Abstract
Introduction
Estrogen inhibits lactation and bisphenol A (BPA) is a high production environmental estrogen. We hypothesize an inhibitory effect of BPA on lactation and aim to analyze the association between third trimester pregnancy urinary BPA and breastfeeding rates one month postpartum.
Methods
Odds ratios (OR) and 95% confidence intervals (95% CI) of breastfeeding and perceived insufficient milk supply (PIM) in relation to maternal peripartum urinary BPA concentrations were calculated in 216 mothers.
Results
97.2% of mothers in the lowest BPA tertile were breastfeeding at one month postpartum, compared to 89.9% in highest (p=0.01). Adjusted ORs (95% CI) for not breastfeeding at one month were 1.9 (0.3, 10.7) and 4.3 (0.8, 21.6) for second and third BPA tertiles, respectively, compared to the lowest (p=0.06, trend). 4.2% reported PIM in the lowest BPA tertile, compared to 8.7% in the highest (p=0.03). Adjusted ORs (95% CI) for PIM were 1.8 (0.4,7.7) and 2.2 (0.5,9.5), for the second and third BPA tertiles, respectively, compared to the lowest (p=0.29, trend).
Discussion
These results suggest an association between maternal BPA exposure and decreased breastfeeding.
Keywords: breastfeeding, Bisphenol A, environmental exposure, infant health
Introduction
Breastfeeding has numerous benefits for child health, including decreased infant mortality and morbidity, such as reduced risk of sudden infant death syndrome, otitis media, gastrointestinal infections, respiratory tract infections, asthma, and atopic dermatitis (Stuebe & Schwarz 2010). Decreased risk of cancers, cardiovascular disease, and diabetes are seen in both mothers who have breastfed and in their children (Stuebe & Schwarz 2010). Six months of exclusive breastfeeding is recommended by the American Academy of Pediatrics and the World Health Organization (American Academy of Pediatrics, 2012; World Health Organization, 2002).
Although the majority of women (74% in the US and 83% in Mexico) initiate breastfeeding, very few accomplish the recommended exclusive six months (12% in the US and 3% in Mexico) (Gonzalez de Cosio, Escobar-Zaragoza, González-Castell, & Rivera-Dommarco 2013; Stuebe & Schwarz 2010). Perceived insufficient milk (PIM) supply is the most commonly reported breastfeeding problem and approximately 35% of early weaning in full term infants is attributed to low milk supply (Gonzalez de Cosio, Escobar-Zaragoza, González-Castell, & Rivera-Dommarco 2013; Stuebe & Schwarz 2010). Women who experienced early undesired weaning due to breastfeeding problems in a large cohort study, including PIM, breastfed for an average of 1.2 months, compared to 7.0 months in mothers who did not experience disrupted lactation (Stuebe et al. 2014). Breastfeeding rates in Mexico have declined in recent years and the most common reason for never breastfeeding in Mexican women was PIM (37.4%), which was cited nearly three times more frequently than any other reason for not breastfeeding (González de Cossío, Escobar-Zaragoza, González-Castell, & Hernández-Ávila 2012).
Lactogenesis and lactation are governed by complex paracrine and endocrine signaling pathways (Neville, McFadden, & Forsyth 2002). Estrogen inhibits lactation by decreasing prolactin levels. The reduction of estrogen at birth, due to the removal of the hormone-secreting placenta, contributes to the initiation of lactogenesis II, the period of colostrum secretion, and subsequent lactation (Neville et al. 2002; Pang & Hartmann 2007). Consequently, estrogen birth control pills are contraindicated in breastfeeding mothers due to their well-established effect of decreasing milk supply (Koetsawang 1987). Nearly three decades ago, Rogan et al. hypothesized an effect of environmental estrogens on breastfeeding, finding a negative association between di-chloro, di-phenyl trichloroethane (DDT) exposure and breastfeeding duration, which was also seen in a more recent retrospective study (Karmaus, Davis, Fussman, & Brooks 2005; Rogan & Ragan 2007). However, studies of other estrogenic persistent organic pollutants, including dichlorodiphenyl dichloroethylene, and a variety of polychlorinated biphenyls, have shown mixed results (Thomas et al., 2001; Weldon et al., 2010).
