Abstract
Social interactions are essential for animals to reproduce, defend their territory, and raise their young. The conserved nature of social behaviors across animal species suggests that the neural pathways underlying the motivation for, and the execution of, specific social responses are also maintained. Modern tools of neuroscience have offered new opportunities for dissecting the molecular and neural mechanisms controlling specific social responses. We will review here recent insights into the neural circuits underlying a particularly fascinating and important form of social interaction, that of parental care. We will discuss how these findings open new avenues to deconstruct infant-directed behavioral control in males and females, and to help understand the neural basis of parenting in a variety of animal species, including humans.
Keywords: hormones, hypothalamus, infants, infanticide, MPOA, neural circuits, neuropeptides, parenting, social behavior
Introduction
Recent advances in neuroscience have made it possible to address fundamental questions about behavioral control at the level of genetically defined neuronal circuits. Among various forms of social interactions, parenting, which includes the nurturing and protection of infants, presents a number of fascinating characteristics for mechanistic inquiries. First, while most social behaviors such as mating or aggression are displayed in short bouts, parental care generally implies a prolonged interaction between infants and parents that may last days, months, or years according to the species. Second, social interactions are often encounters between two individuals, for example during mating or fighting. In contrast, parenting involves one, two, or sometimes a multitude of cooperating adults, in addition to at least one infant. Third, parental care implies the non-reciprocal care of infants by adults. Finally, the circuits underlying parental care are thought to represent an initial neural template from which more widespread intraspecific bonds may have emerged [1]. The development of such bonds between parents and infants might indeed facilitate the involvement and sacrifice of caregivers who do not otherwise receive any immediate benefit from their investment.
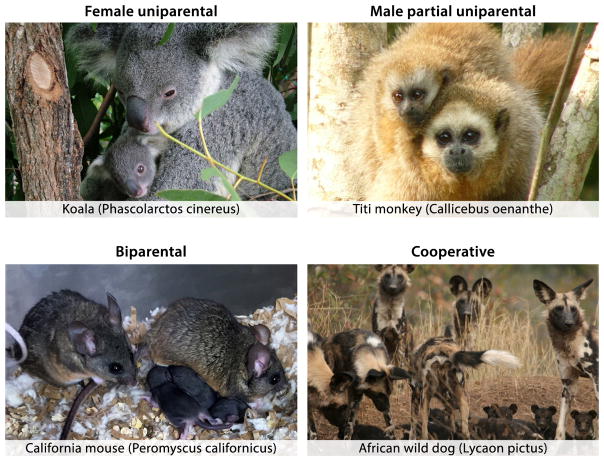
Parental care consists of multiple species-specific stereotypic behaviors such as grooming, nest building, crouching, feeding (including nursing in mothers), carrying, and defense of young. Together, these behaviors increase the likelihood of offspring survival as well as its optimal mental and physical development [2]. In mammals, parenting can be performed by a single parent – usually the mother in uniparental species such as North American deer mice (Peromyscus maniculatus) [3] and Koalas (Phascolarctos cinereus) [4]; by both parents in biparental species, such as California mice (Peromyscus californicus) [5] and titi monkeys (genus Callicebus) [6]; or by related or unrelated group members in alloparental species, such as Barbary macaques (Macaca Sylvanus) [7] or African wild dogs (Lycaon pictus) [8] (Fig. 1). Parenting requires considerable investment of time and resources. Accordingly, adults in many species are known to abandon or even kill offspring when environmental conditions are too challenging, thereby postponing parental investment until more favorable circumstances arise.
Figure 1.
Representative parenting styles in mammalian species. (Upper left) Koalas, as well as the vast majority of marsupials, show female uniparental behavior (photo reproduced with permission from Creative Commons©). (Upper right) In titi monkeys (pictured here: Callicebus oenanthe), male fathers are partially uniparental, performing all aspects of parenting with the exception of nursing (photo reproduced with permission from Anneke DeLuycker©). (Lower left) California mice share parental duties equally between the father and mother (photo courtesy of Andrés Bendesky). (Lower right) African wild dogs live in packs and cooperate in parental care (photo reproduced with permission from the African Wildlife Conservation Fund©).
In humans, parenthood requires protracted dedication and parental planning, and social and cultural norms are important contributors to parent-child interactions. The relationship between parents and their children lasts decades, and children are often cared for by grandparents, siblings, as well as extended family, friends, and nonrelated helpers. Such alloparental care is particularly developed in higher primates and humans (“it takes a village” [9, 10]). Furthermore, while most mammals are reared only until weaning, human parents invest in their offspring through puberty and well into adulthood. This prolonged caregiving leads to particularly strong emotional bonds and complex mutual dependencies.
Together with in utero gestation and nursing of infants, elaborate and robust maternal behavior is a defining feature of mammals. By contrast, male infant-directed behaviors in mammals are more variable – ranging from paternal care to avoidance and aggression [4, 11].
Despite the critical importance of parental care, our understanding of the underlying neural circuits remains rudimentary. In particular, while most of our knowledge comes from the study of maternal behavior, it remains unclear if parental males rely on identical neural mechanisms and circuits. Even less is known about the neural substrates underlying infant-directed aggression and infanticide, which are commonly shown by males [12].
Here we will delineate the core parenting circuits and their modulation in females and discuss their equivalent in males. We will then review evidence suggesting that, rather than being forms of deficient parenting, infant neglect and aggression are instructed by dedicated neural circuits. We will discuss how these circuits might interact to shape appropriate infant-directed behaviors and how a more detailed understanding of their function might inform the understanding of dysfunctional parenting in humans, including parenting-associated mental illnesses such as postpartum psychiatric disorders.
What do we know about the maternal brain?
Most mammalian offspring are born altricial, i.e. unable to fend for themselves after birth [2]. As such, they require considerable parental attention to survive and thrive. In mammals, females disproportionally invest in offspring: they produce large gametes, provide placental nourishment during gestation, and nurse, nurture and protect the young after birth. In contrast, the role of mammalian males is extremely variable. While most males contribute minimally beyond copulation, around 5–10% of mammalian fathers are paternal in the wild [11, 13]. In some species, such as California mice and yellow baboons (Papio cynocephalus), males perform essentially all aspects of parental behavior, with the obvious exception of nursing (although male lactation occurs in extremely rare cases, such as the Dayak fruit bat [Dyacopterus spadiceus] [14]). Some males – for example Djungarian hamsters (Phodopus sungorus) – even assist in the delivery of the young [15]. However, due to the ubiquity of maternal care in mammals, parenting is often considered a female-specific trait, and the overwhelming majority of studies have been performed in females. Also, most of our knowledge on neural circuits underlying parenting has been obtained from a relatively narrow range of animal species, mainly rats and other rodents. We are therefore unlikely to appreciate the full complexity of the neural basis of parental behavior across the animal kingdom [2].
The dissection of the core parental circuit in females
Parenting is a complex behavior that involves (1) the detection and processing of offspring cues, (2) the regulation of parental motivation according to the individual’s sex, physiological state and environment, and finally (3) the execution of specific parental behaviors, such as nest building, retrieving, grooming, and huddling with infants.
A large body of literature (reviewed in [1–3]) has shown that olfactory stimuli activating the main olfactory epithelium (MOE), auditory and tactile infant stimuli promote parental care. In contrast, cues detected by the vomeronasal organ (VNO) and processed by downstream areas such as the medial amygdala (MeA) and bed nucleus of the stria terminalis (BNST) are essential for infant-directed aggression.
