Abstract
Objective
Epilepsy is a chronic disorder, but seizure recordings are usually obtained in the acute setting. The chronic behavior of seizures and the interictal bursts that sometimes initiate them is unknown. We investigate the variability of these electrographic patterns over an extended period of time using chronic intracranial recordings in canine epilepsy.
Methods
Continuous, yearlong intracranial EEG recordings from four dogs with naturally occurring epilepsy were analyzed for seizures and interictal bursts. Following automated detection and clinician verification of interictal bursts and seizures, temporal trends of seizures, burst count, and burst-burst similarities were determined. One dog developed status epilepticus, the recordings of which were also investigated.
Results
Multiple seizure types, determined by onset channels, were observed in each dog, with significant temporal variation between types. The first 14 days of invasive recording, analogous to the average duration of clinical invasive recordings in humans, did not capture the entirety of seizure types. Seizures typically occurred in clusters, and isolated seizures were rare. The count and dynamics of interictal bursts form distinct groups and do not stabilize until several weeks after implantation.
Significance
There is significant temporal variability in seizures and interictal bursts after electrode implantation that requires several weeks to reach steady state. These findings, comparable to those reported in humans implanted with the NeuroPace RNS device 1,2, suggest that transient network changes following electrode implantation may need to be taken into account when interpreting or analyzing intracranial EEG during evaluation for epilepsy surgery. Chronic, ambulatory intracranial EEG may be better suited to accurately map epileptic networks in appropriate individuals.
Keywords: intracranial, EEG, canine, seizure, bursts
INTRODUCTION
Of the 65 million people worldwide with epilepsy, approximately one-third have seizures that cannot be well controlled by anti-epileptic medications 3,4. Many of these medically intractable patients resort to alternative therapies, such as resective surgery and implantable neurodevices for relief. In the absence of obvious causal lesions, candidates for surgery and neurodevice implants are determined based on intracranial EEG (iEEG) and to an increasing extent, neuroimaging (PET, SPECT). The purpose is to localize the seizure onset zone prior to surgical resection or to identify network locations that may be amenable to neuromodulation. Unfortunately, these therapies have less than ideal efficacy: only approximately 50% of patients with neocortical epilepsy will achieve a clinically meaningful improvement in seizure frequency following surgery5. Similarly, median seizure reduction rates are 53% in a recent study of responsive neurostimulation 6. Therefore, there is great interest in the research community in increasing the rate of seizure freedom in this challenging patient population.
New biomarkers in epilepsy are poised to impact patient care, from tracking epileptogenesis, to steering development of new pharmacologic agents, to enriching clinical trials 7. Recently, our group has shown that interictal bursts are dynamically similar to seizure onsets, but occur with much greater frequency 8. These bursts, described in adults as brief potentially ictal rhythmic discharges (B(I)RDs) 9 , have been associated with increased propensity for seizures in the critically ill but their exact significance is still unknown. Studying these patterns in chronic ambulatory recordings will better allow scientists to understand the natural temporal dynamics of epileptic brain.
Canine epilepsy is similar to human epilepsy in its epidemiology, spontaneity, and its response and resistance to therapy 10,11. The similarities make canine models a promising animal model for testing new therapies, particularly neurodevices. In fact, vagal nerve stimulation was first shown to interrupt chemically induced seizures in a canine model 12. We previously presented recordings from a novel implantable device that continuously monitors iEEG deployed in six dogs with naturally occurring epilepsy13. This work further validated canine epilepsy as a promising model of human epilepsy and generated a set of continuous iEEG data of unprecedented length for analysis. This data is the first of its kind to be publicly available on the International Epilepsy Electrophysiology Portal (http://ieeg.org). The recordings, which contained over 200 observed seizures, exhibit remarkable electrographic similarity to human recordings both in seizure onset patterns as well as features of background EEG.
Importantly, this dataset provides epileptic iEEG recordings of an unparalleled length, an average of 14 months across the three main dogs in this study. These recordings are significantly longer than the typical human inpatient iEEG recordings of days to a few weeks in duration14 and provides 1) a greater number observations in time which allow us to describe temporal changes of observed bursts and seizures with greater statistical confidence, and 2) an opportunity to characterize transient post-implantation changes in epileptiform patterns. It is known that chronic iEEG implants and the surgery to implant them for invasive monitoring alters the underlying healthy cortex. The changes they induce, including hemorrhage, ischemia, and inflammation affects overall signal quality, electrode impedance, and possibly the representation of the epileptic network 15. It is unknown how changes in this acute setting influence localization of the epileptic network, as current literature is primarily based on electrographic recordings during this period of inflammation. It is also unclear whether interictal and ictal intracranial electrographic biomarkers currently used for surgical localization are stable within the typical recording period. One recent study analyzing 82 patients implanted chronically with a responsive neurostimulator demonstrates that an accurate representation of the spatial and temporal variability of seizures in these patients require at least 30 days of continuous monitoring2. Another study demonstrates that this prolonged monitoring may reveal patients to have well localized seizures and be good surgical candidates, though this was not apparent during acute monitoring1.
