Abstract
Rheumatic heart disease (RHD) is a disease of poverty, is almost entirely preventable, and is the most common cardiovascular disease worldwide in those under 25 years. RHD is caused by acute rheumatic fever (ARF) which typically results in cumulative valvular lesions that may present clinically after a number of years of subclinical disease. Therapeutic interventions, therefore, typically focus on preventing subsequent ARF episodes (with penicillin prophylaxis). However, not all patients with ARF develop symptoms and not all symptomatic cases present to a physician or are correctly diagnosed. Therefore, if we hope to control ARF and RHD at the population level, we need a more reliable discriminator of subclinical disease. Recent studies have examined the utility of echocardiographic screening, which is far superior to auscultation at detecting RHD. However, there are many concerns surrounding this approach. Despite the introduction of the World Heart Federation diagnostic criteria in 2012, we still do not really know what constitutes the most subtle changes of RHD by echocardiography. This poses serious problems regarding whom to treat and what to do with the rest, both important decisions with widespread implications for already stretched health-care systems. In addition, issues ranging from improving the uptake of penicillin prophylaxis in ARF/RHD-positive patients, improving portable echocardiographic equipment, understanding the natural history of subclinical RHD and how it might respond to penicillin, and developing simplified diagnostic criteria that can be applied by nonexperts, all need to be effectively tackled before routine widespread screening for RHD can be endorsed.
Keywords: Acute rheumatic fever, rheumatic heart disease, screening
INTRODUCTION
Rheumatic heart disease (RHD) remains one of the most preventable causes of heart disease in children and young adults worldwide and is the most common cardiovascular disease in those aged under 25 years.[1] In low- and middle-income nations, and in marginalized people of some wealthy countries, RHD poses a major public health challenge and inflicts severe disability and premature death on many of those affected. As a physical manifestation of poverty, children are particularly vulnerable and hard hit. Worldwide, more than three-quarters of those aged 15 years and younger live in high-prevalence regions,[2] with RHD accounting for the greatest cardiovascular-related loss of disability-adjusted life-years among 10–14-year olds (516.6/100,000 individuals) and the second highest number among children aged 5–9 years old (362/100,000 individuals).[3]
The purpose of this review article is to examine the rationale and issues concerning screening for RHD as a means of reducing the burden of both acute rheumatic fever (ARF) and RHD in endemic areas.
SEARCH STRATEGY
We searched PubMed in English with the search terms “acute rheumatic fever,” “rheumatic heart disease ± screening,” and “subclinical rheumatic heart disease” for papers mostly published in the past 20 years until August 2016. We also relied on our familiarity with key literature. Pertinent review articles, book chapters, proceedings, and papers older than 20 years were used when judged important.
EPIDEMIOLOGY AND PATHOGENESIS
The Global Burden of Disease Estimates in 2013 implied that there are 33 million prevalent cases of RHD worldwide causing 275,000 deaths annually.[4] However, many echocardiographic screening studies put the prevalence of RHD at 8–57 out of 1000 children meaning that the true prevalence may rest closer to 62–78 million individuals worldwide with up to 1.4 million deaths each year.[5,6] By comparison, HIV and tuberculosis, both of which benefit from more intensive research and better funding, each results in 1.5 million deaths annually.[7]
RHD is the only long-term sequela of ARF which, in turn, results from an autoimmune reaction to pharyngeal (and possibly skin)[8,9] infection with Streptococcus pyogenes, the only known Group A Streptococcus (GAS). ARF typically affects children of school-going age with a peak prevalence in the 5–14 years age group, and significant valvular damage is thought to accrue due to repeated episodes of ARF.[10,11] The pathognomonic lesion of RHD (due to its specificity) is commissural fusion leading to mitral stenosis in severe cases. However, nonspecific functional lesions such as mitral regurgitation (MR) and aortic regurgitation (AR) are frequently seen. Given the cumulative nature of valvular damage, classical thinking has been that a substantial period of disease latency of up to 20–30 years needs to be present from the initial ARF episode to clinically symptomatic RHD.[12] However, comprehensive registry data from Australia recently found that 35% of children with ARF had developed RHD by 1 year, and in those who progressed to RHD, 14% developed heart failure within a year of diagnosis, increasing to 27% at 5 years.[13] This shorter latency is also frequently seen in Africa where significant morbidity and mortality is often present in adolescence and young adulthood, possibly through a high burden of ARF recurrences.[14,15,16,17]
RATIONALE FOR RHEUMATIC HEART DISEASE SCREENING
Although there is compelling epidemiological evidence linking GAS pharyngitis, ARF, and RHD, the pathogenesis of these diseases is incompletely understood with potentially more questions than answers.[7] For example, we do not know why only two-thirds of patients with ARF will report a preceding sore throat, why only 40%–60% of ARF cases progress to RHD,[18] and why up to 75% of children with RHD have no memory of symptoms consistent with previous ARF.[19] Therefore, most cases of RHD are not necessarily typified by the classic sequence of GAS pharyngitis resulting in symptomatic ARF with progression to RHD,[7] suggesting that rheumatic carditis frequently occurs at a subclinical level.
This is exemplified by the fact that RHD often presents with moderate-to-severe multivalvular disease (63.9%), heart failure (33.4%), pulmonary hypertension (28.8%), atrial fibrillation (21.8%), stroke (7.1%), and infective endocarditis (4%).[6] In the context of the developing world, such late presentations leave few options for intervention given that surgical and catheter-based treatments are limited by cost and lack of access.[20]
Therefore, treating sore throats with penicillin to prevent ARF (primary prophylaxis) and treating episodes of ARF with long-term penicillin (2–4 weekly intramuscular benzathine penicillin G, [BPG]) to prevent further episodes of ARF (secondary prophylaxis) may not be reliable approaches to disease control on a population scale. Primordial prophylaxis (strategies to avoid GAS infection, e.g., improve housing)[21] and a GAS vaccine that would prevent ARF[22] are potential options too but with significant barriers.
A strategy of secondary prevention relies entirely on case detection and a successful therapeutic strategy. Therefore, if we hope to bridge the gap between the large number of incident RHD cases and the smaller number of patients who present with ARF (there is a 10-fold difference in endemic areas),[7] we need a more robust strategy for detecting early RHD.