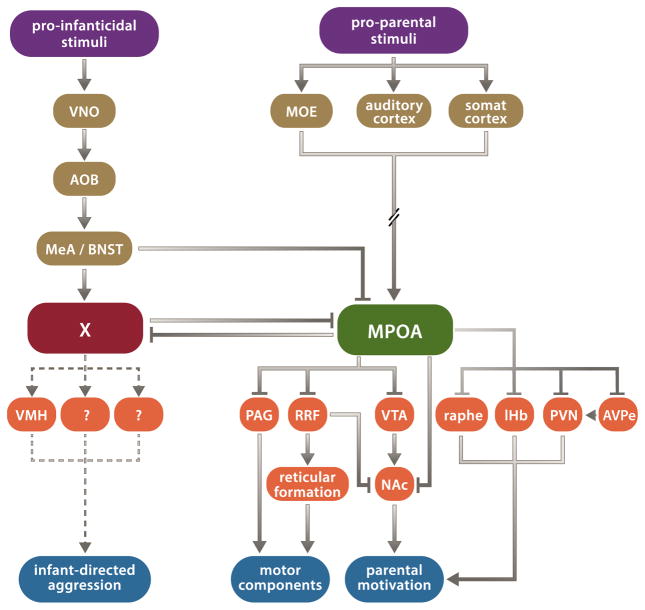
More centrally, the medial preoptic area (MPOA) has long been known as a node for the positive control of parenting. Studies have shown that: (1) MPOA lesions disrupt parenting, particularly its active motor components, e.g. pup retrieval [16], (2) receptors for pregnancy-related hormones and opioids involved in the modulation of parenting (see Table 1) are highly expressed in the MPOA [2] and (3) direct hormonal stimulation of the MPOA facilitates parenting [17, 18]. In order to further understand the role of the MPOA in the central control of parenting, tracing studies have identified brain-wide inputs to, and projections from, this region [19, 20]. In addition, pharmacological manipulations and lesions in female rats have assessed the role of areas connected to the MPOA. These data have led to a working model of brain circuits controlling parental behavior (Fig. 2) [2]: multisensory information (i.e. infant cues) is integrated by parental control neurons in the MPOA [21], which, dependent on the animal’s internal state, instruct the various aspects of parental behavior. Specifically, MPOA projections to the nucleus accumbens (NAc) – or indirect NAc projections via the retrorubral field (RRF) and/or the ventral tegmental area (VTA) – are hypothesized to mediate parental responsiveness [2]. This motivational circuit is likely modulated by projections from the paraventricular nucleus of the hypothalamus (PVN) [22], the lateral habenula (lHb) [23], and by serotonergic inputs from the dorsal raphe nucleus [24]. In contrast, MPOA projections to the periaqueductal grey (PAG), or to the reticular formation via the RRF, have been proposed to mediate motor aspects of parenting [2].
Table 1.
Summary of pharmacological manipulations and gene knockouts affecting parental behavior.
| Pharmacological Intervention | |||
|---|---|---|---|
| Factor | Brain area | Effect on parental behavior | References |
| Estrogen | MPOA | Enhanced retrieval | [17,33] |
| Oxytocin | Auditory cortex | [36] | |
| Oxytocin | VTA and MPOA | [37] | |
| Oxytocin | MPOA and VTA | [121] | |
| Vasopressin | Intracerebroventricular | [37] | |
| Prostaglandin F2α | Intracerebroventricular | [37] | |
| Prolactin | MPOA | [44] | |
| Dopamine receptor antagonist | Nucleus accumbens | Decreased maternal retrieving and grooming | [122–124] |
| GABA receptor agonist | Lateral septum | Increased maternal aggression | [125] |
| Corticotropin releasing factor | Lateral septum | Decreased maternal aggression | [126] |
| Urocortin 1 and Urocortin 3 | Intracerebroventricular | [127] | |
| Mu opioid receptor antagonist | Intracerebroventricular | Slower maternal responses | [128] |
| Gene Knockout | |||
| Gene | Effect on parental behavior | References | |
| Prolactin receptor | Impaired retrieval behavior in virgin females, impaired pup recognition in fathers | [47,129] | |
| Estrogen receptor α | Increased infanticide in virgin females | [66] | |
| TrpC2 | Decreased pup interaction in females, decreased maternal aggression, increased paternal behavior in virgin males | [106] | |
| Peg3 | Reduced retrieval | [130] | |
| Mest | [131] | ||
| GABA receptorƍ | Reduced retrieval and pup survival (lactating dams but not virgin females) | [132] | |
| Corticotropin releasing factor receptor 2 | Decreased maternal aggression | [133] | |
| Dopamine beta-hydroxylase | Decreased maternal retrieval and reduced pup survival | [134] | |
| FosB | Decreased retrieval and grooming | [135] | |
Figure 2.
Circuit diagram for regulation of parental behavior. Parallel pro-parental and pro-infanticidal circuits integrate sensory stimuli and – depending on the animal’s internal state – instruct parental behavior or infant-directed aggression. Abbreviations: AOB, accessory olfactory bulb; AVPe, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; lHb, lateral habenula; MeA, medial amygdala; MOE, main olfactory epithelium; MPOA, medial preoptic area; NAc, nucleus accumbens; PAG, periaqueductal grey; PVN, paraventricular nucleus of the hypothalamus; RRF, retrorubral field; VMH, ventromedial hypothalamus; VNO, vomeronasal organ; VTA, ventral tegmental area.
The MPOA is involved in the control of other social behaviors and homeostatic functions, including reproduction [25–27], aggression [28], and thermoregulation [29]. A fundamental question is therefore how behavioral specificity arises from MPOA activation. One hypothesis is that MPOA neurons controlling these distinct processes each have a specific molecular identity. Indeed, Wu et al. recently showed that a subpopulation of MPOA neurons expressing the neuropeptide Galanin (MPOAGal) are specifically activated during parenting, and that these neurons are both necessary and sufficient for proper execution of parental behavior [30]. These findings offer new opportunities for a circuit-level dissection of parenting using cell-type specific genetic strategies. Already, the identification of MPOAGal neurons as “command-like” neurons for parental care has challenged existing models [2]. Specifically, while the MPOA has been assumed to drive parenting through excitatory (glutamatergic) projections [2], MPOAGal neurons are mostly GABAergic [30], suggesting that the MPOA exerts its overarching control of parenting via inhibition or disinhibition of downstream brain areas. Importantly, non-genetically restricted tracing approaches have targeted all MPOA neurons, including those associated with other behaviors or physiological processes [19, 20]. Moreover, tracers used in these studies can be taken up by fibers of passage, further complicating interpretation of data. In contrast, the molecular identification of MPOA neurons involved in the regulation of parenting now enables a specific assessment of the underlying circuits. Genetic strategies to selectively visualize the connectivity of MPOAGal neurons, which constitute only about 20% of all MPOA neurons, will likely result in a substantially more precise and sparse wiring diagram relevant to parental control.