In this study, we investigate temporal changes of both seizures and interictal bursts over extended iEEG recordings. This dataset provides an unprecedented opportunity to track temporal changes in epileptiform patterns over long periods of time after implantation, and to assess how well acute monitoring represents epileptic networks at steady state. Furthermore, in contrast to existing long-term iEEG findings in literature derived from two recording electrodes recording several minutes per day, our extended dataset contains continuous recordings from a total of 16 contacts. We pay particular attention comparing the initial one to three weeks after intracranial implant to the steady state in these recordings. Lastly, we study quantitative dynamics of interictal bursts, specifically identifying how bursts fluctuate throughout the recordings.
MATERIALS AND METHODS
Dataset
Six dogs with naturally occurring focal epilepsy were originally implanted with four bilateral 4-contact subdural strip electrodes, a total of 8-contacts over each hemisphere, for chronic iEEG recording from a wireless system. Four of these dogs were selected for analysis due to the occurrence of seizures. Dogs 2, 4, 5, and 7 were recorded for 475.7, 329.9, 45.8, and 451.8 days, respectively (Table S1). Dogs were withdrawn from any medications at enrollment. Dog 5 died from status epilepticus, after which all dogs received intermittent (2 mg/kg, twice a day) phenobarbital (PHB) administration (detailed in Figure 2) during monitoring. In all dogs this occurred well after the acute implantation period.
Figure 2.
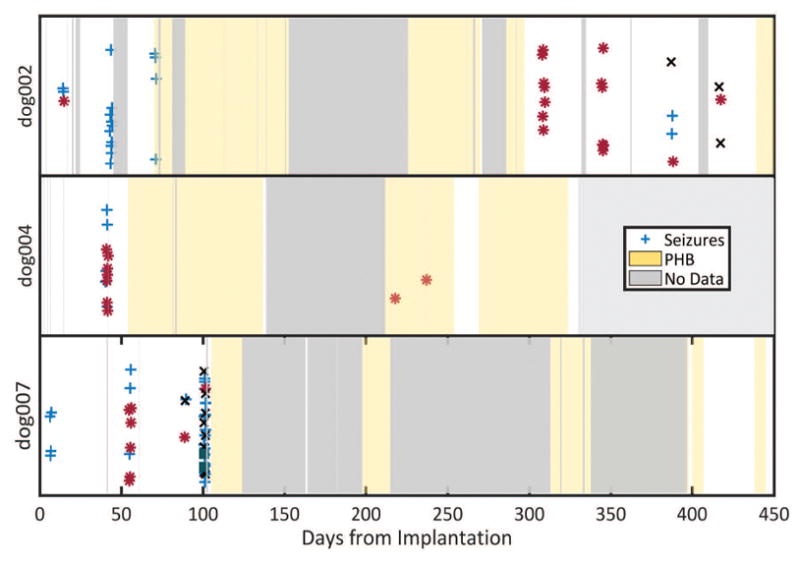
Seizures over the entire recording length across three dogs. Each marker represents a seizure, vertically jittered in time. Each color and symbol denote different seizure types as determined by a board certified epileptologist (KD). Gray represents periods of no data. Yellow represents periods of intermittent phenobarbital (PHB) administration.
Event Detection
Both seizures and bursts were detected as described in prior work 8. Those steps are summarized below.
Seizures
Seizures were identified with an automatic detector based on the line-length feature, which captures both amplitude and time components of the signal and is shown to be robust for seizure detection 16. Of these initial detections, valid electrographic seizures were verified with simultaneous video and the consensus of two board certified epileptologists (BL and GW). They were further subtyped according to localization of the seizure onset zone by a board-certified epileptologist (KD). All dogs were observed to have focal epilepsy of unknown etiology with secondary generalization and exhibited similar seizure symptomatology. Typically, seizures that began focally and secondarily generalized proceeded in four phases. The first phase of typical seizures lasted 5–12 seconds and started with vigorous side-to-side shaking of the head, or jerking of the head followed by shaking, with altered awareness. The second tonic phase lasted 2–15 seconds with extensor rigidity of the jaw and opisthotonus of the head, neck, and limbs. These tonic movements were followed by rhythmic clonic jerking of the limbs, which were rapid for 25–30 seconds, then slowed to resembled post-ictal running movements for 10–50 seconds. In the recovery phase, the dogs lay quietly in lateral recumbency, with occasional jerking movements and hyperventilation.
Interictal bursts
Interictal bursts lasting greater than 500 milliseconds and less than 30 seconds were extracted by using a threshold on the average line-length feature across all the channels. Candidate bursts were then manually culled and verified by an epileptologist (KD). Examples of bursts at different time points are shown in figure 1. To determine the effect of implantation on the number of bursts, a linear mixed model was used to model log burst counts in the first 100 days across all animals, with each dog as a random effect. Residuals were modeled with an autoregressive model to remove autocorrelation.