Auscultation for a pathological murmur has been the traditional approach to screening school-aged children for RHD. However, it is neither sensitive[23] nor specific[24,25] as demonstrated in the seminal paper by Marijon et al. in 2007, who showed that ten times more cases of RHD were detected using echocardiography compared with auscultation.[23] Subsequent studies have also shown a significant (5–50-fold) increase in RHD detection by echocardiography versus auscultation.[24,26,27,28,29,30,31]
The advent of echocardiography thus heralded a new era, revolutionizing RHD screening in the process. The high sensitivity of echocardiography meant researchers were discovering early morphological valvular changes before any clinically detectable functional lesion had developed and the term “subclinical RHD” (or latent RHD) subsequently emerged, recognizing that RHD could be clinically silent.[32] Given the cumulative nature of RHD, this was potentially significant because one would predict that younger patients with early-stage disease have the most to gain from an earlier diagnosis and institution of secondary prophylaxis.
THE WORLD HEART FEDERATION CRITERIA
In 2005, a working group supported by the World Health Organization (WHO) and the National Institutes of Health established case consensus definitions for RHD.[33] These guidelines were not evidence based and the definition of definite RHD required the presence of a heart murmur consistent with MR or AR and echocardiographic evidence of rheumatic valvular damage (or previous history of definite/probable ARF with no echocardiogram having been performed).
Furthermore, these guidelines were not universally accepted and many countries used alternative (sometimes local) guidelines for RHD diagnosis,[24,26,27,34,35,36,37] inadvertently resulting in a diagnostic potpourri that seriously undermined the validity and interchangeability of data from different countries. Inevitably, concerns began to emerge regarding diagnostic specificity:[30] For example, a study retrospectively scoring one sample population with different echocardiographic criteria resulted in an almost 6-fold alteration in disease prevalence.[35]
The push for an evidence-based consensus for echocardiographic diagnostic guidelines started to gain momentum. The 2012 World Heart Federation (WHF) criteria[38] [Box 1] were written to meet these needs and defined the lower limit of what constitutes RHD by echocardiography (although this is highly debatable-see later). Auscultation is no longer required, and the guidelines are intended for screening patients with no history of ARF who live in endemic regions.[38] However, the introduction of the WHF criteria did not solve all problems relating to RHD diagnosis and screening (nor did they intend to) and simultaneously raised additional issues that must be tackled (e.g., borderline RHD).
Box 1.
The abridged World Heart Federation diagnostic screening criteria for rheumatic heart disease[38]

PROBLEMS WITH ECHOCARDIOGRAPHIC SCREENING FOR RHEUMATIC HEART DISEASE
The WHO has recommended echocardiographic screening for RHD in endemic areas since 2004. However, RHD only partially meets the Council of Europe (CoE) criteria for population screening[39] [Box 2]. The first two CoE criteria are met unequivocally: there is a significant burden of disease (CoE criterion 1) with a latent stage (CoE criterion 2). The gaps in our current state of knowledge and how this relates to the remaining three criteria are discussed here.
Box 2.
Council of Europe criteria for population screening[39]

Some general questions, such as the ideal screening age, also remain unanswered and lack concensus in the literature. It has been suggested that screening older age groups to include young adults and pregnant woman would increase pick-up rates of RHD and improve echocardiographic detection of disease.[40,41] At-risk individuals in older age groups, all else being equal, would have had more time to contract ARF and recurrences thereof and are therefore likely to have a higher prevalence of worse valvular involvement, the latter also aiding an already difficult echocardiographic assessment.
However, it is important to remember that RHD screening in its current form with echocardiographic case detection and the institution of secondary prophylaxis aims to prevent ARF recurrences rather than diagnose RHD per se. This explains the rationale for screening children rather than adults as the rate of ARF recurrence in the latter group is very low. Most guidelines support this point of view with recommendations to stop prophylaxis at the age of 18–21 years in individuals with mild valve involvement (and without excessive risk). The ideal timing for screening has to carefully balance picking up more cases (by screening later) with picking up less cases by screening as early as possible to allow maximal time for prophylaxis to make a difference. Unfortunately, the added convenience of screening school children comes at a price as school attendance in poor areas can be <70%,[42] risking underestimating the prevalence of disease in those most likely to be worst affected.
Cost-effectiveness of RHD screening
It remains to be determined if echocardiography screening is cost-effective.[5,43,44] A study by Manji et al.[43] using Markov modeling suggested that primary prophylaxis may be less cost-effective than echocardiographic screening and treatment of early RHD using secondary prophylaxis. The decisions regarding how and where limited resources should be focused in developing countries is an important ethical question[45] and the decision regarding whether to invest in echocardiographic RHD screening programs at the expense of other, possibly more robust evidence-based interventions for other conditions, warrants due consideration.
The use and delivery of secondary prophylaxis
Secondary penicillin prophylaxis (intramuscular BPG is superior to oral penicillin) plays an important role in preventing ARF recurrences[46] and in doing so reduces the severity of RHD by slowing, stopping, or regressing valvular disease[47,48] (CoE criterion 4). In those with mild disease treated with penicillin prophylaxis, for example, the vast majority will have no detectable disease 5–10 years later.[49,50] However, this specific criterion does not necessarily apply to the screened population. The long-term outcome of secondary prophylaxis as a strategy in patients with borderline RHD and definite RHD diagnosed in the absence of a history of ARF (i.e., those diagnoses found on echocardiographic screening) may be different compared to more “traditionally” diagnosed RHD;[30,51,52] there is no evidence that BPG actually slows or halts RHD progression in these conditions. It has also yet to be proven that diagnosing subclinical RHD and instituting prophylaxis through a screening program will lead to better outcomes compared with intervention when the disease becomes clinically symptomatic (CoE criterion 5).[53]
There is still no reported evidence of GAS resistance to penicillin,[54] and the most cost-effective approach is the delivery of secondary prophylaxis within a register-based RHD control program.[55,56] However, the delivery of high-quality secondary prophylaxis remains a significant global challenge in RHD control:[57] adherence is low in many countries including certain parts of Australia,[58] Egypt,[59] Taiwan,[60] Brazil,[61] Uganda,[62] and South Africa,[63] with worldwide use of penicillin in clinically diagnosed ARF and RHD averaging only around 30%.[64,65] Modifying this trend is a difficult task despite significant research and investment, with efforts thus far only really improving patients from poor to moderate adherence.[66]
Reasons for lack of adherence appear to be multifactorial and include factors such as a lack of awareness or understanding of disease and distance to travel for receiving prophylaxis.[67] An important barrier to adherence also relates to BPG itself, i.e., painful injections and frequent administration (prompting some authors to call for a reformulated BPG with an ideal dosing interval of 3–6 months).[66]
Therefore, before embarking on widespread RHD screening programs, more work needs to be done on how best to mobilize initiatives that improve the availability, delivery, uptake, and maintenance of secondary prophylaxis, despite widely divergent views on how to achieve this.[45]
Improving the portability and cost of echocardiographic machines
Standard portable echocardiography (STAND) machines are an overt barrier to the implementation of widespread screening programs in poor regions: They are expensive, cumbersome to transport, and have limited battery capacity. Handheld (HAND) devices help address these issues although their limited functionality (e.g., lacking continuous wave Doppler) means that they are a work in progress. It is now possible to attach probes to smart devices which should help improve portability and reduce costs. The performance of HAND and STAND devices within screening studies is discussed later.