Bisphenol A (BPA) is found in many plastics and in the lining of most canned food products (Rubin 2011). Worldwide exposure to BPA is considered to be nearly universal with eight billion pounds of BPA produced annually (Rubin 2011). Measurable urinary BPA was detected in 92.7% of a U.S. population-based sample (Calafat, Ye, Wong, Reidy, & Needham, 2007) and almost 88% of participants in a Canadian pregnancy cohort (Arbuckle et al., 2014), indicating that, along with the high volume of production, human exposure is nearly ubiquitous. BPA interferes with estrogenic pathways by binding estrogen receptors and consequently has been associated with several reproductive health outcomes, including: altered hormone levels in males and females, sexual dysfunction, decreased sperm quality, impaired oocyte development, miscarriage, preterm birth, and low birth weight (Cantonwine, Hauser, & Meeker 2013). However, to our knowledge, no studies have assessed the relationship between BPA exposure and breastfeeding in humans. Animal studies have found reduced time spent nursing in BPA exposed groups, though the researchers have hypothesized alternative pathways than a direct effect of BPA on lactation (Nakagami et al. 2009; Palanza, Gioiosa, vom Saal, & Parmigiani 2008).
This paper has three main objectives. First, we present a novel hypothesis of the impact of BPA, a synthetic estrogen, on lactation. Next, due to the ubiquity of BPA exposure, the prevalence of reported low milk supply, and the established effects of estrogen on lactation, we investigate the effects of peripartum BPA exposure on breastfeeding rates at one month postpartum, PIM at three months postpartum, and breastfeeding duration. Finally, we provide a call for future research on the relationship between BPA and breastfeeding in larger cohorts and other populations.
Methods
Sample
Women were recruited from maternity hospitals that are part of the Mexican Social Security Institute, a government-run healthcare system for families where at least one member is employed in the private sector. BPA levels were assayed in third trimester urine samples from 250 mothers who were a subset of those enrolled between 1997 and 2004 in the Early Life Exposure in Mexico to Environmental Toxicant (ELEMENT) project and whose children were re-recruited in 2012 (Ferguson et al. 2014). Maternal age was reported at screening. Maternal education and pre-pregnancy BMI were collected by self-report at the first trimester visit. Of the 250 included, 216 had completed study visits at one and three months postpartum and completed interviews about breastfeeding. All participants gave informed consent and the study protocols were approved by the Ethics and Research Committees of the Mexican National Institute of Public Health, Mexican National Institute of Perinatology, and the University of Michigan.
BPA
Total BPA concentration (ng/mL) and specific gravity (SG) were measured in third trimester urine samples by isotope dilution–liquid chromatography–tandem mass spectrometry (ID–LC–MS/MS) at NSF International (Ann Arbor, MI, USA), as described elsewhere (Lewis et al. 2013). Specimen collection in this cohort occurred prior to scientific understanding of the effects of BPA and thus standard collection containers were utilized. The samples were analyzed retrospectively for BPA contamination and no evidence of contamination was found. We calculated values for SG-adjusted BPA concentrations using the following equation: , where BPAadj=BPA, adjusted for SG; BPA= measured third trimester urinary BPA concentration (ng/mL); M=1.013 ng/mL, the median SG for the study population; and SG = measured SG for each individual. Women were classified into tertiles of BPA exposure after adjustment for SG. While a high degree of temporal variability in BPA measures has been shown, classification into the highest tertile using one urine sample has a sensitivity of 0.64 and specificity of 0.74 (Mahalingaiah et al. 2008).
Breastfeeding
Surveys were administered at each of four follow up visits, scheduled at 1, 3, 6, and 12 months post-partum. At each visit, the mothers were asked “Are you breastfeeding now?” If the answer was ‘no’, they were asked “When did you stop?” and “Why did you stop?”
Mothers were categorized as either breastfeeding or not breastfeeding at one month postpartum based on whether they answered ‘yes’ or ‘no’, respectively, to the question of whether they were breastfeeding at the one month post-partum study visit. Not breastfeeding at one month was chosen as a primary outcome because: 1) it was the most proximal to the exposure measure; 2) most women initiate breastfeeding but if lactation is disrupted, they are most likely to quit within the first month (Stuebe et al. 2014); 3) milk supply is established within the first month; and 4) there is less influence from confounders, such as returning to work.
PIM was defined as cessation of breastfeeding by the third month postpartum, with a cited reason of either ‘milk did not come in’ or ‘not enough milk’. All mothers who did not cease breastfeeding for those stated reasons were classified as not having PIM, whether or not they had continued breastfeeding at 3 months.