Another question to consider is whether MPOAGal neurons constitute the only MPOA subpopulation involved in the control of parenting. Using c-fos as a readout of neuronal activation, Wu et al. showed that MPOAGal neurons constitute about 40% of the MPOA neurons activated during parenting [30]. Also, ablation of these neurons significantly impairs all displays of parenting, including crouching, pup retrieving, nest building and overall maternal interactions [30]. However, under the experimental conditions used in this study, optogenetic activation of MPOAGal neurons inhibits infant-mediated aggression, but only elicits some parental behaviors (e.g. pup grooming), at the expense of others, such as crouching or nest building [30]. It is possible that different neuronal stimulation regimens or experimental conditions could lead to a wider range of parenting displays. Alternatively, additional, yet to be defined, neuronal subsets might contribute to the control of parenting.
Interestingly, the number of MPOAGal neurons is not sexually dimorphic and their function in parenting appears to be similar in both males and females [30]. In contrast, other components of the circuit appear to be sex-specific: Scott et al. recently found that tyrosine hydroxylase (TH)-expressing neurons in the anteroventral periventricular nucleus (AVPe) show a sexually dimorphic expression pattern and affect parenting only in females [31]. It will therefore be essential to investigate the interplay between sex-specific and shared elements of the parenting circuit.
Thus, parenting engages cortical areas that process and integrate sensory stimuli including olfactory, tactile, and auditory pup signals. These areas in turn send information to behavioral control centers in the midbrain, which then orchestrate motor actions via their brainstem projections.
How do hormone changes affect the function of maternal circuits?
The expression of maternal behavior is highly dependent on the female’s physiological state, such that mothers are significantly more maternal than virgin females, and stressed females are less maternal. Accordingly, a large number of hormones and neuromodulators have been shown to affect the expression of parenting and the function of the underlying neuronal populations. The neuromodulation of parenting has been almost exclusively investigated in females, and is considerably less well understood in males (see ‘The paternal brain is still poorly understood’). Importantly, these effects have been studied in a wide range of species (rats, mice, gerbils, hamsters, marmosets, etc.), which creates great potential for comparative studies, but has complicated the identification of common regulatory mechanisms. Fundamentally, the appearance of distinct parenting modes might either result from divergent circuit architectures, or from the function of largely identical core circuits that are modulated in a species-specific manner.
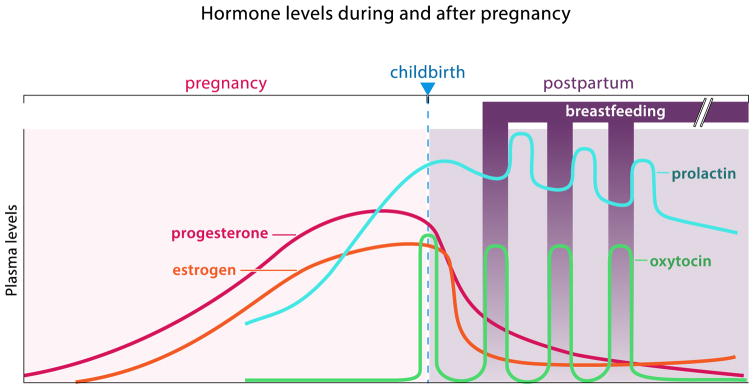
In mothers, the peripartum period is associated with striking fluctuations in steroid hormone levels (Fig. 3), which profoundly affect the expression of maternal behavior. During pregnancy, levels of progesterone and estrogen slowly rise. As progesterone levels peak and then rapidly drop, uterine contractions are triggered by pulsatile release of oxytocin (OXT). Concomitant with parturition, prolactin (PRL) levels rapidly increase to support milk production and OXT stimulates milk ejection in response to the infant’s suckling (Fig. 3). These hormonal changes may coordinate parturition and lactation with the onset of parenting, thus preparing the mother’s body and brain for maternal care. Indeed, early studies found that steroid hormone treatment protocols mimicking pregnancy can induce maternal behavior in ovariectomized rats which normally avoid pups [32]. Further work suggests that hormonal stimulation of the MPOA activates onset of maternal behavior [33]. Even in virgin female mice, which are spontaneously maternal, the quality of parental care increases further after mating. This is presumably due to hormonal changes similar to those described in rats. Prolactin and estrogen receptors, as well as many neuropeptide hormones are expressed in the MPOA and other parts of the core parental circuit. However, neuropeptide release can be paracrine, i.e. outside of synaptic contacts, and thus affect neural processing at a considerable distance. Therefore, the localization of neuropeptide receptors is more relevant for understanding modulatory influences on circuit function. Unfortunately, however, the expression profiles of these receptors are still poorly characterized (see below).
Figure 3.
Hormone levels during and after human pregnancy. Estrogen, progesterone and prolactin steadily increase throughout pregnancy. Childbirth is characterized by a rapid drop in estrogen and progesterone and a surge in oxytocin levels that initiates uterine contractions. During breastfeeding in the postpartum period, pulses of prolactin levels stimulate milk production between feedings, alternating with oxytocin pulses, which lead to milk ejection during breastfeeding in response to the infant’s suckling (let-down reflex).
How does hormone or neuropeptide release affect the function of neural circuits? Steroid and neuropeptide hormones have both short-term (modulation of membrane potential) and long-term (modulation of gene expression) actions. Some of the most striking effects of neuropeptides on the expression of maternal care occur with short latency: central OXT administration can induce maternal behavior within hours in rats [34], and acceptance of an alien lamb within 30 seconds in sheep [35]. Binding of OXT to its receptor (OXTR) in the left auditory cortex of mouse dams increases the salience of pup calls, facilitating retrieval within hours [36]. Effects have also been observed for vasopressin (AVP) [37], but this might be due to off-target AVP binding to OXTRs [38]. The effects of other neuropeptides and neuromodulators, such as estrogen, prostaglandin F2α and corticotropin-releasing factor (CRF) are summarized in Table 1.
Given the impressive and often acute consequences of hormonal neuromodulation, it comes as a surprise that knockouts (KO) of genes encoding neuromodulators or their receptors have rather subtle consequences on parental behavior in mice (see Table 1). For example, despite the well-documented effects of OXT injection on maternal behavior and its central role in milk ejection and uterine contractions, the consequences of OXT removal are mild: Nishimori et al. tested an OXT KO in a mixed C57-129/SvEv genetic background and did not observe any parenting defects [39, 40], while Pedersen et al. found that the same manipulation in a pure-bred C57 strain only slightly decreased retrieval and pup licking [41]. Similarly, parenting is largely unaffected in global and forebrain-specific OXTR KO females [42], although one study found that these manipulations increase the threshold for the initiation of maternal behavior [43]. Furthermore, despite the ability of PRL to shorten the onset of maternal behavior after central administration [44], PRL KO does not prevent female mice from manifesting spontaneous maternal behaviors [45] and PRL receptor KO impairs retrieval and crouching only in some females [46, 47].
Why are the effects of removing hormones and their receptors on maternal behavior so weak? One possible reason is the non-conditional nature of these manipulations: compensatory mechanisms during development might counteract and effectively mask any actual circuit effects. Temporally and spatially specific manipulations, e.g. using conditional Cre recombinase-expressing viral vectors in mice carrying floxed receptor or neuropeptide alleles, may uncover more profound effects of neuromodulators on these circuits.