There is no perfect diagnostic test for rheumatic heart disease
Although echocardiography has become the gold standard for RHD diagnosis, it relies on criteria that must balance sensitivity and specificity and as such invariably remains imperfect at diagnostic categorization (CoE criterion 3). In particular, detecting early valvular lesions that have no diagnostic or prognostic precedent raises many questions including that we still do not truly know what constitutes the lower limit of RHD (the earliest or slightest changes recognizable as being due to RHD) by echocardiography. Part of the problem here is that RHD encompasses pathological changes that exist on a continuum, and delineating the transition point that separates mild disease from a normal variant can be very difficult. Although echocardiography is presently the most discriminating tool, a deeper understanding of the disease mechanisms that underlie morphological changes will no doubt facilitate a more rational diagnostic criteria.[68]
In a disease like RHD, accurate diagnosis is a particularly critical issue: A false-positive diagnosis will expose the patient to inappropriate and lengthy treatment (usually 10 years or longer), potentially create psychological harm and stigmatization by association with a disease (there is even evidence that echocardiographic RHD screening alone lowers quality of life scores for both the caregiver and screened child),[69] and unnecessarily add to the financial and manpower burden of the already stretched healthcare systems of many developing countries. Conversely, a false-negative result risks missing the opportunity to prevent a potentially fatal disease.
The significance of borderline rheumatic heart disease
Understanding the natural history of borderline RHD in particular is crucial because screening studies tend to uncover a burden of disease that is double (or more) that of definite RHD.[42,70] If borderline RHD is indeed confirmed to be associated with an increased risk for ARF recurrences or progression to definite RHD, then this may more than triple the number of individuals who might benefit from secondary prophylaxis and screening programs.[52] Notwithstanding these considerations, however, we do know that patients with mild, clinically evident RHD have an excellent long-term prognosis (even without regular penicillin prophylaxis)[71] and it should follow then that subclinical disease detected by echocardiography might have a potentially even better outcome.[45]
Longitudinal follow-up studies of patients diagnosed with borderline RHD have provided some clues on natural history, but no firm conclusions. Rémond et al.[72] examined Australian children using the WHF criteria in a prospective follow-up study after 2.5–5 years and found that individuals with borderline RHD were 8.8 times more likely to develop ARF, over eight times more likely to experience echocardiographic progression of valve lesions, and that 1 in 6 progressed to definite RHD. However, one-third of these children were receiving secondary prophylaxis, which may have altered the natural course of the disease.
They also demonstrated that patients with nonspecific valvular abnormalities (e.g., one morphological feature of RHD of the mitral valve (MV) and/or aortic valve without pathological MR or AR) were at increased risk of progression to definite RHD, with 1 in 10 progressing. These findings again bring into focus the questions regarding the definition of what constitutes the lower limit of RHD by echocardiography and also raises the issue of how best to manage these patients, whether to treat with secondary prophylaxis, opt for enhanced surveillance or repeat echocardiography, decisions that will have potentially significant implications for already stretched health-care systems.
Another recent study,[73] again using WHF criteria, re-examined 44 South African patients with borderline or definite RHD around 5 years after the initial diagnosis. Half of the participants (52.3%) improved to either borderline RHD or normal status, one-third (31.8%) did not show any change, and 15.9% worsened to definite RHD. In this series, only two patients (4.6%) were on secondary prophylaxis.
Slightly earlier studies from Nicaragua,[27] India,[28,74] Uganda,[75] and New Caledonia[76] also detail the natural history of borderline RHD. Outcomes after 5–43 months from diagnosis were that 49%–69% of possible or borderline RHD (different terminology owing to nonstandard criteria) remained stable, 21%–42% showed disease regression, and 4%–12% showed disease progression. However, the studies from Nicaragua and India used nonstandardized diagnostic criteria (which are associated with widely varying estimates of the prevalence of RHD) and many of these studies reported variable use of secondary prophylaxis, again potentially altering the course of the disease.
One has to question the mechanism of improvement of rheumatic involvement in all these studies and more work is undoubtedly required to tease out true disease improvement from the known measurement variability of mild or subclinical disease. This is an issue because borderline RHD often encompasses minor heart valve abnormalities that are open to subjective assessment (e.g., chordal thickness, anterior MV leaflet thickness, and mild leaflet motion abnormalities). Indeed, one study[77] examining variability in echocardiographic interpretation with serial testing within a 12-month period (and therefore less likely to represent natural progression/regression) demonstrated that there is a large inter-observer and inter-study variability when it comes to the diagnosis of borderline RHD.
Finally, researchers who screened low- and high-risk Australian children for RHD found that high-risk Australians were 3.4 times more likely to have borderline RHD.[42] This shows that almost certainly some cases of borderline RHD represent mild RHD but, as discussed, we have yet to uncover echocardiographically what distinguishes these cases from the normal variants (or alternative pathologies) that also form a significant proportion of the borderline RHD group.