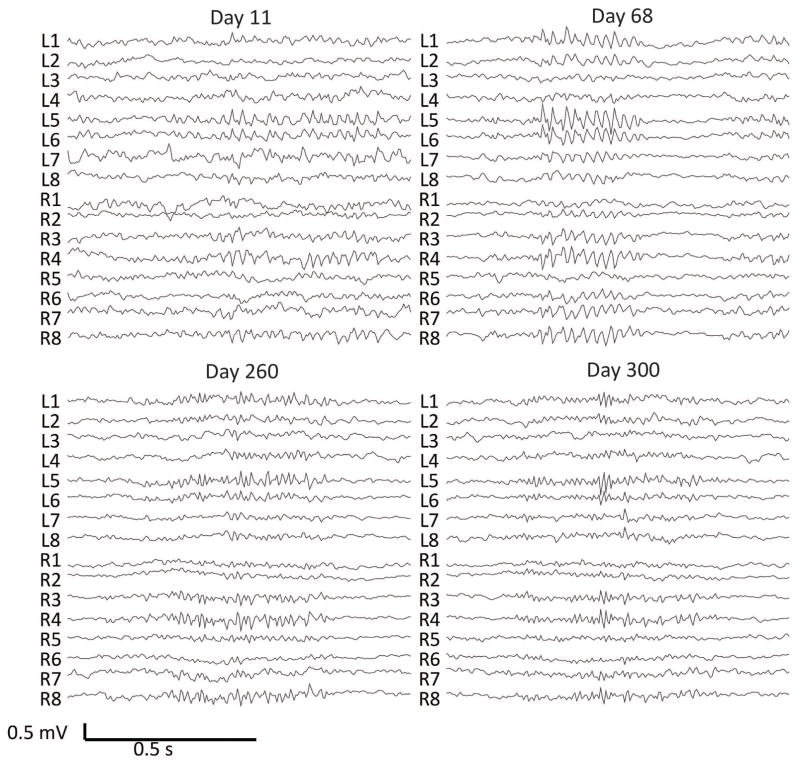
Figure 1.
Examples of interictal bursts detected on the canine iEEG data and corresponding days after implantation from dog 2. There are 8 electrodes on each hemisphere, two parallel strips of four electrodes each. L1–8 are over the left hemisphere and R1–8 are over the right hemisphere. An average referential montage is displayed. (A) Burst of beta activity bilaterally most prominent in L5–6 and R3–4 on Day 11. (B) Burst of diffuse beta activity most marked at channels L5–L6 on Day 68. (C) Burst of rhythmic gamma activity most prominent in L5–6, R3–4,8 on Day 260. (D) Burst of gamma activity most prominent in L5 on Day 300.
To determine how bursts dynamically change, we characterize each time point in a burst by their dynamic frequency content and compare between bursts using a “similarity” metric. First, time points in a burst are modeled as locally stationary autoregressive processes that change states over time with a Markov switching model. In this model, event (burst and seizure) snippets are modeled together across all animals using a spatial autoregressive hidden Markov model (AR-HMM). This model was used to capture the relative spatial locations of the electrodes as well as capturing non-stationarity with a state-switching model. After statistical inference, each time point in each event is assigned a state, and we define a similarity metric as the probability that two events that belong to the same state. Two identical events will have a similarity of 1, and two events with no time points in a common state will have a similarity of 0. This similarity is calculated by determining the number of time points in each burst that share the same state and provides a metric to compare dynamic similarities between one or more bursts.
Similarity matrices were created to show burst-burst similarity for each dog by visualizing the similarity of a given burst to all bursts. To determine stability across time, the similarity of bursts on each day was compared to all future bursts for each dog. Readers are referred to our initial paper 8 as well as prior technical work for a more detailed description of the model 17,18.
RESULTS
Seizures
A total of 37, 14, 91, and 48 seizures were detected for dog 2, 4, 5 and 7, respectively (See Supplementary materials). Seizures electrographically appeared qualitatively indistinguishable in onset and spread patterns compared to human seizures as determined by to two epileptologists (BL and KD). With the exception of the dog in status epilepticus (dog 5), most seizures in each dog occurred in clusters, where all consecutive seizures with an inter-seizure interval (ISI) less than or equal to 24 hours defines a cluster (Figure 2). For Dogs 2, 4, 5, and 7, the mean cluster duration was 29 (sd=7.6), 39 (sd=0), 18 (sd=0), and 25.5 (sd=5.9) hours, respectively. The mean within-cluster ISI was 6.3 (sd=3.5), 3.5 (sd=2.5), 0.79 (sd=1.93), and 2.3 (sd = 3) hours, respectively. Importantly, each dog had multiple seizure types with onset zones arising from bilateral hemispheres independently. The seizure types fluctuated across time. Examples of two seizure subtypes from the same dog on day 14 and day 308 of recording are provided (Figure 3). Within the first 60 days, each dog displayed two seizure types. Across the entire recording there was more variability, as Dogs 2, 4, and 7 had three, two, and four different seizure subtypes, respectively. The first 14 days of recording, analogous to the human inpatient surgical evaluation period, did not capture the full extent of seizure types in any of the dogs. When Dog 5 died from status epilepticus, PHB was intermittently started for dog 2, 4, and 7 on days 54, 74, and 105, respectively. PHB therapy greatly lowered seizure frequency (Figure 2).