In addition, many aspects of hormone and neuropeptide biology are poorly understood, making it difficult to draw firm conclusions from existing data. In particular, hormone and neuropeptide levels are difficult to measure accurately, and central and peripheral concentrations might not be related [48]. OXT has typically been administered either intracerebrally or intravenously. However, its levels are commonly examined from blood plasma using enzyme-linked immunosorbent assay (ELISA) kits, raising the question of whether peripherally measured levels accurately reflect brain concentrations. In addition, the reliability of these assays has been questioned [48]. Another serious challenge for understanding the functional effects of neuromodulators is the fact that neuropeptide receptors are often expressed at levels too low for reliable detection at cellular resolution, even when using sensitive in situ hybridization methods. It is notable, for example, that OTXR expression patterns in the human brain have not yet been characterized [49].
Altogether, obtaining accurate measures for intracerebral hormone levels, identifying the sites of receptor expression in components of parental circuits and determining the physiological effects of these modulators will all be formidable but crucial tasks.
The paternal brain is still poorly understood
As described above, the female contribution to mammalian parenting is extensive. In contrast, levels of paternal care are highly variable and depend on environment, mating strategy, and social group composition. In mammals, the level of paternal contribution can range from (1) complete absence to (2) biparental care, where males provision food to the female and offspring, provide protection and engage in female-like parental care, to (3) partial uniparental care in which males take on primary care of infants, and (4) everything in between these scenarios. The following examples highlight the range and complexity of paternal care in mammalian species. In most marsupials, extremely altricial young attach to a teat within the mother’s pouch to nurse for a prolonged period of postnatal development, and male parenting is almost non-existent [4]. In Hanuman langurs (Semnopithecus entellus), the social structure of groups is such that infants, adolescents, and adult females will form a small group that is protected by one male (the father of all infants in the group) which otherwise takes little part in parental care [50]. Monogamous California mice mate for life, build a burrow and cooperatively nest and care for offspring (Fig. 1) [51]. Finally, in titi monkeys, the female is responsible for nursing the infant, but the father carries the infant for 90% of the time (Fig. 1) [6]. Due to the rarity and variability of paternal care, it is no surprise that, in contrast to our expanding knowledge of the circuit basis of maternal behavior, the paternal brain remains understudied. A fundamental issue is to assess the extent to which the control of parenting in males and females uses identical or sexually dimorphic neural structures and regulatory mechanisms.
Males in species in which paternal behavior has been observed, for example lab mice (Mus musculus), prairie voles (Microtus ochrogaster), California mice, Djungarian hamsters and Mongolian gerbils (Meriones unguiculatus), show most components of parental behavior with the exception of lactation, suggesting that infant stimuli may act on a similar core parenting circuit in male and female brains. Indeed, studies in California mice and Prairie voles demonstrate that lesions in olfactory areas, MPOA and BNST disrupt both paternal and maternal behavior [52]. Moreover, activation of MPOAGal neurons in mice is observed in both parenting males and females, and ablation of this neuronal population abolishes parental behavior in both sexes [30]. Furthermore, artificial activation of MPOAGal neurons in virgin males abolishes pup-directed aggression and triggers pup grooming instead. Similar experiments in fathers also increase parental interactions [30]. Intriguingly, MPOAGal neuron numbers are not sexually dimorphic, although sex differences may exist in their transcriptional profiles and synaptic connectivity.
These observations suggest the existence of shared circuit elements underlying parenting in both sexes. However, this does not imply that all circuit elements are shared: a recent study identified AVPeTH neurons as critical for parental behavior only in females, while the equivalent neurons in males play a role in aggression [31].
Several additional lines of evidence suggest that the regulatory mechanisms underlying the expression of parental behavior are different in males and females. In laboratory mice, virgin females are spontaneously maternal, while virgin males are infanticidal. However, surgical or genetic ablation of the vomeronasal organ in virgin males leads to suppression of infant-mediated aggression and to full display of parental behavior. This implies that parental circuits are normally repressed by pheromonal inputs in virgin male but not female mice.
A fascinating phenomenon described in laboratory mice illustrates how infant-directed behaviors are dramatically modulated by the animal’s internal state in a sex-specific manner. Virgin males become paternal only in the weeks following mating [53, 54]. Intriguingly, this post-mating switch typically occurs within a period corresponding to the female’s gestation time - about three weeks in mice [53], and it remains unclear which sensory stimuli and/or hormonal changes underlie this dramatic behavioral transition.
Altogether, current data indicate that largely non-sexually dimorphic parental circuits are subject to sex-specific hormonal modulation. Maternal behavior is clearly regulated by profound peripartum hormonal changes and while virgin males undergo a similarly dramatic behavioral transition following mating, the underlying hormonal and circuit mechanisms are poorly understood. A more mechanistic understanding of the targets and effects of hormonal modulation in the core parental circuit (see Fig. 2) is needed.
Are there specific neural circuits in males and females underlying infant-directed aggression?
Because of the obvious adaptive value of parental behavior, researchers were reluctant to acknowledge observations of infant-directed aggression or infanticide in both males and females when they first appeared in the literature [50]. Initially regarded as a pathological behavior shown only in exceptional circumstances, Sarah Hrdy’s pioneering work in langurs, followed by numerous studies in other species, suggested that infanticide may in fact be an adaptive behavior [55]. Infant-mediated aggression and infanticide have now been reported in insects, fish, amphibians, birds, rodents, felines, and primates [11, 12]. Even humans occasionally neglect, abuse, or kill offspring, but these behaviors are thought to be driven by cultural norms as well as parental psychopathology, stress, and economic considerations. A striking example is that of Eastern Japanese villages in which infanticide was routinely performed during the late Tokugawa period (1750–1850) [56]. This practice has been characterized as a rational family-planning tool (“post-partum birth control”) and fully ended only in the 1950s with the advent of birth control and legalized abortion [56]. While this well-documented case appears extreme, its scale and patterns are far from exceptional; across most of the world the ethnographic and historical records contain references to habitual infanticide [57].
A number of hypotheses have been put forward to explain the existence of conspecific infanticide in many mammalian species [11, 58]. The benefits of this behavior are most obvious for males: loss of a suckling infant leads to onset of estrus and sexual receptivity in females. Infanticidal males can therefore increase reproductive success by killing a competitor’s offspring [11, 50]. This hypothesis is known as sexually-selected infanticide. An alternative hypothesis postulates that infanticide evolutionarily pre-dates parental care. Since eggs, larvae, and live young represent nutritious and easily accessible food sources, parental care might have evolved as a defense against predatory infanticide [59]. Aggression against intruders is indeed a characteristic of maternal behavior, but such defensive behavior is likely inefficient to prevent infanticide, particularly against a stronger male conspecific. Interestingly, monogamy – in which both partners have reproductive certainty – as well as female promiscuity – which makes it risky for males to kill offspring that might be their own – have both been suggested as effective evolutionary counterstrategies to infanticide [11, 58].
Is infant-directed aggression simply the result of silenced parental circuits, or is there evidence for the existence of dedicated circuits controlling such agonistic interactions? Virgin male mice or rats typically ignore or attack pups, but become parental after perturbations to vomeronasal signaling [30, 60]. Furthermore, while ablation of MPOAGal neurons in mothers and fathers simply abolishes parenting, the same manipulation elicits infanticide in virgin females [30]. Thus, infanticide does not appear to be a simply a consequence of inhibition of parenting, but rather involves the activation of a dedicated circuit. These findings therefore suggest a mutual inhibition between circuits controlling parenting and infanticide. Such a circuit organization would allow for the behavioral flexibility and context-specificity that are hallmarks of parenting.
