidiosis in children.
For protective effects of prior cryptosporidial infection, parametric Poisson regression survival models were used to obtain relative risks and confidence intervals adjusted for repeated infection in the same child. The adjusted relative risks and protective efficacy of prior cryptosporidial infections were adjusted for factors previously reported to be associated either with overall morbidity [6] or cryptosporidiosis [16].
The role of antibody-mediated protection was studied using the effect of presence of serum IgG to gp15 and Cp23 on subsequent infection rates. When there were multiple cryptosporidial infections between 2 sera, analysis was restricted to the first episode. Parametric Poisson regression survival models were used to model the risk of cryptosporidial infection or disease as a function of preexisting antibody levels. Serum IgG levels to gp15 and Cp23 were categorized into quartiles to examine a dose–response relationship between antibody levels and protection. The IgG level was included as a categorical variable with absence of antibody as the reference category. The model was adjusted for age and number of previous infections.
Allele frequencies were counted for analysis of HLA data, with an allele frequency of >10% used for genetic association analysis. The association between HLA markers and occurrence of symptomatic and asymptomatic cryptosporidial infection was measured by calculating the odds ratio, using logistic regression.
Ethics Approval
Written informed consent was obtained from the parents or guardians of children in the study. The study was approved by the institutional review boards of the Christian Medical College, Vellore, India, and the Tufts University Health Sciences campus, Boston, Massachusetts.
RESULTS
Burden of Cryptosporidiosis
Of 497 recruited children, 410 completed 3 years of follow-up contributing 1218 child-years. The most common reason for children not completing the study was permanent migration out of the study area. Of the 410, 397 (97%) had cryptosporidiosis; 237 (59.7%) had only asymptomatic infections and 119 (30%) developed both symptomatic and asymptomatic cryptosporidial infections (Figures 1 and 2). Among the remaining children, 24 (6.0%) children had 1 or more episodes of symptomatic infection but no asymptomatic infection and 17 (4.3%) children had undifferentiated cryptosporidial infection detected only by serology. There was no difference by sex for asymptomatic (P = .10) or symptomatic infections (P = .17).
Figure 1.
Number of children in a birth cohort in Vellore, India, who completed 3 years of follow-up (n = 410) who had cryptosporidiosis detected by polymerase chain reaction (PCR) or serology.
Figure 2.
Number of symptomatic and asymptomatic cryptosporidial infections detected in a birth cohort in Vellore, India, who completed 3 years of follow-up. Abbreviation: PCR, polymerase chain reaction.
Infection occurred by 6 months of age in 165 (40.2%) children, by 2 years in 379 (92.4%), and by 3 years in 397 (97%). Symptomatic cryptosporidiosis was seen in 24 (5.8%), 55 (13.4%), 116 (28.3%), and 143 (35%) children by 6 months and 1, 2, and 3 years of age, respectively. The median age at first cryptosporidial infection was 9 (interquartile range [IQR], 4–17) months, with no difference (P = .75) between asymptomatic (8 [IQR, 4–14]) and symptomatic (9 [IQR, 3–16]) infection (Supplementary Figure 1). Overall, the incidence of cryptosporidiosis was 0.86 episodes (symptomatic 0.16, asymptomatic 0.57) per child-year, with the highest incidence (1.01 episodes per child-year) in the first year of life (Table 1).
Table 1.
Age Distribution and Incidence of Cryptosporidiosis in a Birth Cohort (n = 410) in Vellore, India, Followed up to 3 Years of Age
| Cryptosporidial Infection | 0–1 y | >1–2 y | >2–3 y | 0–3 years |
|---|---|---|---|---|
| Child years of follow-up | 403.5 | 406.09 | 408.55 | 1218.1 |
| Overall infection | ||||
| Episodes | 406 | 358 | 289 | 1053 |
| Incidence rate | 1.01 (.93–1.08) | 0.88 (.81–.96) | 0.71 (.64–.78) | 0.86 (.82–.90) |
| Symptomatic infection | ||||
| Episodes | 66 | 86 | 47 | 199 |
| Incidence rate | 0.16 (.12–.21) | 0.21 (.17–.27) | 0.11 (.09–.15) | 0.16 (.14–.19) |
| Asymptomatic infection | ||||
| Episodes | 223 | 246 | 224 | 693 |
| Incidence rate | 0.55 (.49–.62) | 0.60 (.55–.67) | 0.55 (.49–.62) | 0.57 (.53–.61) |
| Symptomatically undifferentiated infection | ||||
| Episodes | 117 | 26 | 18 | 161 |
| Incidence rate | 0.29 (.25–.34) | 0.06 (.04–.09) | 0.04 (.03–.07) | 0.13 (.11–.15) |
Incidence rates are shown as incidence rate (95% confidence interval) per child-year.