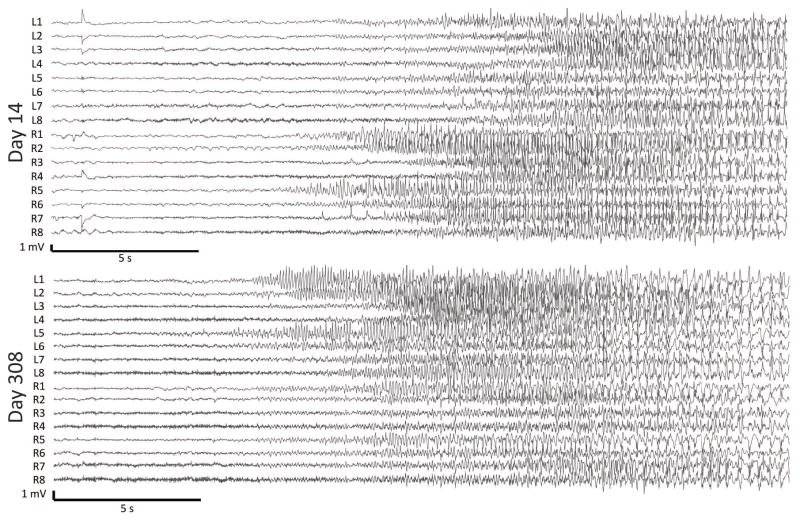
Figure 3.
Two example seizure subtypes at Day 14 and Day 308 from dog 2. In panel A, seizure onset channels were determined to be in R5/R6 and propagating to R1. In panel B, seizure onset channels were on the left side, in channels L5/L6 and propagating to L1.
Interictal Bursts
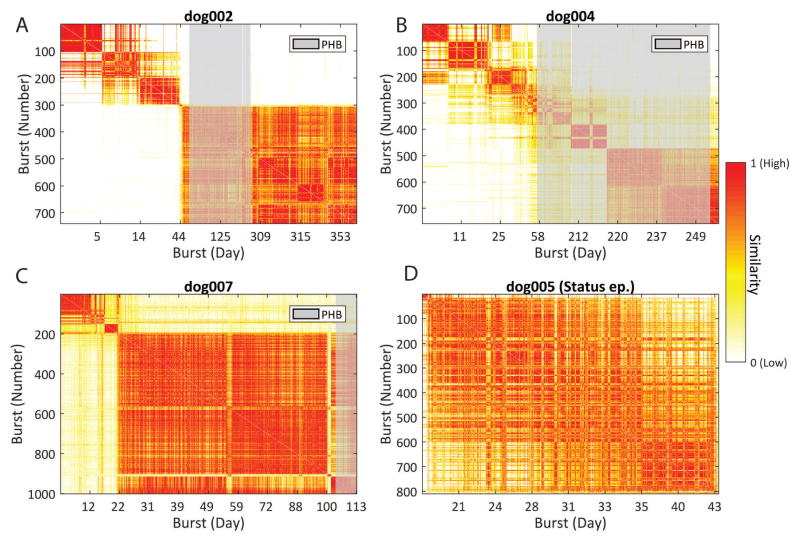
Over 700 bursts were detected in each dog (See Supplementary materials). Examples of bursts and the time of occurrence for one dog are given in Figure 1. In three dogs without status epilepticus, bursts showed temporal variation in similarity and in number. There were a significantly greater number of interictal bursts initially after implantation that decreased over time (P=0.03) (Figure 4). The similarity of bursts approaches that of those at steady state weeks after recording for each dog (Figure 5). A similarity matrix comparing bursts to all bursts show distinct clusters that indicate evolving event dynamics after implantation (Figure 6). It can be observed that for dog 2 (6A), dog 4, (6B), and dog 7 (6C), that there are remarkably distinct groups of bursts that are temporally restricted. For dogs 2 and 7, the largest and most stable group emerges in the first few weeks of recording, while dog 4’s bursts undergo a more gradual, but still observable, transition. Interictal bursts were unaffected during PHB administration, both in count as well as in similarity.
Figure 4.