A valuable opportunity for understanding the conflict between parenting and infanticide at the circuit level is offered by changes in control mechanisms underlying the post-mating switch in males. Although this particular transition between infanticide and parenting has been mainly described in Mus and Peromyscus males under laboratory conditions, its underlying mechanisms may share similarities with the modulation of infanticide by stress or food scarcity, which exists in both sexes [61, 62]. What are the neural substrates underlying the drastic switch in pup-directed behavior? Males obviously lack the periparturial changes (i.e. prolonged increase in gonadal steroids and lactogenic hormones as well as the vaginocervical stimulation of parturition) that are critical for maternal behavior [63]. Post-mating changes in male hormone levels appear to be species-specific: testosterone [63–68], progesterone [67–69], PRL [51, 68, 70], as well as estrogen and glucocorticoids [68] either exhibit conflicting influences on paternal behavior in different rodent and primate species or cannot be causally linked to this behavioral switch. Moreover, hypophysectomized males which lack pituitary secretions still undergo this transition, suggesting that hormonal status is not instructive [63]. However, destruction of the pituitary gland may interfere with infanticidal behavior by disrupting other endocrine processes. It therefore remains uncertain how hormonal modulation affects male parental behavior. Ejaculation, but neither copulation itself nor co-habituation with a pregnant female, is sufficient for the switch in mice [53], while male rats can become parental after continued exposure to pregnant females [71]. Importantly also, males revert and yet again become infanticidal 50–60 days after mating [53]. Vomeronasal signaling is crucial for infanticidal behavior [30, 60, 71], and downstream brain areas in the vomeronasal pathway – accessory olfactory bulb (AOB) and MeA – are more highly activated by pup interaction in virgin males than in fathers [72]. Pup stimuli might therefore inhibit pro-parental MPOAGal neurons either via the AOB→BNST→MPOA or the AOB→MeA→MPOA route (Fig. 2). As we have seen, these potential circuit changes alone would abolish parenting, but by themselves do not lead to infanticide [30]. Concurrent changes must therefore activate agonistic circuits.
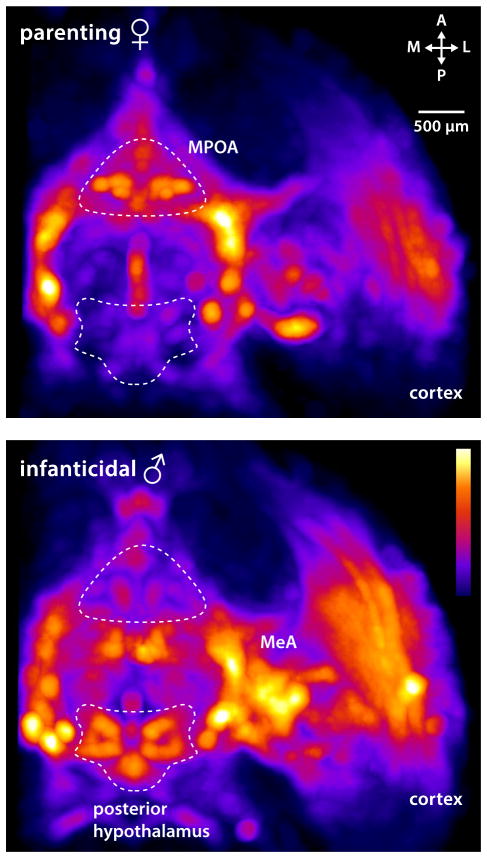
What then are the circuits driving pup-mediated aggression? Common laboratory mouse and rat strains have been selected for low maternal aggression to increase breeding success. Additionally, they display robust strain and sex differences in infanticidal behavior [73, 74]. Thus, our knowledge of brain areas potentially involved in infanticide largely comes from lesion studies in virgin female rats that result in loss of infant aversion. Primarily, areas involved in pup pheromone sensation – olfactory bulb, AOB, cortical amygdala, MeA – have been implicated [75–79], as well as hypothalamic regions [80]. However, these studies did not directly address infanticide as an active behavior or account for potential sexual dimorphisms. A recent study has demonstrated that the latency to commit infanticide is dramatically increased in virgin male mice after lesioning the rhomboid nucleus of the BNST [81]. However, since this manipulation did not abolish infanticidal behavior per se, the underlying circuits remain elusive. Brain-wide activity mapping showed that caudal hypothalamic regions previously implicated in aggression, including the PVN, dorsomedial and ventromedial hypothalamus (DM, VMH), and posterior hypothalamus are more highly active in infanticidal males compared to parental females (Fig. 4) [82]. Since the VMH, a well-described aggression locus [83], is activated in males that attack pups, an important question is whether infanticide recruits circuits for male-male aggression or rather relies on separate, dedicated circuitry.
Figure 4.
Brain areas activated by parenting versus infanticide. Whole brain imaging of the immediate early gene c-fos after behavior reveals that while parental female mice have a high level of activation in the MPOA (top panel), infanticidal males show more neuronal activation in the posterior hypothalamus, as well as MeA and cortex (lower panel). Horizontal brain sections are shown (adapted from Renier et al. [82]).
The circumstances surrounding female infanticide are quite distinct from those described in males. Females will eat infants as a source of nutrition when food is scarce and infants are in poor health. For example, females kill infants of subordinate mothers in cooperative breeding societies when resources are limited, as observed in prairie dogs (Cynomys ludovicianus) [84], African wild dogs (Lycaon pictus) [85], and meerkats (Suricata suricatta) [86]. Virgin females in wild mice have also been shown to be infanticidal [87]. Typically, however, infant-directed aggression by females has mainly been linked to stress. While the negative impact of maternal stress on offspring is established [88], little is known about its effect on parental behavior and the underlying neural substrates. Anecdotal evidence from stressed laboratory dams suggests that stress can inhibit parental, and/or drive infanticidal behavior. Correlational studies have described increased neglect or infant aversion in mothers experiencing social stress or presenting with chronically high cortisol levels in primates such as gorillas (Gorilla gorilla) [89], pigtail macaques (Macaca nemestrina), rhesus macaques (Macaca mulatta) [90], olive and savannah baboons (Papio hamadryas, anubis) [91], common marmosets (Callithrix jacchus) [92] and humans [93]. Dramatic changes in hormone levels and stress responsivity have been observed in pregnant and postpartum females. Accordingly, stress – a key risk factor for postpartum psychiatric illness (see below) – seems to profoundly affect parenting in the early postpartum period [94, 95]. Mechanistic studies on how stress impacts the function of parental circuits will be essential to further understand these effects.
Prospects and emerging questions
Toward a circuit-level dissection of parental behavior
The recent identification of a genetic marker for hypothalamic neurons controlling parenting in males and females [30] is an exciting prospect for a circuit-level dissection of this behavior. Similarly, the molecular identification of neurons involved in feeding [96, 97], sleep [98–101], and reproduction [102] has catalyzed dramatic advances in our understanding of the neural control of these behaviors. For example, the identification of agouti-related peptide (AgRP)-expressing neurons in the arcuate nucleus of the hypothalamus (ArcAgRP) as feeding command neurons was followed by striking and unexpected insights into their role, connectivity, and activity patterns during the motivational and execution phases of feeding behavior [103, 104]. These discoveries highlighted the complexity of brain-wide circuit dynamics and connectivity associated with behavioral control. Work on invertebrate nervous systems with known connectivity has highlighted the fact that wiring diagrams are necessary, but not sufficient, for understanding circuit function, due in part to intrinsic variability in synaptic weights and membrane properties, and the profound influence of neuromodulation [105].