Simplifying the World Heart Federation criteria for rheumatic heart disease screening programs
Implementing the WHF criteria is time-consuming, potentially complex, requires highly trained operators,[38,53] and may be impractical for in-field application.[30,78] Screening programs to date have employed a two-stage process with suspected RHD confirmed following expert review, something which cannot be maintained if we hope to implement large-volume screening given the paucity of expert reviewers and the extra cost this would entail. Therefore, the development of a uniform, simplified criteria with acceptable sensitivity and specificity that allows for a single-stage screening process by nonexperts using HAND, and which can be implemented within a preexisting health care program would significantly improve feasibility.[53] However, some middle-income countries may not be as restricted in terms of budget and might be able to justify using experienced cardiologists to participate more directly in the screening process. This may circumvent some of the criticism regarding simplification of the criteria and echocardiographic interpretation.
Removing the morphological criteria entirely and simplifying the functional criteria (usually measuring MR jet length only) is a strategy that researchers have recently begun to employ, with HAND devices being increasingly used for this purpose. A minimally defined jet length is used as a marker of pathological MR and by extension presence of RHD. A reasonable but unproven assumption here is the rationale that criteria-defined pathological MR found during screening of high-risk RHD populations is more likely to represent RHD than either normal variants or other pathologies.
Possible problems with this simplified approach are that MR has many causes and we are removing the morphological features meant to add specificity, hoping that from a screening perspective, we maintain enough sensitivity to include all possible RHD cases. It is, however, unclear at this stage that the group with isolated morphological change is insignificant and can be ignored.[68] The original WHO Doppler-only criteria were derived from criteria designed to diagnose acute rheumatic carditis during episodes of ARF by differentiating functional from pathological regurgitation and ignored morphological valvular changes that characterize more chronic rheumatic cardiac involvement.[35,79,80] Marijon et al. exposed the lack of sensitivity and specificity of these original criteria by adding important morphological criteria indicative of chronic rheumatic valve involvement and degrading the importance of differentiating functional from pathological valvular regurgitation, although retaining some functional deficits (Marijon Combined Criteria).[35] This study demonstrated the importance of the morphological features or at least getting the balance of morphological and functional features right which may caution against oversimplifying things.
Since 2012, several studies[70,81,82,83] have examined the performance of MR jet length as the single echocardiographic criterion against a reference approach [Table 1]. The definition of pathological MR varied (≥1.5–2.0 cm) between different studies and some included the presence of any degree of AR as a marker of RHD. Sensitivity for all disease (i.e., borderline plus define RHD) varied from 73% to 78.9% and specificity 82.4%–87.3%. Sensitivity for definite RHD was much better, ranging between 97.8% and 97.9% between studies.
Table 1.
Summary of recent screening studies examining the sensitivity and specificity of simplified diagnostic criteria when compared to the reference approach (images obtained using standard portable echocardiography and interpreted by experienced cardiologists with expertise in rheumatic heart disease using the full 2012 World Heart Federation criteria)
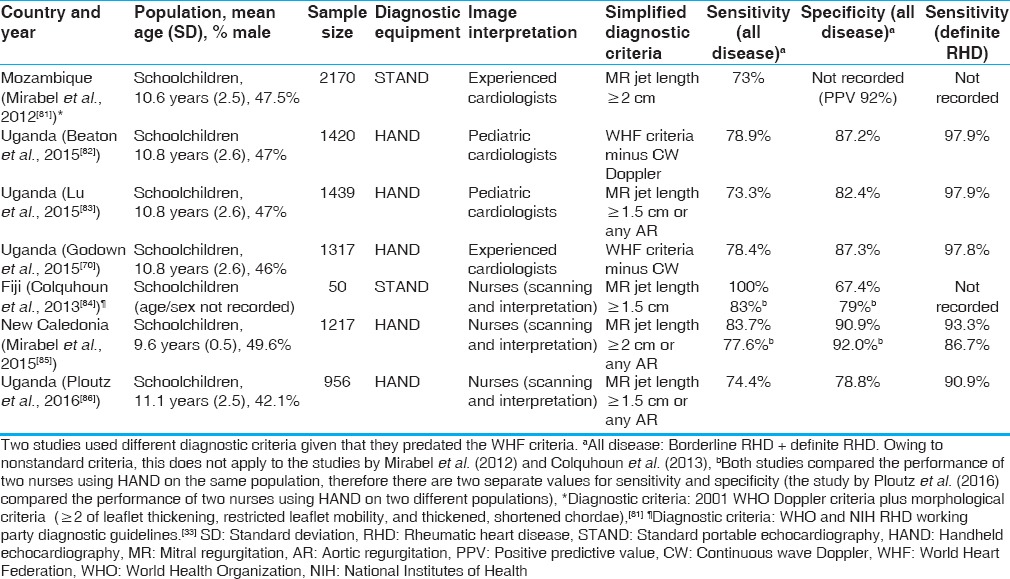
One has to be mindful of the figures in all these studies looking at MR as a single criterion. The gold standard WHF criteria used to define what constitutes RHD in these studies required significant MR as an important diagnostic ingredient for the most common lesion. This risks introducing an important bias into these analyses which become a self-fulfilling prophecy.
However, jet length measurement is quick and reproducible, which suits the requirements of large-volume screening programs and thus remains an important avenue to explore. Cardiology practice guidelines place more importance on the proximal jet width assessment (vena contracta) than length assessment when assessing MR severity. The latter, as a measured marker of MR severity, has all but disappeared from recent guidelines due to its known variability with technical factors (e.g., color scale) and anatomical (e.g., atrial size).[87] However, these guidelines, tasked with shaping patient management, have maximal utility in differentiating moderate from severe lesions,[88] but in the screened population of RHD patients (with mild or very mild MR), the use of MR jet length appears to be quite reasonable as a discriminator of MR severity as long as operators remain mindful of the technical pitfalls when comparing studies.
However, with suboptimal specificity rates, this single criterion may require modification or risk over-treatment. Alternatively, a HAND-positive patient could undergo confirmatory testing with STAND which, although not a flawless approach, will still reduce the number of in-depth echocardiograms that need to be performed. Moreover, when compared to auscultation alone, the case for HAND is very powerful, even if it missed almost one-third of borderline RHD in the Godown study: In developing countries ravaged by disease, some intervention, one could argue, is better than none.
The obvious problem here is that all isolated morphological deficits, even if relatively gross, would be missed by necessity. This again addresses the less obvious sensitivity issues with such a simplified approach. Take the example of a patient with a valve area reduced by half due to rheumatic commissural fusion. Such a valve area may still be above the 2.5 cm2 cutoff for the earliest guideline definition of mitral stenosis[89] but is a clear departure from the normal 5–7 cm2 and even if isolated in terms of not being associated with MR could constitute an important missed group of patients with isolated, milder forms of commissural fusion. The importance of functional versus morphological aspects of the criteria must be weighed carefully and future recommendations based on study evidence.