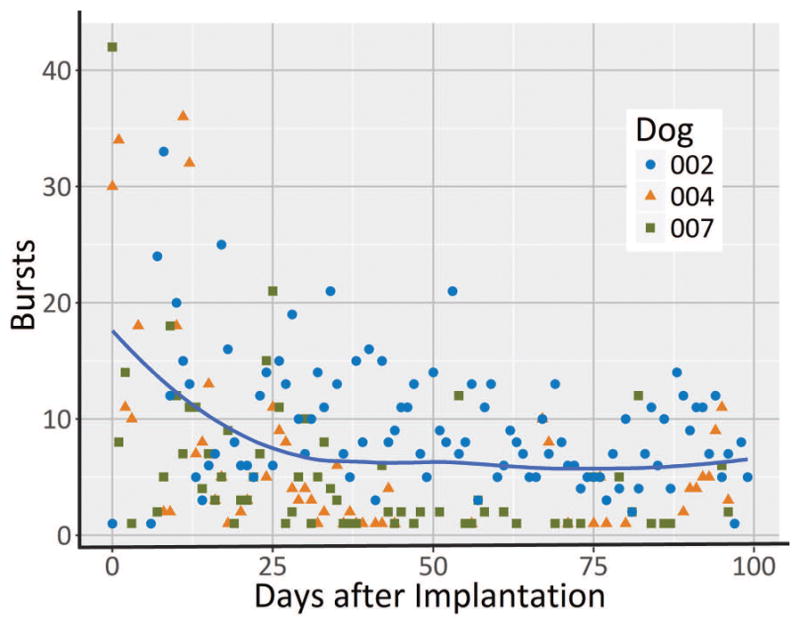
Average number of bursts per day for the first 100 days across three dogs (excluding dog with status epilepticus). The blue line represents the mean number of bursts across three dogs. A linear mixed model fit to log bursts show a significant decrease in burst count (p=0.03).
Figure 5.
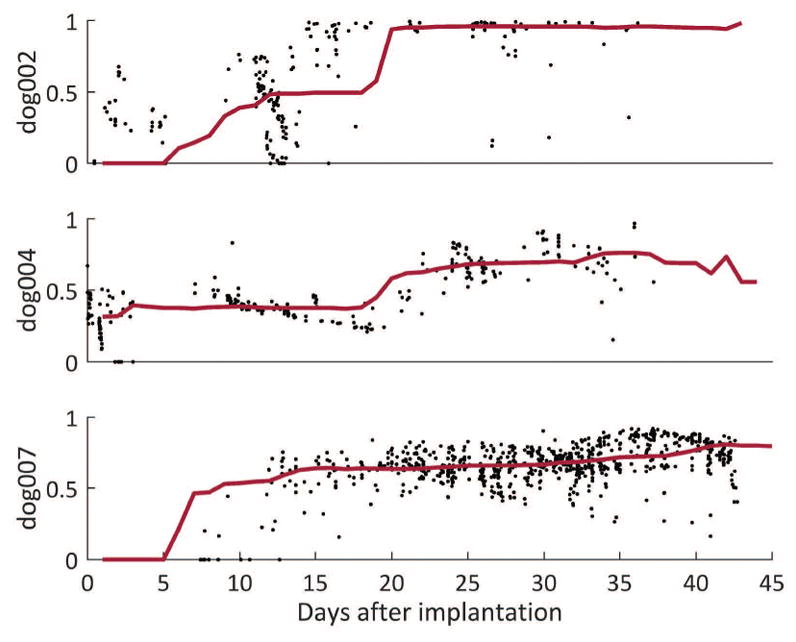
Normalized burst similarity in comparison with all future bursts in three dogs over the first 45 days of recording. Black points represent the similarity of the burst at that time point to future bursts. The red line denotes the median similarity within a 2-week moving window. High similarity represents a value of 1. Of note, early bursts are dissimilar to later bursts and that similarity levels off ~20 days.
Figure 6.
Similarity between bursts over time. Red indicates high similarity, white indicates low similarity. Vertical axes show bursts in order. Horizontal axes show bursts based on day of occurrence. Time periods when dogs received phenobarbital (PHB) are indicated in gray. Panels A, B, and C all show distinct clusters of bursts that are very similar to one another, indicated by well demarcated blocks of high similarity. Panel D displays similarities of all bursts during status epilepticus, showing that most bursts were dynamically similar.
Status epilepticus
One dog died after developing status epilepticus one month following implantation. In this dog, all bursts showed remarkable similarity (Figure 6D), suggesting that a single network generated and sustained status epilepticus in this animal. Following the death of this dog, all other dogs were treated clinically with intermittent PHB.
DISCUSSION
In this work, we investigated the temporal evolution of seizures and interictal bursts in long-term canine recordings and demonstrated that seizure onset location and burst dynamics fluctuate throughout the recording. These findings, while obtained in natural canine epilepsy, may have significant implications for current practice in the management of human epilepsy.
