ABSTRACT
IgA nephropathy (IgAN) is a common autoimmune disease that is characterized by formation and deposition of IgA1-containing immune complexes frequently leading to end-stage kidney disease. The IgA1 in these immune complexes carries aberrantly glycosylated O-glycans. In circulating IgA1 these galactose-deficient mucin-type O-glycans are bound by autoantibodies and thus, contribute to immune complex formation and pathogenesis. Even though the disease is associated with the overproduction of aberrant O-glycans on IgA1, specific structure-function-studies of mucin-type O-glycans are limited. Compared to other expression hosts, plants offer the opportunity for de novo synthesis of O-glycans on recombinant glycoproteins as they are lacking the mammalian O-glycosylation pathway. Recently, we demonstrated that Nicotiana benthamiana are suitable for the generation of distinct O-glycans on recombinant IgA1. Here, we expand our engineering repertoire by in planta generation of galactose-deficient and α2,6-sialylated O-glycans which are the prevailing glycans detected on IgA1 from patients with IgAN.
KEYWORDS: IgA nephropathy, immunoglobulin A, glyco-engineering, glycosylation, O-glycan, post-translational modification, recombinant protein
Introduction
Glycosylation is a common post-translational modification that is important for many biological properties including protein folding, stability and protein-protein interactions.1,2 The most prominent form of protein glycosylation is the linkage of a glycan to an asparagine (N-glycosylation) on newly synthetized proteins. The other abundant type of glycosylation of secreted and membrane bound proteins is O-glycosylation of serine or threonine residues. Notably, both types of glycosylation are altered in patients with different diseases2 and distinct glycans on recombinant glycoprotein therapeutics exhibit optimized efficacy.3 N-glycosylation of the Fc domain from human IgG has, for example, a profound influence on effector functions like antibody dependent cell-mediated cytotoxicity.4 Whereas, a lot is known about the biological role of N-glycans, less effort has been put in structure-function studies of O-glycans. Indeed, when produced in current mammalian expression systems, glycan-structure-function studies are impeded by the high heterogeneity of the resulting O-glycans.5 Obviously, distinct, tailor-made O-glycans on recombinant glycoproteins are required to gain more insight into the function of this type of protein modification. Glyco-engineering of expression hosts has been successfully used to produce recombinant glycoproteins with customized N- and O-glycans.6,7 These engineered glycoproteins display enhanced functions and facilitate the development of novel diagnostic tools for detection of aberrant glycosylation. Recently, we showed that Nicotiana benthamiana plants are a suitable expression host for the generation of distinct O-glycans on recombinant human IgA1.8 In this study, we provide an addition to our O-glycan engineering repertoire and produce a human IgA1 with galactose-deficient and α2,6-sialylated O-glycans. Our engineering approach could be helpful to better understand the influence of distinct O-glycans in IgAN and provide strategies for the development of glycan-related therapeutics.
Mucin-type O-glycan biosynthesis
Depending on the protein-linked sugar there are several types of O-glycosylation. In mucin-type O-glycosylation, N-acetylgalactosamine (GalNAc) is attached to serine or threonine residues.9 The initial step of the mucin-type O-glycan biosynthesis in mammals is mediated mainly in the Golgi by polypeptide N-acetylgalactosaminyltransferases (GalNAc-T). The human GalNAc-T family consists of 20 enzymes which display differences in expression in cells and tissues as well as an individual substrate specificity.9 The resulting GalNAcα-Ser/Thr is known as the Tn antigen (Fig. 1A). Elongations of the Tn antigen are typically performed by glycosyltransferases in the Golgi of mammalian cells. The common T antigen (also classified as core 1 structure: Galβ1–3GalNAcα-Ser/Thr) is synthesized by core 1 β1,3-galactosyltransferase (C1GalT1). Proper expression of active human C1GalT1 is dependent on the specific molecular chaperon Cosmc which promotes folding of human C1GalT1.10 In mammals, core 1 O-glycans are often further modified with sialic acid (N-acetylneuraminic acid: NeuAc) by α2,3- (e.g. ST3Gal-I) and α2,6-sialyltransferases (e.g., ST6GalNAc-III) resulting in the formation of mono- and di-sialylated core 1 O-glycans (Fig. 1A).