Task-shifting in rheumatic heart disease screening
Task-shifting (i.e., delegation of clinical tasks to less specialized health workers) is absolutely vital to the success of any potential RHD screening program in developing nations. However, while reducing costs and freeing physicians to perform other tasks,[90] it may actually require additional resources for successful implementation, particularly in the short term.
Studies examining task-shifting using nurses, having previously received focused echocardiographic training, were first conducted in 2013 [Table 1]. Overall sensitivity for all disease ranged from 74.4% to 100% and specificity was 67.4%–92%. Again, sensitivity for definite RHD was high at 86.7%-93.3%.[84,85,86]
These studies demonstrate that nurses, having received brief and focused training, can follow a simplified screening algorithm using STAND or HAND and achieve reasonable sensitivity and specificity. However, the amount of echocardiographic experience of the nurses (before training for the studies) varied significantly in some cases. Medical students may also make suitable candidates for task-shifting in some developing countries.[91,92] More studies using standardized, high-quality training programs and standardized diagnostic criteria are needed to build a clearer picture of the role of HAND by nonexperts employing simplified criteria.
One possible option for standardized training is an international team of trainers that could act as accredited instructors, implementing these training protocols with competency testing. A cheaper alternative (and which may reach a wider audience) is web-based learning that is open to everyone such as that devised by Engelman et al.,[78] who published their successful training protocol online, which was initially tested on-site (http://www.wiredhealthresources.net/EchoProject/). The WHO guidelines also recommend continuous monitoring and evaluation as vital components of the task-shifting process.[93] In addition, results will vary depending on the skill and motivation of the workers, as well as ability to retain these individuals.[94]
CONCLUSION
The recent proliferation of echocardiographic RHD studies has heralded a much-needed reinvigoration into the study and advancement of this neglected disease, but much work lies ahead. Effective strategies that encourage the regular uptake of secondary prophylaxis, a deeper understanding of the natural history of subclinical RHD and its response to penicillin prophylaxis, advancements in portable echocardiography, and a simplified criteria that is based on disease mechanisms, that can be applied by nonexperts, and adequately balances sensitivity and specificity, are desperately needed. Until then, routine widespread screening for RHD cannot be endorsed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM World Heart Federation. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol. 2013;10:284–92. doi: 10.1038/nrcardio.2013.34. [DOI] [PubMed] [Google Scholar]
- 2.Rothenbühler M, O'Sullivan CJ, Stortecky S, Stefanini GG, Spitzer E, Estill J, et al. Active surveillance for rheumatic heart disease in endemic regions: A systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob Health. 2014;2:e717–26. doi: 10.1016/S2214-109X(14)70310-9. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.GBD Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zühlke L, Mayosi BM. Echocardiographic screening for subclinical rheumatic heart disease remains a research tool pending studies of impact on prognosis. Curr Cardiol Rep. 2013;15:343. doi: 10.1007/s11886-012-0343-1. [DOI] [PubMed] [Google Scholar]
- 6.Zühlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36:1115–22a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bright PD, Mayosi BM, Martin WJ. An immunological perspective on rheumatic heart disease pathogenesis: More questions than answers. Heart. 2016;102:1527–32. doi: 10.1136/heartjnl-2015-309188. [DOI] [PubMed] [Google Scholar]
- 8.McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: A chink in the chain that links the heart to the throat? Lancet Infect Dis. 2004;4:240–5. doi: 10.1016/S1473-3099(04)00975-2. [DOI] [PubMed] [Google Scholar]
- 9.Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. 2012;25:145–53. doi: 10.1097/QCO.0b013e3283511d27. [DOI] [PubMed] [Google Scholar]
- 10.Carapetis JR, Kilburn CJ, MacDonald KT, Walker AR, Currie BJ. Ten-year follow up of a cohort with rheumatic heart disease (RHD) Aust N Z J Med. 1997;27:691–7. doi: 10.1111/j.1445-5994.1997.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 11.Meira ZM, Goulart EM, Colosimo EA, Mota CC. Long term follow up of rheumatic fever and predictors of severe rheumatic valvar disease in Brazilian children and adolescents. Heart. 2005;91:1019–22. doi: 10.1136/hrt.2004.042762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–68. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: Incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. doi: 10.1161/CIRCULATIONAHA.113.001477. [DOI] [PubMed] [Google Scholar]
- 14.Oli K, Asmera J. Rheumatic heart disease in Ethiopia: Could it be more malignant? Ethiop Med J. 2004;42:1–8. [PubMed] [Google Scholar]
- 15.Günther G, Asmera J, Parry E. Death from rheumatic heart disease in rural Ethiopia. Lancet. 2006;367:391. doi: 10.1016/S0140-6736(06)68128-2. [DOI] [PubMed] [Google Scholar]
- 16.Sliwa K, Carrington M, Mayosi BM, Zigiriadis E, Mvungi R, Stewart S. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: Insights from the heart of Soweto study. Eur Heart J. 2010;31:719–27. doi: 10.1093/eurheartj/ehp530. [DOI] [PubMed] [Google Scholar]