Intracranial EEG provides high resolution and fidelity recordings that allow interrogation of the epileptic network. However, most of these recordings are currently collected during clinical monitoring for resective surgery, lasting a few weeks at maximum due to the invasive nature of intracranial electrodes. Trauma to the brain during implantation may result in hemorrhage as well as an acute inflammatory response that activates surrounding glial cells as early as one day post-implantation19. A chronic foreign body reaction is often observed with the formation of a glial scar 20. This reactive tissue at the electrode-tissue interface is presumed to be the cause of increased electrode impedance over time21. Acute implantation of electrodes also alters brain physiology, at least transiently, and even sometimes renders patients seizure free. Despite these findings, the gold standard for seizure onset localization is recorded while the brain is in an acute inflammatory state, and it is important to investigate the validity of inferences drawn from data collected in this period. Although impedance may lead to lower signal to noise ratios, we have shown here that seizure and burst patterns also change when studied over an extended period of time, effects unlikely related to electrode impedance alone.
Seizures
We observed that seizures occur in clusters, a finding previously reported in human seizure diaries and short-term EEG monitoring. Confirmation of this finding in our rigorously annotated data over many months further validates the relevance of naturally epileptic canines as a model of human epilepsy 22,23. It is interesting to note that across each dog there were relatively few clusters despite the relatively large number of seizures (Figure 2).
One interesting finding was the occurrence of multiple seizure types which were not all captured during the first two weeks of recording, a period analogous to inpatient presurgical monitoring. During this period, PHB had not yet been administered to the canines. It is important to note that many seizure clusters consisted primarily of one type of seizure, and that localizing all epileptogenic regions in these animals required recording not multiple seizures, but multiple clusters of seizures. This pattern may hold for humans as well.
Furthermore, while each seizure type included a different set of onset electrodes, there were similarities in each seizure type within individual dogs. The unequivocal electrographic change that localized the seizure onset zone alternates between a finite set of spatial locations, though in each type there were common channels. In other words, each seizure’s onset channels formed an incomplete set of onset channels across all seizures. It is plausible that an underlying propagation pathway could have been kindled in these animals, and that these electrode locations are all part of a single epileptic network which can be ignited to begin from any of these locations. Thus, a patient with focal epilepsy may in fact involve multiple onset regions if enough observations are captured, suggesting that long-term monitoring to capture the full extent of a patient’s network may be key to improving outcome from epilepsy surgery or implantable devices.
This conclusion is supported by the work of DiLorenzo et al., who found that a group of patients who were poorly localized during acute implantation were well localized after chronic implantation of a neuromodulatory device and developed seizure-freedom following resection of their chronically localized network1. It is also possible that the emergence of other seizure patterns after months of chronic electrode implantation could be due to secondary epileptogenesis, though data from King-Stephens, DiLorenzo, and significant relapse rates after resection of well localized unilateral temporal lobe seizure during acute intracranial recordings argue against this conclusion 1,2. The recurrence of early seizure types later in our canine recordings suggests that the initial seizure types are likely not a transient result of tissue reaction post-implantation. The variability observed in seizure types and the associated trend might be also be interpreted as a recovery phase from which a patient’s true network emerges following resolution of the acute trauma, which itself or coupled to acute medication withdrawal might initiate atypical seizures 24.
Since our seizure subtyping was based on electrographic focal onset and spread, one may expect clinical semiology to vary with each type. Interestingly, each seizure’s clinical semiology appeared grossly similar on continuous video recording, and no relationship between subtypes and generalization was observed. Our observations regarding shifting onset patterns may be a feature specific to canine epilepsy, but no detailed IEEG studies of human epilepsy currently exist to allow for adequate comparisons. Our group is actively trying to determine the extent of these findings to human epilepsy in an analogous human dataset.
Interictal Bursts
In addition we found significant variability in the dynamics of interictal bursts throughout the recording. In this study, we found that the number of bursts is significantly elevated in the weeks after implantation (Figure 4). This period of abnormal electrographic activity may represent the decline of the acute inflammatory stage and possibly stabilization through chronic inflammation and a foreign body reaction. Though this may result in increased electrode impedance and reduced voltage readings, we report also a decline in the number of interictal bursts. This is important because the former may necessitate measures to correct for impedance, but the latter suggests more cautious interpretation of traditional epileptiform patterns that may be a transiently distorted by electrode implantation. This effect is unlikely to be medication-driven because the decreasing trend of burst count stabilizes around day 25, before any PHB was administered.
For each dog it is clear that the dynamics of interictal bursts are in flux during the initial stages of a recording. By comparing the similarity of a burst to all future bursts, we observe that the event dynamics of the bursts begin to stabilize days to weeks post-implantation (Figure 5). Distinct groups of bursts are also seen in Figure 6 and it is plausible that they reflect the current state of an underlying seizure generating network. Observationally, the period of time where bursts are heterogeneous correspond to periods where multiple seizure types originate. For example, in the first 50 days dog 4’s burst types are constantly changing, and the seizure cluster on day ~45 consists of two different seizure types. In dog 7, different seizure types were observed before and after day 22. Furthermore, around day 100, many new seizure types are seen, and a break in similarity is also seen near day 100.
PHB does not appear to have any effect on burst dynamics or number, though it did control seizures. In previous work we showed that these bursts are dynamically similar to seizure onsets. This suggests that bursts and seizures may share a similar underlying mechanism, and that the former represents seizures that fail to initiate. It is possible then that PHB preferentially inhibits seizures but not necessarily abnormal interictal patterns, a finding previously reported in children and rodents 25,26.