Breastfeeding duration was determined as the duration reported by the mother, at the first visit in which a mother reported not breastfeeding.
Statistical Analyses
Differences in means were obtained using a t-test comparing log-transformed BPA (SG-adjusted) concentrations between women who were and were not breastfeeding at one month postpartum and those with and without PIM. Fisher’s exact test was used to assess differences in these groups of women by demographic characteristics and BPA tertiles. Unadjusted and adjusted odds ratios (OR) were obtained from logistic regression using SAS Version 9.3 (SAS Institute, Cary, N.C.). Primary predictors included: 1) log-BPA concentration (SG adjusted) and 2) tertiles of SG-adjusted BPA concentration. Outcomes were: not breastfeeding at one month and cessation of breastfeeding by 3 months due to PIM. We employed a confounders approach to covariate selection, limiting our covariate selection to those variables that could influence both the exposure and outcome, to maximize statistical efficiency (Schisterman, Cole, & Platt, 2009). Maternal age, years of education, and pre-pregnancy BMI were included as covariates in adjusted models. Finally, we used Cox proportional hazards models to compare breastfeeding duration between the three tertiles of BPA exposure.
Results
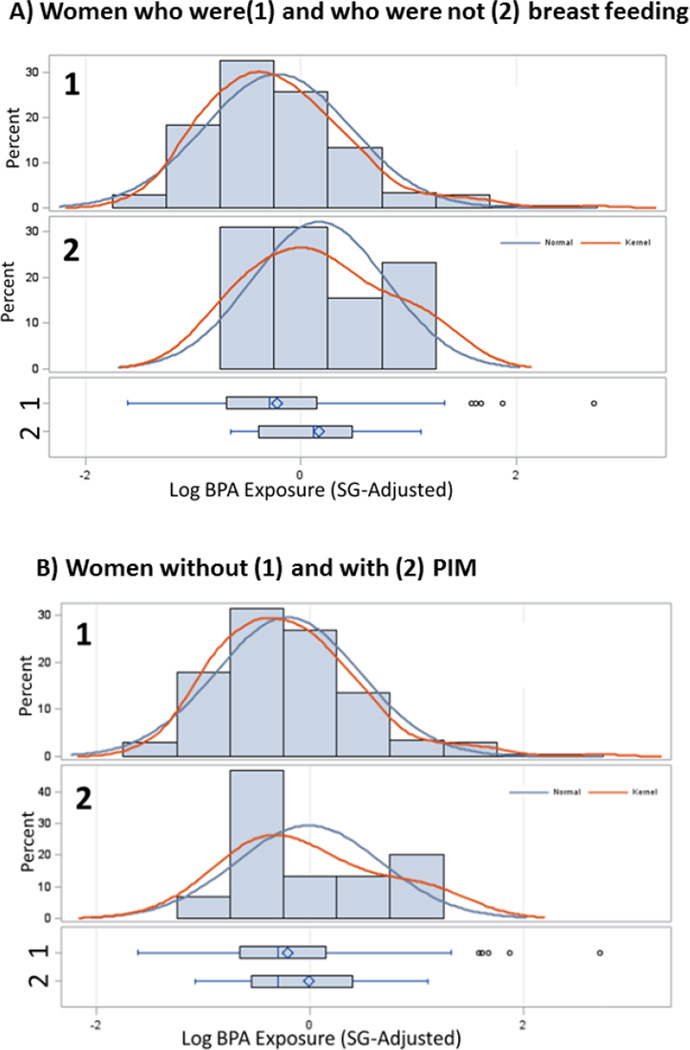
The mean (±SD) age of mothers at delivery was 27.8 (±5.8) years. Mean pre-pregnancy BMI, based on self-report height and weight, was 27.7 (±3.9) kg/m2. The average years of education was 11.1 (±2.9) years. The mean (±SD) of SG-adjusted BPA exposure was 1.07 (±1.33) ng/mL in women who were breastfeeding at one month postpartum, compared to 1.42 (±0.93) ng/mL in women who were not breastfeeding (p, difference of log-adjusted means= 0.05) (Figure 1a). The mean (±SD) of SG-adjusted BPA exposure was 1.08 (±1.34) ng/mL in women without PIM, compared to 1.24 (±0.94) ng/mL in women with PIM (p, difference of log-adjusted means=0.281) (Figure 1b).
Figure 1.