Figure 1.
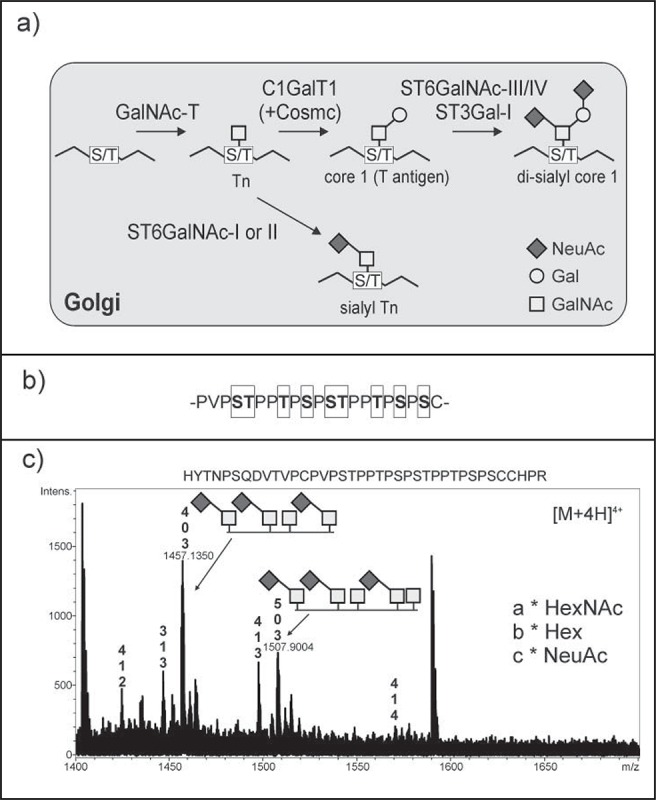
(A) Schematic illustration of the mucin-type O-glycan biosynthesis pathway. Tn and sialyl Tn represent the galactose-deficient O-glycans. GalNAc-T: polypeptide N-acetylgalactosaminyltransferases; C1GalT1: core 1 β1,3-galactosyltransferase; ST6GalNAc-III/IV: α2,6-sialyltransferase III/IV; ST3Gal-I: α2,3-sialyltransferase I; ST6GalNAc-I/II: α2,6-sialyltransferase I/II. Cosmc: core 1 galT1-specific molecular chaperone, required for proper activity of human C1GalT1. (B) IgA1 hinge region. The potential O-glycosylation sites are boxed. (C) In planta generation of sialyl Tn structures on the IgA1 hinge region. Human IgA1 was transiently co-expressed with GCSI-GalNAc-T2, GCSI-ST6GalNAc-II and other proteins required for sialylation in N. benthamiana. Recombinant IgA1 was purified from leaves, digested with trypsin and analyzed by LC-ESI-MS/MS. The highlighted peaks in the mass spectrum indicate the presence of sialyl Tn and Tn O-glycans on the peptide containing the IgA1 hinge region. Details of protein expression, purification and glycopeptide analysis were described previously.8
IgA glycosylation and implications for IgA nephropathy
IgAs are the most prevalent antibody class at mucosal sites in the human body and have an important role in the protection of mucosal surfaces from pathogens.3 There are 2 subclasses of IgA, IgA1 and IgA2. Human IgA1 exists in 2 versions, the monomeric form, mostly found in circulation and the secreted IgA1 form in which 2 antibody monomers are covalently linked to the joining chain (J-chain).11,12 For secretion and transport from the basolateral to the apical pole of exocrine epithelial cells, J-chain-containing IgA1 binds to the polymeric Ig surface-receptor, is released as secretory IgA1 by proteolytic cleavage leaving a glycosylated polypeptide chain, the secretory component, wrapped around the dimeric IgA1.3,11,12 Human monomeric IgA1 harbours 2 conserved N-glycosylation sites, one in the CH2 domain and the second one in the C-terminal tailpiece of the α heavy chain. In contrast to IgA2, IgA1 carries an extended hinge region with 9 O-glycosylation sites located between the constant region domains CH1 and CH2 (Fig. 1B). In healthy individuals, 6 of the 9 O-glycosylation sites of IgA1 are typically occupied by mono- and di-sialylated core 1 structures. Truncated, galactose-deficient mucin-type O-glycans without (Tn antigen) or with terminal sialic acid (sialyl Tn: NeuAcα2–6GalNAcα-Ser/Thr) are observed in diseases.2 Decreased expression or activity of C1GalT1 or its chaperon Cosmc and enhanced expression or activity of N-acetylgalactosaminide α2,6-sialyltransferase I/II (ST6GalNAc-I/II), the enzymes responsible for sialyl Tn formation, are assumed as possible reasons for the presence of aberrantly glycosylated proteins.13
IgAN is seen as the most prevalent form of glomerulonephritis, but the pathogenesis is not well defined.14 In IgAN patients, the increased occurrence of galactose-deficient O-glycans is a consistently observed abnormality.15,16,17 These truncated Tn or sialyl Tn O-glycans serve as epitopes for antiglycan autoantibodies leading to glomerular immune complex deposits. While the analysis of IgA1 from serum of IgAN patients has made tremendous progress by the use of high-resolution mass spectrometry, the assignment of the influence of the