The observations that seizure onset electrodes vary over an extended recording and that the initial weeks after implantation represents an unstable electrographic network may have important implications in the clinical management of human epilepsy. If present in humans, this suggests that chronic outpatient iEEG monitoring during presurgical evaluation may more accurately define the epileptic network and ultimately lead to improved seizure freedom after surgery. In light of our findings, the reliability of potential biomarkers such as high frequency oscillations extracted within the first several weeks after implantation must be interpreted with caution, as the variability of iEEG properties may be too great during the acute and subacute phases to use these features for clinical decision-making.
Dog 5 is an example of canine status epilepticus and provides an interesting case to analyze. The bursts before and during status epilepticus were very similar to each other, even though the first seizure occurred at day 25 (Figure 6D). It is plausible that the bursts were a result of implant injury, or otherwise coincidental during recording, and subsequently evolved into status epilepticus and resulted in death. An alternative explanation is that there was one particular region of the network that gave rise to status epilepticus, and that its particular neurophysiologic composition might have been predisposed to seizures that would not stop spontaneously. Although these observations were made in only one dog, comparison of results with the other three dogs suggests that bursts and seizures that occur during status epilepticus are different than more typical seizures and is supported by prior work in human and other animal models of status epilepticus 27. It deserves further study to determine if there is high burst similarity in human status epilepticus and whether burst variability is mechanistically involved in seizure cessation.
Limitations and Future Directions
The observation that iEEG patterns do not stabilize until weeks after implantation could have significant implications for the interpretation of iEEG both in clinical and research realms. However, there are significant limitations that require further investigation and that must temper this conclusion. First, this study involves a limited number of canines, which may have different etiologies of epilepsy particularly as a result of inbreeding, though many studies demonstrate striking similarities between human and canine epilepsy such as in response rates, refractory rates to therapy, and clinical and electrographic presentations 10. To ultimately determine both the generalizability and applicability of our conclusions, current work in our lab is directed towards verifying and extending these findings to human long-term iEEG. Still, we believe that our findings on a novel long-term ambulatory EEG dataset will help evaluate canines as an experimental model and lend insights into the investigation of similar datasets in humans.
Phenobarbital was also administered intermittently to all dogs following the death of one animal from status epilepticus. While this likely reduced the occurrence of seizures, it did not have an effect on burst-burst similarities as indicated in Figure 6. In addition, although a large portion of time was recorded during PHB administration, this was not started until at minimum 50 days post-implantation, which still allows for interpretation of the initial weeks of recording. This finding is interesting as humans undergoing presurgical evaluation are typically continued on anti-epileptic drugs, which are tapered, during significant periods of iEEG recording.
Finally, the issue of whether the steady state of our recordings represents the true natural state of epileptic networks, or a state of recovery following traumatic electrode implantation, is challenging to answer. DiLorenzo’s data demonstrating more accurate localization of typical seizures and seizure freedom after chronic localization suggest that the patients’ long-term steady state is likely to represent their true baseline. Still, DiLorenzo’s study contains only three patients. The much larger study by King-Stephens et al. is more supportive of our conjecture that our chronic recordings resolve to the patient’s more natural steady state, but outcome data after some of these patients go to surgery will lend useful insights into this issue. In the meantime, there is no alternative to chronically implanted electrodes for obtaining these kinds of recordings, and we believe that studying these types of chronic implants is warranted.
Conclusion
This study provides insight into the stability of iEEG recordings immediately after intracranial electrode implantation in canines, and there is growing evidence that the conclusions may be very relevant to human studies. The observation that seizure onset zones, interictal burst counts, and burst similarities do not stabilize until weeks after implantation of intracranial electrodes suggests that there is initial variability in iEEG recordings that may not accurately delineate an individual’s epileptic network. Rather these recordings may be biased as a result of injury and inflammation from surgery. Though further work is necessary to determine generalizability of our findings to humans, they contribute to a growing literature suggesting that in at least some patients iEEG obtained during standard hospital evaluation of refractory epilepsy with intracranial EEG may not be sufficient to define the epileptic network for optimal therapy.
Supplementary Material
Key Points of Article.
Seizure onset zones fluctuate across chronic iEEG recordings, which is not accurately represented in acute recordings.
Interictal bursts require several weeks to reach steady state, possibly reflecting electrophysiological instability post-implantation.
Long-term ambulatory electrophysiology may be necessary to accurately capture the baseline epileptic network.
Acknowledgments
This study was funded by National Institutes of Health (NIH) and the Mirowski Family Foundation grants through the University of Pennsylvania and Mayo Clinic: NIH P20NS 080181, U01NS073557, and P40OD10939. The International Epilepsy Electrophysiology Portal is funded by the NIH (U24NS063930).