Clustering of Cryptosporidial Infection
Among 410 children, 63 (15.4%) had only 1 infection, 132 (32.2%) had 2, 113 (27.6%) had 3, 64 (15.6%) had 4, and 25 (6.1%) had ≥5 infections. There was clustering of infection (χ2 test for goodness of fit = 1755.21; degrees of freedom = 6; P < .0001). Similarly, among 143 children with symptomatic infection, 99 (69.2%) had only 1 episode, 36 (25.2%) had 2 episodes, and 8 (5.6%) had ≥3 episodes of symptomatic cryptosporidiosis, with evidence of clustering (χ2 test for goodness of fit = 25.04; degrees of freedom = 5; P < .0001).
Clinical Features of Symptomatic Cryptosporidiosis
Of 2134 diarrheal episodes, at least 1 stool sample was obtained for 2121 (99.4%) episodes, and 199 (9.4%) were Cryptosporidium positive by PCR. Most (197 [99%]) were acute diarrhea (≤14 days’ duration), with a median duration of 3 (IQR, 2–4) days, similar to that of noncryptosporidial diarrhea (Table 2). Of the 199 symptomatic cryptosporidial episodes, clinical features and severity did not differ from noncryptosporidial diarrhea (Table 2). There were 48 (24%) episodes of symptomatic cryptosporidiosis where co-pathogens, predominantly Giardia (27 [13%]) and Shigella (12 [6%]), were found.
Table 2.
Clinical Characteristics of Diarrheal Episodes in Which Cryptosporidium Species Was Identified by Polymerase Chain Reaction and Other Episodes in a Birth Cohort in Vellore, India, Followed up to 3 Years of Age
| Clinical Characteristics | Symptomatic Cryptosporidiosis (n = 199) | Noncryptosporidial Diarrhea (n = 1922) |
P Value |
|---|---|---|---|
| Median (IQR) age, mo | 16 (9–24) | 10 (6–20) | <.0001 |
| Median (IQR) duration, d | 3 (2–4) | 3 (2–4) | – |
| Accompanying symptoms, No. (%) | |||
| Vomiting | 45 (22.6) | 455 (23.6) | .86 |
| Fever | 40 (20.1) | 450 (23.4) | .29 |
| Treatment required, No. (%) | |||
| Clinic visits | 117 (58.8) | 1235 (64.3) | .12 |
| Hospitalization | 7 (3.5) | 47 (2.4) | .36 |
| Intravenous fluids | 1 (0.5) | 13 (0.7) | .77 |
| Severity of diarrheal episodesa, No. (%) | |||
| Mild | 95 (47.7) | 955 (49.8) | .76 |
| Moderate | 80 (40.2) | 758 (39.5) | |
| Severe | 24 (12.0) | 203 (10.6) | |
Abbreviation: IQR, interquartile range.
aData not available for 6 episodes.
Protection Conferred by Natural Infection
The incidence of cryptosporidial infection, but not disease, decreased as the number of infections increased (Table 3). The adjusted efficacy after 4 prior infections was 69% against infection, 70% against asymptomatic infection, and 66% against undifferentiated cryptosporidial infection, although the efficacy against symptomatic infection was lower and insignificant (19%). To explore the reason for the lower protection against symptomatic infection, a subgroup analysis was performed excluding 8 children who had >2 symptomatic infections. It showed improved protective efficacy against symptomatic infection, indicating that certain children pushed the protective effect toward the null (Supplementary Table 2).
Table 3.