An interesting paradox emerging from the study of parenting in mice is the existence of shared neuronal circuits between the sexes, which nevertheless drive markedly sexually dimorphic behaviors. Infanticidal virgin males become paternal when unable to detect vomeronasal cues, illustrating the overarching role of the VNO in ensuring appropriate pup interactions [30]. These findings nicely complement previous studies showing that females deficient for vomeronasal sensing display male-like sexual behavior [106]. Together, these observations indicate that similar circuits are present in both sexes, the function of which can be unmasked by removing inhibitory pheromonal input. Increasing evidence from several species, including mice, fish, lizards, and more recently humans, supports an intriguing notion of largely bipotential male and female brains [30, 106–110]. The concept of a core parental circuit that is active under specific physiological conditions illustrates the necessity to understand the hormonal and neuropeptidergic modulation of its constituent neuronal populations, as well as the temporal dynamics and circuit specificity of this modulation.
Implications for parenting in humans
Experiments in rodents have provided us with fundamental insights into the neural architecture of parental care. However, this behavior is considerably more complex in primates, particularly in humans. Human children have the longest period of postnatal development of any primate species, receiving parental care well into young adulthood. This extended process is thought to be related to the prolonged cortical development seen in humans [111, 112] and is accompanied by considerable investments in educating the young. How relevant will a circuit-level understanding of parental behavior in rodents be for human parenting? Core components of the parental circuitry are likely to be conserved among mammals, and most periparturial hormonal changes are shared between rodents and humans (Fig. 3). But in contrast to rodents, alloparental experience seems to play a significant role in the display of parental behavior in non-human primates and humans [113]. This important role for experience in primate parenting may account for the traditions of play parenting and encouragement of alloparenting in juveniles [10].
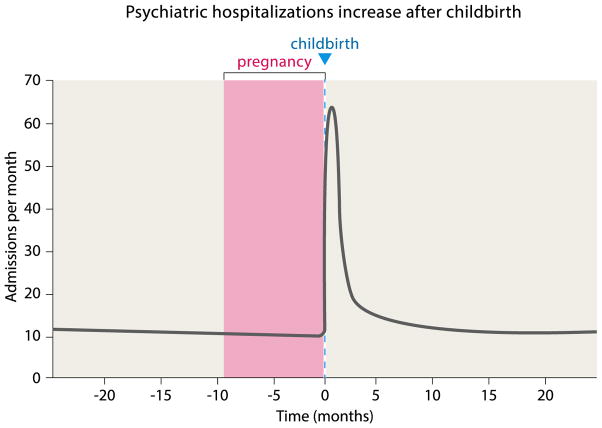
Recent studies of maternal mental illness have revealed both a timeframe of vulnerability to, and range of, psychiatric diseases in the perinatal period much larger than previously appreciated: as many as 20% of mothers are negatively affected within the first year of their child’s life, illustrated by a drastic increase in psychiatric hospitalizations (Fig. 5) [114, 115]. Still, the prevalence of postpartum depression (PPD) has been underestimated until recently, and the fact that PPD affects 5–10% of fathers is largely unappreciated by the public [116, 117]. While hormonal changes presumably underlie these clinical manifestations, our knowledge is still very limited [118, 119].
Figure 5.
Psychiatric hospitalizations increase after childbirth. Psychiatric hospitalizations for women dramatically increase in the early postpartum period. Admissions are shown for the city of Edinburgh catchment area (population 470,000 at the time of the study) within a 12-year period (adapted from Kendell et al. [120]).
Because child neglect and abuse are likely to result from deficiencies in parental responsiveness [2], MPOA→NAc and MPOA→VTA projections might be a useful access point for pharmacological interventions. Due to the conservation of major neurotransmitter and neuromodulator systems between rodents and humans, we anticipate that the experimental strategies outlined here will contribute to the identification of clinical applications benefitting mothers and fathers. From the child’s perspective, healthy parent-infant interactions during a critical period are essential for brain development [111, 112]. Building on our emerging knowledge of parental circuits, we are now in a position to examine new aspects of this behavior in humans.
Acknowledgments
We would like to thank Drs Z. Wu, A. Bendesky, J. Osterhout, L. and T. Newman for helpful comments on the manuscript and R. Hellmiss for help with illustrations. J.K. is supported by a Human Frontier Long-Term Fellowship, an EMBO Long-Term Fellowship and a Sir Henry Wellcome Fellowship. A.E.A. is supported by a NIH Pathway to Independence Award K99 HD 085188 and a NARSAD Young Investigator Award. Some of the work described in the text was supported by the Simons Foundation and NIH grant 1R01HD082131-01A1 to C.D. C.D. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- AVPe
anteroventral periventricular nucleus
- MPOA
medial preoptic area
- OXT(R)
oxytocin (receptor)
- PRL
prolactin
References
- 1.Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- 3.Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–70. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockley P, Hobson L. Paternal care and litter size coevolution in mammals. Proc Biol Sci. 2016:283. doi: 10.1098/rspb.2016.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol. 1987;101:169–77. [PubMed] [Google Scholar]
- 6.Fernandez-Duque E, Valeggia CR, Mendoza SP. The Biology of Paternal Care in Human and Nonhuman Primates. Annu Rev Anthropol. 2009;38:115–30. [Google Scholar]
- 7.Small MF. Alloparental behaviour in Barbary macaques, Macaca sylvanus. Animal Behaviour. 1990;39:297–306. [Google Scholar]
- 8.Creel S, Creel NM. Communal hunting and pack size in African wild dogs, Lycaon pictus. Animal Behaviour. 1995;50:1325–39. [Google Scholar]
- 9.Clinton HR. It takes a village : and other lessons children teach us. New York: Simon & Schuster; 1996. [Google Scholar]
- 10.Hrdy SB. Mothers and others : the evolutionary origins of mutual understanding. Cambridge, Mass: Belknap Press of Harvard University Press; 2009. [Google Scholar]
- 11.Lukas D, Huchard E. Sexual conflict. The evolution of infanticide by males in mammalian societies. Science. 2014;346:841–4. doi: 10.1126/science.1257226. [DOI] [PubMed] [Google Scholar]
- 12.Hausfater G, Hrdy SB. Infanticide : comparative and evolutionary perspectives. New York: Aldine Pub. Co; 1984. [Google Scholar]
- 13.Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–86. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 14.Francis CM, Anthony ELP, Brunton JA, Kunz TH. Lactation in male fruit bats. Nature (London) 1994;367:691–2. [Google Scholar]
- 15.Jones JS, Wynne-Edwards KE. Paternal hamsters mechanically assist the delivery, consume amniotic fluid and placenta, remove fetal membranes, and provide parental care during the birth process. Horm Behav. 2000;37:116–25. doi: 10.1006/hbeh.1999.1563. [DOI] [PubMed] [Google Scholar]
- 16.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–31. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 17.Rosenblatt JS, Ceus K. Estrogen implants in the medial preoptic area stimulate maternal behavior in male rats. Horm Behav. 1998;33:23–30. doi: 10.1006/hbeh.1997.1430. [DOI] [PubMed] [Google Scholar]
- 18.Rosenblatt JS, Olufowobi A, Siegel HI. Effects of pregnancy hormones on maternal responsiveness, responsiveness to estrogen stimulation of maternal behavior, and the lordosis response to estrogen stimulation. Horm Behav. 1998;33:104–14. doi: 10.1006/hbeh.1998.1441. [DOI] [PubMed] [Google Scholar]
- 19.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–42. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 20.Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–42. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- 21.Beach FA, Jaynes J. Studies of maternal retrieving in rats. III. Sensory clues involved in the lactating female’s response to her young. Behaviour. 1956;10:104–25. [Google Scholar]
- 22.Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989;45:1033–41. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 23.Corodimas KP, Rosenblatt JS, Canfield ME, Morrell JI. Neurons in the lateral subdivision of the habenular complex mediate the hormonal onset of maternal behavior in rats. Behav Neurosci. 1993;107:827–43. doi: 10.1037//0735-7044.107.5.827. [DOI] [PubMed] [Google Scholar]
- 24.Barofsky AL, Taylor J, Tizabi Y, Kumar R, et al. Specific neurotoxin lesions of median raphe serotonergic neurons disrupt maternal behavior in the lactating rat. Endocrinology. 1983;113:1884–93. doi: 10.1210/endo-113-5-1884. [DOI] [PubMed] [Google Scholar]