- 17.Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–94. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 18.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 19.Tadele H, Mekonnen W, Tefera E. Rheumatic mitral stenosis in children: More accelerated course in Sub-Saharan patients. BMC Cardiovasc Disord. 2013;13:95. doi: 10.1186/1471-2261-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godown J, Beaton A. Handheld echocardiography: A new tool for rheumatic heart disease screening in the developing world? Transl Pediatr. 2015;4:252–3. doi: 10.3978/j.issn.2224-4336.2015.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. doi: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurice J. Rheumatic heart disease back in the limelight. Lancet. 2013;382:1085–6. doi: 10.1016/s0140-6736(13)61972-8. [DOI] [PubMed] [Google Scholar]
- 23.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 24.Carapetis JR, Hardy M, Fakakovikaetau T, Taib R, Wilkinson L, Penny DJ, et al. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nat Clin Pract Cardiovasc Med. 2008;5:411–7. doi: 10.1038/ncpcardio1185. [DOI] [PubMed] [Google Scholar]
- 25.Roberts KV, Brown AD, Maguire GP, Atkinson DN, Carapetis JR. Utility of auscultatory screening for detecting rheumatic heart disease in high-risk children in Australia's Northern Territory. Med J Aust. 2013;199:196–9. doi: 10.5694/mja13.10520. [DOI] [PubMed] [Google Scholar]
- 26.Bhaya M, Panwar S, Beniwal R, Panwar RB. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography. 2010;27:448–53. doi: 10.1111/j.1540-8175.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 27.Paar JA, Berrios NM, Rose JD, Cáceres M, Peña R, Pérez W, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol. 2010;105:1809–14. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena A, Ramakrishnan S, Roy A, Seth S, Krishnan A, Misra P, et al. Prevalence and outcome of subclinical rheumatic heart disease in India: The RHEUMATIC (Rheumatic Heart Echo Utilisation and Monitoring Actuarial Trends in Indian Children) study. Heart. 2011;97:2018–22. doi: 10.1136/heartjnl-2011-300792. [DOI] [PubMed] [Google Scholar]
- 29.Webb RH, Wilson NJ, Lennon DR, Wilson EM, Nicholson RW, Gentles TL, et al. Optimising echocardiographic screening for rheumatic heart disease in New Zealand: Not all valve disease is rheumatic. Cardiol Young. 2011;21:436–43. doi: 10.1017/S1047951111000266. [DOI] [PubMed] [Google Scholar]
- 30.Roberts K, Colquhoun S, Steer A, Reményi B, Carapetis J. Screening for rheumatic heart disease: Current approaches and controversies. Nat Rev Cardiol. 2013;10:49–58. doi: 10.1038/nrcardio.2012.157. [DOI] [PubMed] [Google Scholar]
- 31.Miranda LP, Camargos PA, Torres RM, Meira ZM. Prevalence of rheumatic heart disease in a public school of Belo Horizonte. Arq Bras Cardiol. 2014;103:89–97. doi: 10.5935/abc.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zühlke LJ, Steer AC. Estimates of the global burden of rheumatic heart disease. Glob Heart. 2013;8:189–95. doi: 10.1016/j.gheart.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Carapetis J, Parr J, Cherian T. Standardization of epidemiologic protocols for surveillance of post-streptococcal sequelae: Acute rheumatic fever, rheumatic heart disease and acute post-streptococcal glomerulonephritis. National Institute of Allergy and Infectious Disease; 1 January. 2006. [Last accessed on 2016 Aug 27]. Available from: https://www.niaidnihgov/topics/strepThroat/Documents/groupasequelaepdf .
- 34.Webb R, Wilson N, Lennon D, Trenholme A, Wilson E, Nicholson R, et al. Population-based Echocardiographic Screening for Rheumatic Heart Disease in High-risk New Zealand Children. World Congress Paediatric Cardiology and Cardiac Surgery, Cairns. 2009 [Google Scholar]
- 35.Marijon E, Celermajer DS, Tafflet M, El-Haou S, Jani DN, Ferreira B, et al. Rheumatic heart disease screening by echocardiography: The inadequacy of World Health Organization criteria for optimizing the diagnosis of subclinical disease. Circulation. 2009;120:663–8. doi: 10.1161/CIRCULATIONAHA.109.849190. [DOI] [PubMed] [Google Scholar]
- 36.Sadiq M, Islam K, Abid R, Latif F, Rehman AU, Waheed A, et al. Prevalence of rheumatic heart disease in school children of urban Lahore. Heart. 2009;95:353–7. doi: 10.1136/hrt.2008.143982. [DOI] [PubMed] [Google Scholar]
- 37.Steer AC, Kado J, Wilson N, Tuiketei T, Batzloff M, Waqatakirewa L, et al. High prevalence of rheumatic heart disease by clinical and echocardiographic screening among children in Fiji. J Heart Valve Dis. 2009;18:327–35. [PubMed] [Google Scholar]
- 38.Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease – An evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Council of Europe, Committee of Ministers, Recommendation No. R (94) 11 on Screening as Tool of Preventive Medicine, 10 October. 1994 [Google Scholar]
- 40.Diao M, Kane A, Ndiaye MB, Mbaye A, Bodian M, Dia MM, et al. Pregnancy in women with heart disease in Sub-Saharan Africa. Arch Cardiovasc Dis. 2011;104:370–4. doi: 10.1016/j.acvd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Kane A, Mirabel M, Touré K, Périer MC, Fazaa S, Tafflet M, et al. Echocardiographic screening for rheumatic heart disease: Age matters. Int J Cardiol. 2013;168:888–91. doi: 10.1016/j.ijcard.2012.10.090. [DOI] [PubMed] [Google Scholar]
- 42.Roberts K, Maguire G, Brown A, Atkinson D, Reményi B, Wheaton G, et al. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation. 2014;129:1953–61. doi: 10.1161/CIRCULATIONAHA.113.003495. [DOI] [PubMed] [Google Scholar]
- 43.Manji RA, Witt J, Tappia PS, Jung Y, Menkis AH, Ramjiawan B. Cost-effectiveness analysis of rheumatic heart disease prevention strategies. Expert Rev Pharmacoecon Outcomes Res. 2013;13:715–24. doi: 10.1586/14737167.2013.852470. [DOI] [PubMed] [Google Scholar]
- 44.Zachariah JP, Samnaliev M. Echo-based screening of rheumatic heart disease in children: A cost-effectiveness Markov model. J Med Econ. 2015;18:410–9. doi: 10.3111/13696998.2015.1006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essop MR, Peters F. Contemporary issues in rheumatic fever and chronic rheumatic heart disease. Circulation. 2014;130:2181–8. doi: 10.1161/CIRCULATIONAHA.114.009857. [DOI] [PubMed] [Google Scholar]