Protective Effect of Prior Cryptosporidial Infection on Subsequent Infection or Disease Graded by the Number of Previous Infections in Birth Cohort (n = 410) Followed up to 3 Years of Age in Vellore, India
| Exposure as No. of Previous Cryptosporidial Infections | Person-years of Follow-up | No. of Infections | Incidence Rate per Child-year (95% CI) | Relative Risk of Subsequent Event (95% CI) | Adjusted Protective Efficacy, % (Range) | ||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Age | Adjusted for Other Factorsa | |||||
| Any cryptosporidial infection | |||||||
| 0 | 336.68 | 397 | 1.18 (1.07–1.29) | 1 | 1 | 1 | 1 |
| 1 | 382.77 | 334 | 0.87 (.79–.95) | 0.74 (.65–.83) | 0.71 (.61–.82) | 0.69 (.60–.80) | 31 (20–40) |
| 2 | 284.89 | 202 | 0.71 (.63–.80) | 0.60 (.52–.69) | 0.56 (.46–.68) | 0.53 (.44–.65) | 47 (35–56) |
| 3 | 142.34 | 89 | 0.62 (.51–.76) | 0.53 (.43–.65) | 0.50 (.38–.65) | 0.45 (.35–.60) | 55 (40–65) |
| 4 | 71.46 | 31 | 0.43 (.31–.63) | 0.37 (.26–.52) | 0.35 (.23–.52) | 0.31 (.20–.46) | 69 (54–80) |
| Asymptomatic infection | |||||||
| 0 | 336.68 | 229 | 0.68 (.60–.77) | 1 | 1 | 1 | 1 |
| 1 | 382.77 | 238 | 0.62 (.56–.69) | 0.91 (.78–1.06) | 0.78 (.65–.94) | 0.76 (.63–.91) | 24 (9–37) |
| 2 | 284.89 | 144 | 0.50 (.43–.59) | 0.74 (.61–.90) | 0.57 (.45–.73) | 0.54 (.43–.69) | 46 (31–57) |
| 3 | 142.34 | 59 | 0.41 (.33–.53) | 0.61 (.46–.80) | 0.45 (.32–.62) | 0.41 (.30–.57) | 59 (43–71) |
| 4 | 71.46 | 23 | 0.32 (.21–.52) | 0.47 (.30–.74) | 0.34 (.21–.56) | 0.30 (.18–.51) | 70 (49–82) |
| Symptomatic infection | |||||||
| 0 | 336.68 | 64 | 0.19 (.15–.24) | 1 | 1 | 1 | 1 |
| 1 | 382.77 | 60 | 0.16 (.12–.20) | 0.82 (.60–1.12) | 0.78 (.54–1.13) | 0.75 (.52–1.09) | 25 (–9 to 48) |
| 2 | 284.89 | 44 | 0.15 (.12–.21) | 0.81 (.55–1.19) | 0.81 (.52–1.29) | 0.75 (.47–1.20) | 25 (–20 to 53) |
| 3 | 142.34 | 23 | 0.16 (.10–.24) | 0.85 (.53–1.35) | 0.96 (.54–1.69) | 0.86 (.47–1.55) | 14 (–55 to 53) |
| 4 | 71.46 | 8 | 0.11 (.05–.27) | 0.59 (.27–1.25) | 0.72 (.30–1.71) | 0.63 (.27–1.48) | 37 (–48 to 73) |
| Moderate to severe symptomatic infection (Vesikari score 6–20) | |||||||
| 0 | 336.68 | 42 | 0.12 (.09–.17) | 1 | 1 | 1 | 1 |
| 1 | 382.77 | 31 | 0.08 (.05–.12) | 0.65 (.43–.97) | 0.66 (.40–1.07) | 0.62 (.38–1.02) | 38 (–2 to 62) |
| 2 | 284.89 | 16 | 0.06 (.03–.09) | 0.45 (.26–.79) | 0.53 (.27–1.01) | 0.47 (.24–.91) | 45 (9–76) |
| 3 | 142.34 | 10 | 0.07 (.04–.14) | 0.56 (.29–1.09) | 0.83 (.37–1.88) | 0.71 (.30–1.68) | 29 (–68 to 70) |
| 4 | 71.46 | 5 | 0.07 (.03–.20) | 0.56 (.24–1.29) | 0.98 (.34–2.83) | 0.81 (.28–2.28) | 19 (–128 to 72) |
| Symptomatically undifferentiated infection | |||||||
| 0 | 336.68 | 104 | 0.31 (.25–.38) | 1 | 1 | 1 | 1 |
| 1 | 382.77 | 36 | 0.09 (.07–.13) | 0.30 (.21–.44) | 0.45 (.31–.65) | 0.44 (.30–.63) | 56 (37–70) |
| 2 | 284.89 | 14 | 0.05 (.03–.09) | 0.16 (.09–.28) | 0.33 (.19–.59) | 0.31 (.18–.56) | 69 (44–82) |
| 3 | 142.34 | 7 | 0.05 (.02–.12) | 0.16 (.07–.34) | 0.37 (.16–.83) | 0.34 (.15–.77) | 66 (23–85) |
| 4 | 71.46 | 0 | – | – | – | – | – |
Abbreviation: CI, confidence interval.
aAdjusted for age, sex, presence of sibling, maternal age <23 years, use of firewood for cooking.