sialyl Tn and Tn antigen formation to the onset of the disease has remained controversial so far. Several studies showed that the galactose-deficient version of IgA1 leads to the formation of immune complexes and therefore has a critical impact on the pathogenesis of IgAN.18,19 In contrast, a recent study concluded that the expression of the Tn antigen alone was not sufficient to cause the disease as it was found in patients as well as in healthy individuals and only the level of the total IgA1 concentration in plasma differed significantly between disease and non-disease suggesting that the overexpression of galactose-deficient IgA1 is critical for the disease progression.16 Due to these inconsistent data, additional studies are needed to investigate the contribution of the galactose-deficient IgA1 O-glycans and antiglycan antibody immune complex formation to disease pathogenesis.
A possible consequence of aberrant O-glycans might be an influence on the physicochemical properties of the antibody which increases the tendency to aggregate in glomeruli.20 The biological roles of sialic acids clustered in O-glycans of IgA1 are of great interest in IgAN but are currently not fully understood.17 Serum IgA1 in healthy controls was found to exhibit more α2,3-sialylated O-glycans than α2,6-sialylated glycans which led to the assumption that distribution and the sites of sialic acid attachment are critically involved in the pathogenesis of IgAN. Apart from that, hypersialylation of IgA1 influences the overall negative charge of the protein leading to enhanced affinity of the antibody to mesangial cells.21 A reduced level of sialic acid and galactose in the IgA1 hinge region was suggested to increase the affinity to extracellular matrix proteins.22 Moreover, sialylated glycans cannot bind to the hepatic asialoglycoprotein receptor which reduces the rate of clearance and prolongs the persistency of immune complexes in the circulation.23 In summary, the ill-defined and even controversial impact of distinct IgA1 O-glycans in IgAN requires further characterization of the carbohydrate structures in order to understand the mechanisms underlying the pathogenesis of IgAN and the production of recombinant IgA1 with defined mucin-type O-glycans that can be used in disease models.17
Engineering of mucin-type-O-glycans on recombinant IgA1 in N. benthamiana plants
In order to get more insight into the pathogenesis of diseases like IgAN, tailored and homogenous mucin-type O-glycans are required to perform specific structure-function studies.14 For that purpose, production hosts other than mammalian cells appear highly suitable. Plants do not show a typical mucin-type O-glycosylation pathway which provides the opportunity for de novo synthesis. However, plants can convert proline residues adjacent to Ser/Thr O-glycosylation sites into hydroxyproline.8 The hydroxyproline residues can then be further extended with arabinose chains or huge arabinogalactans and neighboring serine residues can be modified with a single galactose residue.24,25 Such plant-specific modifications are unwanted and can be eliminated by knockout of the responsible enzymes like prolyl 4-hydroxylases. This engineering approach has been successfully carried out in a moss-based expression system26 and similar strategies are currently developed for higher plants like N. benthamiana.