- 46.Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev. 2002;(3):CD002227. doi: 10.1002/14651858.CD002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taranta A. Factors influencing recurrent rheumatic fever. Annu Rev Med. 1967;18:159–72. doi: 10.1146/annurev.me.18.020167.001111. [DOI] [PubMed] [Google Scholar]
- 48.Tompkins DG, Boxerbaum B, Liebman J. Long-term prognosis of rheumatic fever patients receiving regular intramuscular benzathine penicillin. Circulation. 1972;45:543–51. doi: 10.1161/01.cir.45.3.543. [DOI] [PubMed] [Google Scholar]
- 49.Feinstein AR, Spagnuolo M, Wood HF, Taranta A, Tursky E, Kleinberg E. Rheumatic fever in children and adolescents. A long-term epidemiologic study of subsequent prophylaxis, streptococcal infections, and clinical sequelae. VI. Clinical features of streptococcal infections and rheumatic recurrences. Ann Intern Med. 1964;60(Suppl 5):68–86. [PubMed] [Google Scholar]
- 50.Sanyal SK, Thapar MK, Ahmed SH, Hooja V, Tewari P. The initial attack of acute rheumatic fever during childhood in North India; a prospective study of the clinical profile. Circulation. 1974;49:7–12. doi: 10.1161/01.cir.49.1.7. [DOI] [PubMed] [Google Scholar]
- 51.Saxena A, Zühlke L, Wilson N. Echocardiographic screening for rheumatic heart disease: Issues for the cardiology community. Glob Heart. 2013;8:197–202. doi: 10.1016/j.gheart.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Rémond MG, Maguire GP. Echocardiographic screening for rheumatic heart disease-some answers, but questions remain. Transl Pediatr. 2015;4:206–9. doi: 10.3978/j.issn.2224-4336.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nascimento BR, Nunes MC, Lopes EL, Rezende VM, Landay T, Ribeiro AL, et al. Rheumatic heart disease echocardiographic screening: Approaching practical and affordable solutions. Heart. 2016;102:658–64. doi: 10.1136/heartjnl-2015-308635. [DOI] [PubMed] [Google Scholar]
- 54.Dhanda V, Chaudhary P, Toor D, Kumar R, Chakraborti A. Antimicrobial susceptibility pattern of ß-haemolytic group A, C and G streptococci isolated from North India. J Med Microbiol. 2013;62(Pt 3):386–93. doi: 10.1099/jmm.0.046672-0. [DOI] [PubMed] [Google Scholar]
- 55.Carapetis JR, Zühlke LJ. Global research priorities in rheumatic fever and rheumatic heart disease. Ann Pediatr Cardiol. 2011;4:4–12. doi: 10.4103/0974-2069.79616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watkins DA, Mvundura M, Nordet P, Mayosi BM. A cost-effectiveness analysis of a program to control rheumatic fever and rheumatic heart disease in Pinar del Rio, Cuba. PLoS One. 2015;10:e0121363. doi: 10.1371/journal.pone.0121363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson N. Secondary prophylaxis for rheumatic fever: Simple concepts, difficult delivery. World J Pediatr Congenit Heart Surg. 2013;4:380–4. doi: 10.1177/2150135113497240. [DOI] [PubMed] [Google Scholar]
- 58.Rémond MG, Severin KL, Hodder Y, Martin J, Nelson C, Atkinson D, et al. Variability in disease burden and management of rheumatic fever and rheumatic heart disease in two regions of tropical Australia. Intern Med J. 2013;43:386–93. doi: 10.1111/j.1445-5994.2012.02838.x. [DOI] [PubMed] [Google Scholar]
- 59.Bassili A, Zaher SR, Zaki A, Abdel-Fattah M, Tognoni G. Profile of secondary prophylaxis among children with rheumatic heart disease in Alexandria, Egypt. East Mediterr Health J. 2000;6:437–46. [PubMed] [Google Scholar]
- 60.Lue HC, Chen CL, Wei H. Some problems in long-term prevention of streptococcal infection among children with rheumatic heart disease in Taiwan. Jpn Heart J. 1976;17:550–9. doi: 10.1536/ihj.17.550. [DOI] [PubMed] [Google Scholar]
- 61.Pelajo CF, Lopez-Benitez JM, Torres JM, de Oliveira SK. Adherence to secondary prophylaxis and disease recurrence in 536 Brazilian children with rheumatic fever. Pediatr Rheumatol Online J. 2010;8:22. doi: 10.1186/1546-0096-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musoke C, Mondo CK, Okello E, Zhang W, Kakande B, Nyakoojo W, et al. Benzathine penicillin adherence for secondary prophylaxis among patients affected with rheumatic heart disease attending Mulago Hospital. Cardiovasc J Afr. 2013;24:124–9. doi: 10.5830/CVJA-2013-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker KG, Human DG, De Moor MM, Sprenger KJ. The problem of compliance in rheumatic fever. S Afr Med J. 1987;72:781–3. [PubMed] [Google Scholar]
- 64.Gasse B, Baroux N, Rouchon B, Meunier JM, Frémicourt ID, D'Ortenzio E. Determinants of poor adherence to secondary antibiotic prophylaxis for rheumatic fever recurrence on Lifou, New Caledonia: A retrospective cohort study. BMC Public Health. 2013;13:131. doi: 10.1186/1471-2458-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joseph N, Madi D, Kumar GS, Nelliyanil M, Saralaya V, Rai S. Clinical spectrum of rheumatic Fever and rheumatic heart disease: A 10 year experience in an urban area of South India. N Am J Med Sci. 2013;5:647–52. doi: 10.4103/1947-2714.122307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wyber R, Boyd BJ, Colquhoun S, Currie BJ, Engel M, Kado J, et al. Preliminary consultation on preferred product characteristics of benzathine penicillin G for secondary prophylaxis of rheumatic fever. Drug Deliv Transl Res. 2016;6:572–8. doi: 10.1007/s13346-016-0313-z. [DOI] [PubMed] [Google Scholar]
- 67.Saxena A, Mehta A, Ramakrishnan S. Adherence to benzathine penicillin in children with rheumatic fever/rheumatic heart disease: Results from an Indian pediatric RHD registry. JACC. 2015;65(10) [Google Scholar]
- 68.Herbst P. Screening for asymptomatic rheumatic heart disease: Understanding the mechanisms key to the diagnostic criteria. SA Heart Journal. 2015;12:134–44. [Google Scholar]
- 69.Wyber R, Zühlke L, Carapetis J. The case for global investment in rheumatic heart-disease control. Bull World Health Organ. 2014;92:768–70. doi: 10.2471/BLT.13.134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Godown J, Lu JC, Beaton A, Sable C, Mirembe G, Sanya R, et al. Handheld echocardiography versus auscultation for detection of rheumatic heart disease. Pediatrics. 2015;135:e939–44. doi: 10.1542/peds.2014-2774. [DOI] [PubMed] [Google Scholar]