In children with multiple infections, the severity of diarrhea did not significantly decrease between the first and second infections (P = .93) or between the second and third infections (P = .32). Similarly, in children with multiple symptomatic infections, the severity of diarrhea between the first and second (P = .19) or between the second and third (P = .39) episodes did not significantly decrease.
Association of Serum Antibodies With Protection Against Cryptosporidial Infections and Diarrheal Disease
Serum gp15 and Cp23 IgG levels increased with age till 24 months, after which levels started to decline (Supplementary Figure 2). Geometric mean IgG levels increased with an increasing number of previous infections (Supplementary Figure 3). There was no association between preexisting gp15 or Cp23 IgG levels and subsequent cryptosporidial infection (Supplementary Table 3) or diarrheal disease after adjusting for age and previous infections (Supplementary Table 4).
Distribution of Cryptosporidium Species in the Cohort
Species could be determined for 473 of 733 (64.5%) episodes of cryptosporidiosis detected by stool PCR (77% and 67% of cryptosporidial diarrheal and nondiarrheal stool, respectively). Cryptosporidium hominis predominated and was identified in 347 (73.3%) infections in which species could be identified, followed by C. parvum (81 [17.1%]) (Table 4). Other species were C. meleagridis (5.2%) and C. felis (1%). Mixed infection with both C. parvum and C. hominis was identified in 14 (2.9%) episodes and C. andersoni and C. muris in 1 episode (0.2%). Species could not be identified in 260 (35.5%) episodes, of which 199 (76.5%) episodes were asymptomatic. On the gels, untyped samples generally had weaker bands, which may indicate lower amounts of parasite in samples.
Table 4.
Frequency of Infections Caused by Different Species of Cryptosporidium in a Birth Cohort of Children (n = 410) Followed up to 3 Years of Age in Vellore, India
| Species Distribution | All Infections | Symptomatic Infection | Asymptomatic Infection |
|---|---|---|---|
| Total No. of episodes | 733 | 199 | 534 |
| No. of species determined | 473 | 138 | 335 |
| C. hominis | 347 (73.3) | 99 (71.7) | 248 (74.0) |
| C. parvum | 81 (17.1) | 20 (14.5) | 61 (18.2) |
| C. meleagridis | 25 (5.2) | 6 (4.3) | 19 (5.6) |
| C. felis | 5 (1) | 1 (0.7) | 4 (1.2) |
| Mixed infections | |||
| C. hominis and C. parvum | 14 (2.9) | 12 (8.7) | 2 (0.6) |
| C. andersoni and C. muris | 1 (0.2) | 0 (0) | 1 (0.3) |
Data are presented as No. (%).
To assess species-specific repeated infection and protection against subsequent infection, analysis was restricted to 161 children for whom complete species data were available for all infections. For protection against subsequent infection, we evaluated the risk of primary and subsequent infections with C. hominis or non–C. hominis species (Table 5). There was no decrease in the risk of overall or species-specific asymptomatic or symptomatic cryptosporidiosis in later infections, indicating a lack of species-specific protection.
Table 5.
Rate Ratio for Subsequent Infection to That With Cryptosporidium hominis, According to Species of Primary Infection
| Infection | C. hominis Primary Infection | Non–C. hominis Primary Infection | Rate Ratio (95% CI) | P Value |
|---|---|---|---|---|
| No. of children | 116 | 45 | ||
| Follow-up, child-months | 546.5 | 242.1 | ||
| Any cryptosporidial infection | 12.6 (10.5–15.0) | 12.4 (9.6–15.8) | 1.02 (.7–1.4) | .9 |
| Asymptomatic infection | 7.3 (5.7–9.4) | 9.1 (6.6–12.5) | 0.80 (.5–1.2) | .3 |
| Symptomatic infection | 5.3 (3.7–7.6) | 3.3 (1.6–7.2) | 1.6 (.7–3.5) | .2 |
| Moderate or severe symptomatic infection | 2.2 (1.3–4.0) | 0.8 (.2–7.6) | 2.6 (.6–12.1) | .2 |
| Homotypica infection | 8.4 (6.5–10.8) | NA | ||
| Any infection in children with nonhomotypicb primary infection | NA | 9.5 (7.0–12.9) | 0.88 (.6–1.3) | .5 |
| Homotypic symptomatic infection | 3.5 (2.2–5.5) | NA | ||
| Any symptomatic infection in children with nonhomotypic primary infection | NA | 2.9 (1.3–7.2) | 1.2 (.5–3) | .7 |
Data are shown as percentage (95% CI) unless otherwise indicated.