Distribution of third trimester log-BPA (SG-adjusted) levels in women: A) who were (1) and who were not (2) breastfeeding at one month postpartum and B) without (1) and with (2) PIM
Footnote: Sample sizes for each graph are as follows: A1) n= 213; A2) n=13; B1) n=211; B2) n=15
In the lowest tertile of BPA exposure, 97.2% of mothers were breastfeeding at one month postpartum, as compared to 94.7% and 89.9% in the middle and highest tertiles, respectively (p=0.01) (Table 1). In the lowest tertile, 4.2% of mothers stopped breastfeeding by 3 months due to PIM, compared to 8.0% and 8.7% in the middle and highest tertiles, respectively (p=0.03) (Table 1).
Table 1.
Prevalence of breastfeeding at one month and PIM by maternal characteristics
| N (%) | Not Breastfeeding % (n) |
pa | PIM % (n) |
pa | |
|---|---|---|---|---|---|
| Overall Sample | 216 | 6 (13) | - | 6.9 (15) | - |
| Age at delivery (years) | 0.004 | 0.002 | |||
| <20 | 15 (7.0) | 0 (0) | 0 (0) | ||
| 20–24 | 64 (29.8) | 7.8 (5) | 4.7 (3) | ||
| 25–29 | 69 (32.0) | 5.8 (4) | 8.7 (6) | ||
| 30–34 | 37 (17.2) | 2.7 (1) | 5.4 (2) | ||
| ≥35 | 30 (14.0) | 10 (3) | 13.3 (4) | ||
| Pre-pregnancy BMI | 0.017 | 0.024 | |||
| Normal Weight (>18.5;<25) | 51 (23.6) | 9.8 (5) | 5.9 (3) | ||
| Overweight (≥25; <30) | 112 (51.85) | 3.6 (4) | 5.4 (6) | ||
| Obese (≥30) | 53 (24.5) | 7.5 (4) | 11.3 (6) | ||
| Education (years) | 0.061 | 0.035 | |||
| ≤9 | 75 (31.1) | 6.7 (5) | 5.3 (4) | ||
| 10–15 | 115 (53.7) | 5.2 (6) | 7 (8) | ||
| ≥16 | 24 (11.21) | 8.3 (2) | 12.5 (3) | ||
| Urinary BPA (ng/mL) | 0.014 | 0.03 | |||
| Tertile 1 | 72 (33.3) | 2.8 (2) | 4.2 (3) | ||
| Tertile 2 | 75 (34.7) | 5.3 (4) | 8 (6) | ||
| Tertile 3 | 69 (31.9) | 10.1 (7) | 8.7 (6) |
p-values obtained using Fisher’s exact test
In models with continuous log-BPA as a linear predictor, the unadjusted odds (95% CI) of not breastfeeding by one month increased by 2.05 (1.00,4.21) for each unit increase in log-BPA exposure (SG-adjusted) (p=0.05). The OR was 2.05 (1.00,4.12) adjusted for maternal age, pre-pregnancy BMI, and years of education (p=0.05). The unadjusted odds (95% CI) of PIM increased by 1.48 (0.73,3.01) for each unit increase in log-BPA exposure (SG-adjusted) (p=0.28). The adjusted odds of PIM was 1.52 (0.73,3.17) (p=0.26).