- 71.Joint Report by the Rheumatic Fever Working Party of the Medical Research Council of Great Britain and the Subcommittee of the Principal Investigators of the American Council on Rheumatic Fever and Congenital Heart Disease. The evolution of rheumatic heart disease in children. Circulation. 1960;XXII:503–15. [Google Scholar]
- 72.Rémond M, Atkinson D, White A, Brown A, Carapetis J, Remenyi B, et al. Are minor echocardiographic changes associated with an increased risk of acute rheumatic fever or progression to rheumatic heart disease? Int J Cardiol. 2015;198:117–22. doi: 10.1016/j.ijcard.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Zühlke L, Engel ME, Lemmer CE, van de Wall M, Nkepu S, Meiring A, et al. The natural history of latent rheumatic heart disease in a 5 year follow-up study: A prospective observational study. BMC Cardiovasc Disord. 2016;16:46. doi: 10.1186/s12872-016-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhaya M, Beniwal R, Panwar S, Panwar RB. Two years of follow-up validates the echocardiographic criteria for the diagnosis and screening of rheumatic heart disease in asymptomatic populations. Echocardiography. 2011;28:929–33. doi: 10.1111/j.1540-8175.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 75.Beaton A, Okello E, Aliku T, Lubega S, Lwabi P, Mondo C, et al. Latent rheumatic heart disease: Outcomes 2 years after echocardiographic detection. Pediatr Cardiol. 2014;35:1259–67. doi: 10.1007/s00246-014-0925-3. [DOI] [PubMed] [Google Scholar]
- 76.Mirabel M, Fauchier T, Bacquelin R, Tafflet M, Germain A, Robillard C, et al. Echocardiography screening to detect rheumatic heart disease: A cohort study of schoolchildren in French Pacific Islands. Int J Cardiol. 2015;188:89–95. doi: 10.1016/j.ijcard.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Bacquelin R, Tafflet M, Rouchon B, Guillot N, Marijon E, Jouven X, et al. Echocardiography-based screening for rheumatic heart disease: What does borderline mean? Int J Cardiol. 2016;203:1003–4. doi: 10.1016/j.ijcard.2015.11.110. [DOI] [PubMed] [Google Scholar]
- 78.Engelman D, Kado JH, Reményi B, Colquhoun SM, Watson C, Rayasidamu SC, et al. Teaching focused echocardiography for rheumatic heart disease screening. Ann Pediatr Cardiol. 2015;8:118–21. doi: 10.4103/0974-2069.157024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO. Rheumatic Fever and Rheumatic Heart Disease: Report of a WHO Expert Consultation, Geneva, 29 October-1 November, 2001. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 80.Veasy LG. Time to take soundings in acute rheumatic fever. Lancet. 2001;357:1994–5. doi: 10.1016/S0140-6736(00)05134-5. [DOI] [PubMed] [Google Scholar]
- 81.Mirabel M, Celermajer DS, Ferreira B, Tafflet M, Perier MC, Karam N, et al. Screening for rheumatic heart disease: Evaluation of a simplified echocardiography-based approach. Eur Heart J Cardiovasc Imaging. 2012;13:1024–9. doi: 10.1093/ehjci/jes077. [DOI] [PubMed] [Google Scholar]
- 82.Beaton A, Lu JC, Aliku T, Dean P, Gaur L, Weinberg J, et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: A field study. Eur Heart J Cardiovasc Imaging. 2015;16:475–82. doi: 10.1093/ehjci/jeu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu JC, Sable C, Ensing GJ, Webb C, Scheel J, Aliku T, et al. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J Am Soc Echocardiogr. 2015;28:463–9. doi: 10.1016/j.echo.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Colquhoun SM, Carapetis JR, Kado JH, Reeves BM, Remenyi B, May W, et al. Pilot study of nurse-led rheumatic heart disease echocardiography screening in Fiji – A novel approach in a resource-poor setting. Cardiol Young. 2013;23:546–52. doi: 10.1017/S1047951112001321. [DOI] [PubMed] [Google Scholar]
- 85.Mirabel M, Bacquelin R, Tafflet M, Robillard C, Huon B, Corsenac P, et al. Screening for rheumatic heart disease: Evaluation of a focused cardiac ultrasound approach. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002324. pii: e002324. [DOI] [PubMed] [Google Scholar]
- 86.Ploutz M, Lu JC, Scheel J, Webb C, Ensing GJ, Aliku T, et al. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart. 2016;102:35–9. doi: 10.1136/heartjnl-2015-308236. [DOI] [PubMed] [Google Scholar]
- 87.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012;42:S1–44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 88.Flachskampf FA, Frieske R, Engelhard B, Grenner H, Frielingsdorf J, Beck F, et al. Comparison of transesophageal Doppler methods with angiography for evaluation of the severity of mitral regurgitation. J Am Soc Echocardiogr. 1998;11:882–92. doi: 10.1016/s0894-7317(98)70008-2. [DOI] [PubMed] [Google Scholar]
- 89.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–92. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 90.Joshi R, Alim M, Kengne AP, Jan S, Maulik PK, Peiris D, et al. Task shifting for non-communicable disease management in low and middle income countries – A systematic review. PLoS One. 2014;9:e103754. doi: 10.1371/journal.pone.0103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnes SS, Sim J, Marrone JR, Reddy VD, O'Carroll DC, Sumida L, et al. Echocardiographic screening of schoolchildren in American Samoa to detect rheumatic heart disease: A feasibility study. Pediatr Health Med Ther. 2011;2:21. [Google Scholar]
- 92.Shmueli H, Burstein Y, Sagy I, Perry ZH, Ilia R, Henkin Y, et al. Briefly trained medical students can effectively identify rheumatic mitral valve injury using a hand-carried ultrasound. Echocardiography. 2013;30:621–6. doi: 10.1111/echo.12122. [DOI] [PubMed] [Google Scholar]
- 93.WHO. Task shifting: Rational redistribution of tasks amongst health workforce teams: Global recommendations and guidelines. Geneva: World Health Organization; 2008. [Google Scholar]
- 94.Saxena A. Task shifting rheumatic heart disease screening to non-experts. Lancet Glob Health. 2016;4:e349–50. doi: 10.1016/S2214-109X(16)30077-8. [DOI] [PubMed] [Google Scholar]