Abbreviation: CI, confidence interval; NA, not applicable.
aInfection with the same species as the primary infection.
bInfection with a different species than the primary infection.
HLA Typing
There was no significant association of homozygous and heterozygous HLA class II alleles in children with only asymptomatic or at least 1 symptomatic cryptosporidial infection (Supplementary Tables 5 and 6).
DISCUSSION
This intensive birth cohort study in southern India revealed a high burden of cryptosporidiosis with 97% of children infected by 3 years of age. Both infection and cryptosporidial diarrhea clustered in some children, but limited testing of HLA class II alleles did not find an association with susceptibility or protection. In this cohort, protection conferred by prior infection was slow to develop, required multiple infections for partial protection, and was not associated with the presence of serum antibodies.
The incidence of 0.86 episodes per child-year is higher than reported from cohort studies in Peru (0.22 episodes per child-year) [17] and Guinea-Bissau (0.33 episodes per child-year) [18]. This is because of the inclusion of serology; based on PCR alone, incidence would have been 0.60 episodes per child-year. Among urban Brazilian children followed for 4 years, Israeli Bedouin children followed for 2 years, and an urban Bangladeshi cohort followed for 2 years, approximately 31%, 49%, and 77%, respectively, developed cryptosporidial infection [19–21]. The high rates of infection in this study are likely due to intensive surveillance and use of sensitive diagnostic methods and complementation by serology, as reported for other enteric pathogens [5, 22]. A recent report building on the case-control analysis from the Global Enteric Multicenter Study (GEMS) study also demonstrated the importance of cryptosporidial infection, although the focus was on severe disease and mortality [2]. Similar to this study, the GEMS reanalysis showed that cryptosporidial diarrhea was more common in toddlers (Table 2).
The majority (60%) of children had only asymptomatic cryptosporidial infections, similar to cohorts in Peru (67%) and Bangladesh (72%) [17, 23] and cross-sectional studies in Venezuela (86.3%) [24] and Thailand (64.2%) [25]. However, 2 longitudinal studies from Brazil [19] and Guatemala [26] reported 79% and 65% cryptosporidial diarrhea, respectively. The high proportion of asymptomatic infections may be due to the highly infectious nature of the parasite, with constant exposure resulting in subclinical infections, especially in endemic areas where transmission is through multiple routes [13, 27]. Only 24 (1.1%) episodes of persistent diarrhea were observed in this cohort, of which 2 episodes were associated with Cryptosporidium, differing from longitudinal studies from Brazil [27] and Guinea-Bissau [28], which reported greater persistent diarrhea in children, but are older studies.
During the 3-year follow up, 81% of children had >1 episode of cryptosporidiosis. Although the majority of children (97%) were infected, some children were more at risk for repeated infections and disease, with children with symptomatic cryptosporidiosis tending to have a higher risk of diarrhea when reinfected. This suggests host susceptibility for cryptosporidiosis. Studies by Kirkpatrick in Bangladesh and Haiti reported associations with HLA class II DQB1*0301 and class I B*15 I [29, 30]. In our study there was no significant association of any class II allele with asymptomatic or symptomatic infection, in contrast to the study in Bangladesh in which the class II DQB1*0301 allele was significantly associated with asymptomatic infection. The number of children who were HLA typed was small (41 asymptomatic and 33 symptomatic) but similar to those in Bangladesh (32 asymptomatic and 34 symptomatic). Impaired cell-mediated immunity may be another reason for susceptibility, since CD4 T cells have a crucial role in protection against and resolution of cryptosporidiosis [31]. Previous analysis of data from this and another cohort in the same location that did not include serology or HLA typing showed that presence of older siblings (P = .002) and stunting at 6 months of age (P = .019) were important risk factors for childhood cryptosporidiosis [16].