To produce a recombinant human IgA1 with defined mucin-type O-glycans in N. benthamiana we transiently expressed different mammalian glycosyltransferases and proteins required for the biosynthesis and transport of the precursor CMP-sialic acid together with the recombinant glycoprotein.8 Mucin-type O-glycan formation was efficiently initiated by co-expression of human GalNAc-T2. Since expression of human C1GalT1 with its chaperone Cosmc did not result in any detectable core 1 O-glycan formation,27 we expressed Drosophila melanogaster C1GalT1 which is functional in the absence of a Cosmc-like chaperone. Further expression of human α2,3-sialyltransferase and Mus musculus α2,6-sialyltransferase resulted in the formation of sialylated core 1 O-glycans that are normally found in the hinge region of human IgA1.8 Our study does not only demonstrate the potential of N. benthamiana for the production of tailored mucin-type O-glycans on therapeutically interesting proteins but raise also the opportunity for the generation of pathologically relevant modifications, like the sialyl Tn antigen found on circulating IgA1 in IgAN patients. To this end, we examined whether the co-expression of the sialic acid pathway with human GalNAc-T2 and ST6GalNAc-II will result in the in planta generation of sialyl Tn on recombinant IgA1. The binary vectors required for the expression of recombinant human IgA1, the proteins for the initiation of mucin-type O-glycosylation, CMP-sialic acid biosynthesis as well as transport were described in detail previously.27,28,8 As we wanted to avoid the aforementioned plant-specific O-glycosylation which mainly takes place in the Golgi we used ER-retained versions of GalNAc-T2 and ST6GalNAc-II. Retention of glycosyltransferases and glycosidases in the ER by exchanging the N-terminal targeting regions by that of the ER-resident transmembrane protein α-glucosidase I (GCSI) has been performed previously by our group for GalNAc-T2.29 The GCSI-ST6GalNAc-II construct was generated in a similar way. Following co-expression of IgA1 with ER-retained GCSI-GalNAc-T2, GCSI-ST6GalNAc-II and with the machinery for CMP-sialic acid production and transport, IgA1 was purified, subjected to SDS-PAGE and the corresponding heavy chain band was digested with trypsin. LC-ESI-MS/MS analysis revealed peaks corresponding to glycopeptides with 3 O-glycosylation sites occupied by the sialyl Tn structure and one or 2 additional sites occupied with the Tn antigen (Fig. 1C). Apart from that minor peaks were present with an additional single hexose indicating the presence of small amounts of plant-specific O-glycosylation.
Conclusion and prospects
In addition to our recently plant-produced core 1 structures on recombinant IgA1,8 we have provided now an approach for tailored sialylation of GalNAc-Ser/Thr O-glycans on human IgA1, also identifying human ST6GalNAc-II as a suitable sialyltransferase for this in vivo engineering strategy. Further optimization of targeted O-glycan modifications can be achieved by the use of multi-gene expression cassettes, stable transformed plants and fine-tuned subcellular targeting of non-plant glycosyltransferases. Implementation of these tools will result in the generation of an expression platform that is optimally suited for the reconstruction of physiologic or non-physiologic O-glycan structures on recombinant proteins. This may facilitate a better understanding of disease progression and can lead to the development of glycan-related biomarkers to monitor disease stages.30 Notably, no specific IgAN therapy is currently available. Small glycoproteins or glycopeptides mimicking the galactose-deficient IgA1 hinge region can be produced in our glyco-engineered plants. Such glycopeptides could be tested in a disease-specific therapy to block the formation of IgA1-antiglycan immune complexes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from the Federal Ministry of Transport, Innovation and Technology (bmvit) and Austrian Science Fund (FWF): TRP 242-B20.