- 25.Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–54. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–68. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Jennes L, Conn PM. Gonadotropin-releasing hormone and its receptors in rat brain. Front Neuroendocrinol. 1994;15:51–77. doi: 10.1006/frne.1994.1003. [DOI] [PubMed] [Google Scholar]
- 28.Hammond MA, Rowe FA. Medial preoptic and anterior hypothalamic lesions: influences on aggressive behavior in female hamsters. Physiol Behav. 1976;17:507–13. doi: 10.1016/0031-9384(76)90115-3. [DOI] [PubMed] [Google Scholar]
- 29.McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol. 2010;109:27–33. doi: 10.1007/s00421-009-1295-z. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, et al. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–30. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525:519–22. doi: 10.1038/nature15378. [DOI] [PubMed] [Google Scholar]
- 32.Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav. 1970;5:1373–7. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- 33.Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977;91:146–64. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA. 1979;76:6661–5. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy F, Kendrick KM, Keverne EB, Piketty V, et al. Intracerebral oxytocin is important for the onset of maternal behavior in inexperienced ewes delivered under peridural anesthesia. Behav Neurosci. 1992;106:427–32. doi: 10.1037//0735-7044.106.2.427. [DOI] [PubMed] [Google Scholar]
- 36.Marlin BJ, Mitre M, D’Amour JA, Chao MV, et al. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–50. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 38.Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–54. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- 39.Nishimori K, Young LJ, Guo Q, Wang Z, et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93:11699–704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young WS, 3rd, Shepard E, Amico J, Hennighausen L, et al. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J Neuroendocrinol. 1996;8:847–53. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–81. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 42.Macbeth AH, Stepp JE, Lee HJ, Young WS, 3rd, et al. Normal maternal behavior, but increased pup mortality, in conditional oxytocin receptor knockout females. Behav Neurosci. 2010;124:677–85. doi: 10.1037/a0020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rich ME, deCardenas EJ, Lee HJ, Caldwell HK. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS One. 2014;9:e98839. doi: 10.1371/journal.pone.0098839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bridges RS, Numan M, Ronsheim PM, Mann PE, et al. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87:8003–7. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–35. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ormandy CJ, Camus A, Barra J, Damotte D, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–78. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 47.Lucas BK, Ormandy CJ, Binart N, Bridges RS, et al. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–7. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- 48.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37:1485–92. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Insel TR. Translating Oxytocin Neuroscience to the Clinic: A National Institute of Mental Health Perspective. Biol Psychiatry. 2016;79:153–4. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Hrdy SB. Male-male competition and infanticide among the langurs (Presbytis entellus) of Abu, Rajasthan. Folia Primatol (Basel) 1974;22:19–58. doi: 10.1159/000155616. [DOI] [PubMed] [Google Scholar]
- 51.Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–10. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- 52.Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm Behav. 2016;77:249–59. doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.vom Saal FS. Time-contingent change in infanticide and parental behavior induced by ejaculation in male mice. Physiol Behav. 1985;34:7–15. doi: 10.1016/0031-9384(85)90069-1. [DOI] [PubMed] [Google Scholar]
- 54.vom Saal FS, Howard LS. The regulation of infanticide and parental behavior: implications for reproductive success in male mice. Science. 1982;215:1270–2. doi: 10.1126/science.7058349. [DOI] [PubMed] [Google Scholar]
- 55.Hrdy SB. Infanticide as a primate reproductive strategy. Am Sci. 1977;65:40–9. [PubMed] [Google Scholar]
- 56.Drixler FF. Mabiki : infanticide and population growth in eastern Japan, 1660–1950. Berkeley: University of California Press; 2013. [Google Scholar]
- 57.Hrdy SB. Mother nature : a history of mothers, infants, and natural selection. New York: Pantheon Books; 1999. [DOI] [PubMed] [Google Scholar]
- 58.Opie C, Atkinson QD, Dunbar RI, Shultz S. Male infanticide leads to social monogamy in primates. Proc Natl Acad Sci USA. 2013;110:13328–32. doi: 10.1073/pnas.1307903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klug H, Bonsall MB. When to care for, abandon, or eat your offspring: the evolution of parental care and filial cannibalism. Am Nat. 2007;170:886–901. doi: 10.1086/522936. [DOI] [PubMed] [Google Scholar]
- 60.Mennella JA, Moltz H. Infanticide in the male rat: the role of the vomeronasal organ. Physiol Behav. 1988;42:303–6. doi: 10.1016/0031-9384(88)90087-x. [DOI] [PubMed] [Google Scholar]
- 61.Peters LC, Kristal MB. Suppression of infanticide in mother rats. J Comp Psychol. 1983;97:167–77. [PubMed] [Google Scholar]
- 62.McCarthy MM, vom Saal FS. The influence of reproductive state on infanticide by wild female house mice (Mus musculus) Physiol Behav. 1985;35:843–9. doi: 10.1016/0031-9384(85)90248-3. [DOI] [PubMed] [Google Scholar]
- 63.Perrigo G, Bryant WC, vom Saal FS. Fetal, hormonal and experiential factors influencing the mating-induced regulation of infanticide in male house mice. Physiol Behav. 1989;46:121–8. doi: 10.1016/0031-9384(89)90244-8. [DOI] [PubMed] [Google Scholar]
- 64.Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35:163–76. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- 65.Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa S, Washburn TF, Taylor J, Lubahn DB, et al. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139:5058–69. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 67.Trainor BC, Bird IM, Alday NA, Schlinger BA, et al. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saltzman W, Ziegler TE. Functional significance of hormonal changes in mammalian fathers. J Neuroendocrinol. 2014;26:685–96. doi: 10.1111/jne.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider JS, Burgess C, Horton TH, Levine JE. Effects of progesterone on male-mediated infant-directed aggression. Behav Brain Res. 2009;199:340–4. doi: 10.1016/j.bbr.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brooks PL, Vella ET, Wynne-Edwards KE. Dopamine agonist treatment before and after the birth reduces prolactin concentration but does not impair paternal responsiveness in Djungarian hamsters, Phodopus campbelli. Horm Behav. 2005;47:358–66. doi: 10.1016/j.yhbeh.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Mennella JA, Moltz H. Infanticide in rats: male strategy and female counter-strategy. Physiol Behav. 1988;42:19–28. doi: 10.1016/0031-9384(88)90254-5. [DOI] [PubMed] [Google Scholar]
- 72.Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci. 2013;33:5120–6. doi: 10.1523/JNEUROSCI.2364-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Svare B, Kinsley CH, Mann MA, Broida J. Infanticide: accounting for genetic variation in mice. Physiol Behav. 1984;33:137–52. doi: 10.1016/0031-9384(84)90024-6. [DOI] [PubMed] [Google Scholar]
- 74.Svare B, Broida J. Genotypic influences on infanticide in mice: environmental, situational and experiential determinants. Physiol Behav. 1982;28:171–5. doi: 10.1016/0031-9384(82)90119-6. [DOI] [PubMed] [Google Scholar]