The majority of cryptosporidial infections were associated with C. hominis (73%). Cryptosporidium hominis has been identified as the most common (ranging from 79% to 88%) species in children from the same region [9, 32], India [33–35], and elsewhere [5]. The predominance of the anthroponotic C. hominis species may indicate the primary role of person-to-person transmission of infection in this community [16]. We could not demonstrate species-specific protection in children with primary C. hominis infection, and this may reflect the diversity of C. hominis, with a need for subtyping to determine whether there is subtype-specific protection.
Our study found limited protection (69%) against subsequent infection after 4 prior infections, without significant protection against symptomatic cryptosporidiosis after adjustment for potential confounders, reflecting partial immunity developed over time. Studies on US healthy adult volunteers have demonstrated that prior exposure results in reduction in disease severity and intensity of infection but not rate and duration of illness, indicating that protection is not conferred by a single exposure. However, volunteers with preexisting serum antibodies required higher challenge doses to acquire subsequent infections compared with seronegative adults [36, 37].
Although geometric mean antibody levels increased with number of infections in this cohort (Supplementary Figure 3), indicating a serological response to cryptosporidial infections, preexisting antibodies did not demonstrate any association with protection from subsequent infection or diarrheal disease. This highlights that humoral immunity may not play a major role in protection against cryptosporidial infections and disease, and the role of cell-mediated immunity needs more detailed consideration.
This study provides important insights into the natural history of cryptosporidiosis in an endemic semiurban slum community in southern India. The fact that almost all children in the study acquired cryptosporidial infection by 3 years of age indicates a high rate of transmission in the community. Children with symptomatic infection tended to have a higher probability of repeated diarrhea, suggestive of genetic susceptibility. Further understanding of susceptibility to or protection from disease will require larger studies with more detailed analysis of genetic associations and cell-mediated immunity in conjunction with epidemiological findings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We are grateful to the parents and children from the urban slums of Vellore for their enthusiastic participation and support. We also thank the field workers for their tireless work monitoring the cohort, and all the support staff of the Division of Gastrointestinal Sciences, Christian Medical College, Vellore, who made the study possible.
Author contributions. G. K., H. W., C. W., E. N., J. P. M., and S. S. R. conceived the study. D. K., R. S., V. V., and S. V. managed the cohort and data. S. S. R., S. B., G. K,. N. J., A. D. P., J. C. G., R. P. L., P. D., K. N., and C. G. led the laboratory work. D. K. and P. S. P. led the statistical analysis. All authors contributed to the interpretation of the data and writing of the report, and approved the final manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (R01 A1072222 to H. W.). D. K. was supported by the Fogarty International Center (training grants D43 TW007392 to G. K. and D43 TW009377 to G. K., C. W., and H. W.).
Potential conflicts of interest. All Authors certifies no potential conflicts of interest. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Material
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2. Sow SO, Muhsen K, Nasrin D, et al. The burden of cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 2016; 10:e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarkar R, Tate JE, Ajjampur SS, et al. Burden of diarrhea, hospitalization and mortality due to cryptosporidial infections in Indian children. PLoS Negl Trop Dis 2014; 8:e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shirley DA, Moonah SN, Kotloff KL. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis 2012; 25:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Checkley W, White AC, Jr, Jaganath D, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium . Lancet Infect Dis 2015; 15:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kattula D, Sarkar R, Sivarathinaswamy P, et al. The first 1000 days of life: prenatal and postnatal risk factors for morbidity and growth in a birth cohort in southern India. BMJ Open 2014; 4:e005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259–67. [DOI] [PubMed] [Google Scholar]
- 8. Morris SS, Cousens SN, Lanata CF, Kirkwood BR. Diarrhoea—defining the episode. Int J Epidemiol 1994; 23:617–23. [DOI] [PubMed] [Google Scholar]