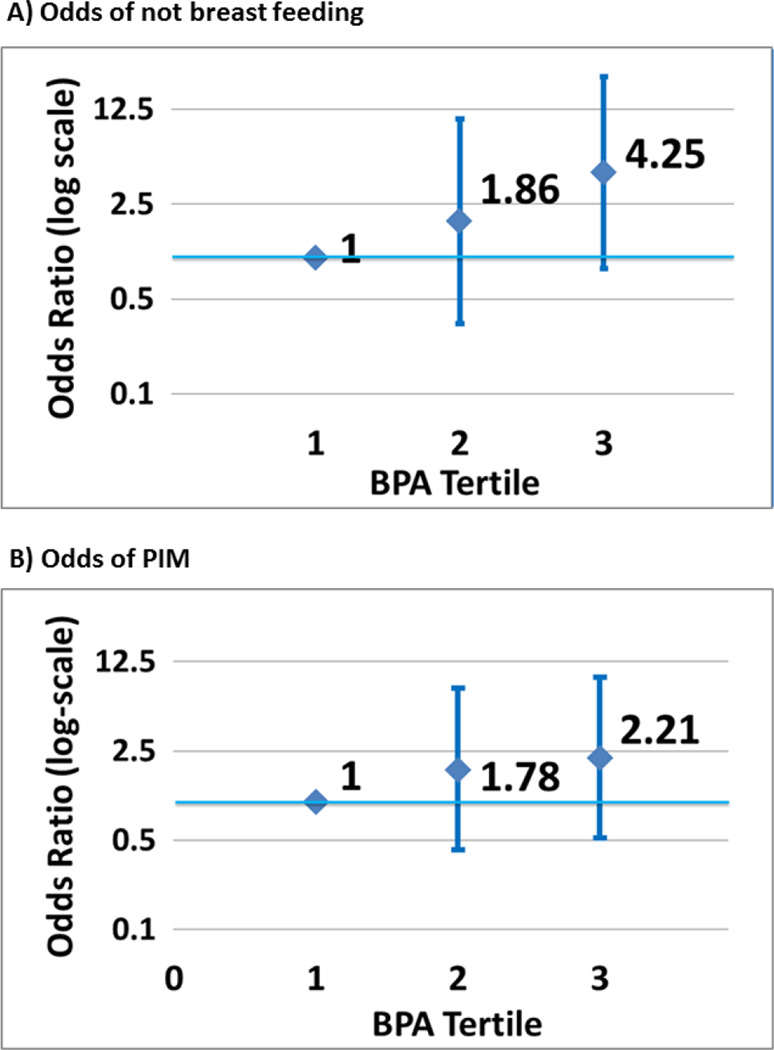
Figure 2 depicts the adjusted odds ratios of not breastfeeding at one month and of PIM by BPA tertiles. Adjusted ORs (95% CI) for not breastfeeding at one month were 1.9 (0.3, 10.7) and 4.3 (0.8, 21.6) in the second and third exposure tertiles, as compared with the first (lowest) (p=0.06, trend) (Figure 2a). Adjusted ORs for cessation due to PIM were 1.8 (0.4,7.7) and 2.2 (0.5,9.5) for the second and third exposure tertiles, compared to the first (p=0.29, trend) (Figure 2b).
Figure 2.
Odds of not breastfeeding at one month postpartum (A) and PIM (B), with 95% confidence intervals, by tertiles of BPA exposure, adjusted for maternal age, education and pre-pregnancy BMI
We did not find any correlation between BPA exposure and duration of breastfeeding (results not shown).
Discussion
In this paper we investigated the relationship between BPA exposure and breastfeeding in a cohort of Mexican women. We found statistically significant associations, between tertiles of third trimester urinary BPA and percent of women who were not breastfeeding at one month postpartum and percent of women with PIM. Mean BPA exposure was higher for women not breastfeeding at one month postpartum and among women with PIM. In adjusted logistic regression models, controlling for maternal age, pre-pregnancy BMI, and educational attainment, the associations between BPA exposure and breastfeeding rates and PIM were not statistically significant. Given the large magnitude of the odds ratios and the limited statistical power of the study due to small sample size and high breastfeeding rates, these results should be interpreted cautiously. In the context of these limitations, the results suggest a potential association and a call for future research on the topic in larger samples is warranted.
While a direct effect of BPA on lactation is the simplest explanation for reduced breastfeeding rates and PIM, some evidence exists for alternative or additional pathways. BPA exposure may increase the rate preterm of birth, which is known to negatively affect breastfeeding (Cantonwine et al. 2010). BPA exposure may reduce thyroid hormone levels which are necessary for lactation (Meeker & Ferguson 2011; Neville et al. 2002). BPA may also influence neurological development and consequently affect an infant’s ability to suckle or latch effectively (Nakagami et al., 2009). BPA might affect the mother-infant relationship through pathways influencing maternal care, as has been suggested by some animal studies (Nakagami et al. 2009; Palanza et al. 2008).
Of note, over 75% of our sample was overweight or obese based on pre-natal BMI. Other studies have found positive associations between urinary BPA concentrations and BMI or obesity risk (Bhandari, Xiao, & Shankar, 2013; Shankar, Teppala, & Sabanayagam, 2012; Trasande, Attina, & Blustein, 2012; Wang et al., 2012). It may be the case that the endocrine disrupting properties of BPA contribute to obesity development or, conversely, that BPA stored in fat cells is released into the bloodstream causing higher measured urinary BPA. Another reason could be that the foods containing high levels of BPA also cause weight gain. These hypotheses for the observed association have different implications for our results. If BPA causes weight gain, then BMI may lie in the causal pathway as a mediator of the relationship between BPA and lactation. In this case, our results would be underestimated. However, if elevated urinary BPA concentrations are a result of increased fat mass or if there is no causal relationship, there is potential for residual confounding to be affecting the results of our study.