- 75.Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav. 1993;27:56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- 76.Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974;86:221–32. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- 77.Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J Comp Physiol Psychol. 1974;86:233–46. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- 78.Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–43. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 79.Sheehan T, Paul M, Amaral E, Numan MJ, et al. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–56. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- 80.Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. J Neuroendocrinol. 1999;11:259–66. doi: 10.1046/j.1365-2826.1999.00322.x. [DOI] [PubMed] [Google Scholar]
- 81.Tsuneoka Y, Tokita K, Yoshihara C, Amano T, et al. Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J. 2015;34:2652–70. doi: 10.15252/embj.201591942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Renier N, Adams EL, Kirst C, Wu Z, et al. Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell. 2016;165:1789–802. doi: 10.1016/j.cell.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin D, Boyle MP, Dollar P, Lee H, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–6. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoogland JL. Infanticide in prairie dogs: lactating females kill offspring of close kin. Science. 1985;230:1037–40. doi: 10.1126/science.230.4729.1037. [DOI] [PubMed] [Google Scholar]
- 85.Lawick Hv. Solo: the story of an African wild dog. Boston: Houghton-Mifflin; 1974. [Google Scholar]
- 86.Young AJ, Clutton-Brock T. Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol Lett. 2006;2:385–7. doi: 10.1098/rsbl.2006.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chalfin L, Dayan M, Levy DR, Austad SN, et al. Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nat Commun. 2014;5:4569. doi: 10.1038/ncomms5569. [DOI] [PubMed] [Google Scholar]
- 88.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 89.Bahr NI, Pryce CR, Dobeli M, Martin RD. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol Behav. 1998;64:429–37. doi: 10.1016/s0031-9384(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 90.Maestripieri D, Lindell SG, Ayala A, Gold PW, et al. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci Biobehav Rev. 2005;29:51–7. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Bardi M, French JA, Ramirez SM, Brent L. The role of the endocrine system in baboon maternal behavior. Biol Psychiatry. 2004;55:724–32. doi: 10.1016/j.biopsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys (Callithrix jacchus) Psychoneuroendocrinology. 2009;34:1222–34. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Besser A, Priel B, Wiznitzer A. Childbearing depressive symptomatology in high-risk pregnancies: The roles of working models and social support. Pers Relationship. 2002;9:395–413. [Google Scholar]
- 94.Maestripieri D. Emotions, stress, and maternal motivation in primates. Am J Primatol. 2011;73:516–29. doi: 10.1002/ajp.20882. [DOI] [PubMed] [Google Scholar]
- 95.Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- 96.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–4. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(00)80949-6. page following 696. [DOI] [PubMed] [Google Scholar]
- 99.de Lecea L, Kilduff TS, Peyron C, Gao X, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin L, Faraco J, Li R, Kadotani H, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 101.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 103.Betley JN, Xu S, Cao ZF, Gong R, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–5. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–90. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 106.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–14. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 107.Zilkha N, Sofer Y, Beny Y, Kimchi T. From classic ethology to modern neuroethology: overcoming the three biases in social behavior research. Curr Opin Neurobiol. 2016;38:96–108. doi: 10.1016/j.conb.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 108.Joel D, Berman Z, Tavor I, Wexler N, et al. Sex beyond the genitalia: The human brain mosaic. Proc Natl Acad Sci USA. 2015;112:15468–73. doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Godwin J, Crews D, Warner RR. Behavioural sex change in the absence of gonads in a coral reef fish. Proc Biol Sci. 1996;263:1683–8. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- 110.Dias BG, Crews D. Regulation of pseudosexual behavior in the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Endocrinology. 2008;149:4622–31. doi: 10.1210/en.2008-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bick J, Zhu T, Stamoulis C, Fox NA, et al. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr. 2015;169:211–9. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nelson CA, Fox NA, Zeanah CH. Romania’s abandoned children : deprivation, brain development, and the struggle for recovery. Cambridge, Massachusetts: Harvard University Press; 2014. [Google Scholar]
- 113.Pryce CR. Socialization, hormones, and the regulation of maternal behavior in nonhuman simian Primates. Advances in the Study of Behavior. 1996;25:423–73. [Google Scholar]
- 114.Wisner KL, Sit DK, McShea MC, Rizzo DM, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–8. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller ES, Chu C, Gollan J, Gossett DR. Obsessive-compulsive symptoms during the postpartum period. A prospective cohort. J Reprod Med. 2013;58:115–22. [PMC free article] [PubMed] [Google Scholar]
- 116.Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303:1961–9. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- 117.Edmondson OJ, Psychogiou L, Vlachos H, Netsi E, et al. Depression in fathers in the postnatal period: assessment of the Edinburgh Postnatal Depression Scale as a screening measure. J Affect Disord. 2010;125:365–8. doi: 10.1016/j.jad.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 119.Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64:311–9. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–73. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- 121.Pedersen CA, Caldwell JD, Walker C, Ayers G, et al. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–71. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 122.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 123.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, et al. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 124.Silva MR, Bernardi MM, Cruz-Casallas PE, Felicio LF. Pimozide injections into the Nucleus accumbens disrupt maternal behaviour in lactating rats. Pharmacol Toxicol. 2003;93:42–7. doi: 10.1034/j.1600-0773.2003.930106.x. [DOI] [PubMed] [Google Scholar]
- 125.Lee G, Gammie SC. GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behav Neurosci. 2009;123:1169–77. doi: 10.1037/a0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D’Anna KL, Gammie SC. Activation of corticotropin-releasing factor receptor 2 in lateral septum negatively regulates maternal defense. Behav Neurosci. 2009;123:356–68. doi: 10.1037/a0014987. [DOI] [PubMed] [Google Scholar]
- 127.D’Anna KL, Stevenson SA, Gammie SC. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci. 2005;119:1061–71. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- 128.Mann PE, Kinsley CH, Bridges RS. Opioid receptor subtype involvement in maternal behavior in lactating rats. Neuroendocrinology. 1991;53:487–92. doi: 10.1159/000125762. [DOI] [PubMed] [Google Scholar]
- 129.Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13:753–8. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- 130.Li L, Keverne EB, Aparicio SA, Ishino F, et al. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284:330–3. doi: 10.1126/science.284.5412.330. [DOI] [PubMed] [Google Scholar]
- 131.Lefebvre L, Viville S, Barton SC, Ishino F, et al. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–9. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 132.Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–13. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gammie SC, Hasen NS, Stevenson SA, Bale TL, et al. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behav Brain Res. 2005;160:169–77. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 134.Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–92. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- 135.Brown JR, Ye H, Bronson RT, Dikkes P, et al. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]