- 9. Ajjampur SS, Gladstone BP, Selvapandian D, et al. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J Clin Microbiol 2007; 45:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiao L, Escalante L, Yang C, et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol 1999; 65:1578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao L, Morgan UM, Limor J, Escalante A, et al. Genetic diyptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol 1999; 65:3386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Houpt E, Gratz J, Kosek M, et al. Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis 2014; 59(suppl 4): S225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarkar R, Ajjampur SS, Prabakaran AD, et al. Cryptosporidiosis among children in an endemic semiurban community in southern India: does a protected drinking water source decrease infection? Clin Infect Dis 2013; 57:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lazarus RP, Ajjampur SSR, Geetha JC, et al. Serum anti-cryptosporidial gp15 antibodies in mothers and children less than 2 years of age in India. Am J Trop Med Hyg 2015; 93:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Priest JW, Kwon JP, Moss DM, et al. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol 1999; 37:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarkar R, Kattula D, Francis MR, et al. Risk factors for cryptosporidiosis among children in a semi urban slum in southern India: a nested case-control study. Am J Trop Med Hyg 2014; 91:1128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cama VA, Bern C, Roberts J, et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis 2008; 14:1567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valentiner-Branth P, Steinsland H, Fischer TK, et al. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J Clin Microbiol 2003; 41:4238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman RD, Sears CL, Moore SR, et al. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J Infect Dis 1999; 180:167–75. [DOI] [PubMed] [Google Scholar]
- 20. Fraser D, Dagan R, Naggan L, et al. Natural history of Giardia lamblia and Cryptosporidium infections in a cohort of Israeli Bedouin infants: a study of a population in transition. Am J Trop Med Hyg 1997; 57:544–9. [DOI] [PubMed] [Google Scholar]
- 21. Korpe PS, Haque R, Gilchrist C, et al. Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: association with severe malnutrition. PLoS Negl Trop Dis 2016; 10:e0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ajjampur SS, Rajendran P, Ramani S, et al. Closing the diarrhoea diagnostic gap in Indian children by the application of molecular techniques. J Med Microbiol 2008; 57:1364–8. [DOI] [PubMed] [Google Scholar]
- 23. Bern C, Ortega Y, Checkley W, et al. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis 2002; 8:581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chacín-Bonilla L, Barrios F, Sanchez Y. Environmental risk factors for Cryptosporidium infection in an island from western Venezuela. Mem Inst Oswaldo Cruz 2008; 103:45–9. [DOI] [PubMed] [Google Scholar]
- 25. Janoff EN, Mead PS, Mead JR, et al. Endemic Cryptosporidium and Giardia lamblia infections in a Thai orphanage. Am J Trop Med Hyg 1990; 43:248–56. [DOI] [PubMed] [Google Scholar]
- 26. Cruz JR, Cano F, Caceres P, et al. Infection and diarrhea caused by Cryptosporidium sp. among Guatemalan infants. J Clin Microbiol 1988; 26:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newman RD, Zu SX, Wuhib T, et al. Household epidemiology of Cryptosporidium parvum infection in an urban community in northeast Brazil. Ann Intern Med 1994; 120:500–5. [DOI] [PubMed] [Google Scholar]
- 28. Mølbak K, Andersen M, Aaby P, et al. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, West Africa. Am J Clin Nutr 1997; 65:149–52. [DOI] [PubMed] [Google Scholar]
- 29. Kirkpatrick BD, Haque R, Duggal P, et al. Association between Cryptosporidium infection and human leukocyte antigen class I and class II alleles. J Infect Dis 2008; 197:474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirkpatrick BD, Huston CD, Wagner D, et al. Serum mannose-binding lectin deficiency is associated with cryptosporidiosis in young Haitian children. Clin Infect Dis 2006; 43:289–94. [DOI] [PubMed] [Google Scholar]
- 31. Borad A, Ward H. Human immune responses in cryptosporidiosis. Future Microbiol 2010; 5:507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ajjampur SS, Sarkar R, Sankaran P, et al. Symptomatic and asymptomatic Cryptosporidium infections in children in a semi-urban slum community in southern India. Am J Trop Med Hyg 2010; 83:1110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ajjampur SS, Liakath FB, Kannan A, et al. Multisite study of cryptosporidiosis in children with diarrhea in India. J Clin Microbiol 2010; 48:2075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gatei W, Das P, Dutta P, et al. Multilocus sequence typing and genetic structure of Cryptosporidium hominis from children in Kolkata, India. Infect Genet Evol 2007; 7:197–205. [DOI] [PubMed] [Google Scholar]
- 35. Nagamani K, Pavuluri PR, Gyaneshwari M, et al. Molecular characterisation of Cryptosporidium: an emerging parasite. Indian J Med Microbiol 2007; 25:133–6. [DOI] [PubMed] [Google Scholar]
- 36. Okhuysen PC, Chappell CL, Sterling CR, et al. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum . Infect Immun 1998; 66:441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chappell CL, Okhuysen PC, Sterling CR, et al. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg 1999; 60:157–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.