The primary strength of this study is that it investigates a novel hypothesis about the relationship between BPA exposure, a synthetic estrogen, and breastfeeding. The sample size for this study led to wide confidence intervals for estimates, despite the large negative effect size of BPA on breastfeeding at one month. Several assumptions were made in the measurement of primary variables. We used third trimester maternal urine BPA concentrations as a proxy for postpartum BPA exposure. Although we adjusted for maternal education, other potentially influential measures of socioecomonic status were not available on the analytic sample. A strength of the study was the concurrent reporting of breastfeeding rather than retrospective collection of this information. We measured ‘any breastfeeding,’ rather than exclusive breastfeeding, however, and therefore could not differentiate between mothers who had no problems with milk supply from those who had limited supply but continued to breastfeed with supplementation. There may have also been mothers with low milk supply who stopped breastfeeding for other reasons or who did not initiate breastfeeding. We used breastfeeding rates at one month and PIM by month three as indicators of insufficient milk production. Although we observed no association between breastfeeding duration and BPA exposure, there are numerous potential confounding factors that may affect a mother’s choice to continue breastfeeding as time progresses after birth. Despite these limitations, we did find suggestive evidence of a relationship between BPA and breastfeeding that should be further investigated in other cohorts.
Conclusions
These results suggest a potential negative association between BPA exposure and breastfeeding at one month postpartum and PIM. Considering these results and the known inhibitory effect of estrogen on lactation, further study is warranted in larger cohorts and different populations. Additionally, future research should explore the potential role of breastfeeding as a mediator between BPA exposure and metabolic and developmental outcomes in children.
Significance.
A few studies have demonstrated decreased breastfeeding rates in women exposed to endocrine disrupting chemicals in the environment. No studies have examined the effect of BPA, an environmental estrogen, on breastfeeding, although estrogen is known to have an inhibitory effect on lactation.
This is the first investigation of the relationship between BPA exposure and lactation. Women with the highest BPA exposure demonstrated greater odds of not breastfeeding one month postpartum and breastfeeding cessation due to perceived insufficient milk supply three months postpartum compared to the lowest BPA exposure.
Acknowledgments
Funding Sources
This research was supported in part by the National Institute of Environmental Health Sciences (NIEHS) and Environmental Protection Agency (EPA) grants R01ES007821, P20 ES018171)/RD83480001, P30 ES017885, P01 ES02284401/RD 83543601 and by the National Institute of Public Health, Ministry of Health, Mexico. The American British Cowdray Hospital provided the facilities used to conduct this research.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- American Academy of Pediatrics. Breastfeeding and the Use of Human Milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. http://doi.org/10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Fraser WD. Phthalate and bisphenol A exposure among pregnant women in Canada-results from the MIREC study. Environment International. 2014;68:55–65. doi: 10.1016/j.envint.2014.02.010. http://doi.org/10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. American Journal of Epidemiology. 2013;177(11):1263–1270. doi: 10.1093/aje/kws391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environmental Health Perspectives. 2007;116(1):39–44. doi: 10.1289/ehp.10753. http://doi.org/10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Hauser R, Meeker JD. Bisphenol A and Human Reproductive Health. Expert Review of Obstetrics & Gynecology. 2013;8(4):329–335. doi: 10.1586/17474108.2013.811939. http://doi.org/10.1586/17474108.2013.811939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine D, Meeker JD, Hu H, Sánchez BN, Lamadrid-Figueroa H, Mercado-García A, Téllez-Rojo MM. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environmental Health. 2010;9:62. doi: 10.1186/1476-069X-9-62. http://doi.org/10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6 Suppl):S18–S24. doi: 10.1210/en.2005-1131. http://doi.org/10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Peterson KE, Lee JM, Mercado-García A, Blank-Goldenberg C, Téllez-Rojo MM, Meeker JD. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reproductive Toxicology. 2014;47:70–76. doi: 10.1016/j.reprotox.2014.06.002. http://doi.org/10.1016/j.reprotox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de Cosio T, Escobar-Zaragoza L, González-castell LD, Rivera-Dommarco JÁ. Prácticas de alimentación infantil y deterioro de la lactancia materna en México. Salud Publica de México. 2013;55(1):170–179. [PubMed] [Google Scholar]