References
- [1].Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science 2001; 291:2364-9; PMID:11269317; http://dx.doi.org/ 10.1126/science.291.5512.2364 [DOI] [PubMed] [Google Scholar]
- [2].Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 2015; 15:540-55; PMID:26289314; http://dx.doi.org/ 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- [3].Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 2007; 25:21-50; PMID:17029568; http://dx.doi.org/ 10.1146/annurev.immunol.25.022106.141702 [DOI] [PubMed] [Google Scholar]
- [4].Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002; 277:26733-40; PMID:11986321; http://dx.doi.org/ 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- [5].Strasser R. Engineering of human-type O-glycosylation in Nicotiana benthamiana plants. Bioengineered 2013; 4:191-6; PMID:23147167; http://dx.doi.org/ 10.4161/bioe.22857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang Z, Wang S, Halim A, Schulz MA, Frodin M, Rahman SH, Vester-Christensen MB, Behrens C, Kristensen C, Vakhrushev SY, et al.. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol 2015; 33:842-4; PMID:26192319; http://dx.doi.org/ 10.1038/nbt.3280 [DOI] [PubMed] [Google Scholar]
- [7].Strasser R, Altmann F, Steinkellner H. Controlled glycosylation of plant-produced recombinant proteins. Curr Opin Biotechnol 2014; 30C:95-100; http://dx.doi.org/ 10.1016/j.copbio.2014.06.008 [DOI] [PubMed] [Google Scholar]
- [8].Dicker M, Tschofen M, Maresch D, König J, Juarez P, Orzaez D, Altmann F, Steinkellner H, Strasser R. Transient Glyco-Engineering to Produce Recombinant IgA1 with Defined N- and O-Glycans in Plants. Front Plant Sci 2016; 7:18; PMID:26858738; http://dx.doi.org/ 10.3389/fpls.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012; 22:736-56; PMID:22183981; http://dx.doi.org/ 10.1093/glycob/cwr182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β 3-galactosyltransferase. Proc Natl Acad Sci U S A 2002; 99:16613-8; PMID:12464682; http://dx.doi.org/ 10.1073/pnas.262438199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Johansen FE, Braathen R, Brandtzaeg P. Role of J chain in secretory immunoglobulin formation. Scand J Immunol 2000; 52:240-8; PMID:10972899; http://dx.doi.org/ 10.1046/j.1365-3083.2000.00790.x [DOI] [PubMed] [Google Scholar]
- [12].Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev 2005; 206:64-82; PMID:16048542; http://dx.doi.org/ 10.1111/j.0105-2896.2005.00290.x [DOI] [PubMed] [Google Scholar]
- [13].Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl 2011; 50:1770-91; PMID:21259410; http://dx.doi.org/ 10.1002/anie.201002313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA, Novak J. The origin and activities of IgA1-Containing immune complexes in IgA Nephropathy. Front Immunol 2016; 7:117; PMID:27148252; http://dx.doi.org/ 10.3389/fimmu.2016.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, Shinzato T, Kobayashi Y, Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 2001; 59:1077-85; PMID:11231363; http://dx.doi.org/ 10.1046/j.1523-1755.2001.0590031077.x [DOI] [PubMed] [Google Scholar]