Footnotes
Disclosures:
None. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.DiLorenzo DJ, Mangubat EZ, Rossi Ma, et al. Chronic unlimited recording electrocorticography-guided resective epilepsy surgery: technology-enabled enhanced fidelity in seizure focus localization with improved surgical efficacy. J Neurosurg. 2014;120:1402–14. doi: 10.3171/2014.1.JNS131592. [DOI] [PubMed] [Google Scholar]
- 2.King-Stephens D, Mirro E, Weber PB, et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56:959–67. doi: 10.1111/epi.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Schachter S, Brodie M. Drug-resistant epilepsy. New Engl J …. 2011:919–26. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- 5.Lee SK, Lee SY, Kim KK, et al. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32. doi: 10.1002/ana.20569. [DOI] [PubMed] [Google Scholar]
- 6.Bergey GK, Morrell MJ, Mizrahi EM, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–7. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel J, Pitkänen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia. 2013;54(Suppl 4):61–9. doi: 10.1111/epi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis KA, Ung H, Wulsin D, et al. Mining continuous intracranial EEG in focal canine epilepsy: Relating interictal bursts to seizure onsets. Epilepsia. 2016;57:89–98. doi: 10.1111/epi.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo JY, Rampal N, Petroff OA, et al. Brief potentially ictal rhythmic discharges in critically ill adults. JAMA Neurol. 2014;71:454–62. doi: 10.1001/jamaneurol.2013.6238. [DOI] [PubMed] [Google Scholar]
- 10.Potschka H, Fischer A, Von Rüden EL, et al. Canine epilepsy as a translational model? Epilepsia. 2013:571–9. doi: 10.1111/epi.12138. [DOI] [PubMed] [Google Scholar]
- 11.Uriarte A, Maestro Saiz I. Canine versus human epilepsy: are we up to date? Journal of Small Animal Practice. 2016:115–21. doi: 10.1111/jsap.12437. [DOI] [PubMed] [Google Scholar]
- 12.Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005–12. doi: 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis KA, Sturges BK, Vite CH, et al. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG. Epilepsy Res. 2011;96:116–22. doi: 10.1016/j.eplepsyres.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noachtar S, Rémi J. The role of EEG in epilepsy: A critical review. Epilepsy and Behavior. 2009:22–33. doi: 10.1016/j.yebeh.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 15.Van Kuyck K, Welkenhuysen M, Arckens L, et al. Histological alterations induced by electrode implantation and electrical stimulation in the human brain: a review. Neuromodulation. 2007;10:244–61. doi: 10.1111/j.1525-1403.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 16.Esteller R, Echauz J, Tcheng T, et al. Line length: an efficient feature for seizure onset detection. 2001 Conf Proc 23rd Annu Int Conf IEEE Eng Med Biol Soc; 2001; pp. 1707–10. [Google Scholar]
- 17.Wulsin DF, Fox EB, Litt B. Parsing Epileptic Events Using a Markov Switching Process for Correlated Time Series. Proceedings of the 30th International Conference on Machine Learning (ICML-13); 2013; pp. 356–64. [Google Scholar]
- 18.Fox E, Sudderth E, Jordan M, et al. Bayesian nonparametric methods for learning markov switching processes. IEEE Signal Process Mag. 2010;27:43–54. [Google Scholar]
- 19.Fong JS, Alexopoulos AV, Bingaman WE, et al. Pathologic findings associated with invasive EEG monitoring for medically intractable epilepsy. Am J Clin Pathol. 2012;138:506–10. doi: 10.1309/AJCPGSNL9VDVNJMX. [DOI] [PubMed] [Google Scholar]
- 20.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Sillay KA, Rutecki P, Cicora K, et al. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 2013;6:718–26. doi: 10.1016/j.brs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Sillanpää M, Schmidt D. Seizure clustering during drug treatment affects seizure outcome and mortality of childhood-onset epilepsy. Brain. 2008;131:938–44. doi: 10.1093/brain/awn037. [DOI] [PubMed] [Google Scholar]
- 23.Haut SR, Swick C, Freeman K, et al. Seizure clustering during epilepsy monitoring. Epilepsia. 2002;43:711–5. doi: 10.1046/j.1528-1157.2002.26401.x. [DOI] [PubMed] [Google Scholar]
- 24.Engel J, Crandall PH. Falsely Localizing Ictal Onsets with Depth EEG Telemetry During Anticonvulsant Withdrawal. Epilepsia. 1983;24:344–55. doi: 10.1111/j.1528-1157.1983.tb04898.x. [DOI] [PubMed] [Google Scholar]
- 25.Libenson MH, Caravale B. Do antiepileptic drugs differ in suppressing interictal epileptiform activity in children? Pediatr Neurol. 2001;24:214–8. doi: 10.1016/s0887-8994(00)00271-x. ST – Do antiepileptic drugs differ in suppr. [DOI] [PubMed] [Google Scholar]
- 26.D’Antuono M, Köhling R, Ricalzone S, et al. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia. 2010;51:423–31. doi: 10.1111/j.1528-1167.2009.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.