- González de Cossío T, Escobar Zaragoza L, González Castell LD, Hernández Ávila M. Deterioro de la lactancia materna : dejoar las fórmulas y apegarse a lo básico. Centro de Investigación en Nutrición y Salud, Instituto Nacional de Salud Pública; 2012. [Google Scholar]
- Karmaus W, Davis S, Fussman C, Brooks K. Maternal concentration of dichlorodiphenyl dichloroethylene (DDE) and initiation and duration of breast feeding. Paediatric and Perinatal Epidemiology. 2005;19(5):388–398. doi: 10.1111/j.1365-3016.2005.00658.x. http://doi.org/10.1111/j.1365-3016.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- Koetsawang S. The effects of contraceptive methods on the quality and quantity of breast milk. International Journal of Gynaecology and Obstetrics. 1987;25(suppl):115–127. doi: 10.1016/0020-7292(87)90401-2. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, Téllez-Rojo MM. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93(10):2390–2398. doi: 10.1016/j.chemosphere.2013.08.038. http://doi.org/10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environmental Health Perspectives. 2008;116:173–178. doi: 10.1289/ehp.10605. http://doi.org/10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Relationship between Urinary Phthalate and Bisphenol A Concentrations and Serum Thyroid Measures in U . S . Adults and Adolescents from the National Health and Nutrition Examination Survey ( NHANES ) 2007 – 2008. Environmental Health Perspectives. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami A, Negishi T, Kawasaki K, Imai N, Nishida Y, Ihara T, Koyama T. Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology. 2009;34:1189–1197. doi: 10.1016/j.psyneuen.2009.03.005. http://doi.org/10.1016/j.psyneuen.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia. 2002;7(1):49–66. doi: 10.1023/a:1015770423167. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12160086. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental Research. 2008;108:150–157. doi: 10.1016/j.envres.2008.07.023. http://doi.org/10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. Journal of Mammary Gland Biology and Neoplasia. 2007;12:211–221. doi: 10.1007/s10911-007-9054-4. http://doi.org/10.1007/s10911-007-9054-4. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Ragan NB. Some evidence of effects of environmental chemicals on the endocrine system in children. International Journal of Hygiene and Environmental Health. 2007;210(5):659–667. doi: 10.1016/j.ijheh.2007.07.005. http://doi.org/10.1016/j.ijheh.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. The Journal of Steroid Biochemistry and Molecular Biology. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. http://doi.org/10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment Bias and Unnecessary Adjustment in Epidemiological Studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. http://doi.org/10.1097/EDE.0b013e3181a819a1.Overadjustment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol A levels and measures of obesity: Results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinology. 2012;2012:965243. doi: 10.5402/2012/965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Horton BJ, Chetwynd E, Watkins S, Grewen K, Meltzer-Brody S. Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. Journal of Women’s Health. 2014;23(5):404–412. doi: 10.1089/jwh.2013.4506. http://doi.org/10.1089/jwh.2013.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe A, Schwarz E. The risks and benefits of infant feeding practices for women and their children. Journal of Perinatology. 2010;30:155–162. doi: 10.1038/jp.2009.107. http://doi.org/10.1038/jp.2009.107. [DOI] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- Thomas AR, Marcus M, Zhang RH, Blanck HM, Tolbert PE, Hertzberg V, Rubin C. Breast-feeding among women exposed to polybrominated biphenyls in Michigan. Environmental Health Perspectives. 2001;109(11):1133–1137. doi: 10.1289/ehp.01109133. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1240474&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Zhou Y, Tang CX, Wu JG, Chen Y, Jiang QW. Association between bisphenol A exposure and body mass index in Chinese school children: A cross-sectional study. Environmental Health. 2012;11:79. doi: 10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon RH, Webster M, Harley KG, Bradman A, Fenster L, Davis MD, Eskenazi B. Serum persistent organic pollutants and duration of lactation among Mexican-American women. Journal of Environmental and Public Health. 2010;2010:1–11. doi: 10.1155/2010/861757. http://doi.org/10.1155/2010/861757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Infant and young child nutrition: Global strategy on infant and young child feeding. Fifty Fifth World Health Assembly. 2002;A55/15:13.10. Retrieved from http://apps.who.int/gb/archive/pdf_files/WHA55/ea5515.pdf. [Google Scholar]