- [16].Lehoux S, Mi R, Aryal RP, Wang Y, Schjoldager KT, Clausen H, van Die I, Han Y, Chapman AB, Cummings RD, et al.. Identification of distinct glycoforms of IgA1 in plasma from patients with immunoglobulin A (IgA) nephropathy and healthy individuals. Mol Cell Proteomics 2014; 13:3097-113; PMID:25071157; http://dx.doi.org/ 10.1074/mcp.M114.039693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takahashi K, Raska M, Stuchlova Horynova M, Hall SD, Poulsen K, Kilian M, Hiki Y, Yuzawa Y, Moldoveanu Z, Julian BA, et al.. Enzymatic sialylation of IgA1 O-glycans: implications for studies of IgA nephropathy. PLoS One 2014; 9:e99026; PMID:24918438; http://dx.doi.org/ 10.1371/journal.pone.0099026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, et al.. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 2009; 119:1668-77; PMID:19478457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 1999; 104:73-81; PMID:10393701; http://dx.doi.org/ 10.1172/JCI5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yoo EM, Morrison SL. IgA: an immune glycoprotein. Clin Immunol 2005; 116:3-10; PMID:15925826; http://dx.doi.org/ 10.1016/j.clim.2005.03.010 [DOI] [PubMed] [Google Scholar]
- [21].Leung JC, Poon PY, Lai KN. Increased sialylation of polymeric immunoglobulin A1: mechanism of selective glomerular deposition in immunoglobulin A nephropathy? J Lab Clin Med 1999; 133:152-60; PMID:9989767; http://dx.doi.org/ 10.1016/S0022-2143(99)90008-2 [DOI] [PubMed] [Google Scholar]
- [22].Hiki Y. O-linked oligosaccharides of the IgA1 hinge region: roles of its aberrant structure in the occurrence and/or progression of IgA nephropathy. Clin Exp Nephrol 2009; 13:415-23; PMID:19365705; http://dx.doi.org/ 10.1007/s10157-009-0173-7 [DOI] [PubMed] [Google Scholar]
- [23].Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology (Carlton) 2007; 12:275-84; PMID:17498123; http://dx.doi.org/ 10.1111/j.1440-1797.2007.00797.x [DOI] [PubMed] [Google Scholar]
- [24].Saito F, Suyama A, Oka T, Yoko-O T, Matsuoka K, Jigami Y, Shimma YI. Identification of Novel Peptidyl Serine α-Galactosyltransferase gene family in plants. J Biol Chem 2014; 289:20405-20; PMID:24914209; http://dx.doi.org/ 10.1074/jbc.M114.553933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karnoup AS, Turkelson V, Anderson WH. O-linked glycosylation in maize-expressed human IgA1. Glyco Biol 2005; 15:965-81; PMID:15901675; http://dx.doi.org/ 10.1093/glycob/cwi077 [DOI] [PubMed] [Google Scholar]
- [26].Parsons J, Altmann F, Graf M, Stadlmann J, Reski R, Decker EL. A gene responsible for prolyl-hydroxylation of moss-produced recombinant human erythropoietin. Sci Rep 2013; 3:3019; PMID:24145658; http://dx.doi.org/ 10.1038/srep03019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Castilho A, Neumann L, Daskalova S, Mason HS, Steinkellner H, Altmann F, Strasser R. Engineering of Sialylated Mucin-type O-Glycosylation in Plants. J Biol Chem 2012; 287:36518-26; PMID:22948156; http://dx.doi.org/ 10.1074/jbc.M112.402685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Juarez P, Huet-Trujillo E, Sarrion-Perdigones A, Falconi EE, Granell A, Orzaez D. Combinatorial analysis of secretory immunoglobulin A (sIgA) expression in plants. Int J Mol Sci 2013; 14:6205-22; PMID:23507755; http://dx.doi.org/ 10.3390/ijms14036205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dicker M, Schoberer J, Vavra U, Strasser R. Subcellular targeting of proteins involved in modification of plant N- and O-Glycosylation. Methods Mol Biol 2015; 1321:249-67; PMID:26082228; http://dx.doi.org/ 10.1007/978-1-4939-2760-9_18 [DOI] [PubMed] [Google Scholar]
- [30].Suzuki Y, Suzuki H, Yasutake J, Tomino Y. Paradigm shift in activity assessment of IgA nephropathy - optimizing the next generation of diagnostic and therapeutic maneuvers via glycan targeting. Expert Opin Biol Ther 2015; 15:583-93; PMID:25604055; http://dx.doi.org/ 10.1517/14712598.2015.1006624 [DOI] [PubMed] [Google Scholar]


